Influence of PHA Substrate Surface Characteristics on the Functional State of Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PHA Film Substrates
2.2. Analysis of Surface
2.3. Endothelial Cell Culture and Immunocytochemistry
2.4. Statistics
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shanmugam, T.; Chokkalingam, L.; Bakthavachalam, P. (Eds.) Trends in Development of Medical Devices, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128209608. [Google Scholar]
- Sastri, V.R. Plastics in Medical Devices, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 9780323851268. [Google Scholar]
- McKavanagh, P.; Zawadowski, G.; Ahmed, N.; Kutryk, M. The Evolution of Coronary Stents. Expert Rev. Cardiovasc. Ther. 2018, 16, 219–228. [Google Scholar] [CrossRef] [PubMed]
- de Mel, A.; Cousins, B.G.; Seifalian, A.M. Surface Modification of Biomaterials: A Quest for Blood Compatibility. Int. J. Biomater. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Gaudière, F.; Masson, I.; Morin-Grognet, S.; Thoumire, O.; Vannier, J.-P.; Atmani, H.; Ladam, G.; Labat, B. Mechano-Chemical Control of Cell Behaviour by Elastomer Templates Coated with Biomimetic Layer-by-Layer Nanofilms. Soft Matter 2012, 8, 8327. [Google Scholar] [CrossRef]
- Al-Barqawi, M.O.; Church, B.; Thevamaran, M.; Thoma, D.J.; Rahman, A. Design and Validation of Additively Manufactured Metallic Cellular Scaffold Structures for Bone Tissue Engineering. Materials 2022, 15, 3310. [Google Scholar] [CrossRef]
- Prokhorov, E.; Bárcenas, G.L.; España Sánchez, B.L.; Franco, B.; Padilla-Vaca, F.; Hernández Landaverde, M.A.; Yáñez Limón, J.M.; López, R.A. Chitosan-BaTiO3 Nanostructured Piezopolymer for Tissue Engineering. Colloids Surf. B Biointerfaces 2020, 196, 111296. [Google Scholar] [CrossRef]
- Augustine, A.; Augustine, R.; Hasan, A.; Raghuveeran, V.; Rouxel, D.; Kalarikkal, N.; Thomas, S. Development of Titanium Dioxide Nanowire Incorporated Poly(Vinylidene Fluoride–Trifluoroethylene) Scaffolds for Bone Tissue Engineering Applications. J. Mater. Sci. Mater. Med. 2019, 30, 96. [Google Scholar] [CrossRef] [PubMed]
- le Clainche, T.; Linklater, D.; Wong, S.; Le, P.; Juodkazis, S.; le Guével, X.; Coll, J.-L.; Ivanova, E.P.; Martel-Frachet, V. Mechano-Bactericidal Titanium Surfaces for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2020, 12, 48272–48283. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Lantada, A.D.; Mager, D.; Korvink, J.G. Carbon-Based Materials for Articular Tissue Engineering: From Innovative Scaffolding Materials toward Engineered Living Carbon. Adv. Healthc. Mater. 2022, 11, 2101834. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Araújo, J.C.; Fangueiro, R.; Ferreira, D.P. Wetspun Polymeric Fibrous Systems as Potential Scaffolds for Tendon and Ligament Repair, Healing and Regeneration. Pharmaceutics 2022, 14, 2526. [Google Scholar] [CrossRef]
- Demina, T.S.; Kuryanova, A.S.; Aksenova, N.A.; Shubnyy, A.G.; Popyrina, T.N.; Sokovikov, Y.v.; Istranova, E.v.; Ivanov, P.L.; Timashev, P.S.; Akopova, T.A. Chitosan- g -Oligo/Polylactide Copolymer Non-Woven Fibrous Mats Containing Protein: From Solid-State Synthesis to Electrospinning. RSC Adv. 2019, 9, 37652–37659. [Google Scholar] [CrossRef]
- Fan, J.; Abedi-Dorcheh, K.; Sadat Vaziri, A.; Kazemi-Aghdam, F.; Rafieyan, S.; Sohrabinejad, M.; Ghorbani, M.; RastegarAdib, F.; Ghasemi, Z.; Klavins, K.; et al. A Review of Recent Advances in Natural Polymer-Based Scaffolds for Musculoskeletal Tissue Engineering. Polymers 2022, 14, 2097. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.S.; Silva, J.C.; Udangawa, R.N.; Cabral, J.M.S.; Ferreira, F.C.; da Silva, C.L.; Linhardt, R.J.; Vashishth, D. Co-Culture Cell-Derived Extracellular Matrix Loaded ElectrospunMicrofibrous Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C 2019, 99, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Fatimi, A.; Okoro, O.V.; Podstawczyk, D.; Siminska-Stanny, J.; Shavandi, A. Natural Hydrogel-Based Bio-Inks for 3D Bioprinting in Tissue Engineering: A Review. Gels 2022, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.H.; Das, P.; DiVito, M.D.; Ivancic, D.; Tan, L.P.; Wertheim, J.A. Nanofibrous PLGA Electrospun Scaffolds Modified with Type I Collagen Influence Hepatocyte Function and Support Viability in Vitro. Acta Biomater. 2018, 73, 217–227. [Google Scholar] [CrossRef]
- Singh, P.; Maparu, A.K.; Shah, S.; Rai, B.; Sivakumar, S. Biomimetic Algal Polysaccharide Coated 3D Nanofibrous Scaffolds Promote Skin Extracellular Matrix Formation. Mater. Sci. Eng. C 2021, 119, 111580. [Google Scholar] [CrossRef]
- Shih, Y.V.; Varghese, S. Tissue Engineered Bone Mi.imetics to Study Bone Disorders Ex Vivo: Role of Bioinspired Materials. Biomaterials 2019, 198, 107–121. [Google Scholar] [CrossRef]
- Formentín, P.; Catalán, Ú.; Pol, L.; Fernández-Castillejo, S.; Solà, R.; Marsal, L.F. Collagen and Fibronectin Surface Modification of Nanoporous Anodic Alumina and Macroporous Silicon for Endothelial Cell Cultures. J. Biol. Eng. 2018, 12, 21. [Google Scholar] [CrossRef]
- Rodriguez-Contreras, A.; Punset, M.; Calero, J.A.; Gil, F.J.; Ruperez, E.; Manero, J.M. Powder Metallurgy with Space Holder for Porous Titanium Implants: A Review. J. Mater. Sci. Technol. 2021, 76, 129–149. [Google Scholar] [CrossRef]
- Agarwal, P.; Zaidel-Bar, R. Mechanosensing in Embryogenesis. Curr. Opin. Cell Biol. 2021, 68, 1–9. [Google Scholar] [CrossRef]
- Duszyc, K.; Gomez, G.A.; Lagendijk, A.K.; Yau, M.-K.; Nanavati, B.N.; Gliddon, B.L.; Hall, T.E.; Verma, S.; Hogan, B.M.; Pitson, S.M.; et al. Mechanotransduction Activates RhoA in the Neighbors of Apoptotic Epithelial Cells to Engage Apical Extrusion. Curr. Biol. 2021, 31, 1326–1336. [Google Scholar] [CrossRef]
- Wang, B.; Ke, W.; Wang, K.; Li, G.; Ma, L.; Lu, S.; Xiang, Q.; Liao, Z.; Luo, R.; Song, Y.; et al. Mechanosensitive Ion Channel Piezo1 Activated by Matrix Stiffness Regulates Oxidative Stress-Induced Senescence and Apoptosis in Human Intervertebral Disc Degeneration. Oxid. Med. Cell Longev. 2021, 2021, 1–13. [Google Scholar] [CrossRef]
- Chi, S.; Cui, Y.; Wang, H.; Jiang, J.; Zhang, T.; Sun, S.; Zhou, Z.; Zhong, Y.; Xiao, B. Astrocytic Piezo1-Mediated Mechanotransduction Determines Adult Neurogenesis and Cognitive Functions. Neuron 2022, 110, 2984–2999.e8. [Google Scholar] [CrossRef]
- Zhou, T.; Gao, B.; Fan, Y.; Liu, Y.; Feng, S.; Cong, Q.; Zhang, X.; Zhou, Y.; Yadav, P.S.; Lin, J.; et al. Piezo1/2 Mediate Mechanotransduction Essential for Bone Formation through Concerted Activation of NFAT-YAP1-ß-Catenin. Elife 2020, 9, e52779. [Google Scholar] [CrossRef]
- Wang, S.; Englund, E.; Kjellman, P.; Li, Z.; Ahnlide, J.K.; Rodriguez-Cupello, C.; Saggioro, M.; Kanzaki, R.; Pietras, K.; Lindgren, D.; et al. CCM3 Is a Gatekeeper in Focal Adhesions Regulating Mechanotransduction and YAP/TAZ Signalling. Nat. Cell Biol. 2021, 23, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.K.; Patel, N.; Pagani, C.A.; Marini, S.; Padmanabhan, K.R.; Matera, D.L.; Said, M.; Hwang, C.; Hsu, G.C.-Y.; Poli, A.A.; et al. Immobilization after Injury Alters Extracellular Matrix and Stem Cell Fate. J. Clin. Investig. 2020, 130, 5444–5460. [Google Scholar] [CrossRef]
- Yoneda, M.; Suzuki, H.; Hatano, N.; Nakano, S.; Muraki, Y.; Miyazawa, K.; Goto, S.; Muraki, K. PIEZO1 and TRPV4, Which Are Distinct Mechano-Sensors in the Osteoblastic MC3T3-E1 Cells, Modify Cell-Proliferation. Int. J. Mol. Sci. 2019, 20, 4960. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Mo, H.; Liu, R.; Niu, Y.; Chen, T.; Xu, Q.; Tu, K.; Yang, N. Matrix Stiffness Modulates Hepatic Stellate Cell Activation into Tumor-Promoting Myofibroblasts via E2F3-Dependent Signaling and Regulates Malignant Progression. Cell Death Dis. 2021, 12, 1134. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Du, J.; Günther, S.; Guo, X.; Wang, S.; Schneider, A.; Zhu, L.; Braun, T. Mechano-Signaling via Piezo1 Prevents Activation and P53-Mediated Senescence of Muscle Stem Cells. Redox Biol. 2022, 52, 102309. [Google Scholar] [CrossRef]
- Song, X.; Sun, Z.; Chen, G.; Shang, P.; You, G.; Zhao, J.; Liu, S.; Han, D.; Zhou, H. Matrix Stiffening Induces Endothelial Dysfunction via the TRPV4/MicroRNA-6740/Endothelin-1 Mechanotransduction Pathway. Acta Biomater. 2019, 100, 52–60. [Google Scholar] [CrossRef]
- VanderBurgh, J.A.; Hotchkiss, H.; Potharazu, A.; Taufalele, P.v.; Reinhart-King, C.A. Substrate Stiffness Heterogeneities Disrupt Endothelial Barrier Integrity in a Micropillar Model of Heterogeneous Vascular Stiffening. Integr. Biol. 2018, 10, 734–746. [Google Scholar] [CrossRef]
- Woodcock, C.-S.C.; Hafeez, N.; Handen, A.; Tang, Y.; Harvey, L.D.; Estephan, L.E.; Speyer, G.; Kim, S.; Bertero, T.; Chan, S.Y. Matrix Stiffening Induces a Pathogenic QKI-MiR-7-SRSF1 Signaling Axis in Pulmonary Arterial Endothelial Cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2021, 320, L726–L738. [Google Scholar] [CrossRef] [PubMed]
- Kohn, J.C.; Zhou, D.W.; Bordeleau, F.; Zhou, A.L.; Mason, B.N.; Mitchell, M.J.; King, M.R.; Reinhart-King, C.A. Cooperative Effects of Matrix Stiffness and Fluid Shear Stress on Endothelial Cell Behavior. Biophys. J. 2015, 108, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Jia, F.; Huang, W.-P.; Li, X.; Hu, D.-F.; Wang, J.; Ren, K.-F.; Fu, G.-S.; Wang, Y.-B.; Ji, J. Substrate Stiffness Differentially Impacts Autophagy of Endothelial Cells and Smooth Muscle Cells. Bioact. Mater. 2021, 6, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Derricks, K.E.; Trinkaus-Randall, V.; Nugent, M.A. Extracellular Matrix Stiffness Modulates VEGF Calcium Signaling in Endothelial Cells: Individual Cell and Population Analysis. Integr. Biol. 2015, 7, 1011–1025. [Google Scholar] [CrossRef]
- Kim, S.; Lee, E.-Y.; Hillman, P.F.; Ko, J.; Yang, I.; Nam, S.-J. Chemical Structure and Biological Activities of Secondary Metabolites from Salicornia Europaea L. Molecules 2021, 26, 2252. [Google Scholar] [CrossRef]
- Lu, J.; Rao, M.P.; MacDonald, N.C.; Khang, D.; Webster, T.J. Improved Endothelial Cell Adhesion and Proliferation on Patterned Titanium Surfaces with Rationally Designed, Micrometer to Nanometer Features. Acta Biomater. 2008, 4, 192–201. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, M.; Yang, Z.; Luo, R.; Lu, X.; Huang, N.; Huang, P.; Leng, Y. Cooperative Control of Blood Compatibility and Re-Endothelialization by Immobilized Heparin and Substrate Topography. Acta Biomater. 2015, 15, 150–163. [Google Scholar] [CrossRef]
- Franco, D.; Klingauf, M.; Bednarzik, M.; Cecchini, M.; Kurtcuoglu, V.; Gobrecht, J.; Poulikakos, D.; Ferrari, A. Control of Initial Endothelial Spreading by Topographic Activation of Focal Adhesion Kinase. Soft Matter 2011, 7, 7313. [Google Scholar] [CrossRef]
- Potthoff, E.; Franco, D.; D’Alessandro, V.; Starck, C.; Falk, V.; Zambelli, T.; Vorholt, J.A.; Poulikakos, D.; Ferrari, A. Toward a Rational Design of Surface Textures Promoting Endothelialization. Nano Lett. 2014, 14, 1069–1079. [Google Scholar] [CrossRef]
- He, W.; Yong, T.; Teo, W.E.; Ma, Z.; Ramakrishna, S. Fabrication and Endothelialization of Collagen-Blended Biodegradable Polymer Nanofibers: Potential Vascular Graft for Blood Vessel Tissue Engineering. Tissue Eng. 2005, 11, 1574–1588. [Google Scholar] [CrossRef]
- Williams, S.F.; Martin, D.P. Applications of Polyhydroxyalkanoates (PHA) in Medicine and Pharmacy. In Biopolymers Online; Doi, Y., Steinbüchel, A., Eds.; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar]
- Zhang, J.; Shishatskaya, E.I.; Volova, T.G.; da Silva, L.F.; Chen, G.-Q. Polyhydroxyalkanoates (PHA) for Therapeutic Applications. Mater. Sci. Eng. C 2018, 86, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Jendrossek, D.; Schirmer, A.; Schlegel, H.G. Biodegradation of Polyhydroxyalkanoic Acids. Appl. Microbiol. Biotechnol. 1996, 46, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Gogolewski, S.; Jovanovic, M.; Perren, S.M.; Dillon, J.G.; Hughes, M.K. Tissue Response Andin Vivo Degradation of Selected Polyhydroxyacids: Polylactides (PLA), Poly(3-Hydroxybutyrate) (PHB), and Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) (PHB/VA). J. Biomed. Mater. Res. 1993, 27, 1135–1148. [Google Scholar] [CrossRef]
- Lipaikin, S.Y.; Yaremenko, I.A.; Terent’ev, A.O.; Volova, T.G.; Shishatskaya, E.I. Development of Biodegradable Delivery Systems Containing Novel 1,2,4-Trioxolane Based on Bacterial Polyhydroxyalkanoates. Adv. Polym. Technol. 2022, 1–14. [Google Scholar] [CrossRef]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The Chemomechanical Properties of Microbial Polyhydroxyalkanoates. Prog. Polym. Sci. 2013, 38, 536–583. [Google Scholar] [CrossRef]
- Volova, T.G.; Shishatskaya, E.; Sinskey, A.J. Degradable Polymers: Production, Properties, Applications. In Degradable Polymers: Production, Properties, Applications; Nova Science Pub Inc.: Hauppauge, NY, USA, 2013; pp. 1–380. [Google Scholar]
- Volova, T.; Kiselev, E.; Nemtsev, I.; Lukyanenko, A.; Sukovatyi, A.; Kuzmin, A.; Ryltseva, G.; Shishatskaya, E. Properties of Degradable Polyhydroxyalkanoates with Different Monomer Compositions. Int. J. Biol. Macromol. 2021, 182, 98–114. [Google Scholar] [CrossRef]
- Menzyanova, N.G.; Pyatina, S.A.; Nikolaeva, E.D.; Shabanov, A.v.; Nemtsev, I.v.; Stolyarov, D.P.; Dryganov, D.B.; Sakhnov, E.v.; Shishatskaya, E.I. Screening of Biopolymeric Materials for Cardiovascular Surgery Toxicity—Evaluation of Their Surface Relief with Assessment of Morphological Aspects of Monocyte/Macrophage Polarization in Atherosclerosis Patients. Toxicol. Rep. 2019, 6, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Chen, J.; Peng, Z.-X.; Chen, J.-N.; Liu, X.; Wu, F.; Zhang, P.; Chen, G.-Q. Enhanced Bone Regeneration via PHA Scaffolds Coated with Polydopamine-Captured BMP2. J. Mater. Chem. B 2022, 10, 6214–6227. [Google Scholar] [CrossRef]
- Basnett, P.; Ching, K.Y.; Stolz, M.; Knowles, J.C.; Boccaccini, A.R.; Smith, C.; Locke, I.C.; Keshavarz, T.; Roy, I. Novel Poly(3-Hydroxyoctanoate)/Poly(3-Hydroxybutyrate) Blends for Medical Applications. React. Funct. Polym. 2013, 73, 1340–1348. [Google Scholar] [CrossRef]
- Rao, U.; Sridhar, R.; Sehgal, P.K. Biosynthesis and Biocompatibility of Poly(3-Hydroxybutyrate-Co-4-Hydroxybutyrate) Produced by CupriavidusNecator from Spent Palm Oil. Biochem. Eng. J. 2010, 49, 13–20. [Google Scholar] [CrossRef]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef] [PubMed]
- Bhat, V.D.; Klitzman, B.; Koger, K.; Truskey, G.A.; Reichert, W.M. Improving Endothelial Cell Adhesion to Vascular Graft Surfaces: Clinical Need and Strategies. J. Biomater. Sci. Polym. Ed. 1998, 9, 1117–1135. [Google Scholar] [CrossRef]
- Volova, T.; Shishatskaya, E. Bacterial Strain VKPM B-10646—A Producer of Polyhydroxyalkanoates and a Method of Their Production). RF Patent No. 2439143, 10 January 2012. [Google Scholar]
- Kaelble, D.H. Dispersion-Polar Surface Tension Properties of Organic Solids. J. Adhes 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Rabel, W. Einige Aspekte der Benetzungstheorie und ihre Anwendung auf die Untersuchung und Veränderung der Oberflächene genschaften von Polymeren. Farbe Lack 1971, 77, 997–1005. [Google Scholar]
- Narayan, D.; Venkatraman, S.S. Effect of Pore Size and Interpore Distance on Endothelial Cell Growth on Polymers. J. Biomed. Mater. Res. A 2008, 87A, 710–718. [Google Scholar] [CrossRef]
- Allahyari, Z.; Gaborski, T.R. Engineering Cell–Substrate Interactions on Porous Membranes for Microphysiological Systems. Lab. Chip 2022, 22, 2080–2089. [Google Scholar] [CrossRef]
- Qu, X.-H.; Wu, Q.; Liang, J.; Qu, X.; Wang, S.-G.; Chen, G.-Q. Enhanced Vascular-Related Cellular Affinity on Surface Modified Copolyesters of 3-Hydroxybutyrate and 3-Hydroxyhexanoate (PHBHHx). Biomaterials 2005, 26, 6991–7001. [Google Scholar] [CrossRef]
- KENNEDY, S.; WASHBURN, N.; SIMONJR, C.; AMIS, E. Combinatorial Screen of the Effect of Surface Energy on Fibronectin-Mediated Osteoblast Adhesion, Spreading and Proliferation☆. Biomaterials 2006, 27, 3817–3824. [Google Scholar] [CrossRef]
- Hao, L.; Li, T.; Wang, L.; Shi, X.; Fan, Y.; Du, C.; Wang, Y. Mechanistic Insights into the Adsorption and Bioactivity of Fibronectin on Surfaces with Varying Chemistries by a Combination of Experimental Strategies and Molecular Simulations. Bioact. Mater. 2021, 6, 3125–3135. [Google Scholar] [CrossRef]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent Advance in Surface Modification for Regulating Cell Adhesion and Behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Wu, Q.; Chen, G.-Q. Reduced Mouse Fibroblast Cell Growth by Increased Hydrophilicity of Microbial Polyhydroxyalkanoates via Hyaluronan Coating. Biomaterials 2003, 24, 4621–4629. [Google Scholar] [CrossRef] [PubMed]
- Bumgardner, J.D.; Wiser, R.; Elder, S.H.; Jouett, R.; Yang, Y.; Ong, J.L. Contact Angle, Protein Adsorption and Osteoblast Precursor Cell Attachment to Chitosan Coatings Bonded to Titanium. J. Biomater. Sci. Polym. Ed. 2003, 14, 1401–1409. [Google Scholar] [CrossRef]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of Surface Energy and Roughness on Cell Adhesion and Growth—Facile Surface Modification for Enhanced Cell Culture. RSC Adv. 2021, 11, 15467–15476. [Google Scholar] [CrossRef]
- Dong, Y.; Li, P.; Chen, C.; Wang, Z.; Ma, P.; Chen, G.-Q. The Improvement of Fibroblast Growth on Hydrophobic Biopolyesters by Coating with Polyhydroxyalkanoate Granule Binding Protein PhaP Fused with Cell Adhesion Motif RGD. Biomaterials 2010, 31, 8921–8930. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.L. Isolation and Culture of Human Umbilical Vein Endothelial Cells (HUVEC). Curr. Protoc. Microbiol. 2007, 4, A-4B. [Google Scholar] [CrossRef]
- Marin, V.; Kaplanski, G.; Grès, S.; Farnarier, C.; Bongrand, P. Endothelial Cell Culture: Protocol to Obtain and Cultivate Human Umbilical Endothelial Cells. J. Immunol. Methods 2001, 254, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Nikolaeva, E.D.; Shishatskaya, E.I.; Mochalov, K.E.; Volova, T.G.; Sinsky, A.J. Comparative Investigation of Polyhydroxyalkanoate Scaffolds with Various Chemical Compositions. Cell Transpl. Tissue Eng. 2011, 6, 54–63. [Google Scholar]
- Nikolaeva, E.D.; Goncharov, D.B.; Shishatskaya, E.I. Effects of H2O2-plasma processing on properties of cellular scaffolds made of «Bioplastotan» resorbing polyesters. Cell Transpl. Tissue Eng. 2011, 6, 65–70. [Google Scholar]
- Surmenev, R.A.; Chernozem, R.V.; Syromotina, D.S.; Oehr, C.; Baumbach, T.; Krause, B.; Boyandin, A.N.; Dvoinina, L.M.; Volova, T.G.; Surmeneva, M.A. Low-Temperature Argon and Ammonia Plasma Treatment of Poly-3-Hydroxybutyrate Films: Surface Topography and Chemistry Changes Affect Fibroblast Cells in Vitro. Eur. Polym. J. 2019, 112, 137–145. [Google Scholar] [CrossRef]
- Volova, T.G.; Golubev, A.I.; Nemtsev, I.V.; Lukyanenko, A.V.; Dudaev, A.E.; Shishatskaya, E.I. Laser Processing of Polymer Films Fabricated from Phas Differing in Their Monomer Composition. Polymers 2021, 13, 1553. [Google Scholar] [CrossRef] [PubMed]
- Grigorieva, O.A.; Vigovskiy, M.A.; Dyachkova, U.D.; Basalova, N.A.; Aleksandrushkina, N.A.; Kulebyakina, M.A.; Zaitsev, I.L.; Popov, V.S.; Efimenko, A.Y. Mechanisms of Endothelial-to-Mesenchymal Transition Induction by Extracellular Matrix Components in Pulmonary Fibrosis. Bull. Exp. Biol. Med. 2021, 171, 523–531. [Google Scholar] [CrossRef]
- Casillo, S.M.; Peredo, A.P.; Perry, S.J.; Chung, H.H.; Gaborski, T.R. Membrane Pore Spacing Can Modulate Endothelial Cell–Substrate and Cell–Cell Interactions. ACS Biomater. Sci. Eng. 2017, 3, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Allahyari, Z.; Gholizadeh, S.; Chung, H.H.; Delgadillo, L.F.; Gaborski, T.R. Micropatterned Poly(Ethylene Glycol) Islands Disrupt Endothelial Cell–Substrate Interactions Differently from Microporous Membranes. ACS Biomater. Sci. Eng. 2020, 6, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Pompe, T.; Keller, K.; Mothes, G.; Nitschke, M.; Teese, M.; Zimmermann, R.; Werner, C. Surface Modification of Poly(Hydroxybutyrate) Films to Control Cell–Matrix Adhesion. Biomaterials 2007, 28, 28–37. [Google Scholar] [CrossRef]
- Qu, X.-H.; Wu, Q.; Chen, G.-Q. In Vitro Study on Hemocompatibility and Cytocompatibility of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate). J. Biomater. Sci. Polym. Ed. 2006, 17, 1107–1121. [Google Scholar] [CrossRef]
- Lee, S.J.; Choi, J.S.; Park, K.S.; Khang, G.; Lee, Y.M.; Lee, H.B. Response of MG63 Osteoblast-like Cells onto Polycarbonate Membrane Surfaces with Different Micropore Sizes. Biomaterials 2004, 25, 4699–4707. [Google Scholar] [CrossRef] [PubMed]
- bin Anwar Fadzil, A.F.; Pramanik, A.; Basak, A.K.; Prakash, C.; Shankar, S. Role of Surface Quality on Biocompatibility of Implants—A Review. Ann. 3D Print. Med. 2022, 8, 100082. [Google Scholar] [CrossRef]
- Brevier, J.; Montero, D.; Svitkina, T.; Riveline, D. The Asymmetric Self-Assembly Mechanism of Adherens Junctions: A Cellular Push–Pull Unit. Phys. Biol. 2008, 5, 016005. [Google Scholar] [CrossRef]
- Barry, A.K.; Wang, N.; Leckband, D.E. Local VE-Cadherin Mechanotransduction Triggers Long-Ranged Remodeling of Endothelial Monolayers. J. Cell Sci. 2015, 128, 1341–1351. [Google Scholar] [CrossRef]
- Gavard, J. Endothelial Permeability and VE-Cadherin. Cell Adh. Migr. 2013, 7, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Birukova, A.A.; Shah, A.S.; Tian, Y.; Gawlak, G.; Sarich, N.; Birukov, K.G. Selective Role of Vinculin in Contractile Mechanisms of Endothelial Permeability. Am. J. Respir. Cell Mol. Biol. 2016, 55, 476–486. [Google Scholar] [CrossRef]
- Gulino-Debrac, D. Mechanotransduction at the Basis of Endothelial Barrier Function. Tissue Barriers 2013, 1, e24180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huveneers, S.; Oldenburg, J.; Spanjaard, E.; van der Krogt, G.; Grigoriev, I.; Akhmanova, A.; Rehmann, H.; de Rooij, J. Vinculin Associates with Endothelial VE-Cadherin Junctions to Control Force-Dependent Remodeling. J. Cell Biol. 2012, 196, 641–652. [Google Scholar] [CrossRef] [PubMed]
- van der Stoel, M.M.; Kotini, M.P.; Schoon, R.M.; Affolter, M.; Belting, H.-G.; Huveneers, S. Vinculin Strengthens the Endothelial Barrier during Vascular Development. Vasc. Biol. 2022, 5, e220012. [Google Scholar] [CrossRef]

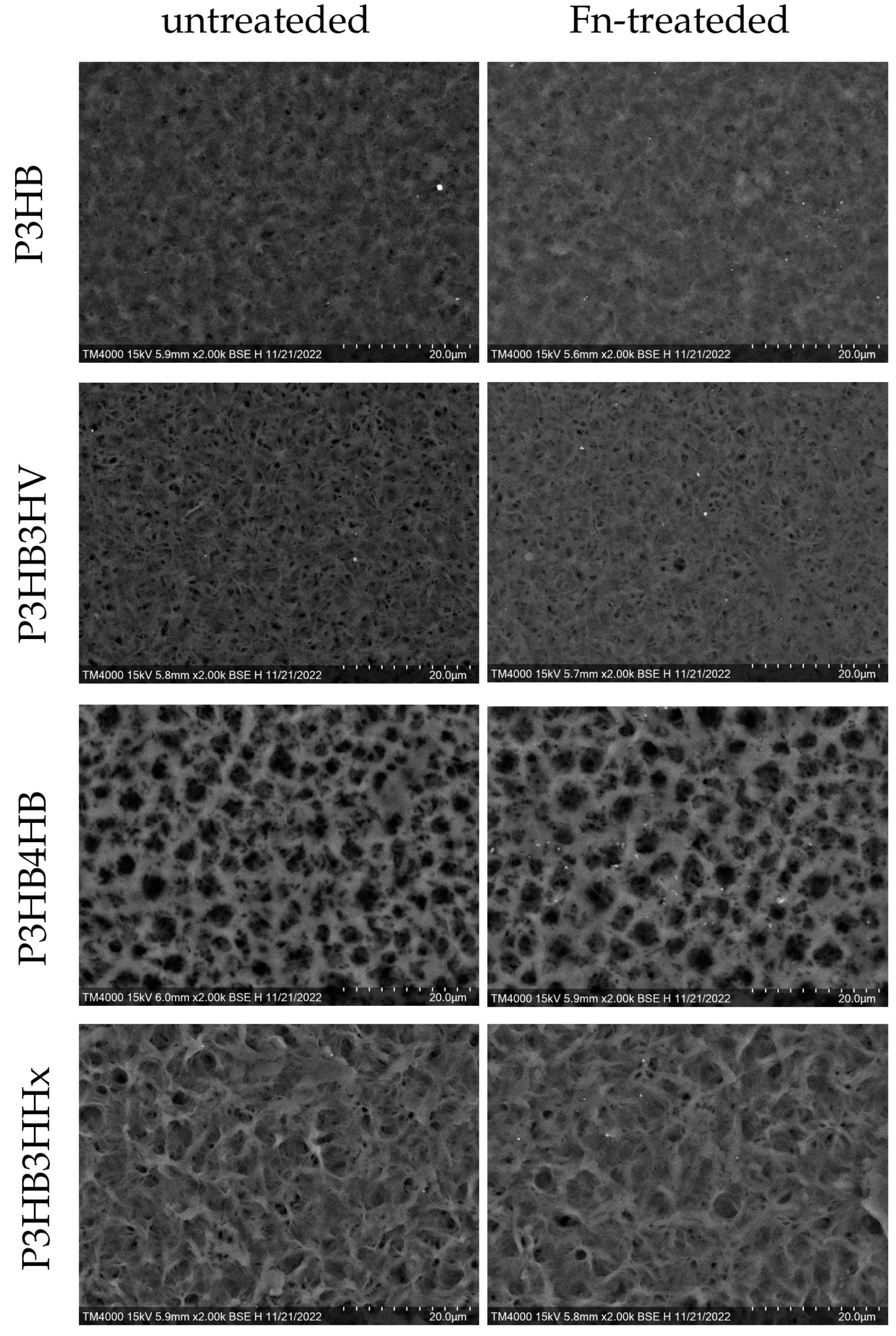
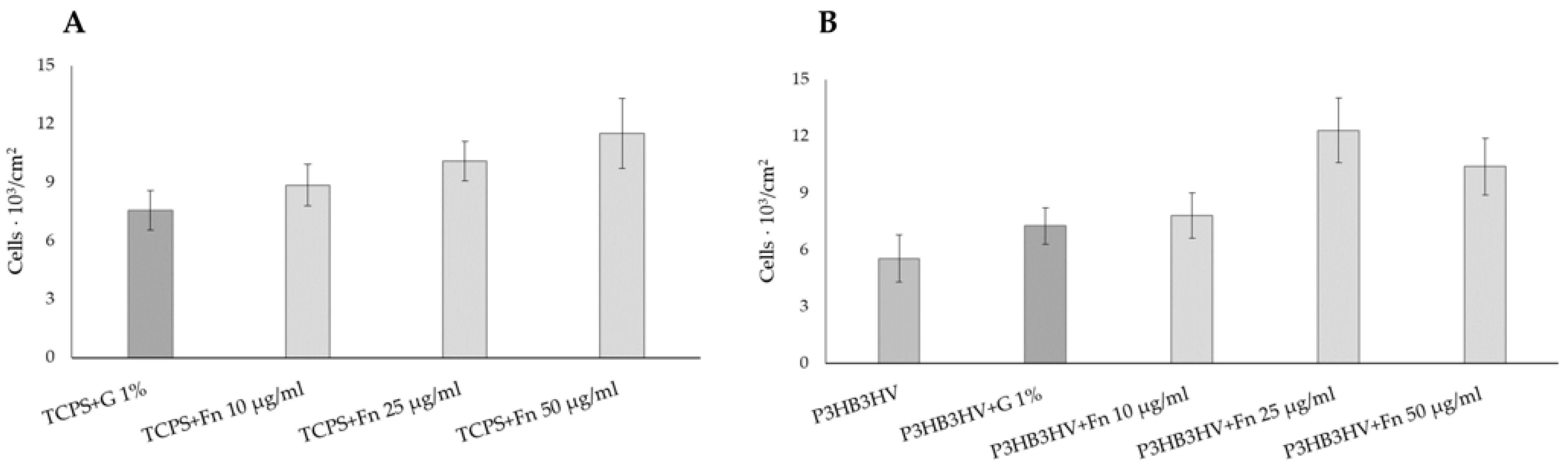

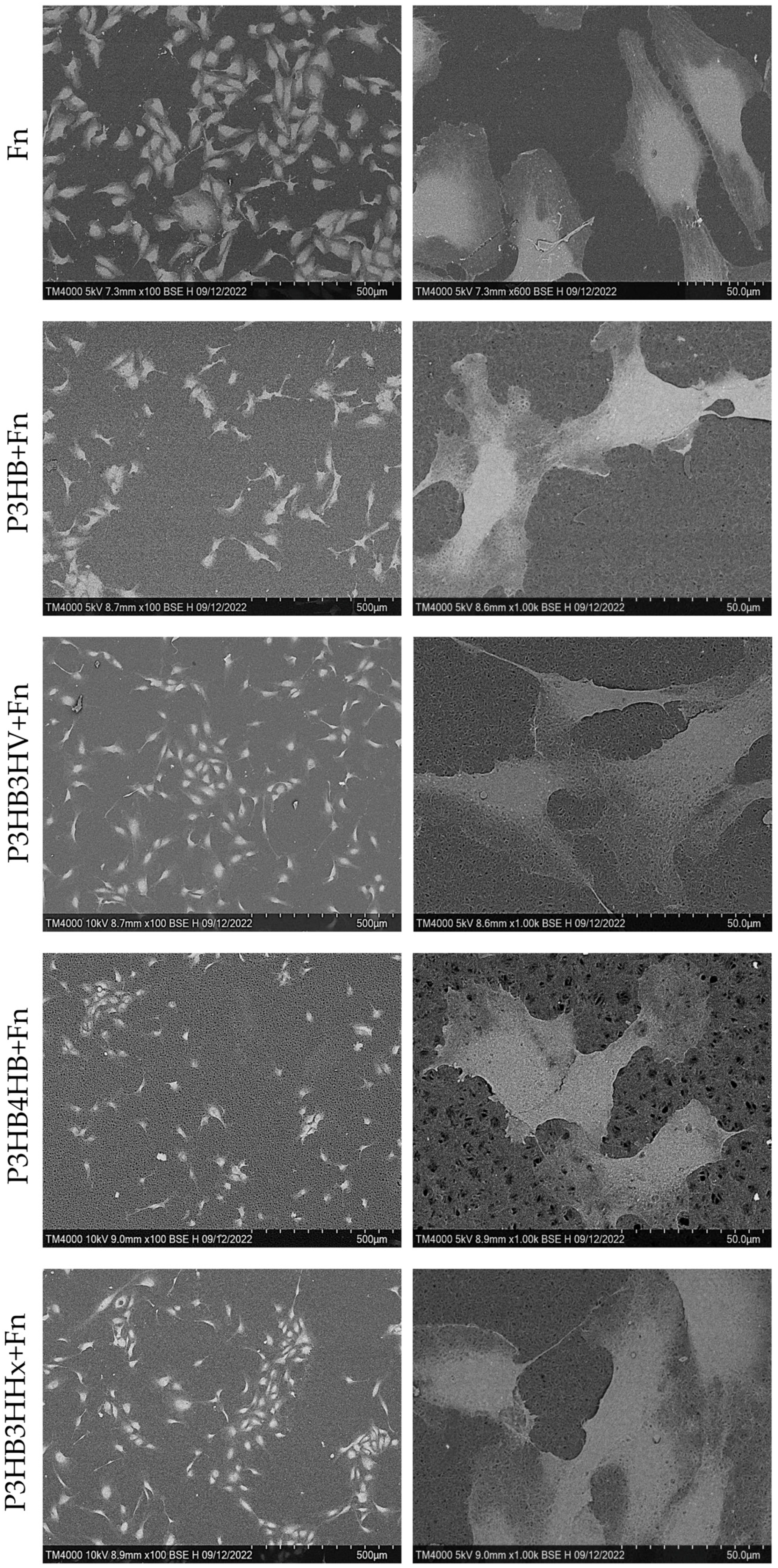

| Samples | Average Molecular Weight (Mw) kDa | Polydispersity (Ð) | Degree of Crystallinity (Cx) % |
|---|---|---|---|
| P3HB | 920 | 2.5 | 78 |
| P3HB3HV | 690 | 2.8 | 65 |
| P3HB4HB | 660 | 3.6 | 22 |
| P3HB3HHx | 486 | 3.7 | 52 |
| Samples | Root Mean Square Roughness (Sq), nm | Arithmetic Mean Surface Roughness (Sa), nm | Peak-to-Valley Height (Sz), nm |
|---|---|---|---|
| P3HB | 180, 26 | 142, 83 | 1255, 77 |
| P3HB3HV | 254, 24 | 206, 92 | 1594, 61 |
| P3HB4HB | 370, 60 | 290, 31 | 2321, 24 |
| P3HB3HHx | 222, 69 | 172, 62 | 1677, 55 |
| Parameters | P3HB | P3HB3HV | P3HB4HB | P3HB3HHx |
|---|---|---|---|---|
| Untreated | ||||
| area, % | 1.519 | 8.686 | 17.470 | 1.248 |
| average size, µm | 0.052 ± 0.032 | 0.056 ± 0.029 | 0.272 ± 0.255 | 0.081 ± 0.070 |
| Fn-treated | ||||
| area, % | 0.785 | 5.920 | 12.364 | 0.756 |
| average size, µm | 0.035 ± 0.010 | 0.047 ± 0.028 | 0.236 ± 0.200 | 0.064 ± 0.047 |
| Parameters | P3HB | P3HB3HV | P3HB4HB | P3HB3HHx |
|---|---|---|---|---|
| Untreated | ||||
| Water contact angle (°) | 94.5 ± 3.7 | 85.3 ± 1.7 | 72.2 ± 7.2 | 83.2 ± 3.2 |
| Surface energy (mN/m) | 35.6 ± 0.3 | 38.6 ± 0.5 | 46.2 ± 1.2 | 36.7 ± 0.5 |
| Dispersion component (mN/m) | 34.7 ± 0.2 | 35.7 ± 0.4 | 39.2 ± 0.7 | 32.5 ± 0.4 |
| Polar component (mN/m) | 0.9 ± 0.1 | 2.9 ± 0.1 | 7.0 ± 0.5 | 4.3 ± 0.2 |
| Fn-treated | ||||
| Water contact angle (°) | 90.7 ± 2.8 | 78.9 ± 4.2 | 77.0 ± 5.4 | 78.5 ± 4.7 |
| Surface energy (mN/m) | 36.5 ± 0.6 | 39.6 ± 0.7 | 43.7 ± 1.6 | 38.8 ± 0.6 |
| Dispersion component (mN/m) | 34.9 ± 0.5 | 34.2 ± 0.5 | 38.4 ± 1.2 | 32.8 ± 0.3 |
| Polar component (mN/m) | 1.6 ± 0.1 | 5.5 ± 0.2 | 5.2 ± 0.4 | 6.0 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryltseva, G.A.; Dudaev, A.E.; Menzyanova, N.G.; Volova, T.G.; Alexandrushkina, N.A.; Efimenko, A.Y.; Shishatskaya, E.I. Influence of PHA Substrate Surface Characteristics on the Functional State of Endothelial Cells. J. Funct. Biomater. 2023, 14, 85. https://doi.org/10.3390/jfb14020085
Ryltseva GA, Dudaev AE, Menzyanova NG, Volova TG, Alexandrushkina NA, Efimenko AY, Shishatskaya EI. Influence of PHA Substrate Surface Characteristics on the Functional State of Endothelial Cells. Journal of Functional Biomaterials. 2023; 14(2):85. https://doi.org/10.3390/jfb14020085
Chicago/Turabian StyleRyltseva, Galina A., Alexey E. Dudaev, Natalia G. Menzyanova, Tatiana G. Volova, Natalia A. Alexandrushkina, Anastasia Yu. Efimenko, and Ekaterina I. Shishatskaya. 2023. "Influence of PHA Substrate Surface Characteristics on the Functional State of Endothelial Cells" Journal of Functional Biomaterials 14, no. 2: 85. https://doi.org/10.3390/jfb14020085
APA StyleRyltseva, G. A., Dudaev, A. E., Menzyanova, N. G., Volova, T. G., Alexandrushkina, N. A., Efimenko, A. Y., & Shishatskaya, E. I. (2023). Influence of PHA Substrate Surface Characteristics on the Functional State of Endothelial Cells. Journal of Functional Biomaterials, 14(2), 85. https://doi.org/10.3390/jfb14020085









