Surface Modified β-Ti-18Mo-6Nb-5Ta (wt%) Alloy for Bone Implant Applications: Composite Characterization and Cytocompatibility Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Characterization of Structural, Physical, and Physicochemical Properties
2.3. Cytocompatibility Studies
2.3.1. Cell Culture
2.3.2. Cell Proliferation and Cell Viability Analysis
2.3.3. Cell Morphology and Cell Adhesion Analysis
2.3.4. Statistical Analysis
3. Results and Discussion
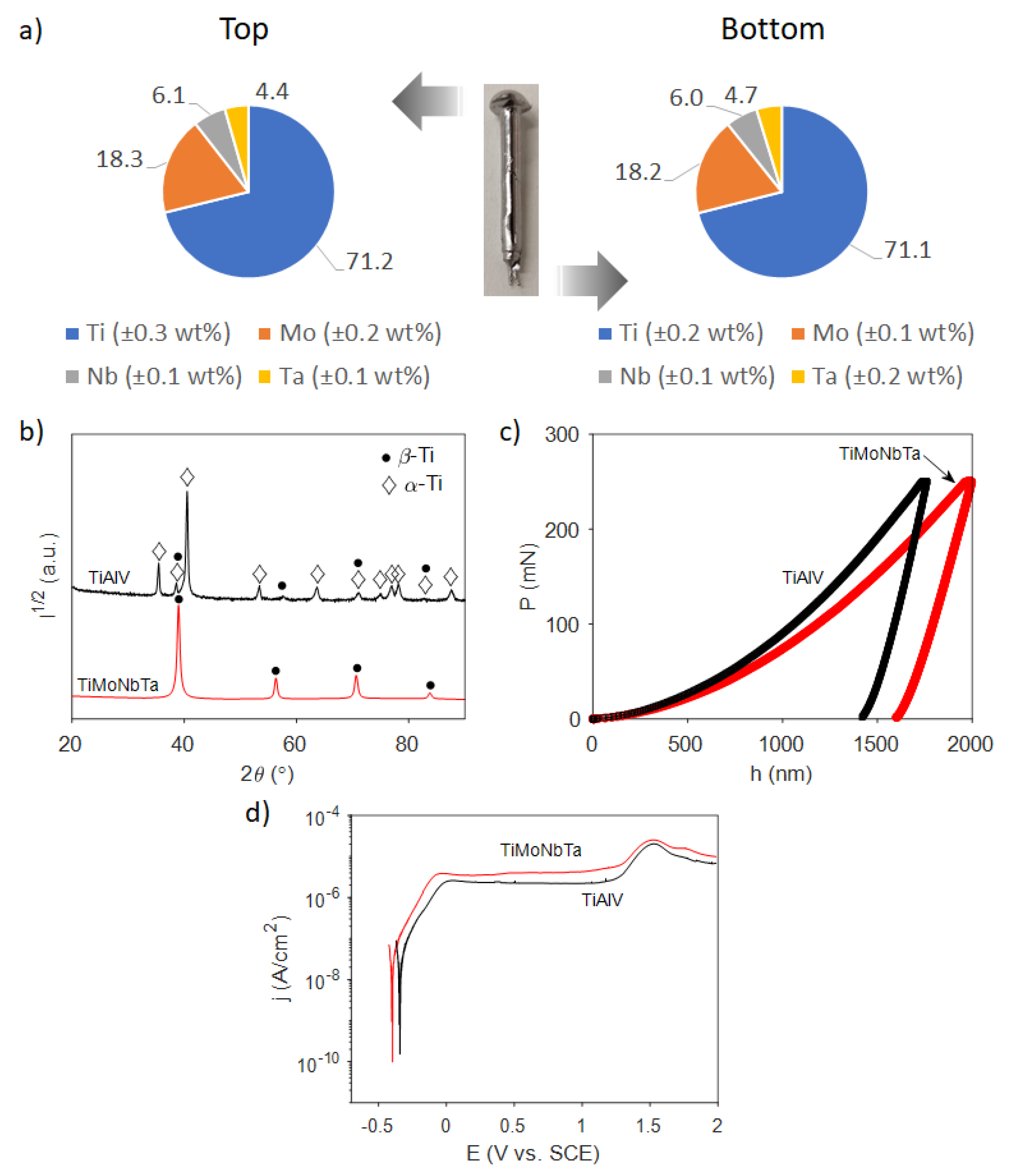
3.1. Fabrication and Characterization of the Ti-18Mo-6Nb-5Ta Alloy
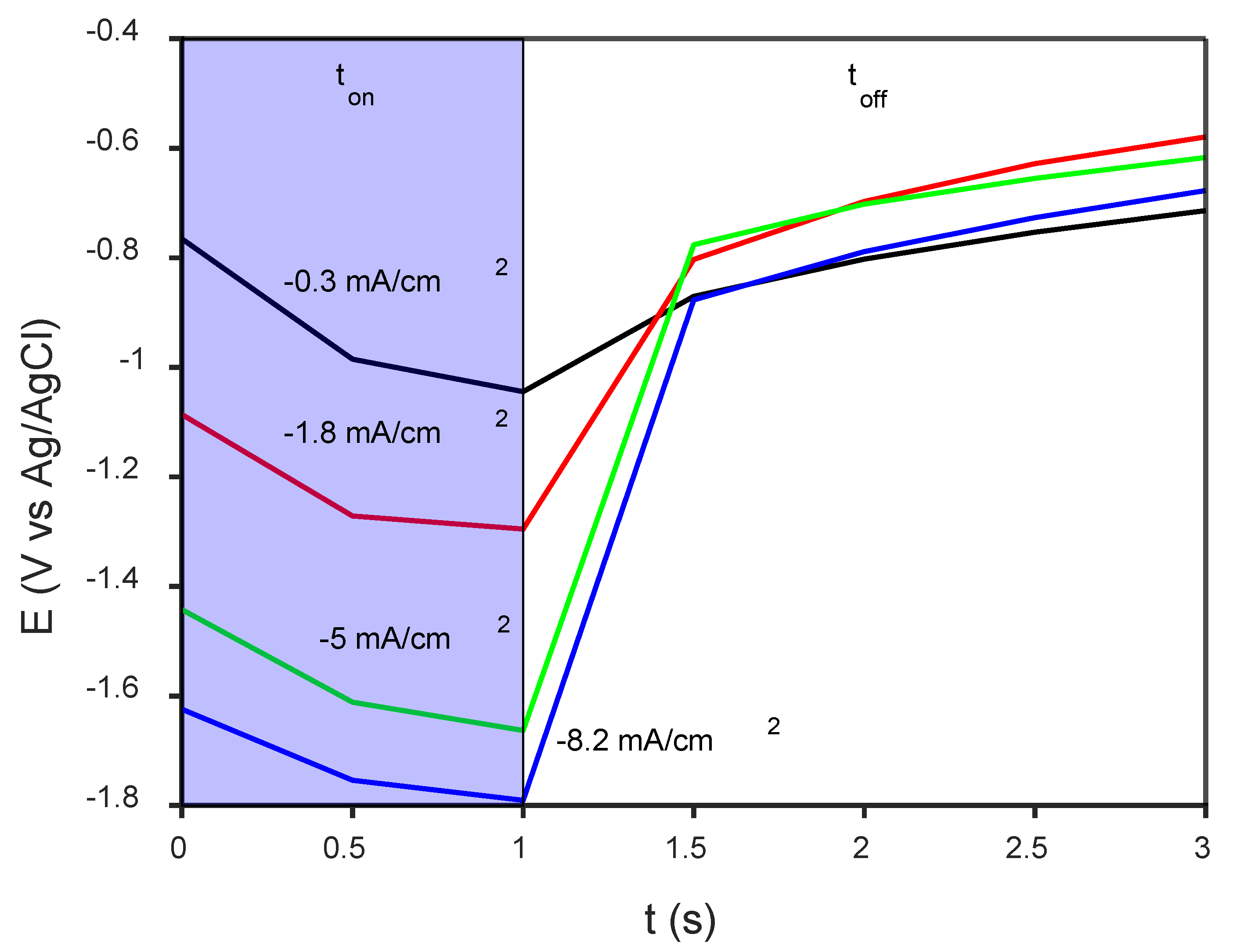
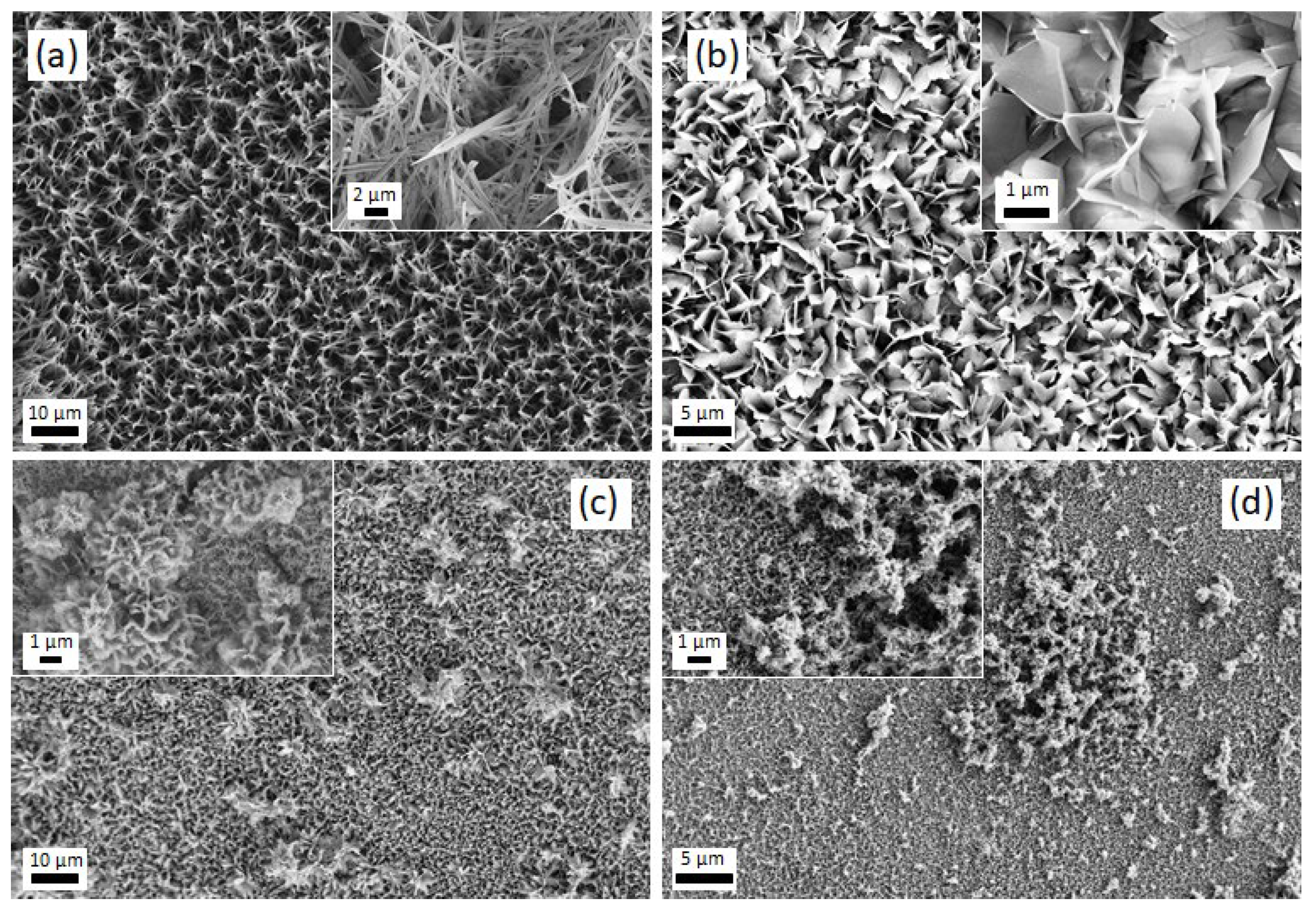
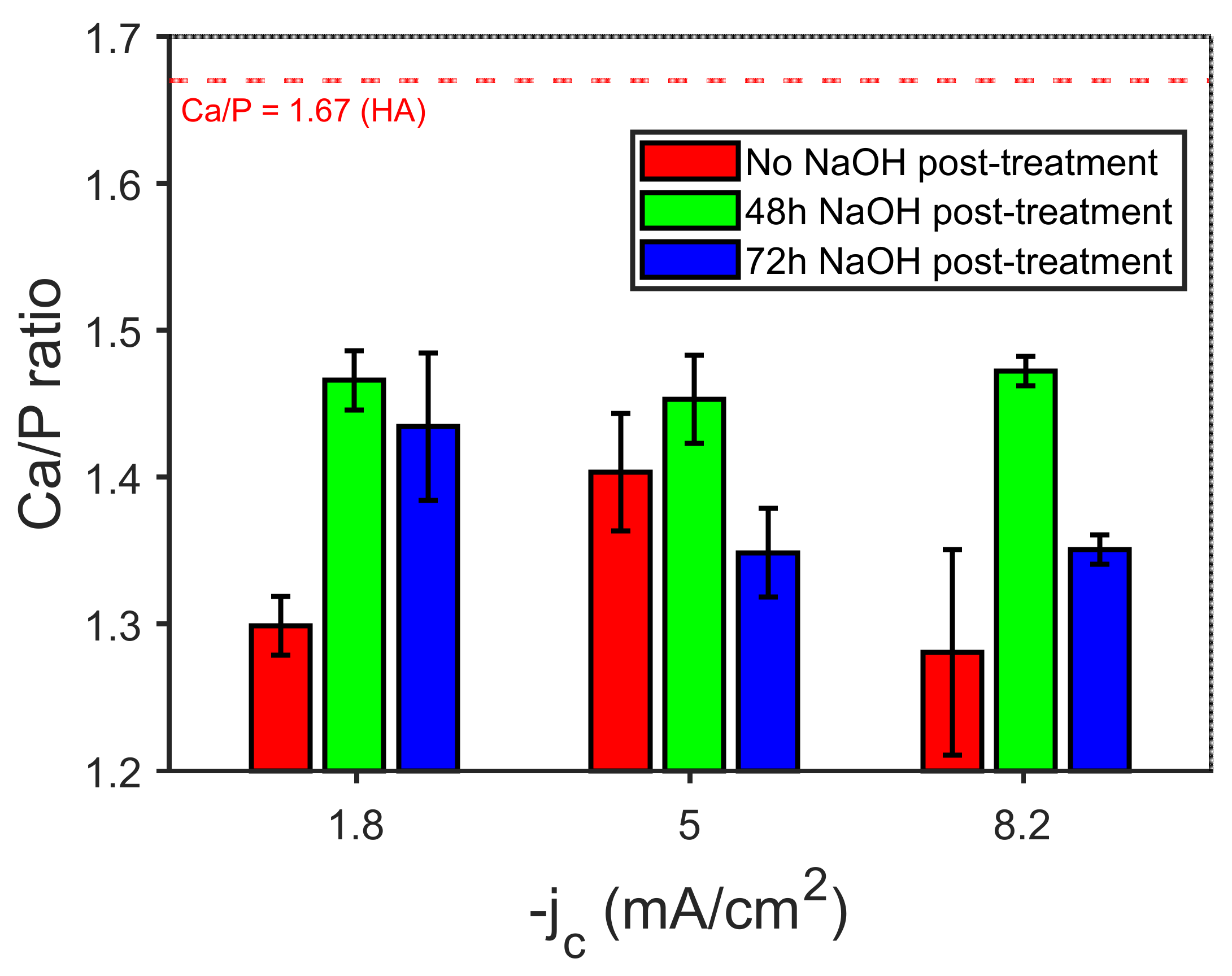
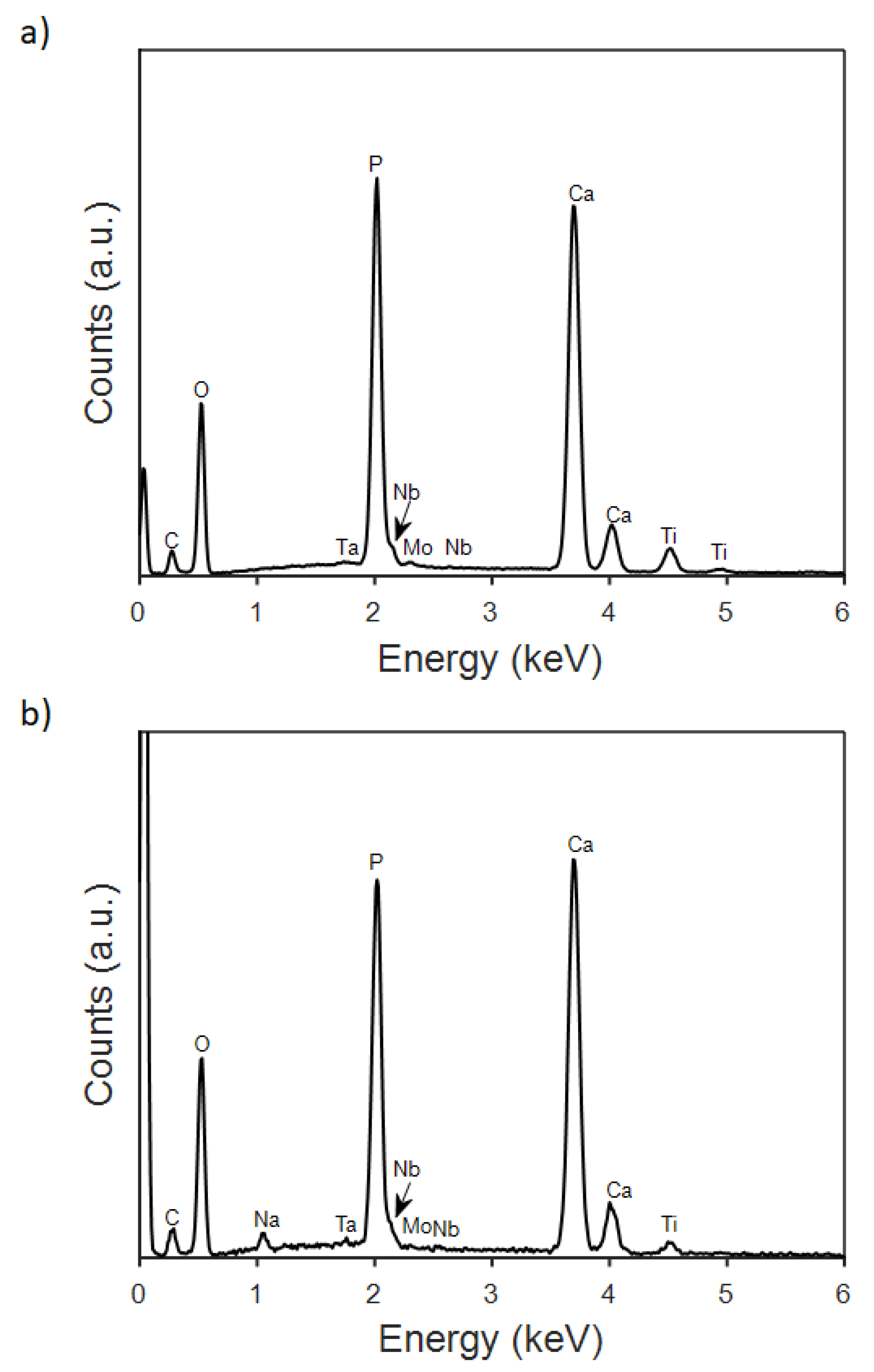
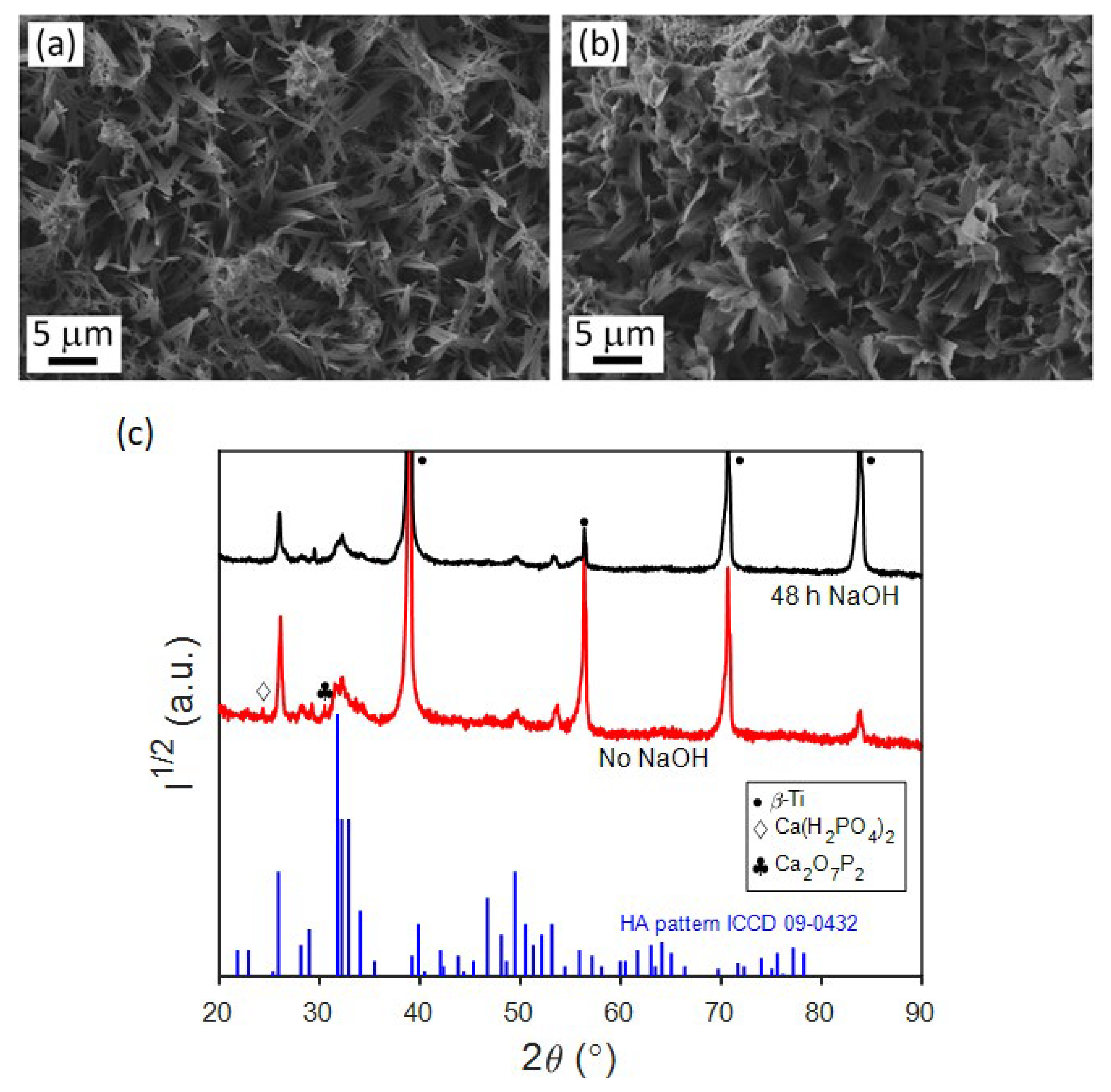
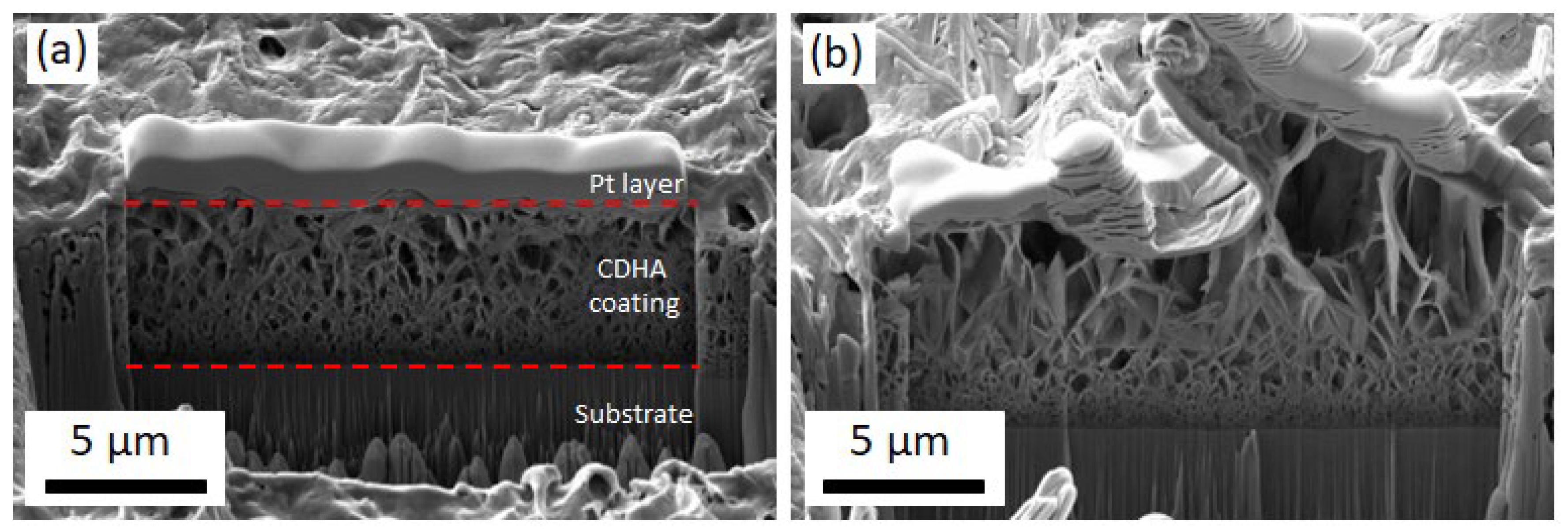
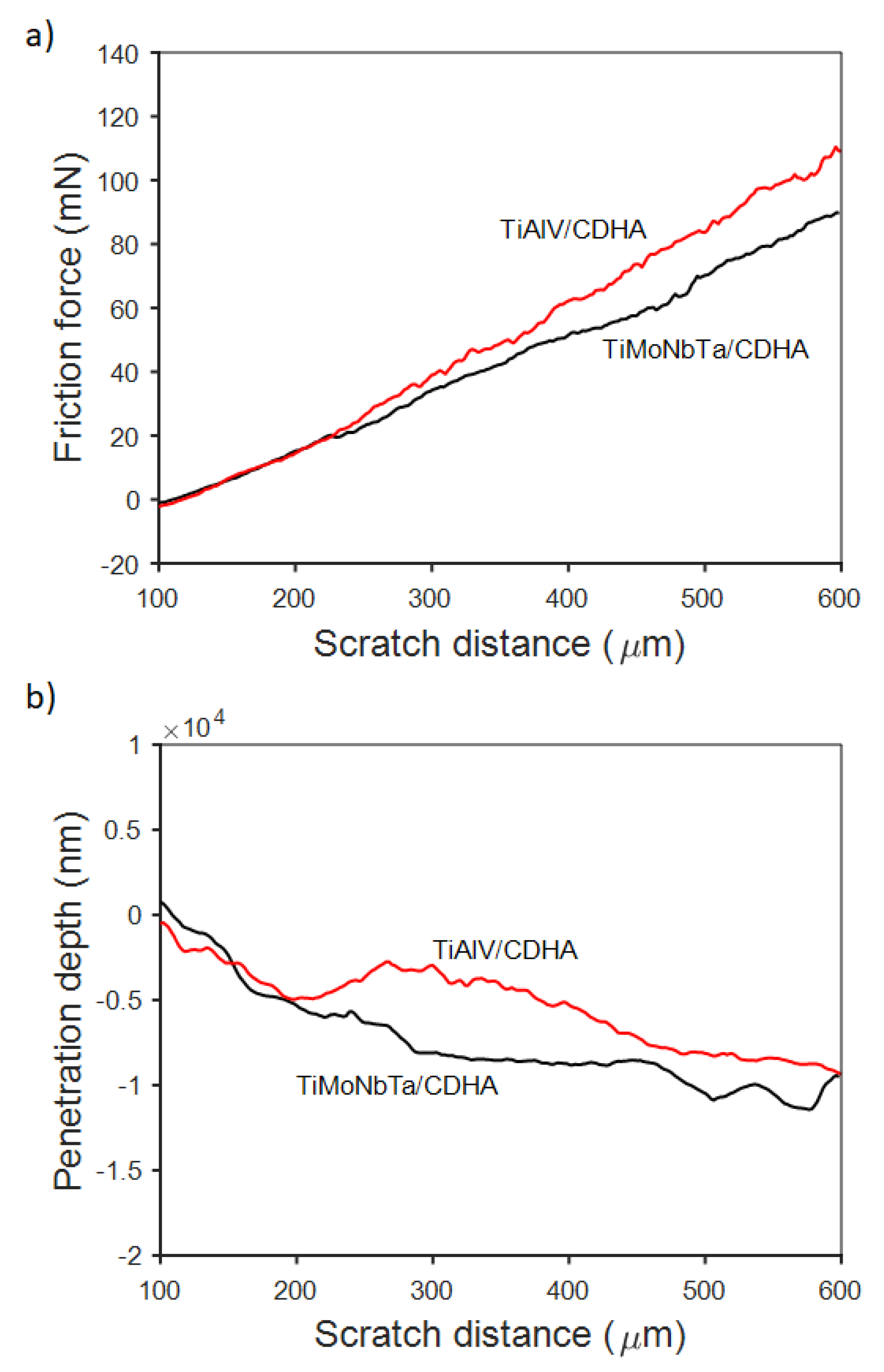
3.2. Electrodeposition of CaP Coatings and Their Characterization
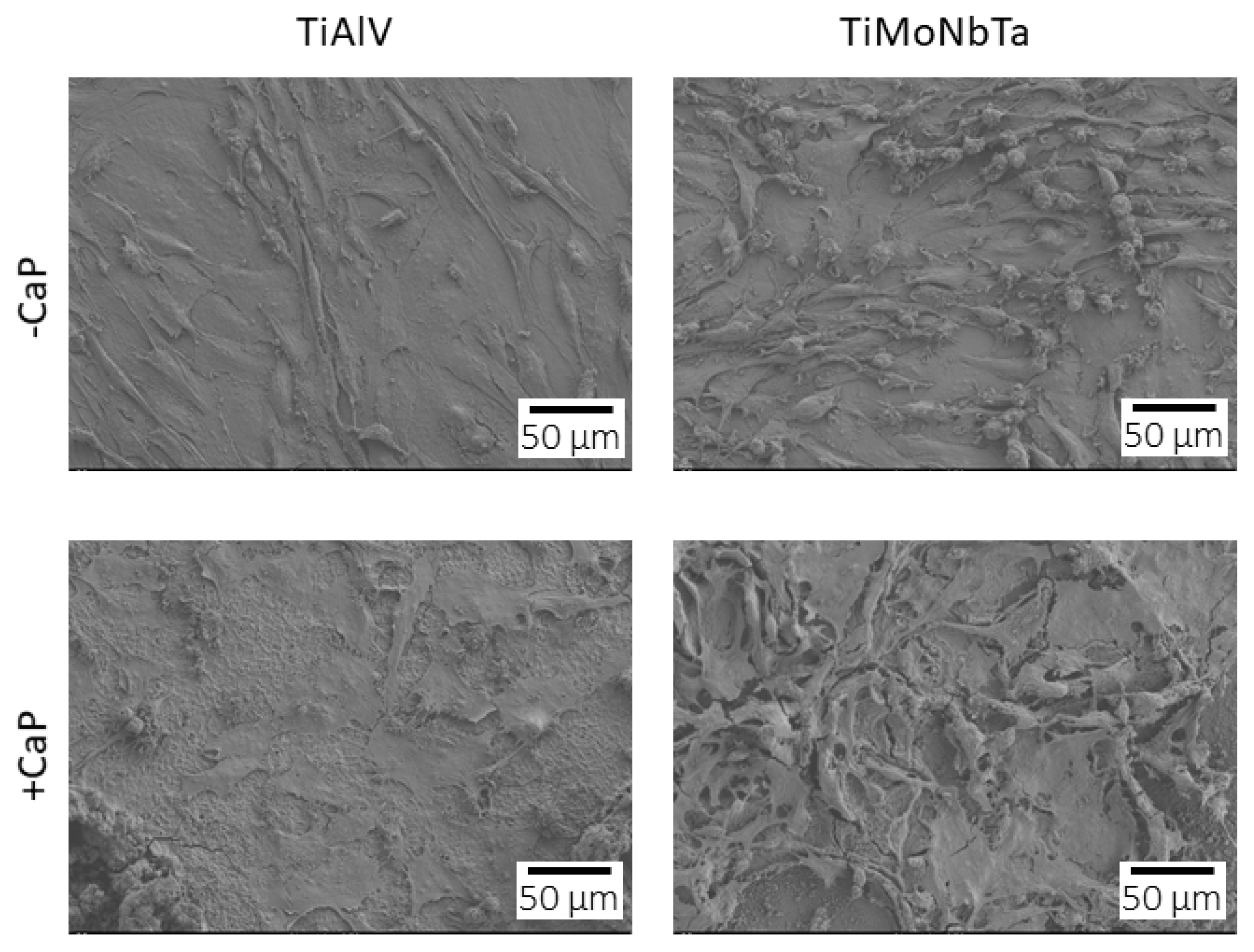
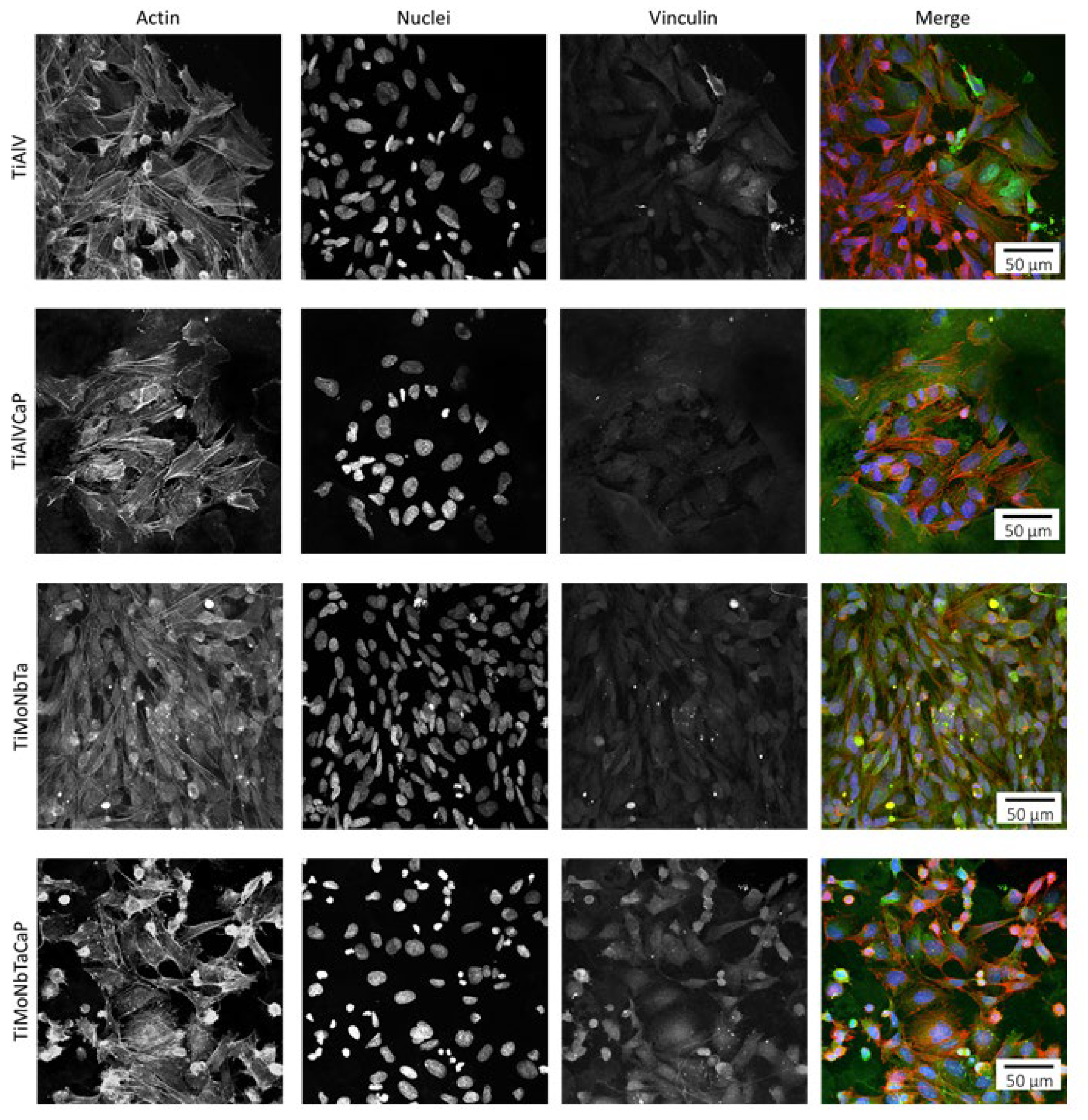
3.3. Cytocompatibility of CaP-Coated and Uncoated Alloys
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tuninetti, V.; Jaramillo, A.F.; Riu, G.; Rojas-Ulloa, C.; Znaidi, A.; Medina, C.; Mateo, A.M.; Roa, J.J. Experimental Correlation of Mechanical Properties of the Ti-6Al-4V Alloy at Different Length Scales. Metals 2021, 11, 104. [Google Scholar] [CrossRef]
- Zysset, P.K.; Edward Guo, X.; Edward Hoffler, C.; Moore, K.E.; Goldstein, S.A. Elastic Modulus and Hardness of Cortical and Trabecular Bone Lamellae Measured by Nanoindentation in the Human Femur. J. Biomech. 1999, 32, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Trincă, L.C.; Mareci, D.; Solcan, C.; Fântânariu, M.; Burtan, L.; Hriţcu, L.; Chiruţă, C.; Fernández-Mérida, L.; Rodríguez-Raposo, R.; Santana, J.J.; et al. New Ti-6Al-2Nb-2Ta-1Mo Alloy as Implant Biomaterial: In Vitro Corrosion and in Vivo Osseointegration Evaluations. Mater. Chem. Phys. 2020, 240, 122229. [Google Scholar] [CrossRef]
- Verma, R.P. Titanium Based Biomaterial for Bone Implants: A Mini Review. Mater. Today Proc. 2020, 26, 3148–3151. [Google Scholar] [CrossRef]
- Kuroda, D.; Niinomi, M.; Morinaga, M.; Kato, Y.; Yashiro, T. Design and Mechanical Properties of New i Type Titanium Alloys for Implant Materials. Mater. Sci. Eng. A 1998, 243, 244–249. [Google Scholar] [CrossRef]
- Xu, D.; Wang, T.; Wang, S.; Jiang, Y.; Wang, Y.; Chen, Y.; Bi, Z.; Geng, S. Antibacterial Effect of the Controlled Nanoscale Precipitates Obtained by Different Heat Treatment Schemes with a Ti-Based Nanomaterial, Ti–7.5Mo–5Cu Alloy. ACS Appl. Bio Mater. 2020, 3, 6145–6154. [Google Scholar] [CrossRef]
- Guo, S.; Meng, Q.; Zhao, X.; Wei, Q.; Xu, H. Design and Fabrication of a Metastable β-Type Titanium Alloy with Ultralow Elastic Modulus and High Strength. Sci. Rep. 2015, 5, 14688. [Google Scholar] [CrossRef]
- Mohammed, M.T.; Khan, Z.A.; Siddiquee, A.N. Beta Titanium Alloys: The Lowest Elastic Modulus for Biomedical Applications: A Review. Int. J. Chem. Nucl. Metall. Mater. Eng. 2014, 8, 726–731. [Google Scholar]
- Kolli, R.; Devaraj, A. A Review of Metastable Beta Titanium Alloys. Metals 2018, 8, 506. [Google Scholar] [CrossRef]
- Pinotti, V.E.; Plaine, A.H.; Romero da Silva, M.; Bolfarini, C. Influence of Oxygen Addition and Aging on the Microstructure and Mechanical Properties of a β-Ti-29Nb–13Ta–4Mo Alloy. Mater. Sci. Eng. A 2021, 819, 141500. [Google Scholar] [CrossRef]
- Barceloux, D.G.; Barceloux, D. Molybdenum. J. Toxicol. Clin. Toxicol. 1999, 37, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, P.; Singh, S.B.; Chakraborty, M. Elastic Modulus of Biomedical Titanium Alloys by Nano-Indentation and Ultrasonic Techniques—A Comparative Study. Mater. Sci. Eng. A 2008, 489, 419–425. [Google Scholar] [CrossRef]
- Sarimov, R.M.; Glinushkin, A.P.; Sevostyanov, M.A.; Konushkin, S.V.; Serov, D.A.; Astashev, M.E.; Lednev, V.N.; Yanykin, D.V.; Sibirev, A.V.; Smirnov, A.A.; et al. Ti-20Nb-10Ta-5Zr Is Biosafe Alloy for Building of Ecofriendly Greenhouse Framework of New Generation. Metals 2022, 12, 2007. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Simakin, A.V.; Konushkin, S.V.; Ivannikov, A.Y.; Nasakina, E.O.; Shatova, L.A.; Kolmakov, A.G.; Sevostyanov, M.A. Preparation, Structural and Microstructural Characterization of Ti–30Nb–10Ta–5Zr Alloy for Biomedical Applications. J. Mater. Res. Technol. 2020, 9, 16018–16028. [Google Scholar] [CrossRef]
- Safavi, M.S.; Walsh, F.C.; Surmeneva, M.A.; Surmenev, R.A.; Khalil-Allafi, J. Electrodeposited Hydroxyapatite-Based Biocoatings: Recent Progress and Future Challenges. Coatings 2021, 11, 110. [Google Scholar] [CrossRef]
- Picard, Q.; Olivier, F.; Delpeux, S.; Chancolon, J.; Warmont, F.; Bonnamy, S. Development and Characterization of Biomimetic Carbonated Calcium-Deficient Hydroxyapatite Deposited on Carbon Fiber Scaffold. J. Carbon Res. 2018, 4, 25. [Google Scholar] [CrossRef]
- Dumelie, N.; Benhayoune, H.; Richard, D.; Laurent-Maquin, D.; Balossier, G. In Vitro Precipitation of Electrodeposited Calcium-Deficient Hydroxyapatite Coatings on Ti6Al4V Substrate. Mater. Charact. 2008, 59, 129–133. [Google Scholar] [CrossRef]
- Li, T.-T.; Ling, L.; Lin, M.-C.; Peng, H.-K.; Ren, H.-T.; Lou, C.-W.; Lin, J.-H. Recent Advances in Multifunctional Hydroxyapatite Coating by Electrochemical Deposition. J. Mater. Sci. 2020, 55, 6352–6374. [Google Scholar] [CrossRef]
- Schmidt, R.; Gebert, A.; Schumacher, M.; Hoffmann, V.; Voss, A.; Pilz, S.; Uhlemann, M.; Lode, A.; Gelinsky, M. Electrodeposition of Sr-Substituted Hydroxyapatite on Low Modulus Beta-Type Ti-45Nb and Effect on in Vitro Sr Release and Cell Response. Mater. Sci. Eng. C 2020, 108, 110425. [Google Scholar] [CrossRef]
- Beaufils, S.; Rouillon, T.; Millet, P.; Le Bideau, J.; Weiss, P.; Chopart, J.-P.; Daltin, A.-L. Synthesis of Calcium-Deficient Hydroxyapatite Nanowires and Nanotubes Performed by Template-Assisted Electrodeposition. Mater. Sci. Eng. C 2019, 98, 333–346. [Google Scholar] [CrossRef]
- Gecim, G.; Dönmez, S.; Erkoc, E. Calcium Deficient Hydroxyapatite by Precipitation: Continuous Process by Vortex Reactor and Semi-Batch Synthesis. Ceram. Int. 2021, 47, 1917–1928. [Google Scholar] [CrossRef]
- Wang, L.; Wang, M.; Li, M.; Shen, Z.; Wang, Y.; Shao, Y.; Zhu, Y. Trace Fluorine Substituted Calcium Deficient Hydroxyapatite with Excellent Osteoblastic Activity and Antibacterial Ability. CrystEngComm 2018, 20, 5744–5753. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, M. Characterization and Thermal Behavior of Calcium Deficient Hydroxyapatite Whiskers with Various Ca/P Ratios. Mater. Chem. Phys. 2011, 126, 642–648. [Google Scholar] [CrossRef]
- Drevet, R.; Benhayoune, H. Electrodeposition of Calcium Phosphate Coatings on Metallic Substrates for Bone Implant Applications: A Review. Coatings 2022, 12, 539. [Google Scholar] [CrossRef]
- Mokabber, T.; Lu, L.Q.; van Rijn, P.; Vakis, A.I.; Pei, Y.T. Crystal Growth Mechanism of Calcium Phosphate Coatings on Titanium by Electrochemical Deposition. Surf. Coat. Technol. 2018, 334, 526–535. [Google Scholar] [CrossRef]
- Fornell, J.; Feng, Y.P.; Pellicer, E.; Suriñach, S.; Baró, M.D.; Sort, J. Mechanical Behaviour of Brushite and Hydroxyapatite Coatings Electrodeposited on Newly Developed FeMnSiPd Alloys. J. Alloys Compd. 2017, 729, 231–239. [Google Scholar] [CrossRef]
- Vidal, E.; Buxadera-Palomero, J.; Pierre, C.; Manero, J.M.; Ginebra, M.-P.; Cazalbou, S.; Combes, C.; Rupérez, E.; Rodríguez, D. Single-Step Pulsed Electrodeposition of Calcium Phosphate Coatings on Titanium for Drug Delivery. Surf. Coat. Technol. 2019, 358, 266–275. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An Improved Technique for Determining Hardness and Elastic Modulus Using Load and Displacement Sensing Indentation Experiments. J. Mater. Res. 1992, 7, 20. [Google Scholar] [CrossRef]
- Hynowska, A.; Blanquer, A.; Pellicer, E.; Fornell, J.; Suriñach, S.; Baró, M.; González, S.; Ibáñez, E.; Barrios, L.; Nogués, C.; et al. Novel Ti–Zr–Hf–Fe Nanostructured Alloy for Biomedical Applications. Materials 2013, 6, 4930–4945. [Google Scholar] [CrossRef]
- Campos-Quirós, A.; Cubero-Sesín, J.M.; Edalati, K. Synthesis of Nanostructured Biomaterials by High-Pressure Torsion: Effect of Niobium Content on Microstructure and Mechanical Properties of Ti-Nb Alloys. Mater. Sci. Eng. A 2020, 795, 139972. [Google Scholar] [CrossRef]
- Leyland, A.; Matthews, A. On the Significance of the H/E Ratio in Wear Control: A Nanocomposite Coating Approach to Optimised Tribological Behaviour. Wear 2000, 246, 1–11. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti Based Biomaterials, the Ultimate Choice for Orthopaedic Implants—A Review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Eliaz, N.; Eliyahu, M. Electrochemical Processes of Nucleation and Growth of Hydroxyapatite on Titanium Supported by Real-Time Electrochemical Atomic Force Microscopy. J. Biomed. Mater. Res. 2007, 80A, 621–634. [Google Scholar] [CrossRef]
- Vladislavić, N.; Rončević, I.Š.; Buzuk, M.; Buljac, M.; Drventić, I. Electrochemical/Chemical Synthesis of Hydroxyapatite on Glassy Carbon Electrode for Electroanalytical Determination of Cysteine. J. Solid State Electrochem. 2021, 25, 841–857. [Google Scholar] [CrossRef]
- Falcone, R.; Sommariva, G.; Verità, M. WDXRF, EPMA and SEM/EDX Quantitative Chemical Analyses of Small Glass Samples. Microchim. Acta 2006, 155, 137–140. [Google Scholar] [CrossRef]
- Sensitivity/Detection Limit of EDS. Available online: https://www.globalsino.com/EM/page4792.html (accessed on 5 November 2022).
- Drevet, R.; Velard, F.; Potiron, S.; Laurent-Maquin, D.; Benhayoune, H. In Vitro Dissolution and Corrosion Study of Calcium Phosphate Coatings Elaborated by Pulsed Electrodeposition Current on Ti6Al4V Substrate. J. Mater. Sci. Mater. Med. 2011, 22, 753–761. [Google Scholar] [CrossRef]
- Victor, S.P.; Kumar, T.S.S. Tailoring Calcium-Deficient Hydroxyapatite Nanocarriers for Enhanced Release of Antibiotics. J. Biomed. Nanotechnol. 2008, 4, 203–209. [Google Scholar] [CrossRef]
- Rao, S.; Ushida, T.; Tateishi, T.; Asao, S. Effect of Ti, AI, and V Ions on the Relative Growth Rate of Fibroblasts (L929) and Osteoblasts (MC3T3-El) Cells. Biomed. Mater. Eng. 1996, 6, 79–86. [Google Scholar] [CrossRef]
- Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J.A. Biomaterials in Orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef]
- Cuijpers, V.M.J.I.; Alghamdi, H.S.; Van Dijk, N.W.M.; Jaroszewicz, J.; Walboomers, X.F.; Jansen, J.A. Osteogenesis Around CaP-Coated Titanium Implants Visualized Using 3D Histology and Micro-Computed Tomography. J. Biomed. Mater. Res. Part A 2015, 103, 3462–3473. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Surmeneva, M.A.; Ivanova, A.A. Significance of Calcium Phosphate Coatings for the Enhancement of New Bone Osteogenesis—A Review. Acta Biomater. 2014, 10, 557–579. [Google Scholar] [CrossRef] [PubMed]

| Bone [2] | Ti-18Mo-6Nb-5Ta | Ti-6Al-4V | |
|---|---|---|---|
| Er (GPa) | 11.4–21.2 | 79.0 ± 3.0 | 106.0 ± 1.0 |
| H (GPa) | 0.23–0.76 | 3.40 ± 0.10 | 4.20 ± 0.1 |
| H/Er | 0.02–0.036 | 0.044 ± 0.002 | 0.040 ± 0.001 |
| H3/Er2 (GPa) | 0.0001–0.001 | 0.0070 ± 0.0010 | 0.0068 ± 0.0005 |
| UEl/UTot | -- | 0.231 ± 0.010 | 0.233 ± 0.004 |
| UPl/UTot | -- | 0.77 ± 0.02 | 0.77 ± 0.01 |
| Material | a (Å) | c (Å) | Crystal Size (nm) | Microstrains |
|---|---|---|---|---|
| Ti-18Mo-6Nb-5Ta (β phase) | 3.2632 | -- | >200 | 5.3·10−4 |
| CDHA (hcp phase) | 9.2911 | 6.8540 | 16 | 11.8·10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobar, M.; Careta, O.; Fernández Navas, N.; Bartkowska, A.; Alberta, L.A.; Fornell, J.; Solsona, P.; Gemming, T.; Gebert, A.; Ibáñez, E.; et al. Surface Modified β-Ti-18Mo-6Nb-5Ta (wt%) Alloy for Bone Implant Applications: Composite Characterization and Cytocompatibility Assessment. J. Funct. Biomater. 2023, 14, 94. https://doi.org/10.3390/jfb14020094
Escobar M, Careta O, Fernández Navas N, Bartkowska A, Alberta LA, Fornell J, Solsona P, Gemming T, Gebert A, Ibáñez E, et al. Surface Modified β-Ti-18Mo-6Nb-5Ta (wt%) Alloy for Bone Implant Applications: Composite Characterization and Cytocompatibility Assessment. Journal of Functional Biomaterials. 2023; 14(2):94. https://doi.org/10.3390/jfb14020094
Chicago/Turabian StyleEscobar, Michael, Oriol Careta, Nora Fernández Navas, Aleksandra Bartkowska, Ludovico Andrea Alberta, Jordina Fornell, Pau Solsona, Thomas Gemming, Annett Gebert, Elena Ibáñez, and et al. 2023. "Surface Modified β-Ti-18Mo-6Nb-5Ta (wt%) Alloy for Bone Implant Applications: Composite Characterization and Cytocompatibility Assessment" Journal of Functional Biomaterials 14, no. 2: 94. https://doi.org/10.3390/jfb14020094
APA StyleEscobar, M., Careta, O., Fernández Navas, N., Bartkowska, A., Alberta, L. A., Fornell, J., Solsona, P., Gemming, T., Gebert, A., Ibáñez, E., Blanquer, A., Nogués, C., Sort, J., & Pellicer, E. (2023). Surface Modified β-Ti-18Mo-6Nb-5Ta (wt%) Alloy for Bone Implant Applications: Composite Characterization and Cytocompatibility Assessment. Journal of Functional Biomaterials, 14(2), 94. https://doi.org/10.3390/jfb14020094










