Conditional Mitigation of Dental-Composite Material-Induced Cytotoxicity by Increasing the Cure Time
Abstract
:1. Introduction
2. Materials and Methods
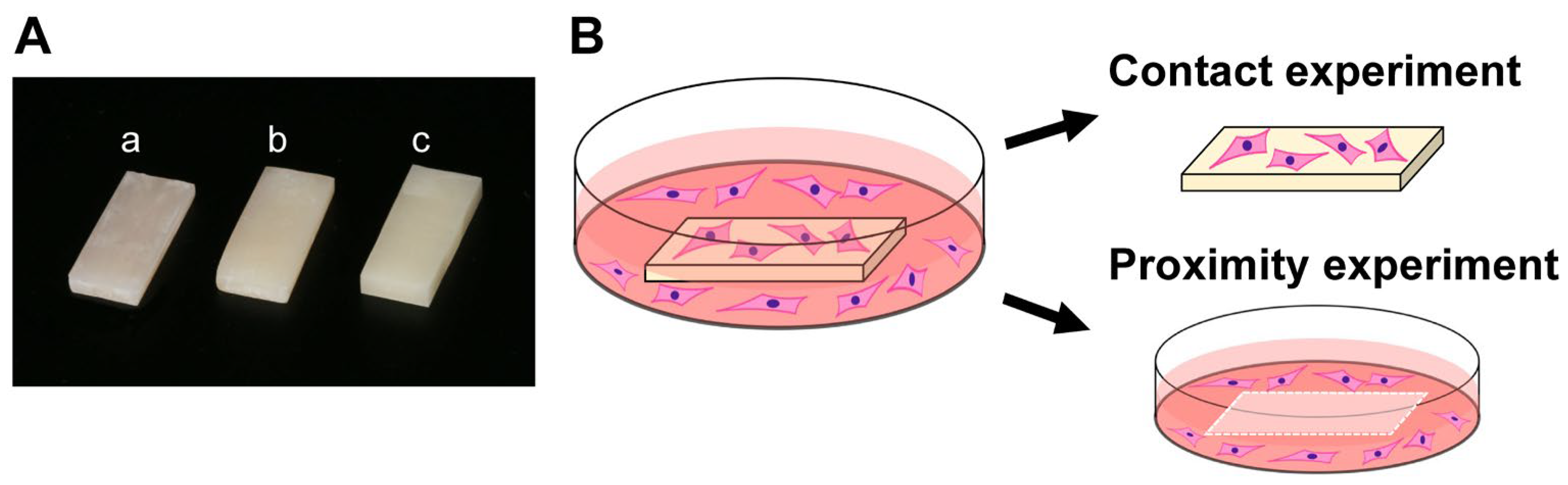
2.1. Material Preparation and Characterization
2.2. Cell Culture
2.3. Quantification of Attached and Propagated Cells
2.4. Fluorescent Microscopy
2.5. Collagen Production
2.6. Statistical Analysis
3. Results
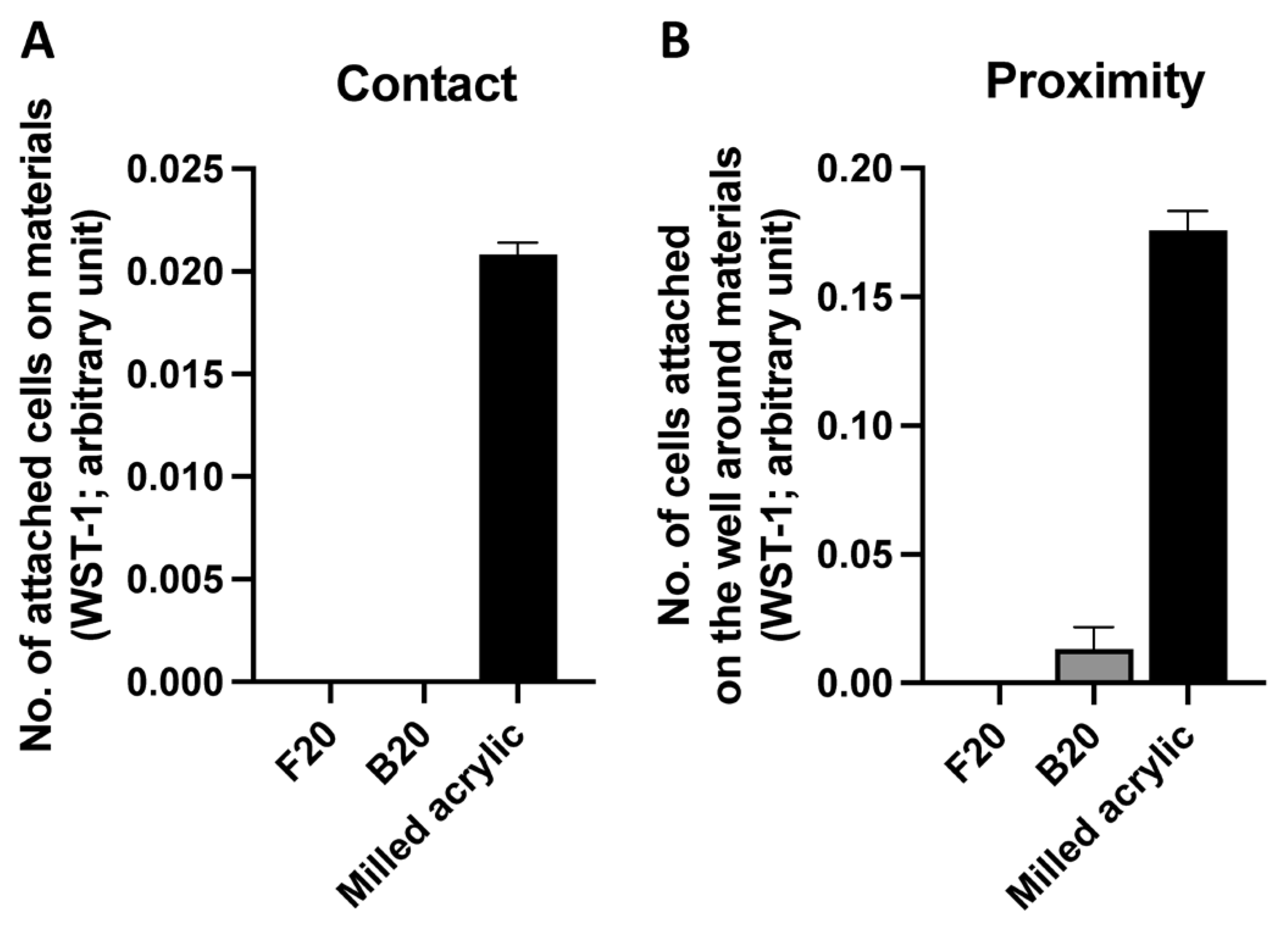
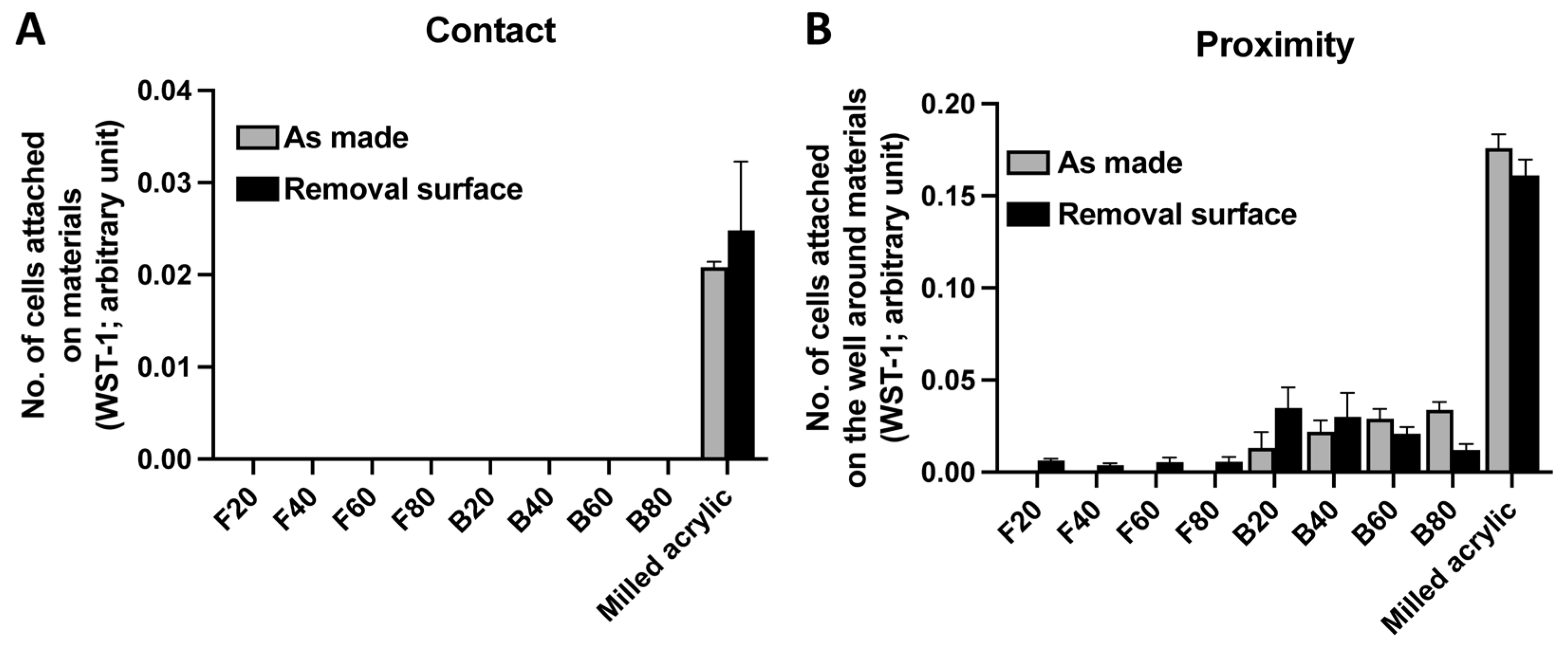
3.1. Initial Cell Attachment
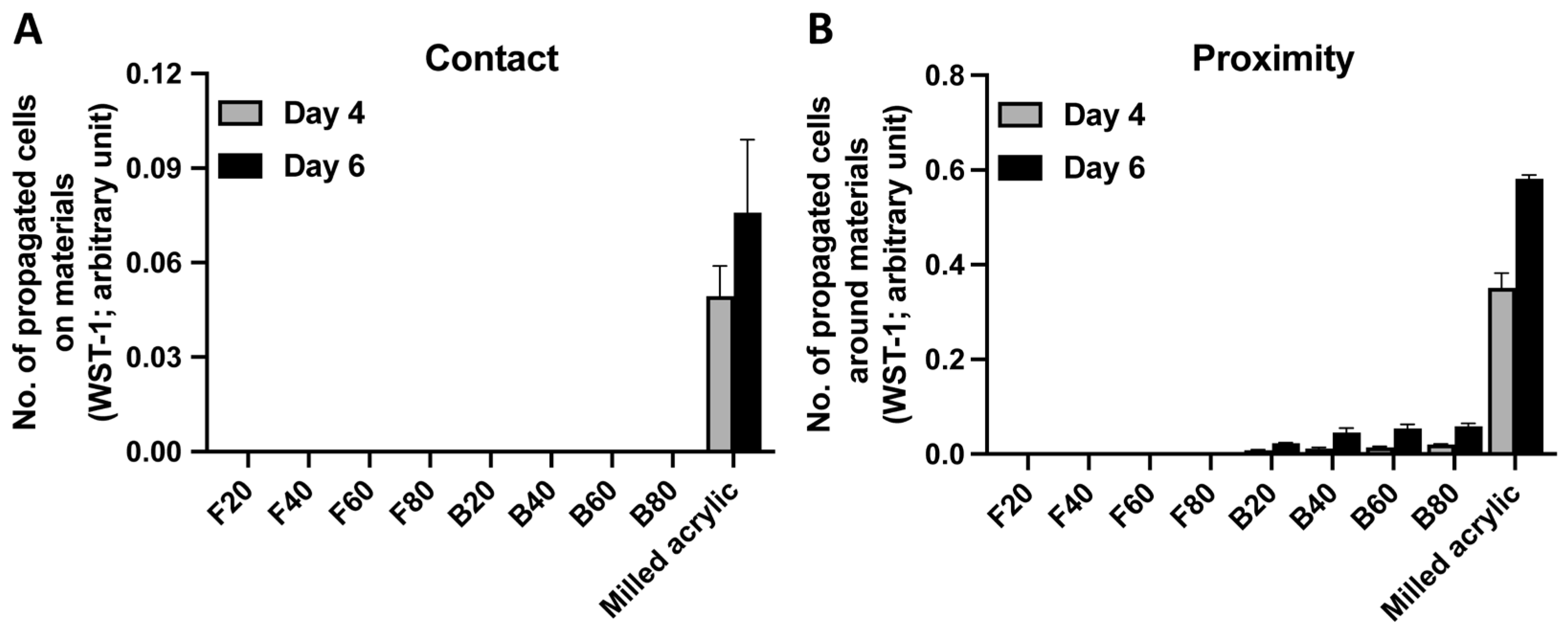
3.2. Cell Proliferation
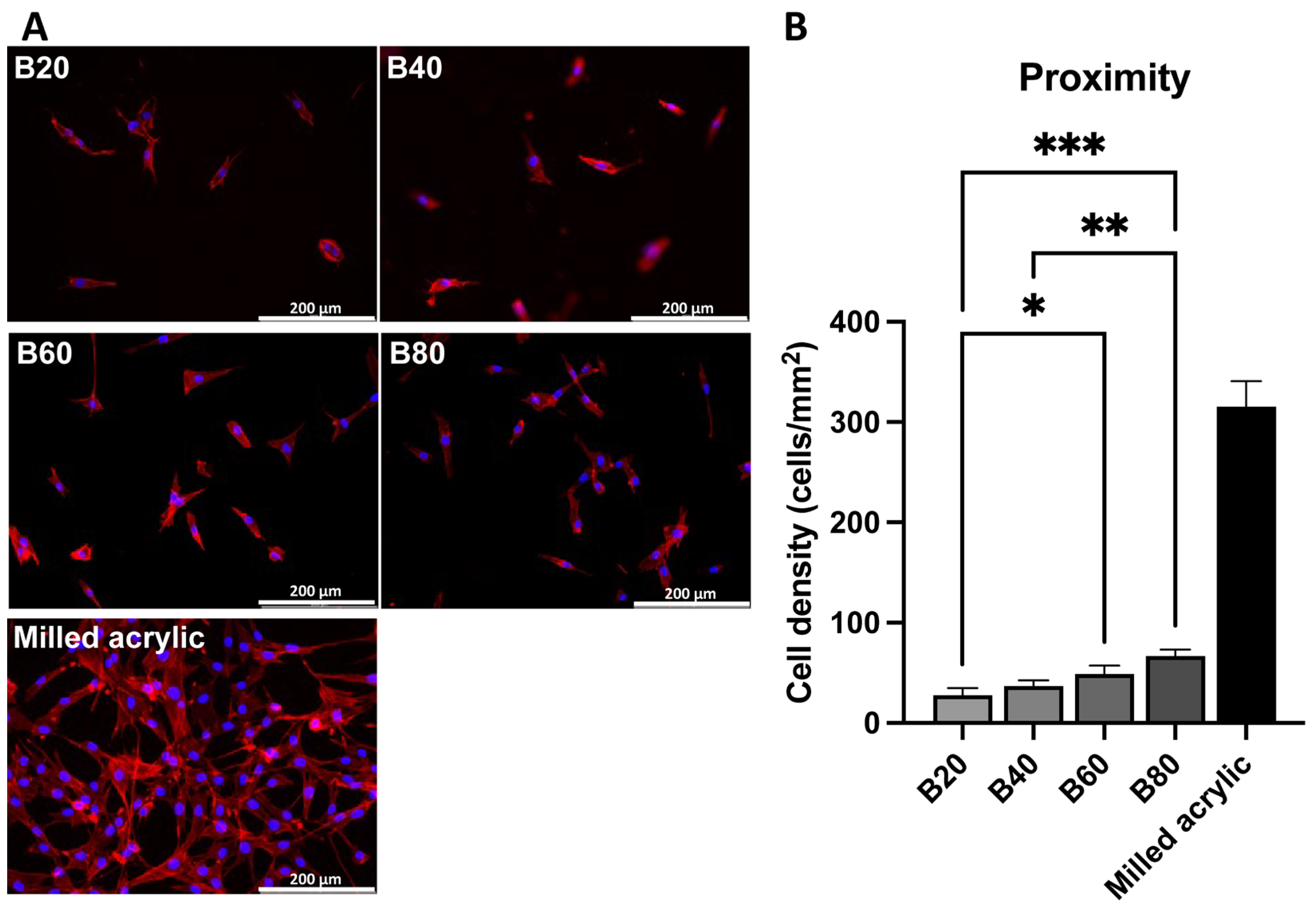
3.3. Cell Visualization
3.4. Collagen Production
3.5. Improvement in Cell Attachment after Surface Removal
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef]
- Studenikin, R.; Niftaliev, S. Fabrication and Use of a Customized Provisional Composite Abutment in Dental Practice. Int. J. Dent. 2021, 2021, 9929803. [Google Scholar] [CrossRef]
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef]
- Peutzfeldt, A. Resin composites in dentistry: The monomer systems. Eur. J. Oral Sci. 1997, 105, 97–116. [Google Scholar] [CrossRef]
- Nicolae, L.C.; Shelton, R.M.; Cooper, P.R.; Martin, R.A.; Palin, W.M. The Effect of UDMA/TEGDMA Mixtures and Bioglass Incorporation on the Mechanical and Physical Properties of Resin and Resin-Based Composite Materials. In Hindawi Publishing Corporation Conference Papers in Science; Hindawi: London, UK, 2014; pp. 1–5. [Google Scholar]
- Boruziniat, A.; Gharaee, S.; Sarraf Shirazi, A.; Majidinia, S.; Vatanpour, M. Evaluation of the efficacy of flowable composite as lining material on microleakage of composite resin restorations: A systematic review and meta-analysis. Quintessence Int. 2016, 47, 93–101. [Google Scholar]
- Van Ende, A.; De Munck, J.; Lise, D.P.; Van Meerbeek, B. Bulk-Fill Composites: A Review of the Current Literature. J. Adhes. Dent. 2017, 19, 95–109. [Google Scholar]
- Stansbury, J.W. Curing dental resins and composites by photopolymerization. J. Esthet. Dent. 2000, 12, 300–308. [Google Scholar] [CrossRef]
- Aita, H.; Tsukimura, N.; Yamada, M.; Hori, N.; Kubo, K.; Sato, N.; Maeda, H.; Kimoto, K.; Ogawa, T. N-acetyl cysteine prevents polymethyl methacrylate bone cement extract-induced cell death and functional suppression of rat primary osteoblasts. J. Biomed. Mater. Res. A 2010, 92, 285–296. [Google Scholar] [CrossRef]
- Att, W.; Yamada, M.; Kojima, N.; Ogawa, T. N-Acetyl cysteine prevents suppression of oral fibroblast function on poly(methylmethacrylate) resin. Acta Biomater. 2009, 5, 391–398. [Google Scholar] [CrossRef]
- Hamajima, K.; Ozawa, R.; Saruta, J.; Saita, M.; Kitajima, H.; Taleghani, S.R.; Usami, D.; Goharian, D.; Uno, M.; Miyazawa, K.; et al. The Effect of TBB, as an Initiator, on the Biological Compatibility of PMMA/MMA Bone Cement. Int. J. Mol. Sci. 2020, 21, 4016. [Google Scholar] [CrossRef]
- Kojima, N.; Yamada, M.; Paranjpe, A.; Tsukimura, N.; Kubo, K.; Jewett, A.; Ogawa, T. Restored viability and function of dental pulp cells on poly-methylmethacrylate (PMMA)-based dental resin supplemented with N-acetyl cysteine (NAC). Dent. Mater. 2008, 24, 1686–1693. [Google Scholar] [CrossRef]
- Nakagawa, K.; Saita, M.; Ikeda, T.; Hirota, M.; Park, W.; Lee, M.C.; Ogawa, T. Biocompatibility of 4-META/MMA-TBB resin used as a dental luting agent. J. Prosthet. Dent. 2015, 114, 114–121. [Google Scholar] [CrossRef]
- Sugita, Y.; Okubo, T.; Saita, M.; Ishijima, M.; Torii, Y.; Tanaka, M.; Iwasaki, C.; Sekiya, T.; Tabuchi, M.; Mohammadzadeh Rezaei, N.; et al. Novel Osteogenic Behaviors around Hydrophilic and Radical-Free 4-META/MMA-TBB: Implications of an Osseointegrating Bone Cement. Int. J. Mol. Sci. 2020, 21, 2405. [Google Scholar] [CrossRef] [Green Version]
- Tsukimura, N.; Yamada, M.; Aita, H.; Hori, N.; Yoshino, F.; Chang-Il Lee, M.; Kimoto, K.; Jewett, A.; Ogawa, T. N-acetyl cysteine (NAC)-mediated detoxification and functionalization of poly(methyl methacrylate) bone cement. Biomaterials 2009, 30, 3378–3389. [Google Scholar] [CrossRef]
- Yamada, M.; Kojima, N.; Att, W.; Hori, N.; Suzuki, T.; Ogawa, T. N-Acetyl cysteine restores viability and function of rat odontoblast-like cells impaired by polymethylmethacrylate dental resin extract. Redox Rep. 2009, 14, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Kojima, N.; Paranjpe, A.; Att, W.; Aita, H.; Jewett, A.; Ogawa, T. N-acetyl cysteine (NAC)-assisted detoxification of PMMA resin. J. Dent. Res. 2008, 87, 372–377. [Google Scholar] [CrossRef]
- Komatsu, K.; Hamajima, K.; Ozawa, R.; Kitajima, H.; Matsuura, T.; Ogawa, T. Novel Tuning of PMMA Orthopedic Bone Cement Using TBB Initiator: Effect of Bone Cement Extracts on Bioactivity of Osteoblasts and Osteoclasts. Cells 2022, 11, 3999. [Google Scholar] [CrossRef]
- Yoshii, E. Cytotoxic effects of acrylates and methacrylates: Relationships of monomer structures and cytotoxicity. J. Biomed. Mater. Res. 1997, 37, 517–524. [Google Scholar] [CrossRef]
- Haugen, H.J.; Marovic, D.; Par, M.; Thieu, M.K.L.; Reseland, J.E.; Johnsen, G.F. Bulk Fill Composites Have Similar Performance to Conventional Dental Composites. Int. J. Mol. Sci. 2020, 21, 5136. [Google Scholar] [CrossRef]
- Schweikl, H.; Spagnuolo, G.; Schmalz, G. Genetic and cellular toxicology of dental resin monomers. J. Dent. Res. 2006, 85, 870–877. [Google Scholar] [CrossRef]
- Kraus, D.; Wolfgarten, M.; Enkling, N.; Helfgen, E.H.; Frentzen, M.; Probstmeier, R.; Winter, J.; Stark, H. In-vitro cytocompatibility of dental resin monomers on osteoblast-like cells. J. Dent. 2017, 65, 76–82. [Google Scholar] [CrossRef]
- Ergun, G.; Mutlu-Sagesen, L.; Karaoglu, T.; Dogan, A. Cytotoxicity of provisional crown and bridge restoration materials: An in vitro study. J. Oral Sci. 2001, 43, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Knezevic, A.; Zeljezic, D.; Kopjar, N.; Tarle, Z. Cytotoxicity of composite materials polymerized with LED curing units. Oper. Dent. 2008, 33, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Goncalves, F.P.; Alves, G.; Guimaraes, V.O.J.; Gallito, M.A.; Oliveira, F.; Scelza, M.Z. Cytotoxicity Evaluation of Two Bis-Acryl Composite Resins Using Human Gingival Fibroblasts. Braz. Dent. J. 2016, 27, 492–496. [Google Scholar] [CrossRef] [Green Version]
- Campaner, M.; Takamiya, A.S.; Bitencourt, S.B.; Mazza, L.C.; de Oliveira, S.H.P.; Shibayama, R.; Barao, V.A.R.; Sukotjo, C.; Pesqueira, A.A. Cytotoxicity and inflammatory response of different types of provisional restorative materials. Arch. Oral Biol. 2020, 111, 104643. [Google Scholar] [CrossRef]
- Tsitrou, E.; Kelogrigoris, S.; Koulaouzidou, E.; Antoniades-Halvatjoglou, M.; Koliniotou-Koumpia, E.; van Noort, R. Effect of extraction media and storage time on the elution of monomers from four contemporary resin composite materials. Toxicol. Int. 2014, 21, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Minamikawa, H.; Yamada, M.; Iwasa, F.; Ueno, T.; Deyama, Y.; Suzuki, K.; Yawaka, Y.; Ogawa, T. Amino acid derivative-mediated detoxification and functionalization of dual cure dental restorative material for dental pulp cell mineralization. Biomaterials 2010, 31, 7213–7225. [Google Scholar] [CrossRef]
- Yamada, M.; Ogawa, T. Chemodynamics underlying N-acetyl cysteine-mediated bone cement monomer detoxification. Acta Biomater. 2009, 5, 2963–2973. [Google Scholar] [CrossRef]
- Collado-Gonzalez, M.; Pecci-Lloret, M.R.; Tomas-Catala, C.J.; Garcia-Bernal, D.; Onate-Sanchez, R.E.; Llena, C.; Forner, L.; Rosa, V.; Rodriguez-Lozano, F.J. Thermo-setting glass ionomer cements promote variable biological responses of human dental pulp stem cells. Dent. Mater. 2018, 34, 932–943. [Google Scholar] [CrossRef]
- Lopez-Garcia, S.; Pecci-Lloret, M.P.; Pecci-Lloret, M.R.; Onate-Sanchez, R.E.; Garcia-Bernal, D.; Castelo-Baz, P.; Rodriguez-Lozano, F.J.; Guerrero-Girones, J. In Vitro Evaluation of the Biological Effects of ACTIVA Kids BioACTIVE Restorative, Ionolux, and Riva Light Cure on Human Dental Pulp Stem Cells. Materials 2019, 12, 3694. [Google Scholar] [CrossRef] [Green Version]
- Lefeuvre, M.; Amjaad, W.; Goldberg, M.; Stanislawski, L. TEGDMA induces mitochondrial damage and oxidative stress in human gingival fibroblasts. Biomaterials 2005, 26, 5130–5137. [Google Scholar] [CrossRef]
- Nakagawa, K.; Ikeda, T.; Saita, M.; Hirota, M.; Tabuchi, M.; Park, W.; Lee, M.; Ogawa, T. Biological and biochemical characterization of 4-META/MMA-TBB resin. J. Dent. Oral Disord. Ther. 2015, 3, 1–7. [Google Scholar]
- Polydorou, O.; Trittler, R.; Hellwig, E.; Kummerer, K. Elution of monomers from two conventional dental composite materials. Dent. Mater. 2007, 23, 1535–1541. [Google Scholar] [CrossRef]
- Vallittu, P.K. Oxygen inhibition of autopolymerization of polymethylmethacrylate-glass fibre composite. J. Mater. Sci. Mater. Med. 1997, 8, 489–492. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Miron, R.J.; Lussi, A.; Gruber, R.; Ilie, N.; Price, R.B.; Schmalz, G. Effect of the degree of conversion of resin-based composites on cytotoxicity, cell attachment, and gene expression. Dent. Mater. 2019, 35, 1173–1193. [Google Scholar] [CrossRef]
- Matsuura, T.; Komatsu, K.; Ogawa, T. N-Acetyl Cysteine-Mediated Improvements in Dental Restorative Material Biocompatibility. Int. J. Mol. Sci. 2022, 23, 15869. [Google Scholar] [CrossRef]
- Matsuura, T.; Komatsu, K.; Chao, D.; Lin, Y.C.; Oberoi, N.; McCulloch, K.; Cheng, J.; Orellana, D.; Ogawa, T. Cell Type-Specific Effects of Implant Provisional Restoration Materials on the Growth and Function of Human Fibroblasts and Osteoblasts. Biomimetics 2022, 7, 243. [Google Scholar] [CrossRef]
- Nakhaei, K.; Ishijima, M.; Ikeda, T.; Ghassemi, A.; Saruta, J.; Ogawa, T. Ultraviolet Light Treatment of Titanium Enhances Attachment, Adhesion, and Retention of Human Oral Epithelial Cells via Decarbonization. Materials 2020, 14, 151. [Google Scholar] [CrossRef]
- Okubo, T.; Tsukimura, N.; Taniyama, T.; Ishijima, M.; Nakhaei, K.; Rezaei, N.M.; Hirota, M.; Park, W.; Akita, D.; Tateno, A.; et al. Ultraviolet treatment restores bioactivity of titanium mesh plate degraded by contact with medical gloves. J. Oral Sci. 2018, 60, 567–573. [Google Scholar] [CrossRef] [Green Version]
- Att, W.; Yamada, M.; Ogawa, T. Effect of titanium surface characteristics on the behavior and function of oral fibroblasts. Int. J. Oral Maxillofac. Implant. 2009, 24, 419–431. [Google Scholar]
- Saruwatari, L.; Aita, H.; Butz, F.; Nakamura, H.K.; Ouyang, J.; Yang, Y.; Chiou, W.A.; Ogawa, T. Osteoblasts generate harder, stiffer, and more delamination-resistant mineralized tissue on titanium than on polystyrene, associated with distinct tissue micro- and ultrastructure. J. Bone Miner. Res. 2005, 20, 2002–2016. [Google Scholar] [CrossRef]
- Takeuchi, K.; Saruwatari, L.; Nakamura, H.K.; Yang, J.M.; Ogawa, T. Enhanced intrinsic biomechanical properties of osteoblastic mineralized tissue on roughened titanium surface. J. Biomed. Mater. Res. A 2005, 72A, 296–305. [Google Scholar] [CrossRef]
- Nguyen, J.F.; Migonney, V.; Ruse, N.D.; Sadoun, M. Resin composite blocks via high-pressure high-temperature polymerization. Dent. Mater. 2012, 28, 529–534. [Google Scholar] [CrossRef]
- Hada, T.; Kanazawa, M.; Iwaki, M.; Katheng, A.; Minakuchi, S. Comparison of Mechanical Properties of PMMA Disks for Digitally Designed Dentures. Polymers 2021, 13, 1745. [Google Scholar] [CrossRef]
- Yilmaz, M.N.; Gul, P. Monomer release from dental restorative materials containing dimethacrylate resin after bleaching. Clin. Oral Investig. 2022, 26, 4647–4662. [Google Scholar] [CrossRef]
- Lang, O.; Kohidai, L.; Kohidai, Z.; Dobo-Nagy, C.; Csomo, K.B.; Lajko, M.; Mozes, M.; Keki, S.; Deak, G.; Tian, K.V.; et al. Cell physiological effects of glass ionomer cements on fibroblast cells. Toxicol. Vitr. 2019, 61, 104627. [Google Scholar] [CrossRef]
- Ogawa, T.; Aizawa, S.; Tanaka, M.; Matsuya, S.; Hasegawa, A.; Koyano, K. Effect of water temperature on the fit of provisional crown margins during polymerization. J. Prosthet. Dent. 1999, 82, 658–661. [Google Scholar] [CrossRef]
- Ogawa, T.; Hasegawa, A. Effect of curing environment on mechanical properties and polymerizing behaviour of methyl-methacrylate autopolymerizing resin. J. Oral Rehabil. 2005, 32, 221–226. [Google Scholar] [CrossRef]
- Ogawa, T.; Tanaka, M.; Matsuya, S.; Aizawa, S.; Koyano, K. Setting characteristics of five autopolymerizing resins measured by an oscillating rheometer. J. Prosthet. Dent. 2001, 85, 170–176. [Google Scholar] [CrossRef]
- Sideridou, I.D.; Achilias, D.S. Elution study of unreacted Bis-GMA, TEGDMA, UDMA, and Bis-EMA from light-cured dental resins and resin composites using HPLC. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 74, 617–626. [Google Scholar] [CrossRef]
- Ciapetti, G.; Granchi, D.; Savarino, L.; Cenni, E.; Magrini, E.; Baldini, N.; Giunti, A. In vitro testing of the potential for orthopedic bone cements to cause apoptosis of osteoblast-like cells. Biomaterials 2002, 23, 617–627. [Google Scholar] [CrossRef]
- De Angelis, F.; Mandatori, D.; Schiavone, V.; Melito, F.P.; Valentinuzzi, S.; Vadini, M.; Di Tomo, P.; Vanini, L.; Pelusi, L.; Pipino, C.; et al. Cytotoxic and Genotoxic Effects of Composite Resins on Cultured Human Gingival Fibroblasts. Materials 2021, 14, 5225. [Google Scholar] [CrossRef]
- Santin, D.C.; Velo, M.; Camim, F.D.S.; Brondino, N.C.M.; Honorio, H.M.; Mondelli, R.F.L. Effect of thickness on shrinkage stress and bottom-to-top hardness ratio of conventional and bulk-fill composites. Eur. J. Oral Sci. 2021, 129, e12825. [Google Scholar] [CrossRef]
- Prati, C.; Chersoni, S.; Montebugnoli, L.; Montanari, G. Effect of air, dentin and resin-based composite thickness on light intensity reduction. Am. J. Dent. 1999, 12, 231–234. [Google Scholar]
- Cidreira Boaro, L.C.; Pereira Lopes, D.; de Souza, A.S.C.; Lie Nakano, E.; Ayala Perez, M.D.; Pfeifer, C.S.; Goncalves, F. Clinical performance and chemical-physical properties of bulk fill composites resin -a systematic review and meta-analysis. Dent. Mater. 2019, 35, e249–e264. [Google Scholar] [CrossRef]
- Larato, D.C. Influence of a composite resin restoration on the gingiva. J. Prosthet. Dent. 1972, 28, 402–404. [Google Scholar] [CrossRef]
- Sirajuddin, S.; Narasappa, K.M.; Gundapaneni, V.; Chungkham, S.; Walikar, A.S. Iatrogenic Damage to Periodontium by Restorative Treatment Procedures: An Overview. Open Dent. J. 2015, 9, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Lozano, F.J.; Serrano-Belmonte, I.; Perez Calvo, J.C.; Coronado-Parra, M.T.; Bernabeu-Esclapez, A.; Moraleda, J.M. Effects of two low-shrinkage composites on dental stem cells (viability, cell damaged or apoptosis and mesenchymal markers expression). J. Mater. Sci. Mater. Med. 2013, 24, 979–988. [Google Scholar] [CrossRef]
- Herrera-Gonzalez, A.M.; Perez-Mondragon, A.A.; Cuevas-Suarez, C.E. Evaluation of bio-based monomers from isosorbide used in the formulation of dental composite resins. J. Mech. Behav. Biomed. Mater. 2019, 100, 103371. [Google Scholar]
- Wedekind, L.; Guth, J.F.; Schweiger, J.; Kollmuss, M.; Reichl, F.X.; Edelhoff, D.; Hogg, C. Elution behavior of a 3D-printed, milled and conventional resin-based occlusal splint material. Dent. Mater. 2021, 37, 701–710. [Google Scholar] [CrossRef]
- Minamikawa, H.; Yamada, M.; Deyama, Y.; Suzuki, K.; Kaga, M.; Yawaka, Y.; Ogawa, T. Effect of N-acetylcysteine on Rat Dental Pulp Cells Cultured on Mineral Trioxide Aggregate. J. Endod. 2011, 37, 637–641. [Google Scholar] [CrossRef]
- Sato, N.; Ueno, T.; Kubo, K.; Suzuki, T.; Tsukimura, N.; Att, W.; Yamada, M.; Hori, N.; Maeda, H.; Ogawa, T. N-Acetyl cysteine (NAC) inhibits proliferation, collagen gene transcription, and redox stress in rat palatal mucosal cells. Dent. Mater. 2009, 25, 1532–1540. [Google Scholar] [CrossRef]
- Ueno, T.; Yamada, M.; Igarashi, Y.; Ogawa, T. N-acetyl cysteine protects osteoblastic function from oxidative stress. J. Biomed. Mater. Res. A 2011, 99, 523–531. [Google Scholar] [CrossRef]
- Yamada, M.; Kubo, K.; Ueno, T.; Iwasa, F.; Att, W.; Hori, N.; Ogawa, T. Alleviation of commercial collagen sponge- and membrane-induced apoptosis and dysfunction in cultured osteoblasts by an amino acid derivative. Int. J. Oral Maxillofac. Implant. 2010, 25, 939–946. [Google Scholar]
- Yamada, M.; Minamikawa, H.; Ueno, T.; Sakurai, K.; Ogawa, T. N-acetyl cysteine improves affinity of beta-tricalcium phosphate granules for cultured osteoblast-like cells. J. Biomater. Appl. 2012, 27, 27–36. [Google Scholar] [CrossRef]
- Yamada, M.; Tsukimura, N.; Ikeda, T.; Sugita, Y.; Att, W.; Kojima, N.; Kubo, K.; Ueno, T.; Sakurai, K.; Ogawa, T. N-acetyl cysteine as an osteogenesis-enhancing molecule for bone regeneration. Biomaterials 2013, 34, 6147–6156. [Google Scholar] [CrossRef]
- Yamada, M.; Ueno, T.; Minamikawa, H.; Sato, N.; Iwasa, F.; Hori, N.; Ogawa, T. N-acetyl cysteine alleviates cytotoxicity of bone substitute. J. Dent. Res. 2010, 89, 411–416. [Google Scholar] [CrossRef]
- Suzuki, T.; Kubo, K.; Hori, N.; Yamada, M.; Kojima, N.; Sugita, Y.; Maeda, H.; Ogawa, T. Nonvolatile buffer coating of titanium to prevent its biological aging and for drug delivery. Biomaterials 2010, 31, 4818–4828. [Google Scholar] [CrossRef]
- Ueno, T.; Yamada, M.; Sugita, Y.; Ogawa, T. N-acetyl cysteine protects TMJ chondrocytes from oxidative stress. J. Dent. Res. 2011, 90, 353–359. [Google Scholar] [CrossRef]
- Yamada, M.; Kojima, N.; Att, W.; Minamikawa, H.; Sakurai, K.; Ogawa, T. Improvement in the osteoblastic cellular response to a commercial collagen membrane and demineralized freeze-dried bone by an amino acid derivative: An in vitro study. Clin. Oral Implant. Res. 2011, 22, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Saruta, J.; Ozawa, R.; Hamajima, K.; Saita, M.; Sato, N.; Ishijima, M.; Kitajima, H.; Ogawa, T. Prolonged Post-Polymerization Biocompatibility of Polymethylmethacrylate-Tri-n-Butylborane (PMMA-TBB) Bone Cement. Materials 2021, 14, 1289. [Google Scholar] [CrossRef]
- Okubo, T.; Ikeda, T.; Saruta, J.; Tsukimura, N.; Hirota, M.; Ogawa, T. Compromised Epithelial Cell Attachment after Polishing Titanium Surface and Its Restoration by UV Treatment. Materials 2020, 13, 3946. [Google Scholar] [CrossRef]
- Ueno, T.; Ikeda, T.; Tsukimura, N.; Ishijima, M.; Minamikawa, H.; Sugita, Y.; Yamada, M.; Wakabayashi, N.; Ogawa, T. Novel antioxidant capability of titanium induced by UV light treatment. Biomaterials 2016, 108, 177–186. [Google Scholar] [CrossRef]
- Kim, S.W.; Ogawa, T.; Tabata, Y.; Nishimura, I. Efficacy and cytotoxicity of cationic-agent-mediated nonviral gene transfer into osteoblasts. J. Biomed. Mater. Res. 2004, 71A, 308–315. [Google Scholar] [CrossRef]
- Rezaei, N.M.; Hasegawa, M.; Ishijima, M.; Nakhaei, K.; Okubo, T.; Taniyama, T.; Ghassemi, A.; Tahsili, T.; Park, W.; Hirota, M.; et al. Biological and osseointegration capabilities of hierarchically (meso-/micro-/nano-scale) roughened zirconia. Int. J. Nanomed. 2018, 13, 3381–3395. [Google Scholar] [CrossRef] [Green Version]
- Kojima, N.; Ozawa, S.; Miyata, Y.; Hasegawa, H.; Tanaka, Y.; Ogawa, T. High-throughput gene expression analysis in bone healing around titanium implants by DNA microarray. Clin. Oral Implant. Res. 2008, 19, 173–181. [Google Scholar] [CrossRef]
- Yamada, M.; Watanabe, J.; Ueno, T.; Ogawa, T.; Egusa, H. Cytoprotective Preconditioning of Osteoblast-Like Cells with N-Acetyl-L-Cysteine for Bone Regeneration in Cell Therapy. Int. J. Mol. Sci. 2019, 20, 5199. [Google Scholar] [CrossRef] [Green Version]


| Materials (Product Name, Manufacturer) | Main Ingredients | Curing Time (seconds) | Notations |
|---|---|---|---|
| Flowable composite (Aeliteflo™, BISCO Inc., Schaumburg, IL, USA) | 20 | F20 | |
| Bis-GMA | 40 | F40 | |
| 60 80 | F60 F80 | ||
| Bulk-fill composite (Aelite™ Aesthetic Enamel, BISCO Inc.) | 20 | B20 | |
| Bis-GMA, UDMA | 40 60 80 | B40 B60 B80 | |
| Milled acrylic (Vivid PMMA Disc, Pearson™ Dental Supply Co.) | |||
| PMMA | – | – | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuura, T.; Komatsu, K.; Choi, K.; Suzumura, T.; Cheng, J.; Chang, T.-L.; Chao, D.; Ogawa, T. Conditional Mitigation of Dental-Composite Material-Induced Cytotoxicity by Increasing the Cure Time. J. Funct. Biomater. 2023, 14, 119. https://doi.org/10.3390/jfb14030119
Matsuura T, Komatsu K, Choi K, Suzumura T, Cheng J, Chang T-L, Chao D, Ogawa T. Conditional Mitigation of Dental-Composite Material-Induced Cytotoxicity by Increasing the Cure Time. Journal of Functional Biomaterials. 2023; 14(3):119. https://doi.org/10.3390/jfb14030119
Chicago/Turabian StyleMatsuura, Takanori, Keiji Komatsu, Kimberly Choi, Toshikatsu Suzumura, James Cheng, Ting-Ling Chang, Denny Chao, and Takahiro Ogawa. 2023. "Conditional Mitigation of Dental-Composite Material-Induced Cytotoxicity by Increasing the Cure Time" Journal of Functional Biomaterials 14, no. 3: 119. https://doi.org/10.3390/jfb14030119
APA StyleMatsuura, T., Komatsu, K., Choi, K., Suzumura, T., Cheng, J., Chang, T.-L., Chao, D., & Ogawa, T. (2023). Conditional Mitigation of Dental-Composite Material-Induced Cytotoxicity by Increasing the Cure Time. Journal of Functional Biomaterials, 14(3), 119. https://doi.org/10.3390/jfb14030119






