Tumor Microenvironment Regulation and Cancer Targeting Therapy Based on Nanoparticles
Abstract
:1. Introduction
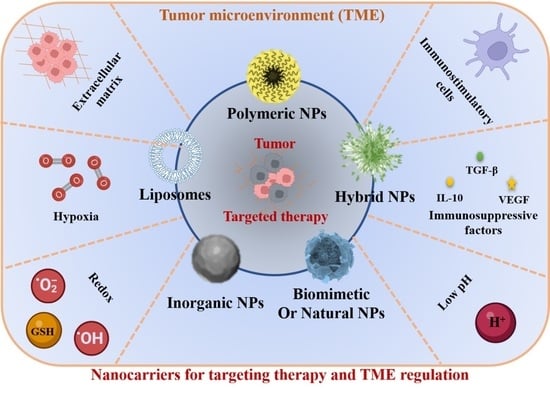
2. The Nanocarriers for Cancer Targeting Therapy
2.1. Inorganic NPs
2.2. Lipid Nanocarriers
2.3. Polymer Nanocarriers
2.4. Hybrid Nanocarriers
2.5. Biomimetic and Natural Nanocarriers
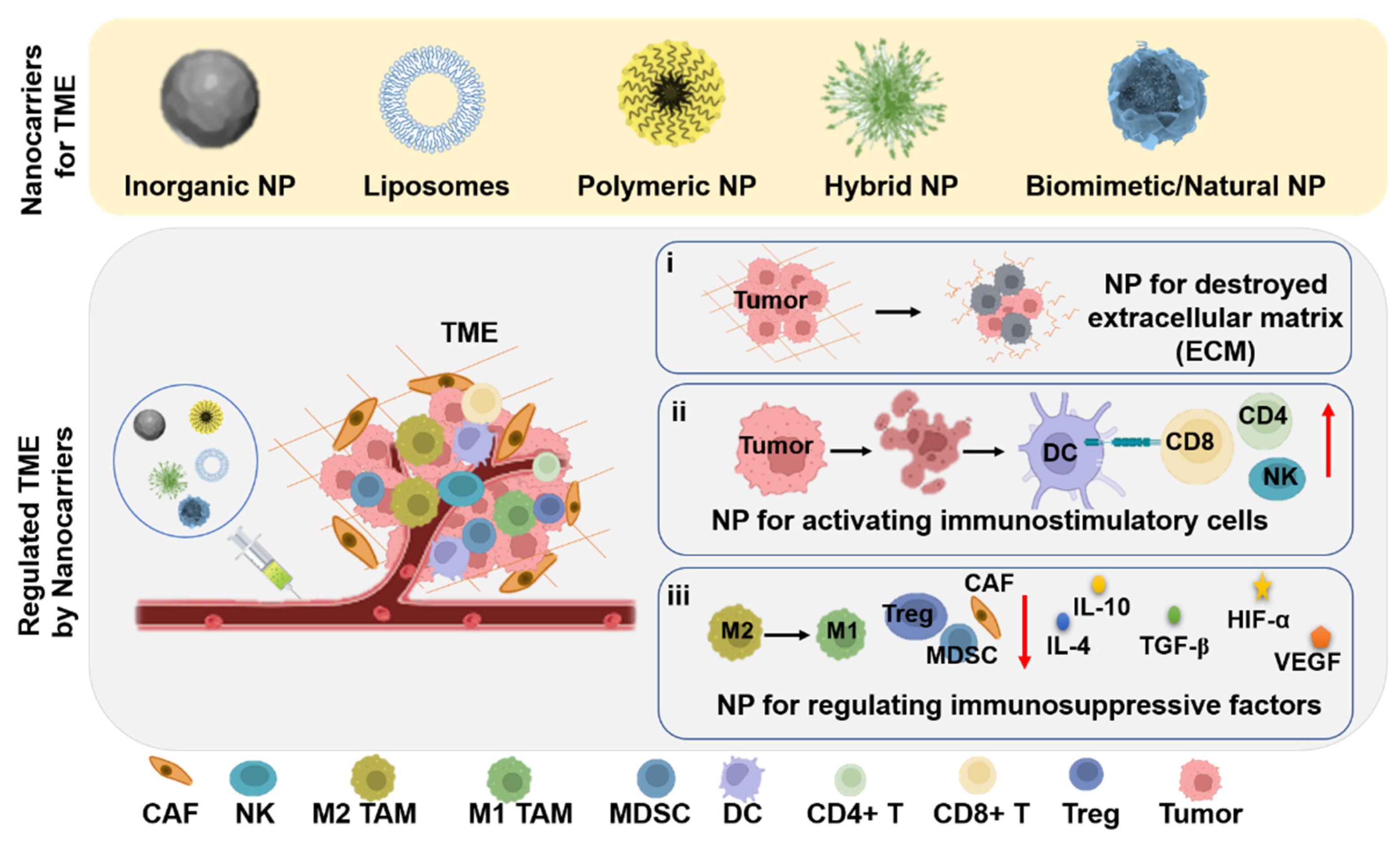
3. Nanocarriers for TME Regulation and Cancer Therapy
3.1. Nanocarriers for Regulating ECM
3.2. Nanocarriers for Activating Immunostimulatory Cells
3.3. Nanocarriers for Decreasing/Regulating Immunosuppressive Factors
3.4. Nanocarriers for Regulating Acidic Environment
3.5. Nanocarriers for Regulating Hypoxia and Redox Environment
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Abbreviation | Full Spelling |
|---|---|
| TME | tumor microenvironment |
| TAM | tumor associated macrophage |
| NPs | nanoparticles |
| PD-1 | programmed cell death protein 1 |
| CTLA-4 | cytotoxic T lymphocyte-associated protein 4 |
| CAR-T | chimeric antigen receptors T cells |
| DCs | dendritic cells |
| Th | T helper (Th) cells |
| CTLs | cytotoxic T lymphocytes |
| NK | natural killer cells |
| MDSCs | myeloid-derived suppressor cells |
| Treg | regulatory T cells |
| CAFs | cancer-associated fibroblasts |
| ECM | extracellular matrix |
| FDA | US Food and Drug Administration |
| BSA | bovine serum albumin |
| ICG | indocyanine green |
| MSNPs | mesoporous silica nanoparticles |
| DOX | doxorubicin |
| FA | folate |
| LPs | liposomes |
| MT1DP | metallothionein 1D |
| ROS | reactive oxygen species |
| OMlips | modified magnetic liposomes |
| OA | oleanolic acid |
| EPR | enhance permeability and retention |
| HCCP | hexachlorocyclic-triphosphonitrile |
| CysM | cysteine derivatives |
| IDO | indoleamine-(2,3)-dioxygenase |
| cRGD | cyclo (Arg-Gly-Asp-D-Tyr-Lys) peptide |
| TRP | l-tryptophan |
| KYN | L-kynurenine |
| SPIONS | supermagnetic iron oxide NPs |
| TZB | trastuzumab |
| PLGA | poly(lactic-co-glycolic acid |
| Mag | magnolol |
| HER2 | human epidermal growth factor-2 |
| NOPD | NO photodonor |
| RBC | red blood cell |
| CCM | cancer cell membrane |
| EVs | extracellular vehicles |
| GBM | anti-glioblastoma |
| ESCs | embryonic stem cells |
| PTX | paclitaxel |
| ECM | extracellular matrix |
| rHuPH20 | recombinant human hyaluronidase PH20 |
| HA | hyaluronidase |
| MMPs | matrix metalloproteinase |
| MRPL | MMP-2 reactive peptide hybrid-liposome |
| PFD | pirfenidone |
| APCs | antigen presenting cells |
| ICD | immunogenic cell death |
| CRT | dead tumor cells calreticulin |
| ATP | adenosine triphosphate |
| HMGB1 | high mobility group protein B1 |
| CGs | cardiac glycosides |
| PDT | Photodynamic therapy |
| IPLB | plumbagin |
| DIH | dihydrotanshinone |
| NVs | nano vesicle |
| TILs | tumor infiltrating lymphocytes |
| PNT | peroxynitrite |
| SUNb PM | polymeric micellar nano delivery system |
| DPPA-1 | programmed cell death-ligand 1 |
| NLG91 | idoleamine 2, 3-dioxygenase |
| NE | nano lotion |
| Frax | fraxinellone |
| VEGF | human vascular endothelial growth factor |
| Hb | hemoglobin |
| PFC | perfluorocarbon |
| GSH | glutathione |
| CPGR | β- CD-b-P (CPTGSH-co-CPTROS-co-OEGMA) |
| PpIX | protoporphyrin |
| BSO | buthionine-sulfoximine |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Goldman, A.; Kulkarni, A.; Kohandel, M.; Pandey, P.; Rao, P.; Natarajan, S.K.; Sabbisetti, V.; Sengupta, S. Rationally Designed 2-in-1 Nanoparticles Can Overcome Adaptive Resistance in Cancer. ACS Nano 2016, 10, 5823–5834. [Google Scholar] [CrossRef]
- Wu, X.; Wu, Y.; Ye, H.; Yu, S.; He, C.; Chen, X. Interleukin-15 and cisplatin co-encapsulated thermosensitive polypeptide hydrogels for combined immuno-chemotherapy. J. Control. Release 2017, 255, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Treat, L.H.; McDannold, N.; Zhang, Y.; Vykhodtseva, N.; Hynynen, K. Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med. Biol. 2012, 38, 1716–1725. [Google Scholar] [CrossRef] [Green Version]
- Coffey, J.C.; Wang, J.H.; Smith, M.J.; Bouchier-Hayes, D.; Cotter, T.G.; Redmond, H.P. Excisional surgery for cancer cure: Therapy at a cost. Lancet Oncol. 2003, 4, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, T.; Cao, L.; Zhang, Y. Chimeric Antigen Receptor T Cell Based Immunotherapy for Cancer. Curr. Stem Cell Res. Ther. 2018, 13, 327–335. [Google Scholar] [CrossRef]
- Kennedy, L.B.; Salama, A.K.S. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, H.T.; Djamgoz, M.B.A. Immuno-Oncology: Emerging Targets and Combination Therapies. Front. Oncol. 2018, 8, 315. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, A.; Lynn, R.C.; Poussin, M.; Eiva, M.A.; Shaw, L.C.; O’Connor, R.S.; Minutolo, N.G.; Casado-Medrano, V.; Lopez, G.; Matsuyama, T.; et al. CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat. Commun. 2021, 12, 877. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, J.; Huang, L. Tackling TAMs for Cancer Immunotherapy: It’s Nano Time. Trends Pharmacol. Sci. 2020, 41, 701–714. [Google Scholar] [CrossRef]
- Wu, D.; Wang, S.; Yu, G.; Chen, X. Cell Death Mediated by the Pyroptosis Pathway with the Aid of Nanotechnology: Prospects for Cancer Therapy. Angew. Chem. Int. Ed. Engl. 2021, 60, 8018–8034. [Google Scholar] [CrossRef]
- Le, Q.V.; Suh, J.; Oh, Y.K. Nanomaterial-Based Modulation of Tumor Microenvironments for Enhancing Chemo/Immunotherapy. AAPS J. 2019, 21, 64. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Wang, W.; Wang, S.; Yang, T.; Zhang, G.; Wang, D.; Ju, R.; Lu, Y.; Wang, H.; Wang, L. Tumor microenvironment remodeling and tumor therapy based on M2-like tumor associated macrophage-targeting nano-complexes. Theranostics 2021, 11, 2892–2916. [Google Scholar] [CrossRef]
- Ho, T.T.B.; Nasti, A.; Seki, A.; Komura, T.; Inui, H.; Kozaka, T.; Kitamura, Y.; Shiba, K.; Yamashita, T.; Yamashita, T.; et al. Combination of gemcitabine and anti-PD-1 antibody enhances the anticancer effect of M1 macrophages and the Th1 response in a murine model of pancreatic cancer liver metastasis. J. Immunother. Cancer 2020, 8, e001367. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Liu, D.; Xu, X.; Ji, J.; Du, Y. Nanomaterials-Based Photodynamic Therapy with Combined Treatment Improves Antitumor Efficacy Through Boosting Immunogenic Cell Death. Int. J. Nanomed. 2021, 16, 4693–4712. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, X.; Jia, J.; Zhang, W.; Yang, T.; Wang, L.; Ma, G. pH-Responsive Poly(D,L-lactic-co-glycolic acid) Nanoparticles with Rapid Antigen Release Behavior Promote Immune Response. ACS Nano 2015, 9, 4925–4938. [Google Scholar] [CrossRef]
- Lv, W.; Cao, M.; Liu, J.; Hei, Y.; Bai, J. Tumor microenvironment-responsive nanozymes achieve photothermal-enhanced multiple catalysis against tumor hypoxia. Acta Biomater. 2021, 135, 617–627. [Google Scholar] [CrossRef]
- Zhu, L.J.; Gu, L.S.; Shi, T.Y.; Zhang, X.Y.; Sun, B.W. Enhanced treatment effect of nanoparticles containing cisplatin and a GSH-reactive probe compound. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Yang, X.; Zhang, Y.; Gao, S.; Liu, S.; Ji, J.; Zhai, G. Transdermal delivery system based on heparin-modified graphene oxide for deep transportation, tumor microenvironment regulation, and immune activation. Nano Today 2022, 46, 101565. [Google Scholar] [CrossRef]
- Liu, Y.; Qiao, L.; Zhang, S.; Wan, G.; Chen, B.; Zhou, P.; Zhang, N.; Wang, Y. Dual pH-responsive multifunctional nanoparticles for targeted treatment of breast cancer by combining immunotherapy and chemotherapy. Acta Biomater. 2018, 66, 310–324. [Google Scholar] [CrossRef]
- Lu, J.; Liu, X.; Liao, Y.P.; Wang, X.; Ahmed, A.; Jiang, W.; Ji, Y.; Meng, H.; Nel, A.E. Breast Cancer Chemo-immunotherapy through Liposomal Delivery of an Immunogenic Cell Death Stimulus Plus Interference in the IDO-1 Pathway. ACS Nano 2018, 12, 11041–11061. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Li, F.; Zhang, L.; Liu, W.; Wang, X.; Zhu, R.; Qiao, Z.A.; Yu, B.; Yu, X. TRAIL-modified, doxorubicin-embedded periodic mesoporous organosilica nanoparticles for targeted drug delivery and efficient antitumor immunotherapy. Acta Biomater. 2022, 143, 392–405. [Google Scholar] [CrossRef]
- Khranovska, N.; Skachkova, O.; Gorbach, O.; Inomistova, M.; Orel, V. Magnetically sensitive nanocomplex enhances antitumor efficacy of dendritic cell-based immunotherapy. Exp. Oncol. 2021, 43, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Wang, W.; Wang, S.; Wang, S.; Ju, R.; Pan, Z.; Yang, T.; Zhang, G.; Wang, H.; Wang, L. Multifunctional biomimetic nanoparticles loading baicalin for polarizing tumor-associated macrophages. Nanoscale 2019, 11, 20206–20220. [Google Scholar] [CrossRef]
- Mulla, M.Z.; Rahman, M.R.T.; Marcos, B.; Tiwari, B.; Pathania, S. Poly Lactic Acid (PLA) Nanocomposites: Effect of Inorganic Nanoparticles Reinforcement on Its Performance and Food Packaging Applications. Molecules 2021, 26, 1967. [Google Scholar] [CrossRef]
- Skoberne, M.; Yewdall, A.; Bahjat, K.S.; Godefroy, E.; Lauer, P.; Lemmens, E.; Liu, W.; Luckett, W.; Leong, M.; Dubensky, T.W.; et al. KBMA Listeria monocytogenes is an effective vector for DC-mediated induction of antitumor immunity. J. Clin. Investig. 2008, 118, 3990–4001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonehill, A.; Van Nuffel, A.M.; Corthals, J.; Tuyaerts, S.; Heirman, C.; François, V.; Colau, D.; van der Bruggen, P.; Neyns, B.; Thielemans, K. Single-step antigen loading and activation of dendritic cells by mRNA electroporation for the purpose of therapeutic vaccination in melanoma patients. Clin. Cancer Res. 2009, 15, 3366–3375. [Google Scholar] [CrossRef] [Green Version]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.; Figdor, C. Immunotherapy: Cancer vaccine triggers antiviral-type defences. Nature 2016, 534, 329–331. [Google Scholar] [CrossRef]
- Wang, S.; Guo, J.; Bai, Y.; Sun, C.; Wu, Y.; Liu, Z.; Liu, X.; Wang, Y.; Wang, Z.; Zhang, Y.; et al. Bacterial outer membrane vesicles as a candidate tumor vaccine platform. Front. Immunol. 2022, 13, 987419. [Google Scholar] [CrossRef]
- Ye, B.; Hu, Y.; Zhang, M.; Huang, H. Research advance in lipid nanoparticle-mRNA delivery system and its application in CAR-T cell therapy. Zhejiang Da Xue Xue Bao Yi Xue Ban 2022, 51, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; Xue, C.; Hu, Y.; Zhao, Y.; Cai, K.; Li, M.; Luo, Z. A protein-based cGAS-STING nanoagonist enhances T cell-mediated anti-tumor immune responses. Nat. Commun. 2022, 13, 5685. [Google Scholar] [CrossRef]
- Wang, C.; Ye, Y.; Hochu, G.M.; Sadeghifar, H.; Gu, Z. Enhanced Cancer Immunotherapy by Microneedle Patch-Assisted Delivery of Anti-PD1 Antibody. Nano Lett. 2016, 16, 2334–2340. [Google Scholar] [CrossRef]
- Zhang, N.; Song, J.; Liu, Y.; Liu, M.; Zhang, L.; Sheng, D.; Deng, L.; Yi, H.; Wu, M.; Zheng, Y.; et al. Photothermal therapy mediated by phase-transformation nanoparticles facilitates delivery of anti-PD1 antibody and synergizes with antitumor immunotherapy for melanoma. J. Control. Release 2019, 306, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ramishetti, S.; Tseng, Y.C.; Guo, S.; Wang, Y.; Huang, L. Multifunctional nanoparticles co-delivering Trp2 peptide and CpG adjuvant induce potent cytotoxic T-lymphocyte response against melanoma and its lung metastasis. J. Control. Release 2013, 172, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huo, M.; Xu, Z.; Wang, Y.; Huang, L. Nanoparticle delivery of CDDO-Me remodels the tumor microenvironment and enhances vaccine therapy for melanoma. Biomaterials 2015, 68, 54–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huo, M.; Zhao, Y.; Satterlee, A.B.; Wang, Y.; Xu, Y.; Huang, L. Tumor-targeted delivery of sunitinib base enhances vaccine therapy for advanced melanoma by remodeling the tumor microenvironment. J. Control. Release 2017, 245, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Sasso, M.S.; Lollo, G.; Pitorre, M.; Solito, S.; Pinton, L.; Valpione, S.; Bastiat, G.; Mandruzzato, S.; Bronte, V.; Marigo, I.; et al. Low dose gemcitabine-loaded lipid nanocapsules target monocytic myeloid-derived suppressor cells and potentiate cancer immunotherapy. Biomaterials 2016, 96, 47–62. [Google Scholar] [CrossRef]
- Zhan, X.; Jia, L.; Niu, Y.; Qi, H.; Chen, X.; Zhang, Q.; Zhang, J.; Wang, Y.; Dong, L.; Wang, C. Targeted depletion of tumour-associated macrophages by an alendronate-glucomannan conjugate for cancer immunotherapy. Biomaterials 2014, 35, 10046–10057. [Google Scholar] [CrossRef]
- Wang, L.; Niu, M.; Zheng, C.; Zhao, H.; Niu, X.; Li, L.; Hu, Y.; Zhang, Y.; Shi, J.; Zhang, Z. A Core-Shell Nanoplatform for Synergistic Enhanced Sonodynamic Therapy of Hypoxic Tumor via Cascaded Strategy. Adv. Healthc. Mater. 2018, 7, e1800819. [Google Scholar] [CrossRef]
- Qin, L.; Gao, H. The application of nitric oxide delivery in nanoparticle-based tumor targeting drug delivery and treatment. Asian J. Pharm. Sci. 2019, 14, 380–390. [Google Scholar] [CrossRef]
- Cao, J.; Zheng, M.; Sun, Z.; Li, Z.; Qi, X.; Shen, S. One-Step Fabrication of Multifunctional PLGA-HMME-DTX@MnO(2) Nanoparticles for Enhanced Chemo-Sonodynamic Antitumor Treatment. Int. J. Nanomed. 2022, 17, 2577–2591. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.X.; Zhang, J.; Xue, F.; Liu, W.; Kuang, Y.; Gu, B.; Song, S.; Chen, H. In situ forming oxygen/ROS-responsive niche-like hydrogel enabling gelation-triggered chemotherapy and inhibition of metastasis. Bioact. Mater. 2023, 21, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, F.; Han, X.; Zhang, L.; Hu, Z.; Jiang, Q.; Wang, Z.; Ran, H.; Wang, D.; Li, P. Nanosonosensitizers for Highly Efficient Sonodynamic Cancer Theranostics. Theranostics 2018, 8, 6178–6194. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ma, W.; Yang, Y.; He, M.; Li, A.; Bai, L.; Yu, B.; Yu, Z. Cancer nanotechnology: Enhancing tumor cell response to chemotherapy for hepatocellular carcinoma therapy. Asian J. Pharm. Sci. 2019, 14, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Ji, J.; Liu, Z. Multifunctional MnO(2) nanoparticles for tumor microenvironment modulation and cancer therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1720. [Google Scholar] [CrossRef]
- Srivatsan, A.; Jenkins, S.V.; Jeon, M.; Wu, Z.; Kim, C.; Chen, J.; Pandey, R.K. Gold nanocage-photosensitizer conjugates for dual-modal image-guided enhanced photodynamic therapy. Theranostics 2014, 4, 163–174. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, Y.; Wang, H.; Du, H. Synergistic Integration of Layer-by-Layer Assembly of Photosensitizer and Gold Nanorings for Enhanced Photodynamic Therapy in the Near Infrared. ACS Nano 2015, 9, 8744–8754. [Google Scholar] [CrossRef]
- Kumthekar, P.; Ko, C.H.; Paunesku, T.; Dixit, K.; Sonabend, A.M.; Bloch, O.; Tate, M.; Schwartz, M.; Zuckerman, L.; Lezon, R.; et al. A first-in-human phase 0 clinical study of RNA interference-based spherical nucleic acids in patients with recurrent glioblastoma. Sci. Transl. Med. 2021, 13, eabb3945. [Google Scholar] [CrossRef]
- Haque, S.; Norbert, C.C.; Acharyya, R.; Mukherjee, S.; Kathirvel, M.; Patra, C.R. Biosynthesized Silver Nanoparticles for Cancer Therapy and In Vivo Bioimaging. Cancers 2021, 13, 6114. [Google Scholar] [CrossRef]
- He, Y.; Du, Z.; Ma, S.; Liu, Y.; Li, D.; Huang, H.; Jiang, S.; Cheng, S.; Wu, W.; Zhang, K.; et al. Effects of green-synthesized silver nanoparticles on lung cancer cells in vitro and grown as xenograft tumors in vivo. Int. J. Nanomed. 2016, 11, 1879–1887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Qu, Q.; Zhao, Y. Targeted delivery of 5-aminolevulinic acid by multifunctional hollow mesoporous silica nanoparticles for photodynamic skin cancer therapy. ACS Appl. Mater. Interfaces 2015, 7, 10671–10676. [Google Scholar] [CrossRef]
- Tu, J.; Wang, T.; Shi, W.; Wu, G.; Tian, X.; Wang, Y.; Ge, D.; Ren, L. Multifunctional ZnPc-loaded mesoporous silica nanoparticles for enhancement of photodynamic therapy efficacy by endolysosomal escape. Biomaterials 2012, 33, 7903–7914. [Google Scholar] [CrossRef]
- Mrozek, E.; Rhoades, C.A.; Allen, J.; Hade, E.M.; Shapiro, C.L. Phase I trial of liposomal encapsulated doxorubicin (Myocet; D-99) and weekly docetaxel in advanced breast cancer patients. Ann. Oncol. 2005, 16, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, D.D.; Pyle, L.; Allen, M.; Vaughan, M.; Webb, A.; Johnston, S.R.; Gore, M.E. A phase I dose-finding study of a combination of pegylated liposomal doxorubicin (Doxil), carboplatin and paclitaxel in ovarian cancer. Br. J. Cancer 2002, 86, 1379–1384. [Google Scholar] [CrossRef] [Green Version]
- James, N.D.; Coker, R.J.; Tomlinson, D.; Harris, J.R.; Gompels, M.; Pinching, A.J.; Stewart, J.S. Liposomal doxorubicin (Doxil): An effective new treatment for Kaposi’s sarcoma in AIDS. Clin. Oncol. R. Coll. Radiol. 1994, 6, 294–296. [Google Scholar] [CrossRef]
- Li, G.; Liu, D.; Kimchi, E.T.; Kaifi, J.T.; Qi, X.; Manjunath, Y.; Liu, X.; Deering, T.; Avella, D.M.; Fox, T.; et al. Nanoliposome C6-Ceramide Increases the Anti-tumor Immune Response and Slows Growth of Liver Tumors in Mice. Gastroenterology 2018, 154, 1024–1036.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasey, P.A.; Kaye, S.B.; Morrison, R.; Twelves, C.; Wilson, P.; Duncan, R.; Thomson, A.H.; Murray, L.S.; Hilditch, T.E.; Murray, T.; et al. Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin]: First member of a new class of chemotherapeutic agents-drug-polymer conjugates. Cancer Research Campaign Phase I/II Committee. Clin. Cancer Res. 1999, 5, 83–94. [Google Scholar]
- Park, K.; Lee, S.; Kang, E.; Kim, K.; Choi, K.; Kwon, I.C. New Generation of Multifunctional Nanoparticles for Cancer Imaging and Therapy. Adv. Funct. Mater. 2009, 19, 1553–1566. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kim, D.W.; Chung, J.Y.; Shin, S.G.; Kim, S.C.; Heo, D.S.; Kim, N.K.; Bang, Y.J. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin. Cancer Res. 2004, 10, 3708–3716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamboni, W.C. Liposomal, nanoparticle, and conjugated formulations of anticancer agents. Clin. Cancer Res. 2005, 11, 8230–8234. [Google Scholar] [CrossRef] [Green Version]
- Xiong, S.; Yu, B.; Wu, J.; Li, H.; Lee, R.J. Preparation, therapeutic efficacy and intratumoral localization of targeted daunorubicin liposomes conjugating folate-PEG-CHEMS. Biomed. Pharmacother. 2011, 65, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Xiong, M.H.; Wang, Y.C.; Zhu, J.; Wang, J. N-acetylgalactosamine functionalized mixed micellar nanoparticles for targeted delivery of siRNA to liver. J. Control. Release 2013, 166, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Teo, J.Y.; Chin, W.; Ke, X.; Gao, S.; Liu, S.; Cheng, W.; Hedrick, J.L.; Yang, Y.Y. pH and redox dual-responsive biodegradable polymeric micelles with high drug loading for effective anticancer drug delivery. Nanomedicine 2017, 13, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Itaka, K.; Nomoto, T.; Ishii, T.; Suma, T.; Ikegami, M.; Miyata, K.; Oba, M.; Nishiyama, N.; Kataoka, K. Modulated protonation of side chain aminoethylene repeats in N-substituted polyaspartamides promotes mRNA transfection. J. Am. Chem. Soc. 2014, 136, 12396–12405. [Google Scholar] [CrossRef] [Green Version]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Verschraegen, C.F.; Skubitz, K.; Daud, A.; Kudelka, A.P.; Rabinowitz, I.; Allievi, C.; Eisenfeld, A.; Singer, J.W.; Oldham, F.B. A phase I and pharmacokinetic study of paclitaxel poliglumex and cisplatin in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2009, 63, 903–910. [Google Scholar] [CrossRef]
- Caster, J.M.; Patel, A.N.; Zhang, T.; Wang, A. Investigational nanomedicines in 2016: A review of nanotherapeutics currently undergoing clinical trials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1416. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Akbarzadeh, A.; Samiei, M.; Joo, S.W.; Anzaby, M.; Hanifehpour, Y.; Nasrabadi, H.T.; Davaran, S. Synthesis, characterization and in vitro studies of doxorubicin-loaded magnetic nanoparticles grafted to smart copolymers on A549 lung cancer cell line. J. Nanobiotechnol. 2012, 10, 46. [Google Scholar] [CrossRef] [Green Version]
- Oroojalian, F.; Beygi, M.; Baradaran, B.; Mokhtarzadeh, A.; Shahbazi, M.A. Immune Cell Membrane-Coated Biomimetic Nanoparticles for Targeted Cancer Therapy. Small 2021, 17, e2006484. [Google Scholar] [CrossRef] [PubMed]
- Bol, K.F.; Schreibelt, G.; Gerritsen, W.R.; de Vries, I.J.; Figdor, C.G. Dendritic Cell-Based Immunotherapy: State of the Art and Beyond. Clin. Cancer Res. 2016, 22, 1897–1906. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Hu, Y.; Luo, S.; Wang, Y.; Gong, T.; Sun, X.; Fu, Y.; Zhang, Z. Neutrophil-mimicking therapeutic nanoparticles for targeted chemotherapy of pancreatic carcinoma. Acta Pharm. Sin. B 2019, 9, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Zhao, Q.; Yu, J.; Zhang, F.; Zhang, H.; Wang, Z. Nanoparticle Targeting of Neutrophils for Improved Cancer Immunotherapy. Adv. Healthc. Mater. 2016, 5, 1088–1093. [Google Scholar] [CrossRef] [Green Version]
- Choi, B.; Park, W.; Park, S.B.; Rhim, W.K.; Han, D.K. Recent trends in cell membrane-cloaked nanoparticles for therapeutic applications. Methods 2020, 177, 2–14. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, J.; Chen, X.; Liu, W.; Chen, T. Cell Membrane Coating Technology: A Promising Strategy for Biomedical Applications. Nanomicro. Lett. 2019, 11, 100. [Google Scholar] [CrossRef] [Green Version]
- Bahmani, B.; Gong, H.; Luk, B.T.; Haushalter, K.J.; DeTeresa, E.; Previti, M.; Zhou, J.; Gao, W.; Bui, J.D.; Zhang, L.; et al. Intratumoral immunotherapy using platelet-cloaked nanoparticles enhances antitumor immunity in solid tumors. Nat. Commun. 2021, 12, 1999. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, W.; Ye, Y.; Hu, Q.; Bomba, H.N.; Gu, Z. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nat. Biomed. Eng. 2017, 1, 11. [Google Scholar] [CrossRef]
- Hu, Q.; Li, H.; Archibong, E.; Chen, Q.; Ruan, H.; Ahn, S.; Dukhovlinova, E.; Kang, Y.; Wen, D.; Dotti, G.; et al. Inhibition of post-surgery tumour recurrence via a hydrogel releasing CAR-T cells and anti-PDL1-conjugated platelets. Nat. Biomed. Eng. 2021, 5, 1038–1047. [Google Scholar] [CrossRef]
- Lv, Y.; Li, F.; Wang, S.; Lu, G.; Bao, W.; Wang, Y.; Tian, Z.; Wei, W.; Ma, G. Near-infrared light-triggered platelet arsenal for combined photothermal-immunotherapy against cancer. Sci. Adv. 2021, 7, eabd7614. [Google Scholar] [CrossRef]
- Lu, Q.; Ye, H.; Wang, K.; Zhao, J.; Wang, H.; Song, J.; Fan, X.; Lu, Y.; Cao, L.; Wan, B.; et al. Bioengineered Platelets Combining Chemotherapy and Immunotherapy for Postsurgical Melanoma Treatment: Internal Core-Loaded Doxorubicin and External Surface-Anchored Anti-PD-L1 Antibody Backpacks. Nano Lett. 2022, 22, 3141–3150. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, D.; Mei, D.; Zhang, H.; Wang, Z.; He, B.; Dai, W.; Zhang, H.; Wang, X.; Zhang, Q. Macrophage mediated biomimetic delivery system for the treatment of lung metastasis of breast cancer. J. Control. Release 2015, 204, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, K.; Li, T.; Chen, Z.; Wen, Y.; Liu, X.; Jia, X.; Zhang, Y.; Xu, Y.; Han, M.; et al. Monocyte-mediated chemotherapy drug delivery in glioblastoma. Nanomedicine 2018, 13, 157–178. [Google Scholar] [CrossRef]
- Tang, K.; Zhang, Y.; Zhang, H.; Xu, P.; Liu, J.; Ma, J.; Lv, M.; Li, D.; Katirai, F.; Shen, G.X.; et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat. Commun. 2012, 3, 1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanaihara, N.; Caplen, N.; Bowman, E.; Seike, M.; Kumamoto, K.; Yi, M.; Stephens, R.M.; Okamoto, A.; Yokota, J.; Tanaka, T.; et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell 2006, 9, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Saari, H.; Lazaro-Ibanez, E.; Viitala, T.; Vuorimaa-Laukkanen, E.; Siljander, P.; Yliperttula, M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J. Control. Release 2015, 220, 727–737. [Google Scholar] [CrossRef] [Green Version]
- Ohno, S.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 2013, 21, 185–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivoltini, L.; Chiodoni, C.; Squarcina, P.; Tortoreto, M.; Villa, A.; Vergani, B.; Burdek, M.; Botti, L.; Arioli, I.; Cova, A.; et al. TNF-Related Apoptosis-Inducing Ligand (TRAIL)-Armed Exosomes Deliver Proapoptotic Signals to Tumor Site. Clin. Cancer Res. 2016, 22, 3499–3512. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Korovin, M.S.; Fomenko, A.N. Application of nanodimensional particles and aluminum hydroxide nanostructures for cancer diagnosis and therapy. AIP Conf. Proc. 2017, 1882, 020036. [Google Scholar]
- Dykman, L.A.; Khlebtsov, N.G. Gold nanoparticles in biology and medicine: Recent advances and prospects. Acta Nat. 2011, 3, 34–55. [Google Scholar] [CrossRef] [Green Version]
- Sakthi Devi, R.; Girigoswami, A.; Siddharth, M.; Girigoswami, K. Applications of Gold and Silver Nanoparticles in Theranostics. Appl. Biochem. Biotechnol. 2022, 194, 4187–4219. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, Y.; Du, H.; Ren, L.; Wang, H. Colloidal plasmonic gold nanoparticles and gold nanorings: Shape-dependent generation of singlet oxygen and their performance in enhanced photodynamic cancer therapy. Int. J. Nanomed. 2018, 13, 2065–2078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, T.; Lee, S.; Amatya, R.; Cheong, H.; Moon, C.; Kwak, H.D.; Min, K.A.; Shin, M.C. ICG-Loaded PEGylated BSA-Silver Nanoparticles for Effective Photothermal Cancer Therapy. Int. J. Nanomed. 2020, 15, 5459–5471. [Google Scholar] [CrossRef]
- Teodor, E.D.; Radu, G.L. Phyto-synthesized Gold Nanoparticles as Antitumor Agents. Pharm. Nanotechnol. 2021, 9, 51–60. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Herdade, A.M.; Guerra, C.; Peixoto, D.; Pereira-Silva, M.; Zeinali, M.; Mascarenhas-Melo, F.; Paranhos, A.; Veiga, F. Plant-mediated green synthesis of metal-based nanoparticles for dermopharmaceutical and cosmetic applications. Int. J. Pharm. 2021, 597, 120311. [Google Scholar] [CrossRef]
- Vijayan, R.; Joseph, S.; Mathew, B. Anticancer, antimicrobial, antioxidant, and catalytic activities of green-synthesized silver and gold nanoparticles using Bauhinia purpurea leaf extract. Bioprocess Biosyst. Eng. 2019, 42, 305–319. [Google Scholar] [CrossRef]
- Naraginti, S.; Li, Y. Preliminary investigation of catalytic, antioxidant, anticancer and bactericidal activity of green synthesized silver and gold nanoparticles using Actinidia deliciosa. J. Photochem. Photobiol. B 2017, 170, 225–234. [Google Scholar] [CrossRef]
- Anand, K.; Tiloke, C.; Naidoo, P.; Chuturgoon, A.A. Phytonanotherapy for management of diabetes using green synthesis nanoparticles. J. Photochem. Photobiol. B 2017, 173, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Baghbani-Arani, F.; Movagharnia, R.; Sharifian, A.; Salehi, S.; Shandiz, S.A.S. Photo-catalytic, anti-bacterial, and anti-cancer properties of phyto-mediated synthesis of silver nanoparticles from Artemisia tournefortiana Rchb extract. J. Photochem. Photobiol. B 2017, 173, 640–649. [Google Scholar] [CrossRef]
- Sharma, R.; Srivastava, N. Plant Mediated Silver Nanoparticles and Mode of Action in Cancer Therapy: A Review. Anticancer Agents Med. Chem. 2021, 21, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Barkat, A.; Beg, S.; Panda, S.K.; Alharbi, K.S.; Rahman, M.; Ahmed, F.J. Functionalized mesoporous silica nanoparticles in anticancer therapeutics. Semin. Cancer Biol. 2021, 69, 365–375. [Google Scholar] [CrossRef]
- Tarn, D.; Ashley, C.E.; Xue, M.; Carnes, E.C.; Zink, J.I.; Brinker, C.J. Mesoporous silica nanoparticle nanocarriers: Biofunctionality and biocompatibility. Acc. Chem. Res. 2013, 46, 792–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, S.; Singh, R.K.; Khanal, D.; Patel, K.D.; Lee, E.J.; Leong, K.W.; Chrzanowski, W.; Kim, H.W. Smart multifunctional drug delivery towards anticancer therapy harmonized in mesoporous nanoparticles. Nanoscale 2015, 7, 14191–14216. [Google Scholar] [CrossRef]
- Kumar, P.; Tambe, P.; Paknikar, K.M.; Gajbhiye, V. Folate/N-acetyl glucosamine conjugated mesoporous silica nanoparticles for targeting breast cancer cells: A comparative study. Colloids Surf. B Biointerfaces 2017, 156, 203–212. [Google Scholar] [CrossRef]
- Gu, F.X.; Karnik, R.; Wang, A.Z.; Alexis, F.; Levy-Nissenbaum, E.; Hong, S.; Langer, R.S.; Farokhzad, O.C. Targeted nanoparticles for cancer therapy. Nano Today 2007, 2, 14–21. [Google Scholar] [CrossRef]
- Hong, E.J.; Choi, D.G.; Shim, M.S. Targeted and effective photodynamic therapy for cancer using functionalized nanomaterials. Acta Pharm. Sin. B 2016, 6, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Apostolova, N.; Victor, V.M. Molecular strategies for targeting antioxidants to mitochondria: Therapeutic implications. Antioxid. Redox Signal. 2015, 22, 686–729. [Google Scholar] [CrossRef] [Green Version]
- Tuo, J.; Xie, Y.; Song, J.; Chen, Y.; Guo, Q.; Liu, X.; Ni, X.; Xu, D.; Huang, H.; Yin, S.; et al. Development of a novel berberine-mediated mitochondria-targeting nano-platform for drug-resistant cancer therapy. J. Mater. Chem. B 2016, 4, 6856–6864. [Google Scholar] [CrossRef]
- Lo, Y.L.; Wang, C.S.; Chen, Y.C.; Wang, T.Y.; Chang, Y.H.; Chen, C.J.; Yang, C.P. Mitochondrion-Directed Nanoparticles Loaded with a Natural Compound and a microRNA for Promoting Cancer Cell Death via the Modulation of Tumor Metabolism and Mitochondrial Dynamics. Pharmaceutics 2020, 12, 756. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liang, J.; Gao, C.; Wang, A.; Xia, J.; Hong, C.; Zhong, Z.; Zuo, Z.; Kim, J.; Ren, H.; et al. Multifunctional ginsenoside Rg3-based liposomes for glioma targeting therapy. J. Control. Release 2021, 330, 641–657. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, Y.; Liu, S.; Cai, J.; Zhang, Q.; Li, K.; Liu, Z.; Shi, M.; Wang, J.; Cui, H. Aptamer-functionalized quercetin thermosensitive liposomes for targeting drug delivery and antitumor therapy. Biomed. Mater. 2022, 17, 65003. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, J.; Jiang, J.; He, Y.; Zhang, W.; Mo, X.; Kang, X.; Xu, Q.; Wang, B.; Huang, Y. Remodeling tumor immune microenvironment (TIME) for glioma therapy using multi-targeting liposomal codelivery. J. Immunother. Cancer 2020, 8, e000207. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Q.; Zhang, X.; Luo, L.; He, Y.; Zhu, R.; Gao, D. Dual-targeting liposomes for enhanced anticancer effect in somatostatin receptor II-positive tumor model. Nanomedicine 2018, 13, 2155–2169. [Google Scholar] [CrossRef] [PubMed]
- Gai, C.; Liu, C.; Wu, X.; Yu, M.; Zheng, J.; Zhang, W.; Lv, S.; Li, W. MT1DP loaded by folate-modified liposomes sensitizes erastin-induced ferroptosis via regulating miR-365a-3p/NRF2 axis in non-small cell lung cancer cells. Cell Death Dis. 2020, 11, 751. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, X.; Zheng, G.; Yao, H.; Liang, H. In vitro and in vivo antitumor effects of lupeol-loaded galactosylated liposomes. Drug Deliv. 2021, 28, 709–718. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Meng, J.; Guo, F.; Xu, H.; Liang, W.; Wang, C.; Yang, X.D. Combination Therapy using Co-encapsulated Resveratrol and Paclitaxel in Liposomes for Drug Resistance Reversal in Breast Cancer Cells in vivo. Sci. Rep. 2016, 6, 22390. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Sun, Z.; Shen, Q.; Huang, Z.; Wang, S.; Yang, N.; Li, G.; Wu, Q.; Wang, W.; Li, L.; et al. Rational design of nanocarriers for mitochondria-targeted drug delivery. Chin. Chem. Lett. 2022, 33, 4146–4156. [Google Scholar] [CrossRef]
- Pijeira, M.S.O.; Viltres, H.; Kozempel, J.; Sakmár, M.; Vlk, M.; İlem-Özdemir, D.; Ekinci, M.; Srinivasan, S.; Rajabzadeh, A.R.; Ricci-Junior, E.; et al. Radiolabeled nanomaterials for biomedical applications: Radiopharmacy in the era of nanotechnology. EJNMMI Radiopharm. Chem. 2022, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, S.; Owrang, M.; Javaheri, F.; Farjadian, F. Spermine Modified PNIPAAm Nano-Hydrogel Serving as Thermo Responsive System for Delivery of Cisplatin. Macromol. Res. 2022, 30, 314–324. [Google Scholar] [CrossRef]
- He, Q.; Yan, R.; Hou, W.; Wang, H.; Tian, Y. A pH-Responsive Zwitterionic Polyurethane Prodrug as Drug Delivery System for Enhanced Cancer Therapy. Molecules 2021, 26, 74. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Bai, T.; Du, J.; Kong, J. One-pot synthesis of glutathione-responsive amphiphilic drug self-delivery micelles of doxorubicin-disulfide-methoxy polyethylene glycol for tumor therapy. J. Mater. Chem. B 2018, 6, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Kim, T.H.; Wu, W.C.; Huang, C.M.; Wei, H.; Mount, C.W.; Tian, Y.; Jang, S.H.; Pun, S.H.; Jen, A.K. pH-dependent, thermosensitive polymeric nanocarriers for drug delivery to solid tumors. Biomaterials 2013, 34, 4501–4509. [Google Scholar] [CrossRef] [Green Version]
- Alotaibi, K.M.; Almethen, A.A.; Beagan, A.M.; Alfhaid, L.H.; Ahamed, M.; El-Toni, A.M.; Alswieleh, A.M. Poly(oligo(ethylene glycol) methyl ether methacrylate) Capped pH-Responsive Poly(2-(diethylamino)ethyl methacrylate) Brushes Grafted on Mesoporous Silica Nanoparticles as Nanocarrier. Polymers 2021, 13, 823. [Google Scholar] [CrossRef]
- Hou, S.L.; Chen, S.S.; Huang, Z.J.; Lu, Q.H. Dual-responsive polyphosphazene as a common platform for highly efficient drug self-delivery. J. Mater. Chem. B 2019, 7, 4319–4327. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, J.; Zhao, X.; Zhang, Y.; Zhong, Z.; Deng, C. A polymeric IDO inhibitor based on poly(ethylene glycol)-b-poly(L-tyrosine-co-1-methyl-D-tryptophan) enables facile trident cancer immunotherapy. Biomater. Sci. 2022, 10, 5731–5743. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Renukuntla, J. Thermo- and pH dual responsive polymeric micelles and nanoparticles. Chem. Biol. Interact. 2018, 295, 20–37. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, B.; Luo, Z.; Ding, X.; Li, J.; Dai, L.; Zhou, J.; Zhao, X.; Ye, J.; Cai, K. Enzyme responsive mesoporous silica nanoparticles for targeted tumor therapy in vitro and in vivo. Nanoscale 2015, 7, 3614–3626. [Google Scholar] [CrossRef]
- Li, B.; Pang, S.; Li, X.; Li, Y. PH and redox dual-responsive polymeric micelles with charge conversion for paclitaxel delivery. J. Biomater. Sci. Polym. Ed. 2020, 31, 2078–2093. [Google Scholar] [CrossRef]
- Whitlow, J.; Pacelli, S.; Paul, A. Polymeric Nanohybrids as a New Class of Therapeutic Biotransporters. Macromol. Chem. Phys. 2016, 217, 1245–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Qin, Y.; Zhang, Z.; Fan, F.; Huang, C.; Lu, L.; Wang, H.; Jin, X.; Zhao, H.; Kong, D.; et al. Dual pH/reduction-responsive hybrid polymeric micelles for targeted chemo-photothermal combination therapy. Acta Biomater. 2018, 75, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Mottaghitalab, F.; Farokhi, M.; Fatahi, Y.; Atyabi, F.; Dinarvand, R. New insights into designing hybrid nanoparticles for lung cancer: Diagnosis and treatment. J. Control. Release 2019, 295, 250–267. [Google Scholar] [CrossRef]
- Mignani, S.; Shi, X.; Cena, V.; Rodrigues, J.; Tomas, H.; Majoral, J.P. Engineered non-invasive functionalized dendrimer/dendron-entrapped/complexed gold nanoparticles as a novel class of theranostic (radio)pharmaceuticals in cancer therapy. J. Control. Release 2021, 332, 346–366. [Google Scholar] [CrossRef]
- Pfaff, A.; Schallon, A.; Ruhland, T.M.; Majewski, A.P.; Schmalz, H.; Freitag, R.; Muller, A.H. Magnetic and fluorescent glycopolymer hybrid nanoparticles for intranuclear optical imaging. Biomacromolecules 2011, 12, 3805–3811. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, L.; Peng, C.; Shen, M.; Shi, X.; Zhang, G. Folic acid-modified dendrimer-entrapped gold nanoparticles as nanoprobes for targeted CT imaging of human lung adencarcinoma. Biomaterials 2013, 34, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Elhabak, M.; Osman, R.; Mohamed, M.; El-Borady, O.M.; Awad, G.A.S.; Mortada, N. Near IR responsive targeted integrated lipid polymer nanoconstruct for enhanced magnolol cytotoxicity in breast cancer. Sci. Rep. 2020, 10, 8771. [Google Scholar] [CrossRef]
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review. Eur. J. Pharm. Biopharm. 2013, 85, 427–443. [Google Scholar] [CrossRef]
- Gautier, J.; Allard-Vannier, E.; Herve-Aubert, K.; Souce, M.; Chourpa, I. Design strategies of hybrid metallic nanoparticles for theragnostic applications. Nanotechnology 2013, 24, 432002. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, X.; Jia, X.; Bai, J.; Jiang, X. Folate-Conjugated Polylactic Acid-Silica Hybrid Nanoparticles as Degradable Carriers for Targeted Drug Delivery, On-Demand Release and Simultaneous Self-Clearance. Chempluschem 2016, 81, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, J.C.; Patel, A.K.; Kauffman, K.J.; Fenton, O.S.; Webber, M.J.; Heartlein, M.W.; DeRosa, F.; Anderson, D.G. Polymer-Lipid Nanoparticles for Systemic Delivery of mRNA to the Lungs. Angew. Chem. Int. Ed. Engl. 2016, 55, 13808–13812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Ren, X.; Xu, S.; Zhang, D.; Han, T. Optimization of Lipid Nanoformulations for Effective mRNA Delivery. Int. J. Nanomed. 2022, 17, 2893–2905. [Google Scholar] [CrossRef]
- Fraix, A.; Conte, C.; Gazzano, E.; Riganti, C.; Quaglia, F.; Sortino, S. Overcoming Doxorubicin Resistance with Lipid-Polymer Hybrid Nanoparticles Photoreleasing Nitric Oxide. Mol. Pharm. 2020, 17, 2135–2144. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, D.; Song, Q.; Wu, T.; Zhuang, X.; Bao, Y.; Kong, M.; Qi, Y.; Tan, S.; Zhang, Z. Erythrocyte Membrane-Enveloped Polymeric Nanoparticles as Nanovaccine for Induction of Antitumor Immunity against Melanoma. ACS Nano 2015, 9, 6918–6933. [Google Scholar] [CrossRef]
- Mandal, B.; Bhattacharjee, H.; Mittal, N.; Sah, H.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Core-shell-type lipid-polymer hybrid nanoparticles as a drug delivery platform. Nanomedicine 2013, 9, 474–491. [Google Scholar] [CrossRef]
- Reuven, E.M.; Leviatan Ben-Arye, S.; Yu, H.; Duchi, R.; Perota, A.; Conchon, S.; Bachar Abramovitch, S.; Soulillou, J.P.; Galli, C.; Chen, X.; et al. Biomimetic Glyconanoparticle Vaccine for Cancer Immunotherapy. ACS Nano 2019, 13, 2936–2947. [Google Scholar] [CrossRef]
- Peng, H.; Xu, Z.; Wang, Y.; Feng, N.; Yang, W.; Tang, J. Biomimetic Mesoporous Silica Nanoparticles for Enhanced Blood Circulation and Cancer Therapy. ACS Appl. Bio Mater. 2020, 3, 7849–7857. [Google Scholar] [CrossRef]
- Pitchaimani, A.; Nguyen, T.D.T.; Aryal, S. Natural killer cell membrane infused biomimetic liposomes for targeted tumor therapy. Biomaterials 2018, 160, 124–137. [Google Scholar] [CrossRef]
- Parodi, A.; Molinaro, R.; Sushnitha, M.; Evangelopoulos, M.; Martinez, J.O.; Arrighetti, N.; Corbo, C.; Tasciotti, E. Bio-inspired engineering of cell- and virus-like nanoparticles for drug delivery. Biomaterials 2017, 147, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Pan, H.; Li, W.; Chen, Z.; Ma, A.; Yin, T.; Liang, R.; Chen, F.; Ma, Y.; Jin, Y.; et al. T Cell Membrane Mimicking Nanoparticles with Bioorthogonal Targeting and Immune Recognition for Enhanced Photothermal Therapy. Adv. Sci. 2019, 6, 1900251. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Zhang, W.; Tang, Q.; Zhou, Y.; Li, Y.; Rong, T.; Wang, H.; Chen, Y. Isolated cell-bound membrane vesicles (CBMVs) as a novel class of drug nanocarriers. J. Nanobiotechnol. 2020, 18, 69. [Google Scholar] [CrossRef]
- Hu, C.M.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Mei, C.; Tian, Y.; Nie, T.; Liu, Z.; Chen, T. Bioinspired tumor-homing nanosystem for precise cancer therapy via reprogramming of tumor-associated macrophages. NPG Asia Mater. 2018, 10, 1002–1015. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Liu, W.; Hu, Y.; Li, W.; Di, W. Bioinspired tumor-homing nanoplatform for co-delivery of paclitaxel and siRNA-E7 to HPV-related cervical malignancies for synergistic therapy. Theranostics 2020, 10, 3325–3339. [Google Scholar] [CrossRef]
- Liu, J.; Wu, F.; Zhou, H. Macrophage-derived exosomes in cancers: Biogenesis, functions and therapeutic applications. Immunol. Lett. 2020, 227, 102–108. [Google Scholar] [CrossRef]
- Zhang, Y.; Guoqiang, L.; Sun, M.; Lu, X. Targeting and exploitation of tumor-associated neutrophils to enhance immunotherapy and drug delivery for cancer treatment. Cancer Biol. Med. 2020, 17, 32–43. [Google Scholar] [CrossRef]
- Zhao, L.; Gu, C.; Gan, Y.; Shao, L.; Chen, H.; Zhu, H. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J. Control. Release 2020, 318, 1–15. [Google Scholar] [CrossRef]
- Zuo, B.; Qi, H.; Lu, Z.; Chen, L.; Sun, B.; Yang, R.; Zhang, Y.; Liu, Z.; Gao, X.; You, A.; et al. Alarmin-painted exosomes elicit persistent antitumor immunity in large established tumors in mice. Nat. Commun. 2020, 11, 1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Q.; Ling, X.; Yang, Y.; Zhang, J.; Li, Q.; Niu, X.; Hu, G.; Chen, B.; Li, H.; Wang, Y.; et al. Embryonic Stem Cells-Derived Exosomes Endowed with Targeting Properties as Chemotherapeutics Delivery Vehicles for Glioblastoma Therapy. Adv. Sci. 2019, 6, 1801899. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, J.; Huang, L. Modulation of tumor microenvironment for immunotherapy: Focus on nanomaterial-based strategies. Theranostics 2020, 10, 3099–3117. [Google Scholar] [CrossRef] [PubMed]
- Phuengkham, H.; Ren, L.; Shin, I.W.; Lim, Y.T. Nanoengineered Immune Niches for Reprogramming the Immunosuppressive Tumor Microenvironment and Enhancing Cancer Immunotherapy. Adv. Mater. 2019, 31, e1803322. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef] [PubMed]
- Upreti, M.; Jyoti, A.; Sethi, P. Tumor microenvironment and nanotherapeutics. Transl. Cancer Res. 2013, 2, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhang, B.; Shen, S.; Tuo, Y.; Luo, Z.; Hu, Y.; Pang, Z.; Jiang, X. Tumor Microenvironment Modulation by Cyclopamine Improved Photothermal Therapy of Biomimetic Gold Nanorods for Pancreatic Ductal Adenocarcinomas. ACS Appl. Mater. Interfaces 2017, 9, 31497–31508. [Google Scholar] [CrossRef]
- Zhang, D.; Feng, F.; Li, Q.; Wang, X.; Yao, L. Nanopurpurin-based photodynamic therapy destructs extracellular matrix against intractable tumor metastasis. Biomaterials 2018, 173, 22–33. [Google Scholar] [CrossRef]
- Paardekooper, L.M.; Vos, W.; van den Bogaart, G. Oxygen in the tumor microenvironment: Effects on dendritic cell function. Oncotarget 2019, 10, 883–896. [Google Scholar] [CrossRef] [Green Version]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Zhao, Y.; Ma, H.; Sun, Y.; Cao, J. How to improve photodynamic therapy-induced antitumor immunity for cancer treatment? Theranostics 2022, 12, 4629–4655. [Google Scholar] [CrossRef]
- Gupta, Y.H.; Khanom, A.; Acton, S.E. Control of Dendritic Cell Function Within the Tumour Microenvironment. Front. Immunol. 2022, 13, 733800. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, Y.; He, Y.; Du, Y.; Wang, W.; Shi, X.; Gao, F. The use of HA oligosaccharide-loaded nanoparticles to breach the endogenous hyaluronan glycocalyx for breast cancer therapy. Biomaterials 2013, 34, 6829–6838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, X.; Nie, G. Responsive and activable nanomedicines for remodeling the tumor microenvironment. Nat. Protoc. 2021, 16, 405–430. [Google Scholar] [CrossRef] [PubMed]
- Huels, D.J.; Medema, J.P. Think About the Environment: Cellular Reprogramming by the Extracellular Matrix. Cell Stem Cell 2018, 22, 7–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawk, M.A.; Gorsuch, C.L.; Fagan, P.; Lee, C.; Kim, S.E.; Hamann, J.C.; Mason, J.A.; Weigel, K.J.; Tsegaye, M.A.; Shen, L.; et al. RIPK1-mediated induction of mitophagy compromises the viability of extracellular-matrix-detached cells. Nat. Cell Biol. 2018, 20, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, D.; Igaz, N.; Marton, A.; Ronavari, A.; Belteky, P.; Bodai, L.; Spengler, G.; Tiszlavicz, L.; Razga, Z.; Hegyi, P.; et al. Core-shell nanoparticles suppress metastasis and modify the tumour-supportive activity of cancer-associated fibroblasts. J. Nanobiotechnol. 2020, 18, 18. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Zhou, H.; Fan, Z.; Deng, J.; Lemons, P.K.; Arhontoulis, D.C.; Bowne, W.B.; Cheng, H. Hyaluronidase Embedded in Nanocarrier PEG Shell for Enhanced Tumor Penetration and Highly Efficient Antitumor Efficacy. Nano Lett. 2016, 16, 3268–3277. [Google Scholar] [CrossRef]
- Gong, H.; Chao, Y.; Xiang, J.; Han, X.; Song, G.; Feng, L.; Liu, J.; Yang, G.; Chen, Q.; Liu, Z. Hyaluronidase To Enhance Nanoparticle-Based Photodynamic Tumor Therapy. Nano Lett. 2016, 16, 2512–2521. [Google Scholar] [CrossRef]
- Guan, X.; Chen, J.; Hu, Y.; Lin, L.; Sun, P.; Tian, H.; Chen, X. Highly enhanced cancer immunotherapy by combining nanovaccine with hyaluronidase. Biomaterials 2018, 171, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Lang, J.; Wang, J.; Cai, R.; Zhang, Y.; Qi, F.; Zhang, L.; Zhao, X.; Wu, W.; Hao, J.; et al. Designing Liposomes To Suppress Extracellular Matrix Expression To Enhance Drug Penetration and Pancreatic Tumor Therapy. ACS Nano 2017, 11, 8668–8678. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yu, Z.; Sun, D.; Zou, Y.; Liu, Y.; Huang, L. Two nanoformulations induce reactive oxygen species and immunogenetic cell death for synergistic chemo-immunotherapy eradicating colorectal cancer and hepatocellular carcinoma. Mol. Cancer 2021, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Bi, S.; Guo, T.; Sun, D.; Zou, Y.; Wang, L.; Song, L.; Chu, D.; Liao, A.; Song, X.; et al. Nano co-delivery of Plumbagin and Dihydrotanshinone I reverses immunosuppressive TME of liver cancer. J. Control. Release 2022, 348, 250–263. [Google Scholar] [CrossRef]
- Dudek, A.M.; Garg, A.D.; Krysko, D.V.; De Ruysscher, D.; Agostinis, P. Inducers of immunogenic cancer cell death. Cytokine Growth Factor Rev. 2013, 24, 319–333. [Google Scholar] [CrossRef]
- Yu, X.; Fang, C.; Zhang, K.; Su, C. Recent Advances in Nanoparticles-Based Platforms Targeting the PD-1/PD-L1 Pathway for Cancer Treatment. Pharmaceutics 2022, 14, 1581. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Wang, J.; Hu, Q.; Langworthy, B.; Ye, Y.; Sun, W.; Lin, J.; Wang, T.; Fine, J.; et al. PD-1 Blockade Cellular Vesicles for Cancer Immunotherapy. Adv. Mater. 2018, 30, e1707112. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Wang, J.; Ruan, H.; Zhang, X.; Ye, Y.; Shen, S.; Wang, C.; Lu, W.; Cheng, K.; et al. Conjugation of haematopoietic stem cells and platelets decorated with anti-PD-1 antibodies augments anti-leukaemia efficacy. Nat. Biomed. Eng. 2018, 2, 831–840. [Google Scholar] [CrossRef]
- Komohara, Y.; Fujiwara, Y.; Ohnishi, K.; Takeya, M. Tumor-associated macrophages: Potential therapeutic targets for anti-cancer therapy. Adv. Drug Deliv. Rev. 2016, 99, 180–185. [Google Scholar] [CrossRef]
- Ngambenjawong, C.; Gustafson, H.H.; Pun, S.H. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv. Drug Deliv. Rev. 2017, 114, 206–221. [Google Scholar] [CrossRef] [Green Version]
- Peranzoni, E.; Lemoine, J.; Vimeux, L.; Feuillet, V.; Barrin, S.; Kantari-Mimoun, C.; Bercovici, N.; Guerin, M.; Biton, J.; Ouakrim, H.; et al. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment. Proc. Natl. Acad. Sci. USA 2018, 115, E4041–E4050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.; Rao, L.; Yao, H.; Wang, Z.; Ning, P.; Chen, X. Engineering Macrophages for Cancer Immunotherapy and Drug Delivery. Adv. Mater. 2020, 32, e2002054. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, C.; Zhang, X.; Chen, G.; Hu, Q.; Li, H.; Wang, J.; Wen, D.; Zhang, Y.; Lu, Y.; et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 2019, 14, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Liu, T.; Guo, Z.; Zhuang, R.; Zhang, X.; Chen, X. Reprogramming Tumor-Associated Macrophages by Nanoparticle-Based Reactive Oxygen Species Photogeneration. Nano Lett. 2018, 18, 7330–7342. [Google Scholar] [CrossRef] [PubMed]
- Riera-Domingo, C.; Audigé, A.; Granja, S.; Cheng, W.C.; Ho, P.C.; Baltazar, F.; Stockmann, C.; Mazzone, M. Immunity, Hypoxia, and Metabolism-the Ménage à Trois of Cancer: Implications for Immunotherapy. Physiol. Rev. 2020, 100, 1–102. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Barbi, J.; Pan, F. The regulation of immune tolerance by FOXP3. Nat. Rev. Immunol. 2017, 17, 703–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Li, Y.; Shi, S.; Dong, C. Exploiting a New Approach to Destroy the Barrier of Tumor Microenvironment: Nano-Architecture Delivery Systems. Molecules 2021, 26, 2703. [Google Scholar] [CrossRef]
- Ou, W.; Thapa, R.K.; Jiang, L.; Soe, Z.C.; Gautam, M.; Chang, J.H.; Jeong, J.H.; Ku, S.K.; Choi, H.G.; Yong, C.S.; et al. Regulatory T cell-targeted hybrid nanoparticles combined with immuno-checkpoint blockage for cancer immunotherapy. J. Control. Release 2018, 281, 84–96. [Google Scholar] [CrossRef]
- Cheng, K.; Ding, Y.; Zhao, Y.; Ye, S.; Zhao, X.; Zhang, Y.; Ji, T.; Wu, H.; Wang, B.; Anderson, G.J.; et al. Sequentially Responsive Therapeutic Peptide Assembling Nanoparticles for Dual-Targeted Cancer Immunotherapy. Nano Lett. 2018, 18, 3250–3258. [Google Scholar] [CrossRef]
- Hou, L.; Liu, Q.; Shen, L.; Liu, Y.; Zhang, X.; Chen, F.; Huang, L. Nano-delivery of fraxinellone remodels tumor microenvironment and facilitates therapeutic vaccination in desmoplastic melanoma. Theranostics 2018, 8, 3781–3796. [Google Scholar] [CrossRef]
- Cecchini, A.; Raffa, V.; Canfarotta, F.; Signore, G.; Piletsky, S.; MacDonald, M.P.; Cuschieri, A. In Vivo Recognition of Human Vascular Endothelial Growth Factor by Molecularly Imprinted Polymers. Nano Lett. 2017, 17, 2307–2312. [Google Scholar] [CrossRef] [Green Version]
- Becker, H.M.; Deitmer, J.W. Transport Metabolons and Acid/Base Balance in Tumor Cells. Cancers 2020, 12, 899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imtiyaz, Z.; He, J.; Leng, Q.; Agrawal, A.K.; Mixson, A.J. pH-Sensitive Targeting of Tumors with Chemotherapy-Laden Nanoparticles: Progress and Challenges. Pharmaceutics 2022, 14, 2427. [Google Scholar] [CrossRef] [PubMed]
- Siriwibool, S.; Kaekratoke, N.; Chansaenpak, K.; Siwawannapong, K.; Panajapo, P.; Sagarik, K.; Noisa, P.; Lai, R.Y.; Kamkaew, A. Near-Infrared Fluorescent pH Responsive Probe for Targeted Photodynamic Cancer Therapy. Sci. Rep. 2020, 10, 1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Som, A.; Raliya, R.; Tian, L.; Akers, W.; Ippolito, J.E.; Singamaneni, S.; Biswas, P.; Achilefu, S. Monodispersed calcium carbonate nanoparticles modulate local pH and inhibit tumor growth in vivo. Nanoscale 2016, 8, 12639–12647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.X.; Chen, S.Y.; Yi, N.B.; Li, X.; Chen, S.L.; Lei, Z.; Cheng, D.B.; Sun, T. Research progress on tumor hypoxia-associative nanomedicine. J. Control. Release 2022, 350, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Luo, H.; Liu, Y.; Zhang, W.; Li, H.; Luo, T.; Zhang, K.; Zhao, Y.; Liu, J. Oxygen-Self-Produced Nanoplatform for Relieving Hypoxia and Breaking Resistance to Sonodynamic Treatment of Pancreatic Cancer. ACS Nano 2017, 11, 12849–12862. [Google Scholar] [CrossRef]
- Zhao, P.; Zheng, M.; Luo, Z.; Fan, X.; Sheng, Z.; Gong, P.; Chen, Z.; Zhang, B.; Ni, D.; Ma, Y.; et al. Oxygen Nanocarrier for Combined Cancer Therapy: Oxygen-Boosted ATP-Responsive Chemotherapy with Amplified ROS Lethality. Adv. Healthc. Mater. 2016, 5, 2161–2167. [Google Scholar] [CrossRef]
- Tian, H.; Luo, Z.; Liu, L.; Zheng, M.; Chen, Z.; Ma, A.; Liang, R.; Han, Z.; Lu, C.; Cai, L. Cancer Cell Membrane-Biomimetic Oxygen Nanocarrier for Breaking Hypoxia-Induced Chemoresistance. Adv. Funct. Mater. 2017, 27, 1703197. [Google Scholar] [CrossRef]
- Song, G.; Ji, C.; Liang, C.; Song, X.; Yi, X.; Dong, Z.; Yang, K.; Liu, Z. TaOx decorated perfluorocarbon nanodroplets as oxygen reservoirs to overcome tumor hypoxia and enhance cancer radiotherapy. Biomaterials 2017, 112, 257–263. [Google Scholar] [CrossRef]
- Chen, M.; Liu, D.; Liu, F.; Wu, Y.; Peng, X.; Song, F. Recent advances of redox-responsive nanoplatforms for tumor theranostics. J. Control. Release 2021, 332, 269–284. [Google Scholar] [CrossRef]
- Guan, X.; Yin, H.H.; Xu, X.H.; Xu, G.; Zhang, Y.; Zhou, B.G.; Yue, W.W.; Liu, C.; Sun, L.P.; Xu, H.X.; et al. Tumor Metabolism-Engineered Composite Nanoplatforms Potentiate Sonodynamic Therapy via Reshaping Tumor Microenvironment and Facilitating Electron–Hole Pairs’ Separation. Adv. Funct. Mater. 2020, 30, 2000326. [Google Scholar] [CrossRef]
- Hu, B.; Yu, M.; Ma, X.; Sun, J.; Liu, C.; Wang, C.; Wu, S.; Fu, P.Y.; Yang, Z.; He, Y.; et al. Interferon-a potentiates anti-PD-1 efficacy by remodeling glucose metabolism in the hepatocellular carcinoma microenvironment. Cancer Discov. 2022, 12, 1718–1741. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, X.; Yong, T.; Bie, N.; Zhan, G.; Li, X.; Liang, Q.; Li, J.; Yu, J.; Huang, G.; et al. Boosting anti-PD-1 therapy with metformin-loaded macrophage-derived microparticles. Nat. Commun. 2021, 12, 440. [Google Scholar] [CrossRef]
- Dai, Q.; Wilhelm, S.; Ding, D.; Syed, A.M.; Sindhwani, S.; Zhang, Y.; Chen, Y.Y.; MacMillan, P.; Chan, W.C.W. Quantifying the Ligand-Coated Nanoparticle Delivery to Cancer Cells in Solid Tumors. ACS Nano 2018, 12, 8423–8435. [Google Scholar] [CrossRef]





| Type | Nanomaterial and Drug | Cancer and Method | Efficacy | Clinical Stage and Ref. |
|---|---|---|---|---|
| Inorganic NPs | Gold and PS | CRC, Breast PTT | Effective delivery and enhances PS’ phototoxicity | Preclinical [48,49] |
| Gold and siRNA | GBM Gene therapy | Reduced tumor-associated Bcl2 protein expression | Phase 0 [50] | |
| Silver and Chinese herb extracts | GBM Undefined | Inhibiting blood vessel formation | Preclinical [51] | |
| Silver and longan peel powder | Lung Chemotherapy | Down-regulated NF-κB and up-regulated Bcl2, caspase-3 and survival rate of mice. | Preclinical [52] | |
| Silica and 5-ALA, ZnPc | Skin, Liver PDT | Enhances PS’ phototoxicity, endolysosomal escape | Preclinical [53,54] | |
| Lipid NPs | Phospholipid, cholesterol and Doxil, carboplatin, paclitaxel | Kaposi’s Sarcoma, ovarian, breast chemotherapy | Inhibit DNA synthesis and induce apoptosis | Phase 1, phase 3 [55,56,57] |
| Phospholipid, Rg3-based liposomes and Ceramide, PTX | HCC, breast immunotherapy | ↓ROS and M2-TAM, ↑M1-TAM and CD8+T; ↓MDSCs, TME remodeling | Preclinical [58] | |
| Polymeric NPs | HPMA, PLGA, PEG-PLA, and DOX, paclitaxel, camptotheci-n | CRC, breast, pancreatic, NSCLCs, ovarian chemotherapy | Inhibit DNA synthesis and induce apoptosis | Phase 1 to 3 [59,60,61,62] |
| Folate-PEG-Chems, GalNAc, PEG-b-PCL, PCL-b-PPEEA and Daunorubici-n, siRNA | Leukemia tumor, liver, Pancreatic Chemotherapy, Gene therapy | Enhanced the endocytosis of cells in vitro and in vivo, hepatocyte-specific gene delivery | Preclinical [63,64,65,66] | |
| Hybrid NPs | Albumin and Paclitaxel | NSCLCs Chemotherapy | Increase drug solubility, improve bioavailability, and promote absorption of drugs by cancer cells | FDA approved [67] |
| PEG, polyglutamic acid, mPEG and D,L-PLA and Camptothecin, paclitaxel | NSCLCs Chemotherapy | Increase drug solubility, improve bioavailability, and promote absorption of drugs by cancer cells | Phase 0 to 2 [68,69,70] | |
| SPIONs, PNIPAAm-MAA and DOX | Lung cancer Chemotherapy, imaging | pH-dependent manner, time-dependent manner | Preclinical [71] | |
| Biomimetic NPs | DC, tumor antigen, and sunitinib | GBM, melanoma, prostate, immunotherapy + radiochemotherapy, immunotherapy + chemotherapy | Activate T-cells’ immune response | FDA approve [72,73] |
| Neutrophil, RBC, Platelets and Celastrol, tumor antigen, CpG, R848 | Pancreatic cancer, melanoma chemotherapy, immunotherapy | Targeting tumor site, prevent liver metastasis of tumor, improve the survival rate of tumor bearing mice, activate immune response | Preclinical [14,74,75,76,77,78] | |
| Natural NPs | Platelets and PD-L1 | Melanomas, breast cancer, immunotherapy | Delivery of anti-PDL1 to the surgical bed and target CTCs, reduce the risk of cancer regrowth and metastatic spread | Preclinical [79,80,81,82] |
| Macrophages Chemical drugs | breast, GBM chemotherapy | Targeted to cancer cells, inhibit tumor invasion | Preclinical [83,84] | |
| Exosomes mi-RNA, chemical drugs, proteins | GBM, breast, ovarian cancer Gene therapy, chemotherapy, immunotherapy | Activate T cell response, inhibit tumor growth | Preclinical [85,86,87,88,89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Chi, Y.; Yang, Z.; Ma, J.; Wang, L. Tumor Microenvironment Regulation and Cancer Targeting Therapy Based on Nanoparticles. J. Funct. Biomater. 2023, 14, 136. https://doi.org/10.3390/jfb14030136
Han S, Chi Y, Yang Z, Ma J, Wang L. Tumor Microenvironment Regulation and Cancer Targeting Therapy Based on Nanoparticles. Journal of Functional Biomaterials. 2023; 14(3):136. https://doi.org/10.3390/jfb14030136
Chicago/Turabian StyleHan, Shulan, Yongjie Chi, Zhu Yang, Juan Ma, and Lianyan Wang. 2023. "Tumor Microenvironment Regulation and Cancer Targeting Therapy Based on Nanoparticles" Journal of Functional Biomaterials 14, no. 3: 136. https://doi.org/10.3390/jfb14030136
APA StyleHan, S., Chi, Y., Yang, Z., Ma, J., & Wang, L. (2023). Tumor Microenvironment Regulation and Cancer Targeting Therapy Based on Nanoparticles. Journal of Functional Biomaterials, 14(3), 136. https://doi.org/10.3390/jfb14030136