Realizing Both Antibacterial Activity and Cytocompatibility in Silicocarnotite Bioceramic via Germanium Incorporation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
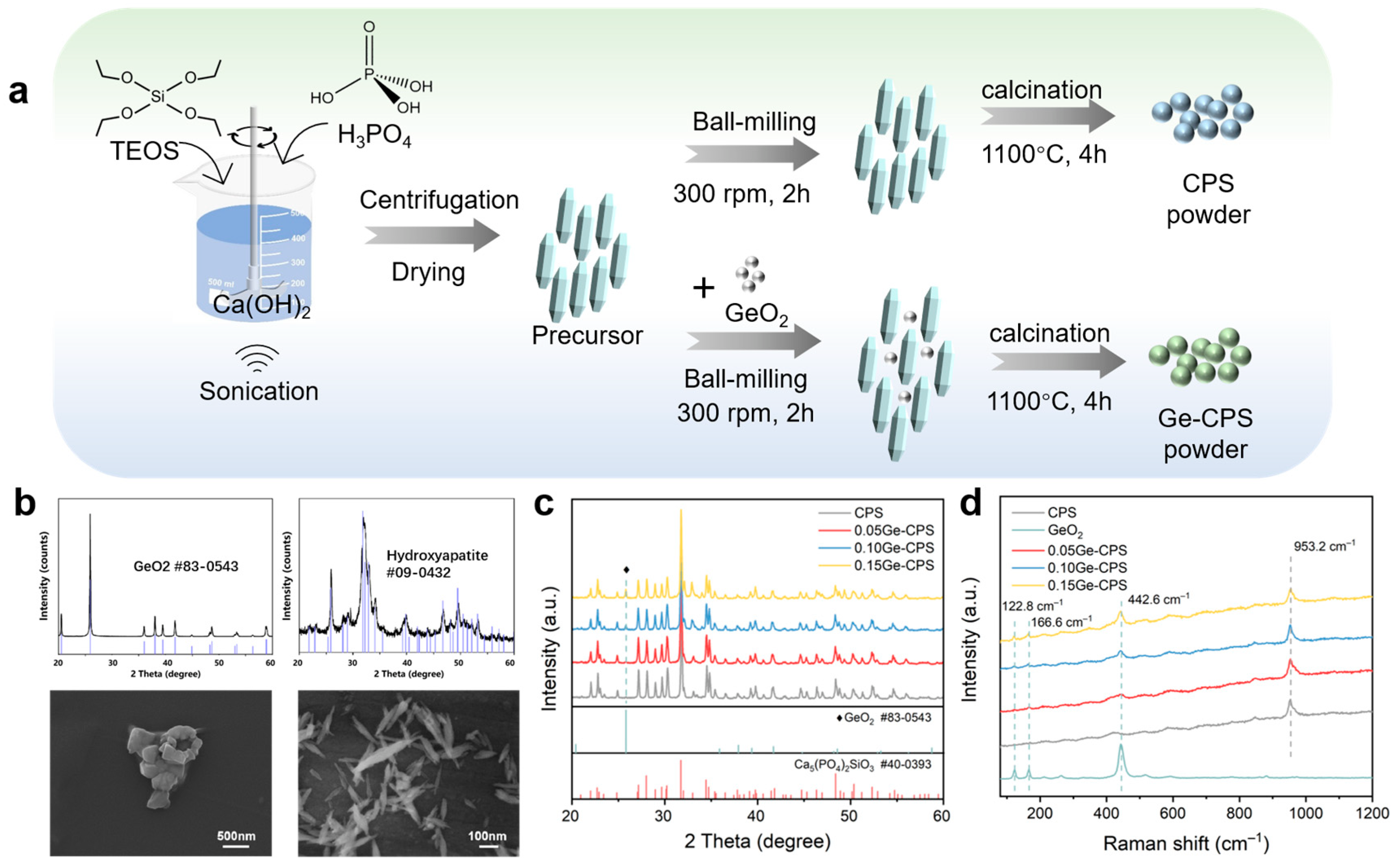
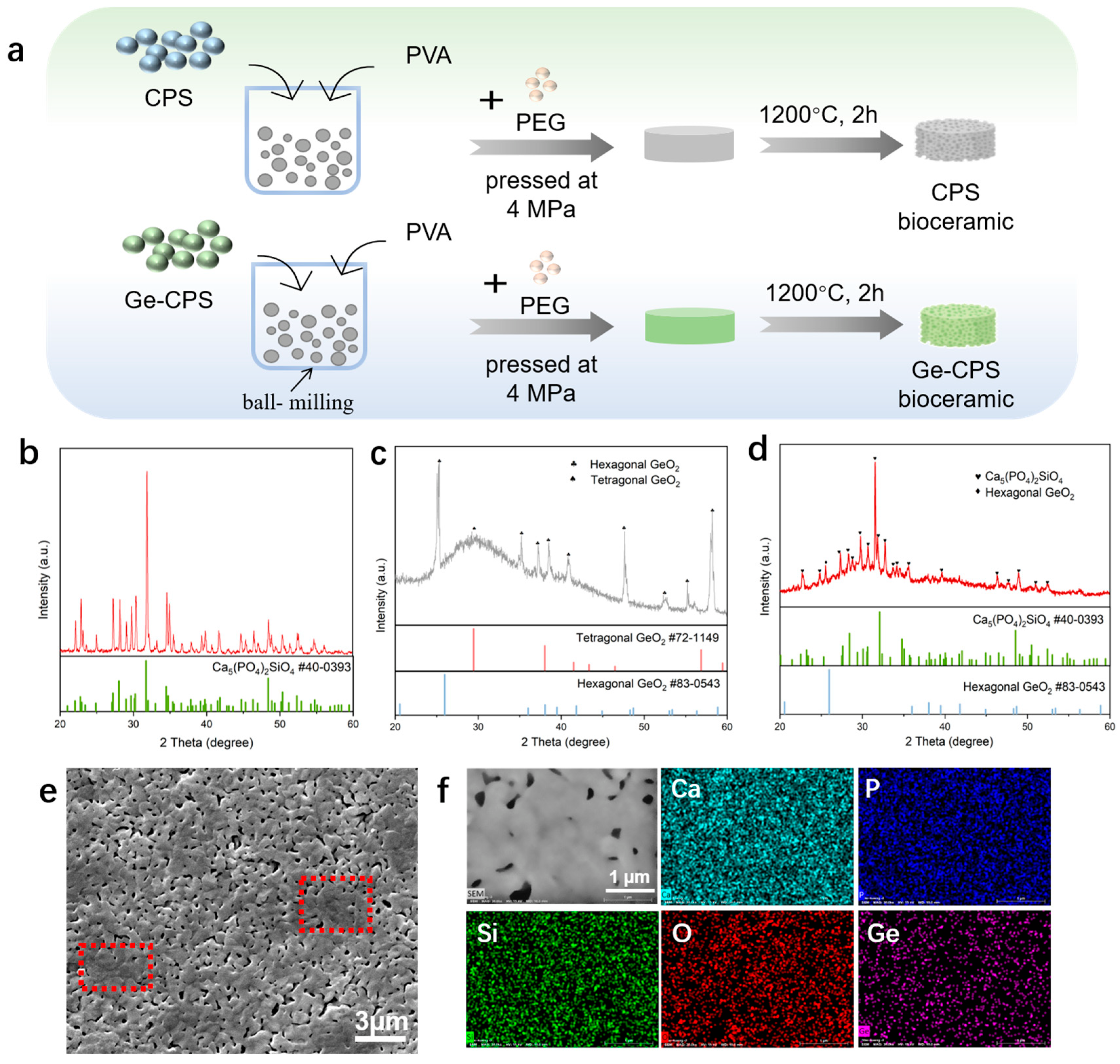
2.2. Synthesis of Ge–CPS Powders and Bioceramics
2.3. Characterization of the Ge–CPS Powders and Bioceramics
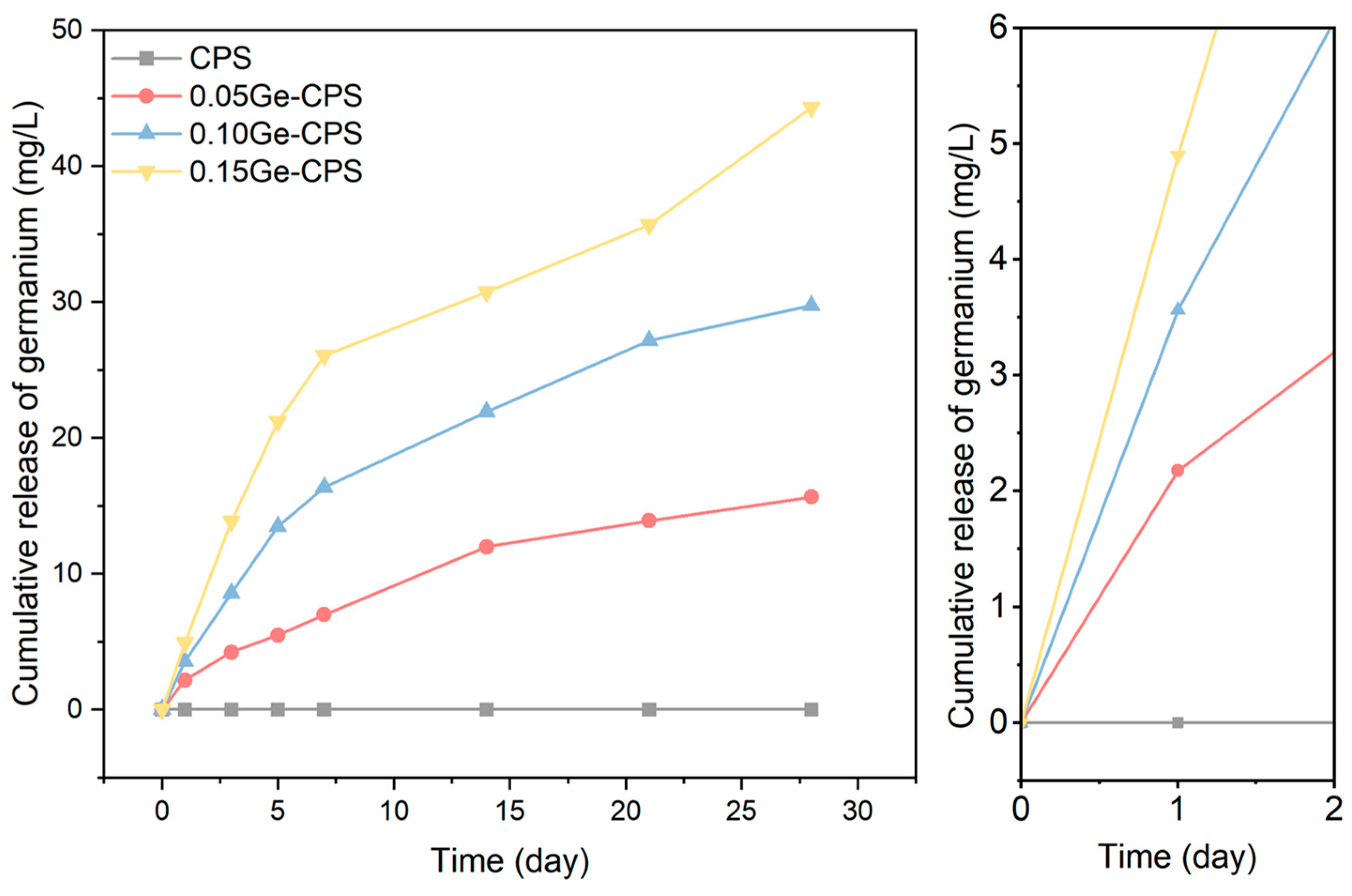
2.4. Release of Germanium in Vitro
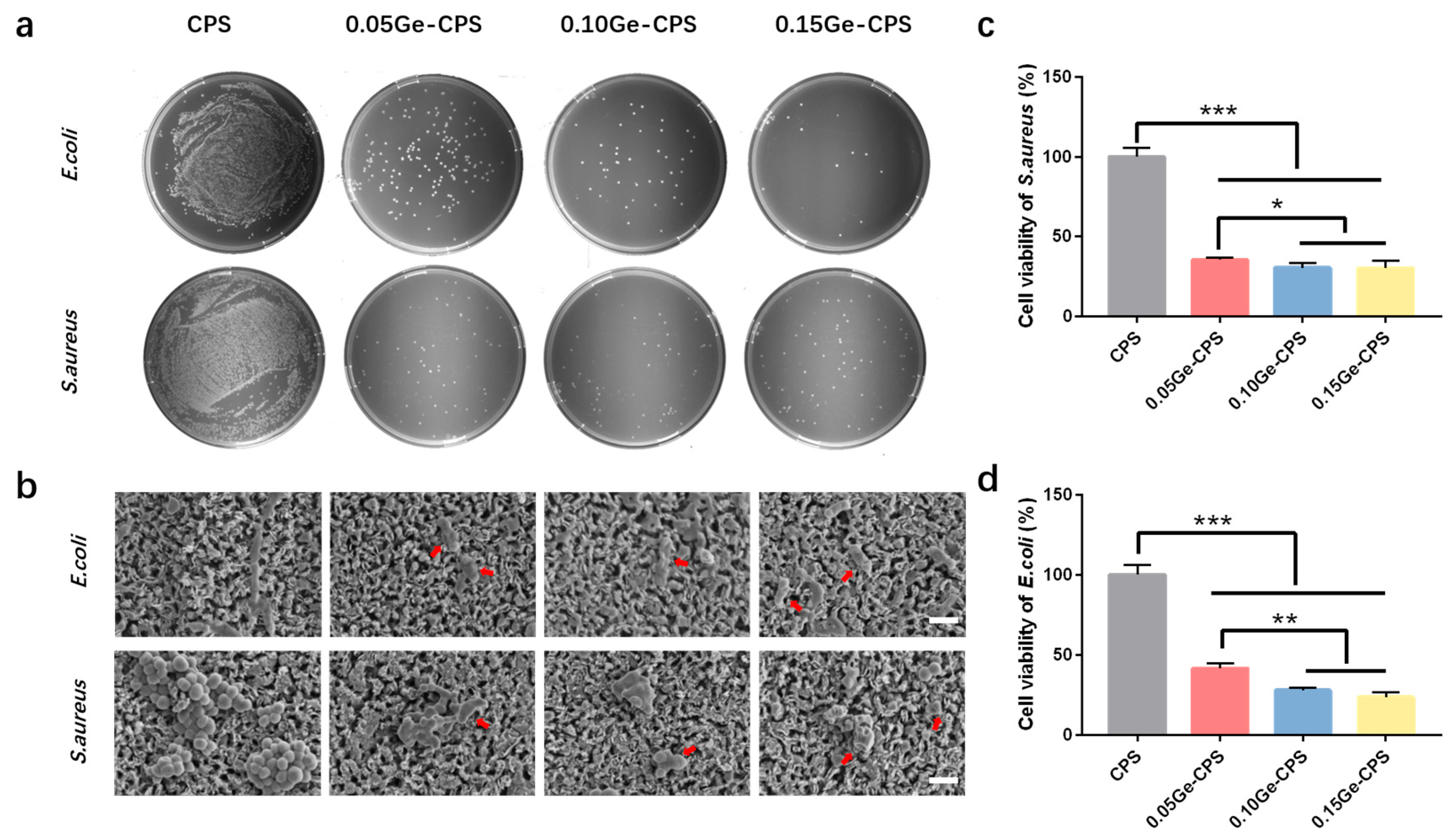
2.5. Bacterial Activity Assays
2.5.1. Bacteria Strains
2.5.2. Viability of Bacteria
2.5.3. Morphology of Bacteria
2.5.4. Bacteria Live/Dead Staining
2.6. Cytocompatibility Assessment of Ge–CPS Bioceramics
2.6.1. rBMSCs Culture
2.6.2. Cell Viability Assay
2.6.3. Cell Adhesion
2.7. Statistical Analysis
3. Results
3.1. Characterization of the Ge–CPS Powders
3.2. Characterization of the CPS and Ge–CPS Bioceramics
3.3. Release of Ge in PBS
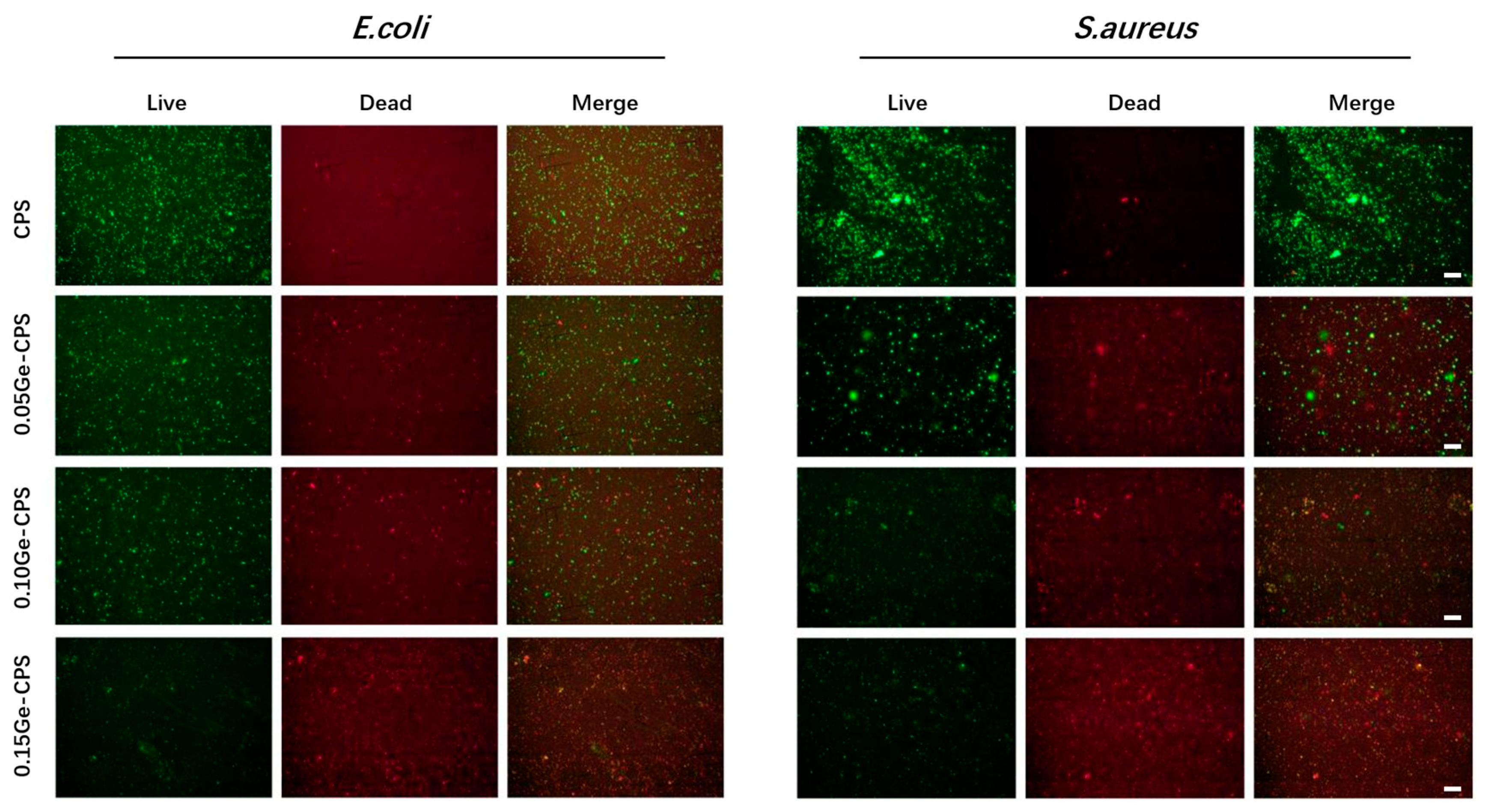
3.4. Antibacterial Activity of Ge–CPS Bioceramics
3.5. Cytocompatibility of the Ge–CPS Bioceramics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bal, B.S.; Rahaman, M.N. Orthopedic applications of silicon nitride ceramics. Acta Biomater. 2012, 8, 2889–2898. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Fielding, G.; Tarafder, S.; Bandyopadhyay, A. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 2013, 31, 594–605. [Google Scholar] [CrossRef]
- Albuquerque, J.S.V.; Franca, I.W.L.; Silva, G.F.; Ferreira, A.L.O.; Nogueira, R.E.F.Q. Macroporous Calcium Phosphate Bioceramics as Drug Release Agents: A Kinetics Study of Ampicillin Release. In Key Engineering Materials; Bioceramics 21; Prado, M., Zavaglia, C., Eds.; Trans Tech Publications Ltd.: Buzios, Brazil, 2008; pp. 675–678. [Google Scholar]
- Cioni, B.; Lazzeri, A.; Gallone, G.; Levita, G. Synthesis of Bioactive Hydroxyapatite-Zirconia Toughened Composites for Bone Replacement. In Biomedical Applications of Smart Materials, Nanotechnology and Micro/Nano Engineering; Vincenzini, P., De Rossi, D., Eds.; Trans Tech Publications Ltd.: Acrieale, Italy, 2008; pp. 31–36. [Google Scholar]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Akagi, H.; Ochi, H.; Soeta, S.; Kanno, N.; Yoshihara, M.; Okazaki, K.; Yogo, T.; Harada, Y.; Amasaki, H.; Hara, Y. A Comparison of the Process of Remodeling of Hydroxyapatite/Poly-D/L-Lactide and Beta-Tricalcium Phosphate in a Loading Site. Biomed Res. Int. 2015, 2015, 730105. [Google Scholar] [CrossRef]
- Gokcekaya, O.; Ueda, K.; Narushima, T.; Ergun, C. Synthesis and characterization of Ag-containing calcium phosphates with various Ca/P ratios. Mater. Sci. Eng. C-Mater. Biol. Appl. 2015, 53, 111–119. [Google Scholar] [CrossRef]
- Bohner, M. Silicon-substituted calcium phosphates—A critical view. Biomaterials 2009, 30, 6403–6406. [Google Scholar] [CrossRef]
- Hench, L. The story of Bioglass. J. Mater. Sci.-Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Xu, Y.B.; Lu, T.L.; He, F.P.; Ma, N.; Ye, J.D.; Wu, T.T. Enhancing the Cell-Biological Performances of Hydroxyapatite Bioceramic by Constructing Silicate-Containing Grain Boundary Phases via Sol Infiltration. ACS Biomater. Sci. Eng. 2018, 4, 3154–3162. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Brunner, E.; Simon, P.; Bazhenov, V.V.; Botting, J.P.; Tabachnick, K.R.; Springer, A.; Kummer, K.; Vyalikh, D.V.; Molodtsov, S.L.; et al. Calcite Reinforced Silica-Silica Joints in the Biocomposite Skeleton of Deep-Sea Glass Sponges. Adv. Funct. Mater. 2011, 21, 3473–3481. [Google Scholar] [CrossRef]
- Vandiver, J.; Dean, D.; Patel, N.; Botelho, C.; Best, S.; Santos, J.D.; Lopes, M.A.; Bonfield, W.; Ortiz, C. Silicon addition to hydroxyapatite increases nanoscale electrostatic, van der Waals, and adhesive interactions. J. Biomed. Mater. Res. Part A 2006, 78, 352–363. [Google Scholar] [CrossRef]
- Ehrlich, H.; Simon, P.; Carrillo-Cabrera, W.; Bazhenov, V.V.; Botting, J.P.; Ilan, M.; Ereskovsky, A.V.; Muricy, G.; Worch, H.; Mensch, A.; et al. Insights into Chemistry of Biological Materials: Newly Discovered Silica-Aragonite-Chitin Biocomposites in Demosponges. Chem. Mater. 2010, 22, 1462–1471. [Google Scholar] [CrossRef]
- Ewald, A.; Kaeppel, C.; Vorndran, E.; Moseke, C.; Gelinsky, M.; Gbureck, U. The effect of Cu(II)-loaded brushite scaffolds on growth and activity of osteoblastic cells. J. Biomed. Mater. Res. Part A 2012, 100, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Ghijsen, J.; Tjeng, L.H.; Vanelp, J.; Eskes, H.; Westerink, J.; Sawatzky, G.A.; Czyzyk, M.T. Electronic-structure of Cu2O and CuO. Phys. Rev. B 1988, 38, 11322–11330. [Google Scholar] [CrossRef] [PubMed]
- Gross, T.M.; Lahiri, J.; Golas, A.; Luo, J.; Verrier, F.; Kurzejewski, J.L.; Baker, D.E.; Wang, J.; Novak, P.F.; Snyder, M.J. Copper-containing glass ceramic with high antimicrobial efficacy. Nat. Commun. 2019, 10, 1979. [Google Scholar] [CrossRef]
- Fielding, G.; Bose, S. SiO2 and ZnO dopants in three-dimensionally printed tricalcium phosphate bone tissue engineering scaffolds enhance osteogenesis and angiogenesis in vivo. Acta Biomater. 2013, 9, 9137–9148. [Google Scholar] [CrossRef]
- Fielding, G.A.; Smoot, W.; Bose, S. Effects of SiO2, SrO, MgO, and ZnO dopants in tricalcium phosphates on osteoblastic Runx2 expression. J. Biomed. Mater. Res. Part A 2014, 102, 2417–2426. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wu, Q.; Wu, J.; Kou, H.; Zhu, Y.; Ning, C.; Dai, K. Ultrasound-assisted synthesis of nanocrystallized silicocarnotite biomaterial with improved sinterability and osteogenic activity. J. Mater. Chem. B 2020, 8, 3092–3103. [Google Scholar] [CrossRef]
- Madhumathi, K.; Rubaiya, Y.; Doble, M.; Venkateswari, R.; Kumar, T.S.S. Antibacterial, anti-inflammatory, and bone-regenerative dual-drug-loaded calcium phosphate nanocarriers-in vitro and in vivo studies. Drug Deliv. Transl. Res. 2018, 8, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Krieg, P.; Killinger, A.; Gadow, R.; Burtscher, S.; Bernstein, A. High velocity suspension flame spraying (HVSFS) of metal doped bioceramic coatings. Bioact. Mater. 2017, 2, 162–169. [Google Scholar] [CrossRef]
- Chazono, M.; Tanaka, T.; Komaki, H.; Fujii, K. Bone formation and bioresorption after implantation of injectable beta-tricalcium phosphate granules-hyaluronate complex in rabbit bone defects. J. Biomed. Mater. Res. Part A 2004, 70, 542–549. [Google Scholar] [CrossRef]
- Durham, P.G.; Sidders, A.E.; Beam, J.E.; Kedziora, K.M.; Dayton, P.A.; Conlon, B.P.; Papadopoulou, V.; Rowe, S.E. Harnessing ultrasound-stimulated phase change contrast agents to improve antibiotic efficacy against methicillin-resistant Staphylococcus aureus biofilms. Biofilm 2021, 3, 100049. [Google Scholar] [CrossRef]
- Vucic, D.; Cvitkusic-Lukenda, K.; Dunder, I.; Gabaldo, K.; Knezevic-Pravecek, M.; Miskic, B. Diagnostic complexity of rifampicin-induced coagulopathy in a patient with spontaneous muscle bleeding: A case report. Medicine 2021, 100, e26234. [Google Scholar] [CrossRef] [PubMed]
- Touri, M.; Mortarzadeh, F.; Abu Osman, N.A.; Dehghan, M.M.; Mozafari, M. Optimisation and biological activities of bioceramic robocast scaffolds provided with an oxygen-releasing coating for bone tissue engineering applications. Ceram. Int. 2019, 45, 805–816. [Google Scholar] [CrossRef]
- Saidin, S.; Jumat, M.A.; Amin, N.A.A.M.; Al-Hammadi, A.S.S. Organic and inorganic antibacterial approaches in combating bacterial infection for biomedical application. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 118, 111382. [Google Scholar] [CrossRef]
- Heshmatpour, F.; Lashteneshaee, S.H.; Samadipour, M. Study of In Vitro Bioactivity of Nano Hydroxyapatite Composites Doped by Various Cations. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2063–2068. [Google Scholar] [CrossRef]
- Lin, X.; Yang, S.F.; Lai, K.; Yang, H.L.; Webster, T.J.; Yang, L. Orthopedic implant biomaterials with both osteogenic and anti-infection capacities and associated in vivo evaluation methods. Nanomed.-Nanotechnol. Biol. Med. 2017, 13, 123–142. [Google Scholar] [CrossRef]
- Shimada, Y.; Sato, K.; Masaki, M.; Nakamura, T.; Tokuji, Y. Quantitative assessment of the interactions between the organogermanium compound and saccharides using an NMR reporter molecule. Carbohydr. Res. 2021, 499, 108199. [Google Scholar] [CrossRef]
- Khader, B.A.; Rodriguez, O.; Towler, M.R. Incorporating Germanium Oxide into the Glass Phase of Novel Zinc/Magnesium-Based GPCs Designed for Bone Void Filling: Evaluating their Physical and Mechanical Properties. J. Funct. Biomater. 2018, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, C.; Suzuki, H.; Ito, M.; Okumura, M.; Oda, T. Propagermanium: A nonspecific immune modulator for chronic hepatitis B. J. Gastroenterol. 2003, 38, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Amtul, Z.; Follmer, C.; Mahboob, S.; Ur, R.A.; Mazhar, M.; Khan, K.M.; Siddiqui, R.A.; Muhammad, S.; Kazmi, S.A.; Choudhary, M.I. Germa-gamma-lactones as novel inhibitors of bacterial urease activity. Biochem. Biophys. Res. Commun. 2007, 356, 457–463. [Google Scholar] [CrossRef]
- Katakura, T.; Yoshida, T.; Kobayashi, M.; Herndon, D.N.; Suzuki, F. Immunological control of methicillin-resistant Staphylococcus aureus (MRSA) infection in an immunodeficient murine model of thermal injuries. Clin. Exp. Immunol. 2005, 142, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Chen, S.-S.; Lin, Y.-C.; Lee, Y.-S.; Chen, T.-J. Germanium dioxide induces mitochondria-mediated apoptosis in Neuro-2A cells. Neurotoxicology 2006, 27, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Avanzato, C.P.; Follieri, J.M.; Banerjee, I.A.; Fath, K.R. Biomimetic Synthesis and Antibacterial Characteristics of Magnesium Oxide-Germanium dioxide Nanocomposite Powders. J. Compos. Mater. 2009, 43, 897–910. [Google Scholar] [CrossRef]
- Chauhan, N.P.S.; Gholipourmalekabadi, M.; Mozafari, M. Fabrication of newly developed pectin—GeO2 nanocomposite using extreme biomimetics route and its antibacterial activities. J. Macromol. Sci. Part A-Pure Appl. Chem. 2017, 54, 655–661. [Google Scholar] [CrossRef]
- Jeon, H.J.; Yi, S.C.; Oh, S.G. Preparation and antibacterial effects of Ag-SiO2 thin films by sol-gel method. Biomaterials 2003, 24, 4921–4928. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Fan, C.; Zheng, X.; Zhang, Y.; Chen, Y. In vitro antibacterial and osteogenic properties of plasma sprayed silver-containing hydroxyapatite coating. Chin. Sci. Bull. 2009, 54, 4438–4445. [Google Scholar] [CrossRef]
- McNulty, D.; Geaney, H.; Buckley, D.; O’Dwyer, C. High capacity binder-free nanocrystalline GeO2 inverse opal anodes for Li-ion batteries with long cycle life and stable cell voltage. Nano Energy 2018, 43, 11–21. [Google Scholar] [CrossRef]
- Cho, J.M.; Chae, J.; Jeong, S.R.; Moon, M.J.; Shin, D.Y.; Lee, J.H. Immune activation of Bio-Germanium in a randomized, double-blind, placebo-controlled clinical trial with 130 human subjects: Therapeutic opportunities from new insights. PLoS ONE 2020, 15, e0240358. [Google Scholar] [CrossRef]
- Deng, F.; Rao, J.; Ning, C. Ferric oxide: A favorable additive to balance mechanical strength and biological activity of silicocarnotite bioceramic. J. Mech. Behav. Biomed. Mater. 2020, 109, 103819. [Google Scholar] [CrossRef]
- Deng, F.; Wang, F.; Liu, Z.; Kou, H.; Cheng, G.; Ning, C. Enhanced mechanical property of Ca5(PO4)2SiO4 bioceramic by a biocompatible sintering aid of zinc oxide. Ceram. Int. 2018, 44, 18352–18362. [Google Scholar] [CrossRef]
- Micoulaut, M.; Cormier, L.; Henderson, G.S. The structure of amorphous, crystalline and liquid GeO2. J. Phys.-Condens. Matter 2006, 18, R753–R784. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, C.; Thouas, G.A. Progress and challenges in biomaterials used for bone tissue engineering: Bioactive glasses and elastomeric composites. Prog. Biomater. 2012, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Feng, C.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018, 79, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wu, Q.; Guo, Y.; Ning, C.; Dai, K. Copper containing silicocarnotite bioceramic with improved mechanical strength and antibacterial activity. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 118, 111493. [Google Scholar] [CrossRef]
- Zeng, J.; Guo, J.; Sun, Z.; Deng, F.; Ning, C.; Xie, Y. Osteoblastic and anti-osteoclastic activities of strontium-substituted silicocarnotite ceramics: In vitro and in vivo studies. Bioact. Mater. 2020, 5, 435–446. [Google Scholar] [CrossRef]
- Ou, S.-F.; Huang, M.-S.; Chiou, S.-Y.; Ou, K.-L. Research of antibacterial activity on silver containing yttria-stabilized-zirconia bioceramic. Ceram. Int. 2013, 39, 3591–3596. [Google Scholar] [CrossRef]
- Munitic, M.S.; Budimir, A.; Jakovljevic, S.; Anic, I.; Bago, I. Short-Term Antibacterial Efficacy of Three Bioceramic Root Canal Sealers against Enterococcus faecalis Biofilms. Acta Stomatol. Croat. 2020, 54, 3–9. [Google Scholar] [CrossRef]
- Schauss, A.G. Nephrotoxicity and neurotoxicity in humans from organogermanium compounds and germanium dioxide. Biol. Trace Elem. Res. 1991, 29, 267–280. [Google Scholar] [CrossRef]
- Krapf, R.; Schaffner, T.; Iten, P.X. Abuse of germanium associated with fatal lactic-acidosis. Nephron 1992, 62, 351–356. [Google Scholar] [CrossRef]
- Das, A.; Shukla, M. Multifunctional hydroxyapatite and hopeite coatings on SS254 by laser rapid manufacturing for improved osseointegration and antibacterial character: A comparative study. Proc. Inst. Mech. Eng. Part H-J. Eng. Med. 2020, 234, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Kharouf, N.; Arntz, Y.; Eid, A.; Zghal, J.; Sauro, S.; Haikel, Y.; Mancino, D. Physicochemical and Antibacterial Properties of Novel, Premixed Calcium Silicate-Based Sealer Compared to Powder-Liquid Bioceramic Sealer. J. Clin. Med. 2020, 9, 3096. [Google Scholar] [CrossRef] [PubMed]
- Uskokovic, V.; Ignjatovic, N.; Skapin, S.; Uskokovic, D.P. Germanium-doped hydroxyapatite: Synthesis and characterization of a new substituted apatite. Ceram. Int. 2022, 48, 27693–27702. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Yang, S.; Sun, J.; Ning, C. Realizing Both Antibacterial Activity and Cytocompatibility in Silicocarnotite Bioceramic via Germanium Incorporation. J. Funct. Biomater. 2023, 14, 154. https://doi.org/10.3390/jfb14030154
Ji Y, Yang S, Sun J, Ning C. Realizing Both Antibacterial Activity and Cytocompatibility in Silicocarnotite Bioceramic via Germanium Incorporation. Journal of Functional Biomaterials. 2023; 14(3):154. https://doi.org/10.3390/jfb14030154
Chicago/Turabian StyleJi, Yingqi, Shun Yang, Jian Sun, and Congqin Ning. 2023. "Realizing Both Antibacterial Activity and Cytocompatibility in Silicocarnotite Bioceramic via Germanium Incorporation" Journal of Functional Biomaterials 14, no. 3: 154. https://doi.org/10.3390/jfb14030154
APA StyleJi, Y., Yang, S., Sun, J., & Ning, C. (2023). Realizing Both Antibacterial Activity and Cytocompatibility in Silicocarnotite Bioceramic via Germanium Incorporation. Journal of Functional Biomaterials, 14(3), 154. https://doi.org/10.3390/jfb14030154






