Degassing a Decellularized Scaffold Enhances Wound Healing and Reduces Fibrosis during Tracheal Defect Reconstruction: A Preliminary Animal Study
Abstract
:1. Introduction
2. Materials and Methods
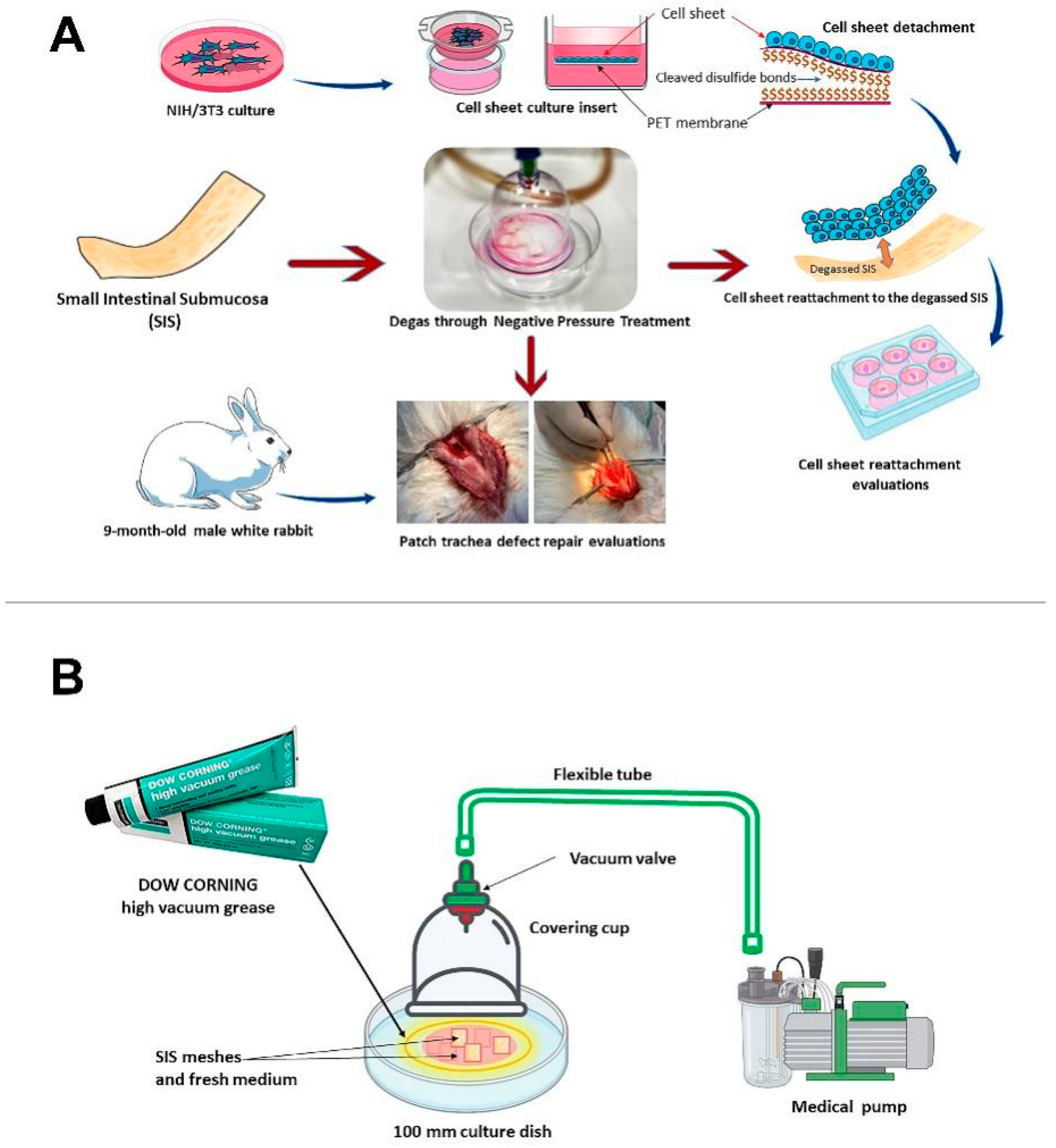
2.1. SIS Mesh Preparation and Degassing
2.1.1. SIS Mesh Preparation
2.1.2. Degassing of the SIS Scaffold
2.2. Cell Sheet Fabrication
2.2.1. Preparation of the Cell Culture Inserts
2.2.2. Cell Sheet Culture
2.2.3. NIH/3T3 Cell Sheet Reattachment to the Scaffold
2.3. In Vitro Evaluation of Degassed SIS Mesh Cell Sheet Attachment
2.3.1. Reattached Cell Sheet Surface Analysis
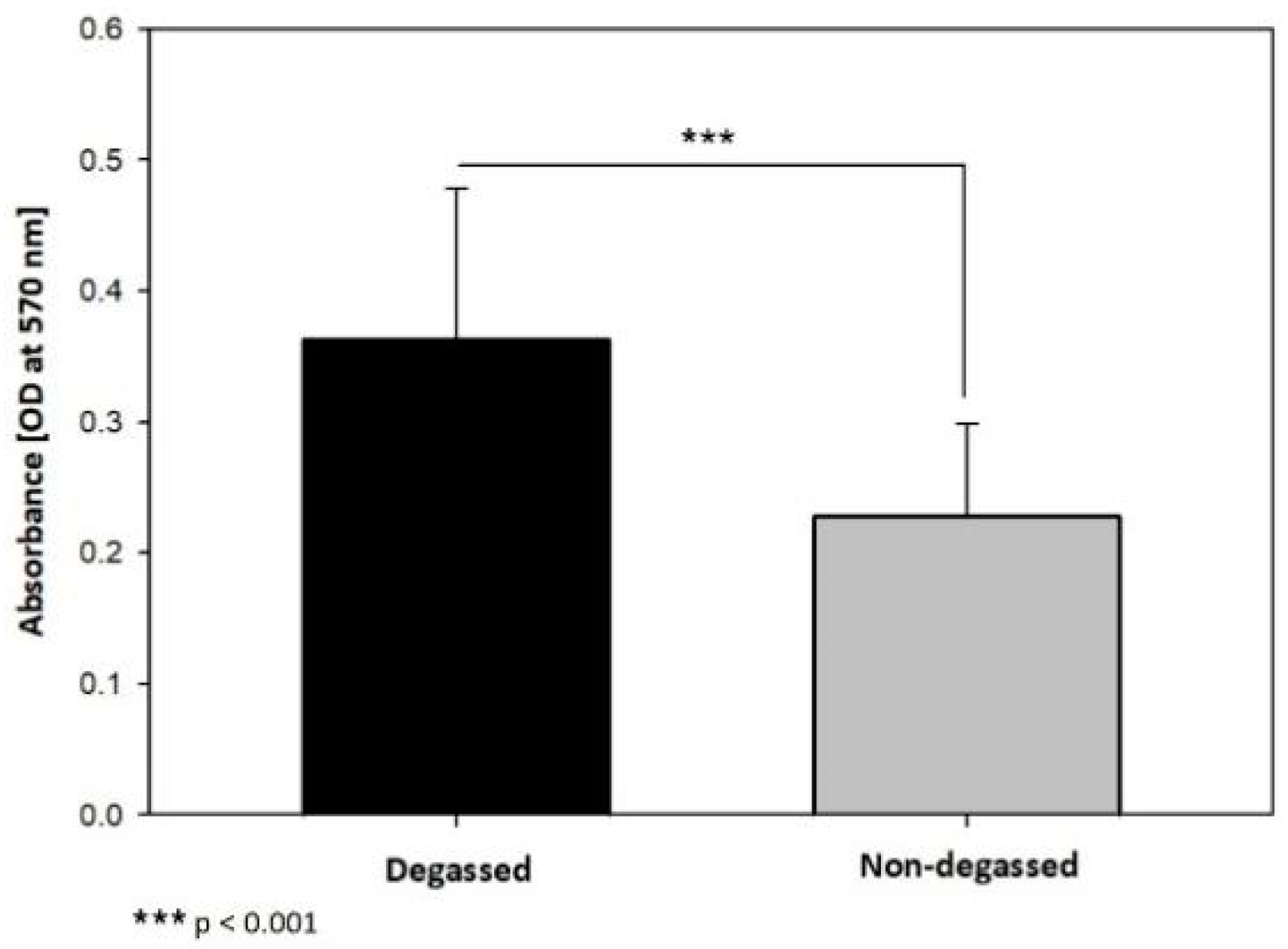
2.3.2. Reattached Cell Sheet MTT Assay
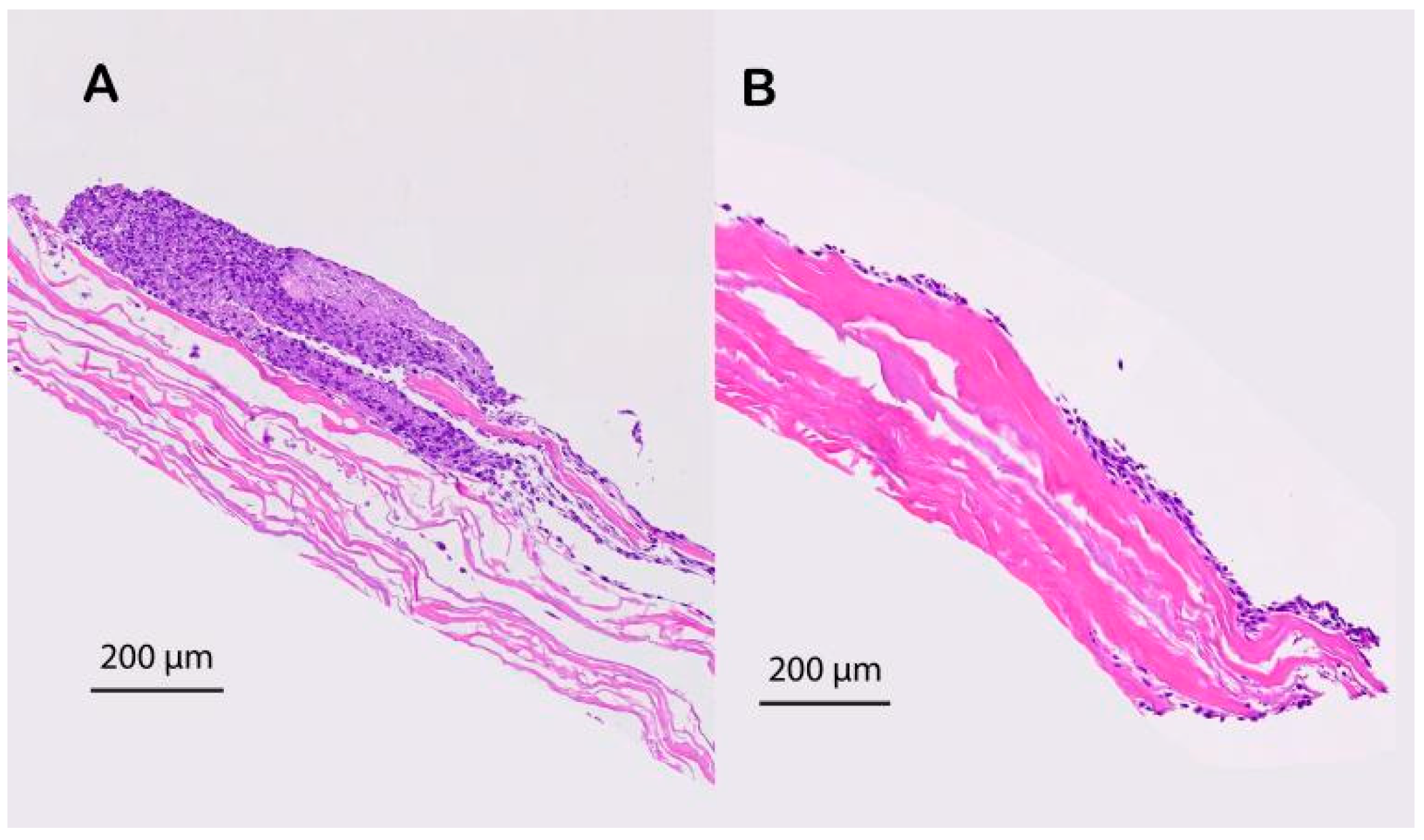
2.3.3. H&E Staining
2.4. In Vivo Evaluation of the Degassed SIS Mesh in a Trachea Patch Repair Model
2.4.1. Ethics Statement and Animal Use
2.4.2. Patch Model
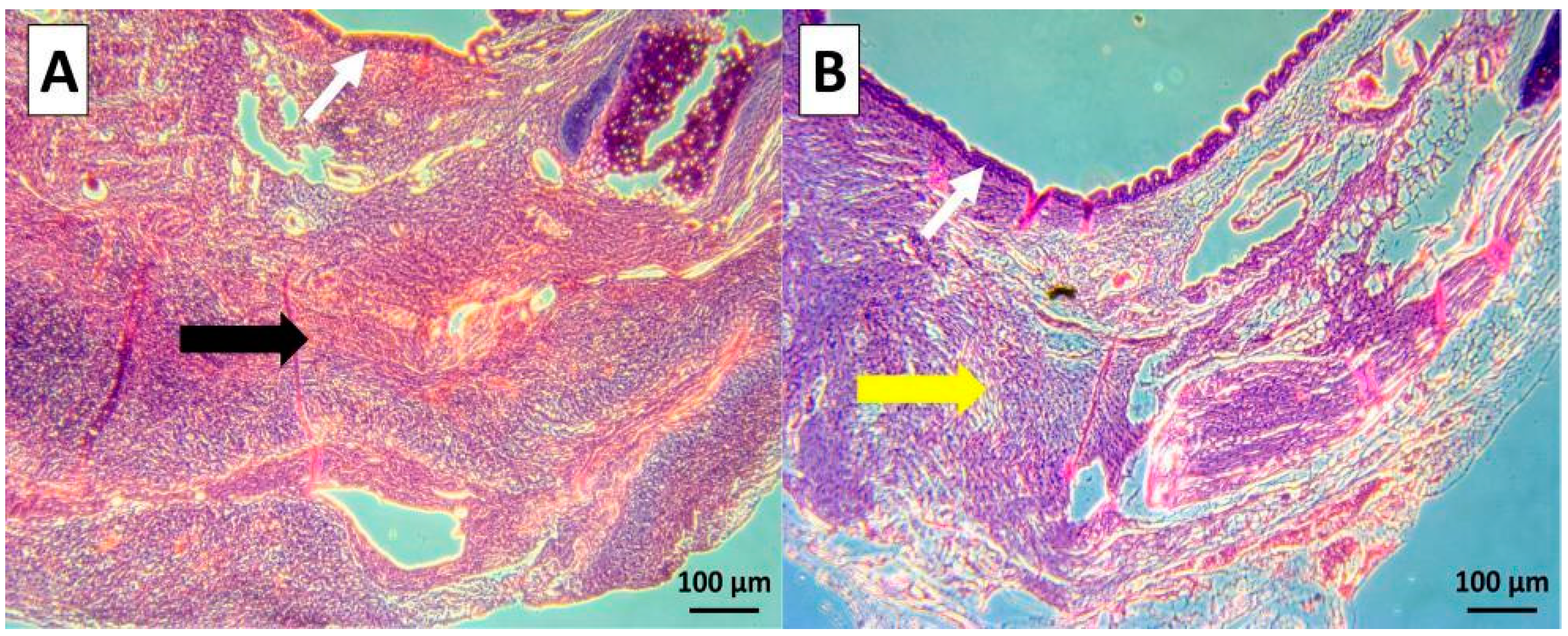
2.4.3. Histological Analysis
2.5. Statistical Analysis
3. Results
3.1. In Vitro Evaluation of the Ability of the Degassed SIS Mesh Cell Sheet to Attach
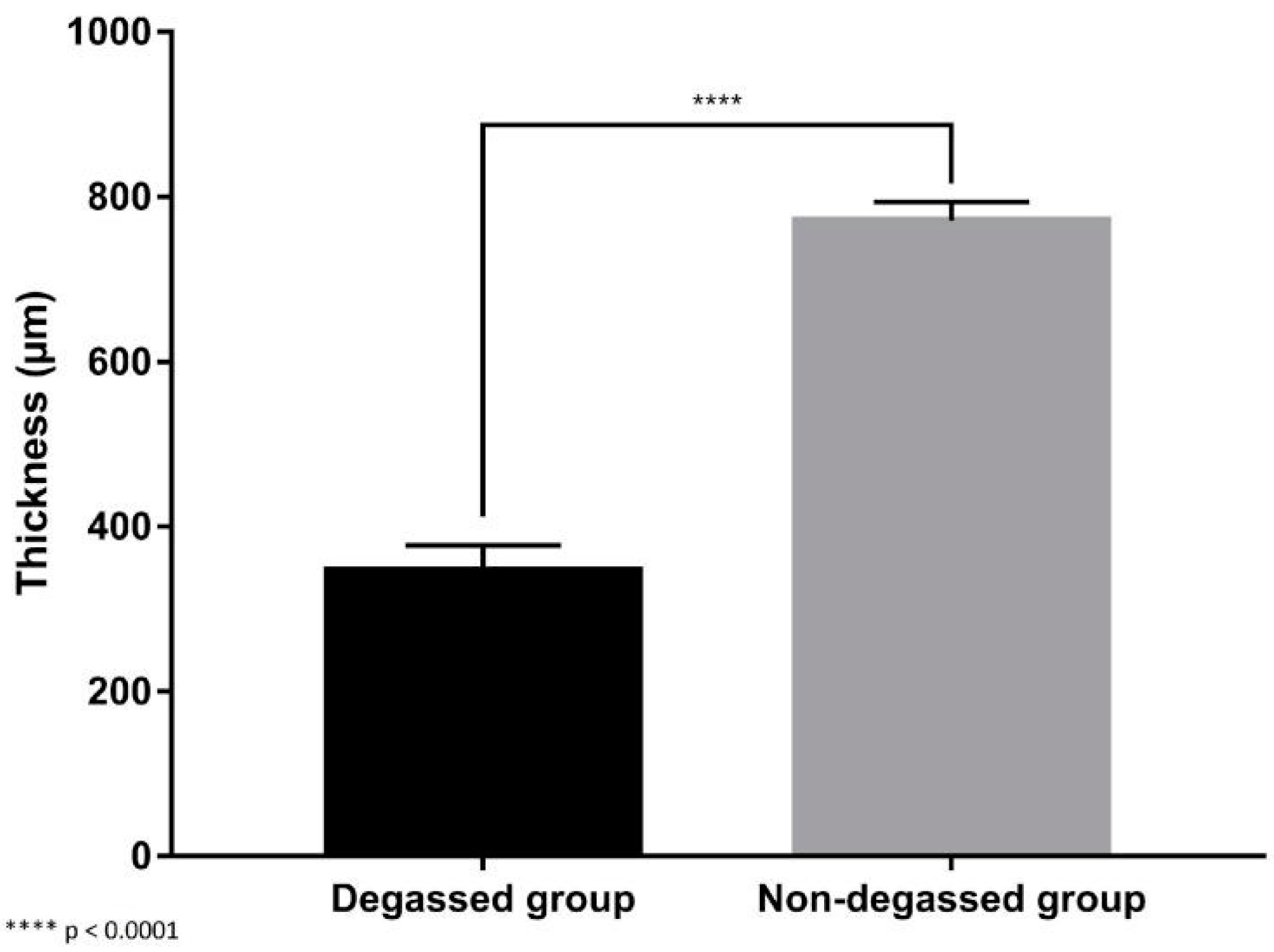
3.2. In Vivo Evaluation of the Degassed SIS Mesh in a Trachea Patch Repair Model
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, Y.; Zhang, X.; Zhang, Y.; Shen, F.; Ding, J.; Hua, K. Cervicovaginal reconstruction with small intestinal submucosa graft in congenital cervicovaginal atresia: A report of 38 cases. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 267, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Guevara, C.G.; Suarez, M.C.; Raymo, A.; Ransford, G.A.; Nassau, D.E.; Alam, A.; Labbie, A.S.; Castellan, M.A.; Gosalbez, R. Small Intestinal Submucosa for corporeal body grafting in patients with proximal hypospadias and severe chordee: Long term follow-up assessing erectile function and genital self-perception. J. Pediatr. Urol. 2022, 18, 758.e1–758.e7. [Google Scholar] [CrossRef]
- Kobayashi, H.; Imai, Y.; Hirao, T.; Nakao, K.; Kajinaka, H.; Kishi, K. Histopathological Analysis of Decellularized Porcine Small Intestinal Submucosa after Treatment of Skin Ulcer. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3967. [Google Scholar] [CrossRef] [PubMed]
- Pollaers, K.; Bumbak, P.; Kuthubutheen, J. Outcomes following tympanoplasty surgery using porcine-derived small intestinal submucosa. J. Laryngol. Otol. 2022, 136, 304–308. [Google Scholar] [CrossRef]
- Shi, L.; Ronfard, V. Biochemical and biomechanical characterization of porcine small intestinal submucosa (SIS): A mini review. Int. J. Burn. Trauma 2013, 3, 173. [Google Scholar]
- Clarke, K.M.; Lantz, G.C.; Salisbury, S.K.; Badylak, S.F.; Hiles, M.C.; Voytik, S.L. Intestine submucosa and polypropylene mesh for abdominal wall repair in dogs. J. Surg. Res. 1996, 60, 107–114. [Google Scholar] [CrossRef]
- Casarin, M.; Fortunato, T.M.; Imran, S.; Todesco, M.; Sandrin, D.; Borile, G.; Toniolo, I.; Marchesan, M.; Gerosa, G.; Bagno, A. Porcine Small Intestinal Submucosa (SIS) as a Suitable scaffold for the creation of a tissue-engineered urinary conduit: Decellularization, biomechanical and biocompatibility characterization using new approaches. Int. J. Mol. Sci. 2022, 23, 2826. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, V.; Teleman, P.; Rudnicki, M. Efficacy and safety of pelvic organ prolapse surgery with porcine small intestinal submucosa graft implantation. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 267, 18–22. [Google Scholar] [CrossRef]
- Niezgoda, J.A.; Van Gils, C.C.; Frykberg, R.G.; Hodde, J.P.; Group, O.D.U.S. Randomized clinical trial comparing OASIS Wound Matrix to Regranex Gel for diabetic ulcers. Adv. Ski. Wound Care 2005, 18, 258–266. [Google Scholar] [CrossRef]
- Brown-Etris, M.; Milne, C.T.; Hodde, J.P. An extracellular matrix graft (Oasis® wound matrix) for treating full-thickness pressure ulcers: A randomized clinical trial. J. Tissue Viability 2019, 28, 21–26. [Google Scholar] [CrossRef]
- Sengupta, A.; Beroukhim, R.; Baird, C.W.; Del Nido, P.J.; Geva, T.; Gauvreau, K.; Marcus, E.; Sanders, S.P.; Nathan, M. Outcomes of repair of congenital aortic valve lesions using autologous pericardium vs porcine intestinal submucosa. J. Am. Coll. Cardiol. 2022, 80, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, W.; Liu, Y.; Wang, X.; Tang, L.; You, P.; Han, J.; Li, B.; Zhang, Y.; Wang, M. Bone augmentation of peri-implant dehiscence defects using multilaminated small intestinal submucosa as a barrier membrane: An experimental study in dogs. BioMed Res. Int. 2019, 2019, 8962730. [Google Scholar] [CrossRef] [Green Version]
- Fujii, M.; Tanaka, R. Porcine Small Intestinal Submucosa Alters the Biochemical Properties of Wound Healing: A Narrative Review. Biomedicines 2022, 10, 2213. [Google Scholar] [CrossRef]
- Butler, C.R.; Hynds, R.E.; Crowley, C.; Gowers, K.H.; Partington, L.; Hamilton, N.J.; Carvalho, C.; Platé, M.; Samuel, E.R.; Burns, A.J. Vacuum-assisted decellularization: An accelerated protocol to generate tissue-engineered human tracheal scaffolds. Biomaterials 2017, 124, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Lange, P.; Greco, K.; Partington, L.; Carvalho, C.; Oliani, S.; Birchall, M.; Sibbons, P.; Lowdell, M.; Ansari, T. Pilot study of a novel vacuum-assisted method for decellularization of tracheae for clinical tissue engineering applications. J. Tissue Eng. Regen. Med. 2017, 11, 800–811. [Google Scholar] [CrossRef]
- Negishi, J.; Funamoto, S.; Hashimoto, Y.; Yanagisawa, K. PLA-collagen composite scaffold fabrication by vacuum pressure impregnation. Tissue Eng. Part C Methods 2019, 25, 742–747. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, L. Bioprosthetic heart valves with reduced immunogenic residuals using vacuum-assisted decellularization treatment. Biomed. Mater. 2020, 15, 065012. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Chen, Y.; Li, M.; Zhang, T.; Ding, S.; Xu, Y.; Hu, J.; Chen, C.; Lu, H. Designing a novel vacuum aspiration system to decellularize large-size enthesis with preservation of physicochemical and biological properties. Ann. Transl. Med. 2020, 8, 1364. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, F.; Lu, Y.; Zhang, B.; Zhang, G.; Shi, H. Rapid preparation method for preparing tracheal decellularized scaffolds: Vacuum assistance and optimization of DNase I. ACS Omega 2021, 6, 10637–10644. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, X.; Fang, Q.; Wang, F.; Ao, Q.; Wang, X.; Tian, X.; Tong, H.; Bai, S.; Fan, J. Surface modification of small intestine submucosa in tissue engineering. Regen Biomater 2020, 7, 339–348. [Google Scholar] [CrossRef]
- Dang, L.H.; Hung, S.-H.; Tseng, Y.; Quang, L.X.; Le, N.T.N.; Fang, C.-L.; Tseng, H. Partial decellularized scaffold combined with autologous nasal epithelial cell sheet for tracheal tissue engineering. Int. J. Mol. Sci. 2021, 22, 10322. [Google Scholar] [CrossRef]
- Tseng, H.; Tsai, J.-K.; Ou, K.-L.; Chen, P.-N. Supports for Cell Culture and Cell Sheet Detachment and Methods for Cell Sheet Detachment; Taipei Medical University TMU: Taipei, Taiwan, 2017. [Google Scholar]
- Badylak, S.; Kokini, K.; Tullius, B.; Simmons-Byrd, A.; Morff, R. Morphologic study of small intestinal submucosa as a body wall repair device. J. Surg. Res. 2002, 103, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.E.; Treviño, J.M.; Portillo, G.; Vela, I.; Glass, J.L.; González, J.J. The use of porcine small intestinal submucosa as a prosthetic material for laparoscopic hernia repair in infected and potentially contaminated fields: Long-term follow-up. Surg. Endosc. 2008, 22, 1941–1946. [Google Scholar] [CrossRef] [PubMed]
- Helton, W.S.; Fisichella, P.M.; Berger, R.; Horgan, S.; Espat, N.J.; Abcarian, H. Short-term outcomes with small intestinal submucosa for ventral abdominal hernia. Arch. Surg. 2005, 140, 549–562. [Google Scholar] [CrossRef] [Green Version]
- Gubbels, S.P.; Richardson, M.; Trune, D.; Bascom, D.A.; Wax, M.K. Tracheal reconstruction with porcine small intestine submucosa in a rabbit model. Otolaryngol. Head Neck Surg. 2006, 134, 1028–1035. [Google Scholar] [CrossRef]
- Bergonse Neto, N.; Jorge, L.F.; Francisco, J.C.; Erbano, B.O.; Barboza, B.E.G.; Da Silva, L.L.G.; Olandoski, M.; De Carvalho, K.A.T.; Moreira, L.F.P.; Faria Neto, J.R. Regeneration of tracheal tissue in partial defects using porcine small intestinal submucosa. Stem Cells Int. 2018, 2018, 5102630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.F.; Kwon, S.K.; Song, J.-J.; Cho, C.G.; Park, S.-W. Tracheal reconstruction by mesenchymal stem cells with small intestine submucosa in rabbits. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 345–351. [Google Scholar] [CrossRef]
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The pros and cons of mesenchymal stem cell-based therapies. Cell Transplant. 2019, 28, 801–812. [Google Scholar] [CrossRef] [Green Version]
- Joo, H.; Park, J.; Sutthiwanjampa, C.; Kim, H.; Bae, T.; Kim, W.; Choi, J.; Kim, M.; Kang, S.; Park, H. Surface coating with hyaluronic acid-gelatin-crosslinked hydrogel on gelatin-conjugated poly (dimethylsiloxane) for implantable medical device-induced fibrosis. Pharmaceutics 2021, 13, 269. [Google Scholar] [CrossRef]
- Akdoğan, E.; Şirin, H.T. Plasma surface modification strategies for the preparation of antibacterial biomaterials: A review of the recent literature. Mater. Sci. Eng. C 2021, 131, 112474. [Google Scholar] [CrossRef]
- Jacobs, T.; Morent, R.; De Geyter, N.; Dubruel, P.; Leys, C. Plasma surface modification of biomedical polymers: Influence on cell-material interaction. Plasma Chem. Plasma Process. 2012, 32, 1039–1073. [Google Scholar] [CrossRef]
- Lu, T.; Qiao, Y.; Liu, X. Surface modification of biomaterials using plasma immersion ion implantation and deposition. Interface Focus 2012, 2, 325–336. [Google Scholar] [CrossRef] [Green Version]
- Yohe, S.T.; Freedman, J.D.; Falde, E.J.; Colson, Y.L.; Grinstaff, M.W. A mechanistic study of wetting superhydrophobic porous 3D meshes. Adv. Funct. Mater. 2013, 23, 3628–3637. [Google Scholar] [CrossRef] [PubMed]
- McKenna, E.; Klein, T.J.; Doran, M.R.; Futrega, K. Integration of an ultra-strong poly (lactic-co-glycolic acid)(PLGA) knitted mesh into a thermally induced phase separation (TIPS) PLGA porous structure to yield a thin biphasic scaffold suitable for dermal tissue engineering. Biofabrication 2019, 12, 015015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, L.H.; Tseng, Y.; Tseng, H.; Hung, S.-H. Partial decellularization for segmental tracheal scaffold tissue engineering: A preliminary study in rabbits. Biomolecules 2021, 11, 866. [Google Scholar] [CrossRef] [PubMed]
- Sofu, H.; Camurcu, Y.; Ucpunar, H.; Ozcan, S.; Yurten, H.; Sahin, V. Clinical and radiographic outcomes of chitosan-glycerol phosphate/blood implant are similar with hyaluronic acid-based cell-free scaffold in the treatment of focal osteochondral lesions of the knee joint. Knee Surg. Sport. Traumatol. Arthrosc. 2019, 27, 773–781. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viet-Nhi, N.-K.; Chen, Y.-C.; Dang, L.H.; Tseng, H.; Hung, S.-H. Degassing a Decellularized Scaffold Enhances Wound Healing and Reduces Fibrosis during Tracheal Defect Reconstruction: A Preliminary Animal Study. J. Funct. Biomater. 2023, 14, 147. https://doi.org/10.3390/jfb14030147
Viet-Nhi N-K, Chen Y-C, Dang LH, Tseng H, Hung S-H. Degassing a Decellularized Scaffold Enhances Wound Healing and Reduces Fibrosis during Tracheal Defect Reconstruction: A Preliminary Animal Study. Journal of Functional Biomaterials. 2023; 14(3):147. https://doi.org/10.3390/jfb14030147
Chicago/Turabian StyleViet-Nhi, Nguyen-Kieu, Yen-Chun Chen, Luong Huu Dang, How Tseng, and Shih-Han Hung. 2023. "Degassing a Decellularized Scaffold Enhances Wound Healing and Reduces Fibrosis during Tracheal Defect Reconstruction: A Preliminary Animal Study" Journal of Functional Biomaterials 14, no. 3: 147. https://doi.org/10.3390/jfb14030147
APA StyleViet-Nhi, N. -K., Chen, Y. -C., Dang, L. H., Tseng, H., & Hung, S. -H. (2023). Degassing a Decellularized Scaffold Enhances Wound Healing and Reduces Fibrosis during Tracheal Defect Reconstruction: A Preliminary Animal Study. Journal of Functional Biomaterials, 14(3), 147. https://doi.org/10.3390/jfb14030147








