Current Status and Future Outlook of Additive Manufacturing Technologies for the Reconstruction of the Trachea
Abstract
:1. Introduction
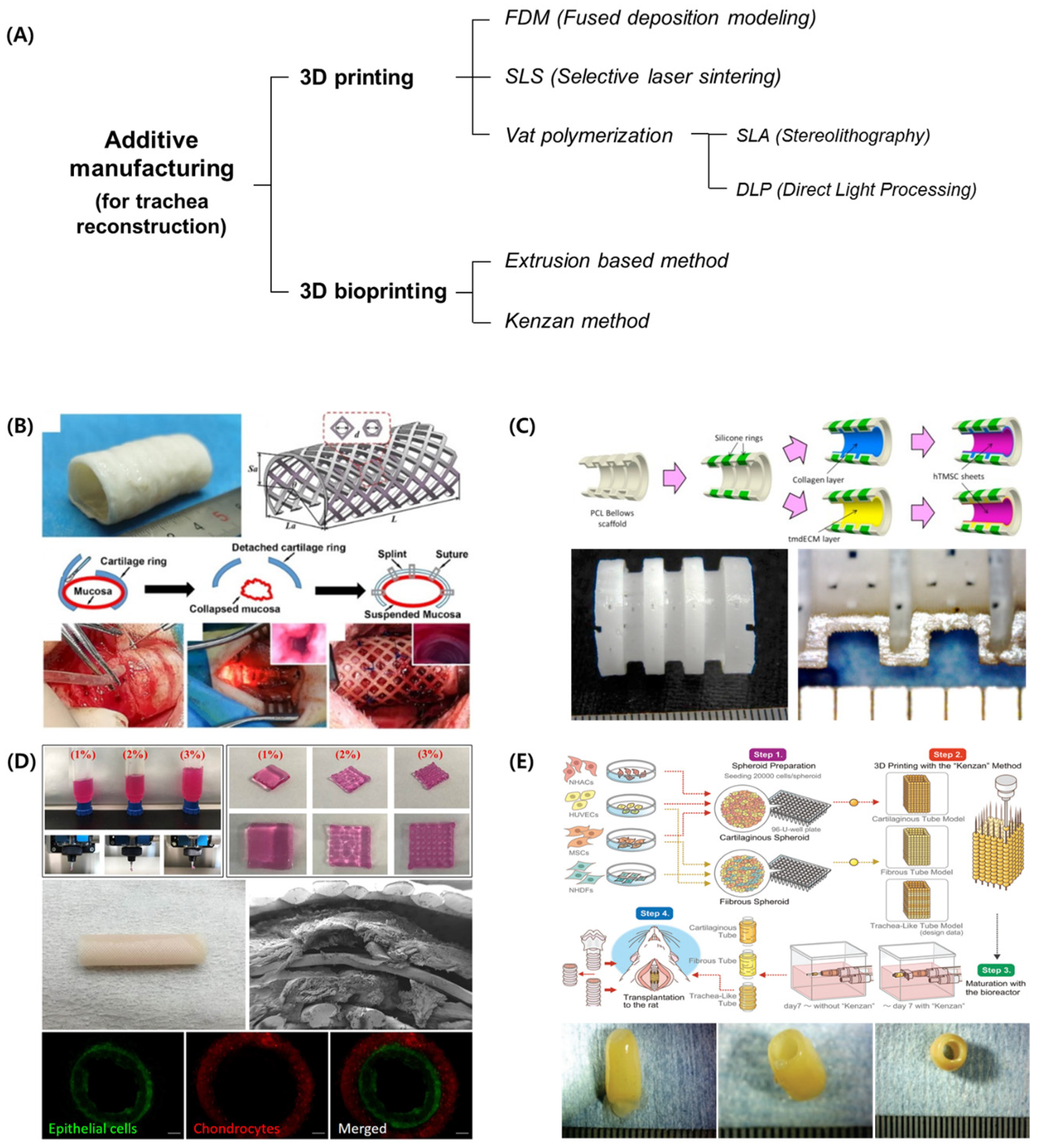
2. 3D-Printing-Based Technologies for Tracheal Reconstruction
2.1. 3D Printing
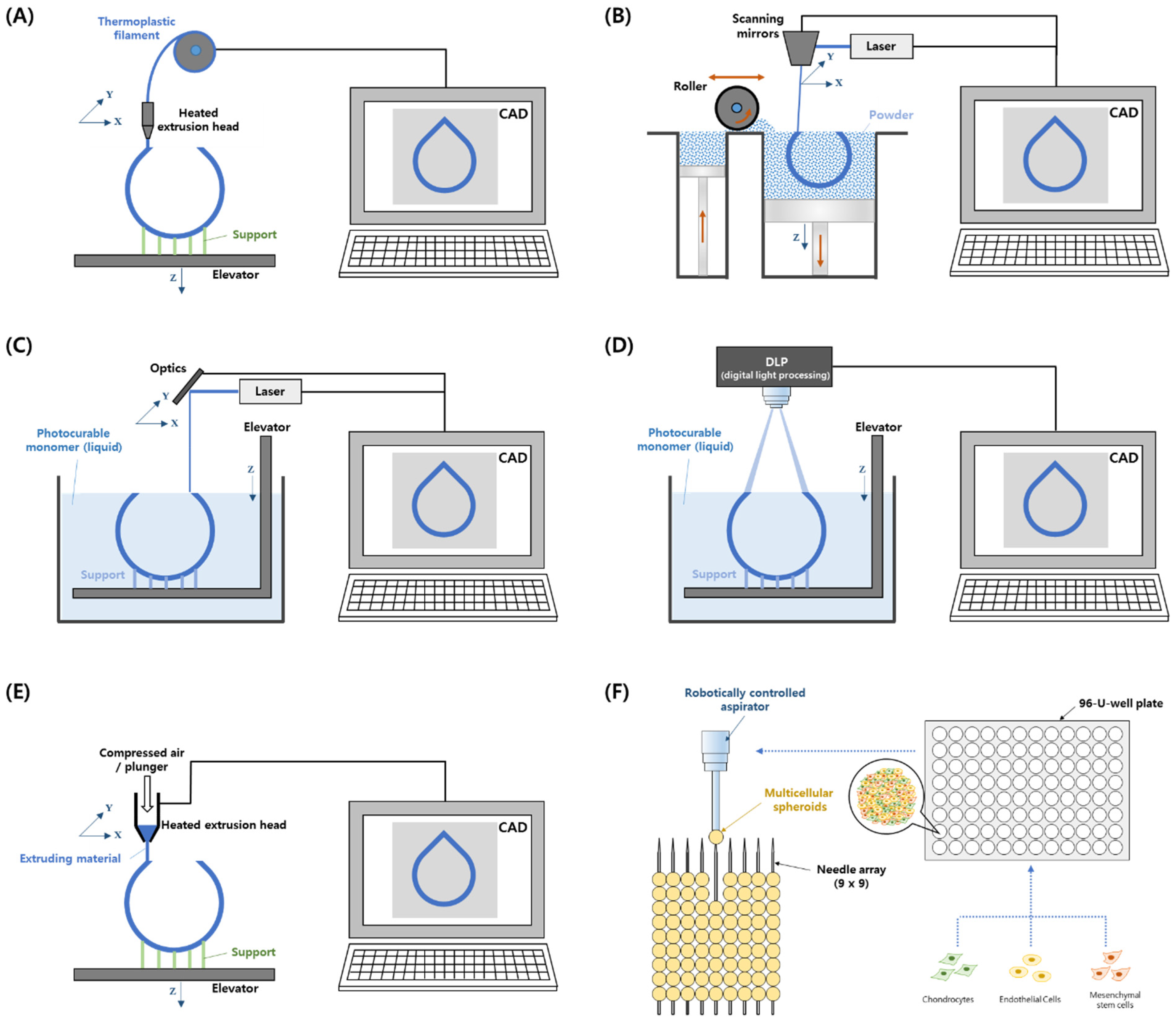
2.1.1. FDM
2.1.2. SLS
2.1.3. Vat Photopolymerization–Stereolithography (SLA) and Direct Light Processing (DLP)
2.1.4. Electrospinning
2.2. 3D Bioprinting
2.2.1. Extrusion-Based Bioprinting
2.2.2. Kenzan Method
3. Tracheal Reconstruction Using 3D Printing
3.1. External Splint
3.2. Circumferential Graft
3.3. Segmental Graft
4. Prospect
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Benjamin, B.; Pitkin, J.; Cohen, D. Congenital tracheal stenosis. Ann. Otol. Rhinol. Laryngol. 1981, 90, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Hertegård, S. Tissue engineering in the larynx and airway. Curr. Opin. Otolaryngol. Head Neck Surg. 2016, 24, 469–476. [Google Scholar] [CrossRef]
- Law, J.X.; Liau, L.L.; Aminuddin, B.S.; Ruszymah, B.H. Tissue-engineered trachea: A review. Int. J. Pediatr. Otorhinolaryngol. 2016, 91, 55–63. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Lu, Z.; Zhu, L.; Du, X.; Wang, H.; Xu, Z. Slide tracheoplasty in 81 children: Improved outcomes with modified surgical technique and optimal surgical age. Medicine 2017, 96, e8013. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.J.; De Coppi, P.; Speggiorin, S.; Roebuck, D.; Butler, C.R.; Samuel, E.; Crowley, C.; McLaren, C.; Fierens, A.; Vondrys, D.; et al. Stem-cell based, tissue engineered tracheal replacement in a child: A 2-year follow-up study. Lancet 2012, 380, 994–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, M.J.; Butler, C.R.; Varanou-Jenkins, A.; Partington, L.; Carvalho, C.; Samuel, E.; Crowley, C.; Lange, P.; Hamilton, N.J.; Hynds, R.E.; et al. Tracheal replacement therapy with a stem cell-seeded graft: Lessons from compassionate use application of a GMP compliant tissue-engineered medicine. Stem Cells Transl. Med. 2017, 6, 1458–1464. [Google Scholar] [CrossRef]
- Hamilton, N.J.; Kanani, M.; Roebuck, D.J.; Hewitt, R.J.; Cetto, R.; Culme-Seymour, E.J.; Toll, E.; Bates, A.J.; Comerford, A.P.; McLaren, C.A.; et al. Tissue engineered tracheal replacement in a child: A 4-year follow up study. Am. J. Transplant. 2015, 15, 2750–2757. [Google Scholar] [CrossRef]
- Dharmadhikari, S.; Best, C.A.; King, N.; Henderson, M.; Johnson, J.; Breuer, C.K.; Chiang, T. Mouse model of tracheal replacement with electrospun nanofiber scaffolds. Ann. Otol. Rhinol. Laryngol. 2019, 128, 391–400. [Google Scholar] [CrossRef]
- Omori, K.; Tada, Y.; Suzuki, T.; Nomoto, Y.; Matsuzuka, T.; Kobayashi, K.; Nakamura, T.; Kanemaru, S.; Yamashita, M.; Asato, R. Clinical application of in situ tissue engineering using a scaffolding technique for reconstruction of the larynx and trachea. Ann. Otol. Rhinol. Laryngol. 2008, 117, 673–678. [Google Scholar] [CrossRef]
- Greaney, A.M.; Niklason, L.E. The history of engineered trachea replacements: Interpreting the past and guiding the future. Tissue Eng. Part B 2021, 27, 341–352. [Google Scholar] [CrossRef]
- Zopf, D.A.; Hollister, S.J.; Nelson, M.E.; Ohye, R.G.; Green, G.E. Bioresorbable airway splint created with a three-dimensional printer. N. Engl. J. Med. 2013, 368, 2043–2045. [Google Scholar] [CrossRef]
- Morrison, R.J.; Hollister, S.J.; Niedner, M.F.; Mahani, M.G.; Park, A.H.; Mehta, D.K.; Ohye, R.G.; Green, G.E. Mitigation of tracheobronchomalacia with 3D-printed personalized medical devices in pediatric patients. Sci. Transl. Med. 2015, 7, 285ra64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, R.J.; Sengupta, S.; Flanangan, C.L.; Ohye, R.G.; Hollister, S.J.; Green, G.E. Successful treatment of severe acquired tracheomalacia with a patient-specific 3D printed permanent tracheal splint. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 523–525. [Google Scholar]
- Les, A.S.; Ohye, R.G.; Filbrun, A.G.; Mahani, M.G.; Flanagan, C.L.; Daniels, R.C.; Kidwell, K.M.; Zopf, D.A.; Hollister, S.J.; Green, G.E. 3D-Printed, externally-implanted, bioresorbable airway splints for severe tracheobronchomalacia. Laryngoscope 2019, 129, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Rehmani, S.S.; Al-Ayoubi, A.M.; Ayub, A.; Barsky, M.; Lewis, E.; Flores, R.; Lebovics, R.; Bhora, F.Y. Three-dimensional-printed bioengineered tracheal grafts: Preclinical results and potential for human use. Ann. Thorac. Surg. 2017, 104, 998–1004. [Google Scholar] [CrossRef] [Green Version]
- Xia, D.; Jin, D.; Wang, Q.; Gao, M.; Zhang, J.; Zhang, H.; Bai, J.; Feng, B.; Chen, M.; Huang, Y.; et al. Tissue-engineered trachea from a 3D-printed scaffold enhances whole-segment tracheal repair in a goat model. J. Tissue Eng. Regen. Med. 2019, 13, 694–703. [Google Scholar] [CrossRef]
- Kaye, R.; Goldstein, T.; Grande, D.A.; Zeltsman, D.; Smith, L.P. A 3-dimensional bioprinted tracheal segment implant pilot study: Rabbit tracheal resection with graft implantation. Int. J. Pediatr. Otorhinolaryngol. 2019, 117, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Yoon, J.K.; Lee, J.B.; Shin, Y.M.; Lee, K.W.; Bae, S.W.; Lee, J.; Yu, J.; Jung, C.-R.; Youn, Y.-N.; et al. Experimental tracheal replacement using 3-dimensional bioprinted artificial trachea with autologous epithelial cells and chondrocytes. Sci. Rep. 2019, 9, 2103. [Google Scholar] [CrossRef]
- Taniguchi, D.; Matsumoto, K.; Tsuchiya, T.; Machino, R.; Takeoka, Y.; Elgalad, A.; Gunge, K.; Takagi, K.; Taura, Y.; Hatachi, G.; et al. Scaffold-free trachea regeneration by tissue engineering with bio-3D printing. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 745–752. [Google Scholar] [CrossRef]
- Machino, R.; Matsumoto, K.; Taniguchi, D.; Tsuchiya, T.; Takeoka, Y.; Taura, Y.; Moriyama, M.; Tetsuo, T.; Oyama, S.; Takagi, K.; et al. Replacement of rat tracheas by layered, trachea-like, scaffold-free structures of human cells using a bio-3D printing system. Adv. Healthc. Mater. 2019, 8, e1800983. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Ahn, M.; Park, S.H.; Kim, H.; Bae, M.; Park, W.; Hollister, S.J.; Kim, S.W.; Cho, D.-W. 3D Bioprinting of a trachea-mimetic cellular construct of a clinically relevant size. Biomaterials 2021, 279, 121246. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Meng, Z.; Zheng, K.; Wang, L.; Zhang, C.; Ji, J.; Li, X.; He, J.; Zhao, J. Development of three-dimensional printed biodegradable external airway splints with native-like shape and mechanical properties for tracheomalacia treatment. Mater. Des. 2021, 210, 110105. [Google Scholar] [CrossRef]
- Park, J.H.; Park, J.Y.; Nam, I.C.; Ahn, M.; Lee, J.Y.; Choi, S.H.; Kim, S.W.; Cho, D.-W. A rational tissue engineering strategy based on three-dimensional (3D) printing for extensive circumferential tracheal reconstruction. Biomaterials 2018, 185, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Klebe, R.J. Cytoscribing: A method for micro positioning cells and the construction of two and three-dimensional synthetic tissues. Exp. Cell Res. 1988, 179, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Derby, B. Printing and prototyping of tissues and scaffolds. Science 2012, 338, 921–926. [Google Scholar] [CrossRef] [Green Version]
- Murphy, S.V.; Atala, A. 3D Bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.V.; Hernandez, A. A review of additive manufacturing. Int. Sch. Res. Not. 2012, 2012, 208760. [Google Scholar] [CrossRef] [Green Version]
- ISO/ASTM 52900; Additive Manufacturing–General Principles–Terminology. ISO: Geneva, Switzerland, 2015.
- Olejnik, P.; Juskanic, D.; Patrovic, L.; Halaj, M. First printed 3D heart model based on cardiac magnetic resonance imaging data in Slovakia. Bratisl. Lek. Listy 2018, 119, 781–784. [Google Scholar] [CrossRef]
- Xia, Z.; Jin, S.; Ye, K. Tissue and organ 3D bioprinting. SLAS Technol. 2018, 23, 301–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Park, J.K.; Son, K.H.; Lee, J.W. PCL/Sodium-alginate based 3D-printed dual drug delivery system with antibacterial activity for osteomyelitis therapy. Gels 2022, 8, 163. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Baik, J.M.; Yu, Y.S.; Kim, J.H.; Ahn, C.B.; Son, K.H.; Choi, E.S.; Lee, J.W. Development of a heat labile antibiotic eluting 3D printed scaffold for the treatment of osteomyelitis. Sci. Rep. 2020, 10, 7554. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, J.H.; Ahn, C.B.; Hong, J.H.; Son, K.H.; Lee, J.W. Development of a 3D-printed drug-eluting stent for treating obstructive salivary gland disease. ACS Biomater. Sci. Eng. 2019, 5, 3572–3581. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, J.W.; Yun, W.-S. Fabrication and tissue engineering application of a 3D PPF/DEF scaffold using Blu-ray based 3D printing system. J. Mech. Sci. Technol. 2017, 31, 2581–2587. [Google Scholar] [CrossRef]
- Gauvin, R.; Chen, Y.-C.; Lee, J.W.; Soman, P.; Zorlutuna, P.; Nichol, J.W.; Bae, H.; Chen, S.; Khademhosseini, A. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials 2012, 33, 3824–3834. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Jin, J.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing of viable mammalian cells. Biomaterials 2005, 26, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.S.; Ahn, C.B.; Son, K.H.; Lee, J.W. Motility improvement of biomimetic trachea scaffold via hybrid 3D-bioprinting technology. Polymers 2021, 13, 971. [Google Scholar] [CrossRef]
- Ahn, C.B.; Lee, J.H.; Kim, J.H.; Kim, T.H.; Jun, H.S.; Son, K.H.; Lee, J.W. Development of a 3D subcutaneous construct containing insulin-producing beta cells to treat type I diabetes. Bio-Des. Manuf. 2022, 5, 265–276. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, Y.-J.; Yong, W.-J.; Pati, F.; Shim, J.-H.; Kang, K.S.; Kang, I.-H.; Park, J.; Cho, D.-W. Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication 2016, 8, 015007. [Google Scholar] [CrossRef]
- Choi, W.S.; Kim, J.H.; Ahn, C.B.; Lee, J.H.; Kim, Y.J.; Son, K.H.; Lee, J.W. Development of a multi-layer skin substitute using human hair keratinic extract-based hybrid 3D printing. Polymers 2021, 13, 2584. [Google Scholar] [CrossRef]
- Ahn, C.B.; Kim, J.H.; Lee, J.H.; Park, K.Y.; Son, K.H.; Lee, J.W. Development of multi-layer tubular vascular scaffold to enhance compliance by exhibiting a negative Poisson’s ratio. Int. J. Precis. Eng. Manuf.—Green Technol. 2021, 8, 841–853. [Google Scholar] [CrossRef]
- Wu, W.; Geng, P.; Li, G.; Zhao, D.; Zhang, H.; Zhao, J. Influence of layer thickness and raster angle on the mechanical properties of 3Dprinted peek and a comparative mechanical study between PEEK and ABS. Materials 2015, 8, 5834–5846. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Yan, M.; Wang, Y.; Fu, J.; Suo, H. 3D Bioprinting of low-concentration cell-laden gelatin methacrylate (GelMA) bioinks with a two-step cross-linking strategy. ACS Appl. Mater. Interfaces 2018, 10, 6849–6857. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.B.; Mishra, A.; Lin, P.T.P.; Ng, S.H.; Yeong, W.Y.; Kim, Y.-J.; Yoon, Y.-J. 3D Printed polycaprolactone carbon nanotube composite scaffolds for cardiac tissue engineering. Macromol. Biosci. 2017, 17, 1600250. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, K.; Deckers, J.; Zhang, Z.; Kruth, J.-P.; Vleugels, J. Additive manufacturing of zirconia parts by indirect selective laser sintering. J. Eur. Ceram. Soc. 2014, 34, 81–89. [Google Scholar] [CrossRef]
- Bae, E.-J.; Kim, J.-H.; Kim, W.-C.; Kim, H.-Y. Bond and fracture strength of metal-ceramic restorations formed by selective laser sintering. J. Adv. Prosthodont. 2014, 6, 266–271. [Google Scholar] [CrossRef] [Green Version]
- Liao, H.-T.; Lee, M.-Y.; Tsai, W.-W.; Wang, H.-C.; Lu, W.-C. Osteogenesis of adipose-derived stem cells on polycaprolactone–β-tricalcium phosphate scaffold fabricated via selective laser sintering and surface coating with collagen type I. J. Tissue Eng. Regen. Med. 2016, 10, 337–353. [Google Scholar] [CrossRef]
- Sun, H.; He, S.; Wu, P.; Gao, C.; Feng, P.; Xiao, T.; Deng, Y.; Shuai, C. A novel mgo-cao-sio2 system for fabricating bone scaffolds with improved overall performance. Materials 2016, 9, 287. [Google Scholar] [CrossRef] [Green Version]
- Mazzoli, A. Selective laser sintering in biomedical engineering. Med. Biol. Eng. Comput. 2013, 51, 245–256. [Google Scholar] [CrossRef]
- Lee, J.W.; Kang, K.S.; Lee, S.H.; Kim, J.-Y.; Lee, B.-K.; Cho, D.-W. Bone regeneration using a microstereolithography-produced customized poly(propylene fumarate) / diethyl fumarate photopolymer 3D scaffold incorporating BMP-2 loaded PLGA microspheres. Biomaterials 2011, 32, 744–752. [Google Scholar] [CrossRef]
- Fozdar, D.Y.; Soman, P.; Lee, J.W.; Han, L.-H.; Chen, S. Three-dimensional polymer constructs exhibiting a tunable negative Poisson ratio. Adv. Funct. Mater. 2011, 21, 2712–2720. [Google Scholar] [CrossRef]
- Soman, P.; Lee, J.W.; Phadke, A.; Varghese, S.; Chen, S. Spatial tuning of negative and positive Poisson ratio in a multi-layer scaffold. Acta Biomater. 2012, 8, 2587–2594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aisenbrey, E.A.; Tomaschke, A.; Kleinjan, E.; Muralidharan, A.; Pascual-Garrido, C.; McLeod, R.R.; Ferguson, V.L.; Bryant, S.J. A stereolithography-based 3D printed hybrid scaffold for in situ cartilage defect repair. Macromol. Biosci. 2018, 18, 1700267. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, O.; Geven, M.; Sprecher, C.; Stadelmann, V.; Grijpma, D.; Tang, T.; Qin, L.; Lai, Y.; Alini, M.; De Bruijn, J.D.; et al. Surface enrichment with hydroxyapatite nanoparticles in stereolithography fabricated composite polymer scaffolds promotes bone repair. Acta Biomater. 2017, 54, 386–398. [Google Scholar] [CrossRef]
- Kishan, A.P.; Cosgriff-Hernandez, E.M. Recent advancements in electrospinning design for tissue engineering applications: A review. J. Biomed. Mater. Res. A 2017, 105, 2892–2905. [Google Scholar] [CrossRef] [PubMed]
- Jun, I.; Han, H.-S.; Edwards, J.R.; Jeon, H. Electrospun fibrous scaffolds for tissue engineering: Viewpoints on architecture and fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef] [Green Version]
- Li, B.Y.; Meng, D. Research progress of 3D bioprinting in bone tissue engineering. Chin. J. Prosthodont. 2021, 22, 138–143. [Google Scholar]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, T.; Fortunato, G.M.; Hann, S.Y.; Ayan, B.; Vajanthri, K.Y.; Presutti, D.; Cui, H.; Chan, A.H.P.; Costantini, M.; Onesto, V.; et al. Recent advances in bioprinting technologies for engineering cardiac tissue. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 124, 112057. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent advances in bioprinting techniques: Approaches, applications and future prospects. J. Transl. Med. 2016, 14, 271. [Google Scholar] [CrossRef] [Green Version]
- Gaebel, R.; Ma, N.; Liu, J.; Guan, J.; Koch, L.; Klopsch, C.; Gruene, M.; Toelk, A.; Wang, W.; Mark, P.; et al. Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials 2011, 32, 9218–9230. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar]
- Serpooshan, V.; Mahmoudi, M.; Hu, D.A.; Hu, J.B.; Wu, S.M. Bioengineering cardiac constructs using 3D printing. J. 3D Print. Med. 2017, 1, 123–139. [Google Scholar] [CrossRef]
- Cui, H.; Nowicki, M.; Fisher, J.P.; Zhang, L.G. 3D Bioprinting for organ regeneration. Adv. Healthc. Mater. 2017, 6, 1601118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Wang, L.; He, J.; Zhao, J.; Zhong, D.; Yang, G.; Guo, T.; Yan, X.; Zhang, L.; Li, D.; et al. Tracheal suspension by using 3-dimensional printed personalized scaffold in a patient with tracheomalacia. J. Thorac. Dis. 2016, 8, 3323–3328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zopf, D.A.; Flanagan, C.L.; Wheeler, M.; Hollister, S.J.; Green, G.E. Treatment of severe porcine tracheomalacia with a 3-dimensionally printed, bioresorbable, external airway splint. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 66–71. [Google Scholar]
- Kaye, R.; Goldstein, T.; Aronowitz, D.; Grande, D.A.; Zeltsman, D.; Smith, L.P. Ex vivo tracheomalacia model with 3D-printed external tracheal splint. Laryngoscope 2017, 127, 950–955. [Google Scholar] [CrossRef]
- Ott, L.M.; Zabel, T.A.; Walker, N.K.; Farris, A.L.; Chakroff, J.T.; Ohst, D.G.; Johnson, J.K.; Gehrke, S.H.; Weatherly, R.A.; Detamore, M.S. Mechanical evaluation of gradient electrospun scaffolds with 3D printed ring reinforcements for tracheal defect repair. Biomed. Mater. 2016, 11, 025020. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Dharmadhikari, S.; Spector, B.M.; Tan, Z.H.; Curen, C.E.; Agarwa, R.; Nyirjesy, S.; Shontz, K.; Sperber, S.A.; Breuer, C.K.; et al. Tissue-engineered composite tracheal grafts create mechanically stable and biocompatible airway replacements. J. Tissue Eng. 2022, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jin, Z.; Yang, L.; Liu, Y.; Liu, J.; Cai, S.; Shen, Y.; Guo, S. A self-expandable C-shaped 3D printing tracheal stent for combinatorial controlled paclitaxel release and tracheal support. Mater. Today Chem. 2022, 24, 100760. [Google Scholar] [CrossRef]
- She, Y.; Fan, Z.; Wang, L.; Li, Y.; Sun, W.; Tang, H.; Zhang, L.; Wu, L.; Zheng, H.; Chen, C. 3D Printed biomimetic PCL scaffold as framework interspersed with collagen for long segment tracheal replacement. Front. Cell Dev. Biol. 2021, 9, 629796. [Google Scholar] [PubMed]
- Hsieh, C.-T.; Liao, C.-Y.; Dai, N.-T.; Tseng, C.-S.; Yen, B.L.; Hsu, S. 3D Printing of tubular scaffolds with elasticity and complex structure from multiple waterborne polyurethanes for tracheal tissue engineering. Appl. Mater. Today 2018, 12, 330–341. [Google Scholar]
- Lee, D.Y.; Park, S.A.; Lee, S.J.; Kim, T.H.; Oh, S.H.; Lee, J.H.; Kwon, S.K. Segmental tracheal reconstruction by 3D-printed scaffold: Pivotal role of asymmetrically porous membrane. Laryngoscope 2016, 126, E304–E309. [Google Scholar] [PubMed]
- Bhora, F.Y.; Lewis, E.E.; Rehmani, S.S.; Ayub, A.; Raad, W.; Al-Ayoubi, A.M.; Lebovics, R.S. Circumferential three-dimensional-printed tracheal grafts: Research model feasibility and early results. Ann. Thorac. Surg. 2017, 104, 958–963. [Google Scholar] [CrossRef] [Green Version]
- Park, H.S.; Park, H.J.; Lee, J.; Kim, P.; Lee, J.S.; Lee, Y.J.; Seo, Y.B.; Kim, D.Y.; Ajiteru, O.; Lee, O.J.; et al. A 4-axis technique for three-dimensional printing of an artificial trachea. Tissue Eng. Regen. Med. 2018, 15, 415–425. [Google Scholar] [CrossRef]
- Pan, S.; Zhong, Y.; Shan, Y.; Liu, X.; Xiao, Y.; Shi, H. Selection of the optimum 3D-printed pore and the surface modification techniques for tissue engineering tracheal scaffold in vivo reconstruction. J. Biomed. Mater. Res. A 2019, 107, 360–370. [Google Scholar]
- Weber, J.F.; Rehmani, S.S.; Baig, M.Z.; Lebovics, R.; Raad, W.; Connery, C.; Bhora, F.Y. Novel composite trachea grafts using 3-dimensional printing. JTCVS Open 2021, 5, 152–160. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, H.; Dong, W.; Bai, J.; Gao, B.; Xia, D.; Feng, B.; Chen, M.; He, X.; Yin, M.; et al. Tissue-engineered trachea from a 3D-printed scaffold enhances whole-segment tracheal repair. Sci. Rep. 2017, 7, 5246. [Google Scholar]
- Kim, I.G.; Park, S.A.; Lee, S.H.; Choi, J.S.; Cho, H.; Lee, S.J.; Kwon, Y.W.; Kwon, S.K. Transplantation of a 3D-printed tracheal graft combined with iPS cell-derived MSCs and chondrocytes. Sci. Rep. 2020, 10, 4326. [Google Scholar]
- Park, H.S.; Lee, J.S.; Jung, H.; Kim, D.Y.; Kim, S.W.; Sultan, M.T.; Park, C.H. An omentum-cultured 3D-printed artificial trachea: In vivo bioreactor. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 3), S1131–S1140. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Jing, H.; Luo, K.; Shi, B.; Luo, Q.; Zhu, Z.; He, X.; Zheng, J. Exosomes from 3T3-J2 promote expansion of tracheal basal cells to facilitate rapid epithelization of 3D-printed double-layer tissue engineered trachea. Mater. Sci. Eng. C 2021, 129, 112371. [Google Scholar]
- Gao, B.; Jing, H.; Gao, M.; Wang, S.; Fu, W.; Zhang, X.; He, X.; Zheng, J. Long-segmental tracheal reconstruction in rabbits with pedicled tissue-engineered trachea based on a 3D-printed scaffold. Acta Biomater. 2019, 97, 177–186. [Google Scholar]
- Frejo, L.; Goldstein, T.; Swami, P.; Patel, N.A.; Grande, D.A.; Zeltsman, D.; Smith, L.P. A two-stage in vivo approach for implanting a 3D printed tissue-engineered tracheal replacement graft: A proof of concept. Int. J. Pediatr. Otorhinolaryngol. 2022, 155, 111066. [Google Scholar]
- Ke, D.; Yi, H.; Est-Witte, S.; George, S.; Kengla, C.; Lee, S.J.; Atala, A.; Murphy, S.V. Bioprinted trachea constructs with patient-matched design, mechanical and biological properties. Biofabrication 2020, 12, 015022. [Google Scholar]
- Huo, Y.; Xu, Y.; Wu, X.; Gao, E.; Zhan, A.; Chen, Y.; Zhang, Y.; Hua, Y.; Swieszkowski, W.; Zhang, Y.S.; et al. Functional trachea reconstruction using 3D-bioprinted native-like tissue architecture based on designable tissue-specific bioinks. Adv. Sci. 2022, 9, 2202181. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, C.; Qiao, Y.; Gu, J.; Zhang, H.; Peijs, T.; Kong, J.; Zhang, G.; Shi, X. Tissue-engineered trachea consisting of electrospun patterned sc-PLA/GO-g-IL fibrous membranes with antibacterial property and 3D-printed skeletons with elasticity. Biomacromolecules. 2019, 20, 1765–1776. [Google Scholar] [CrossRef]
- Ahn, C.B.; Son, K.H.; Yu, Y.S.; Kim, T.H.; Lee, J.I.; Lee, J.W. Development of a flexible 3D printed scaffold with a cell-adhesive surface for artificial trachea. Biomed. Mater. 2019, 14, 055001. [Google Scholar] [CrossRef]
- Paunović, N.; Bao, Y.; Coulter, F.B.; Masania, K.; Geks, A.K.; Klein, K.; Rafsanjani, A.; Cadalbert, J.; Kronen, P.W.; Kleger, N.; et al. Digital light 3D printing of customized bioresorbable airway stents with elastomeric properties. Sci. Adv. 2021, 7, eabe9499. [Google Scholar]
- Kandi, R.; Pandey, P.M.; Majood, M.; Mohanty, S. Fabrication and characterization of customized tubular scaffolds for tracheal tissue engineering by using solvent based 3D printing on predefined template. Rapid Prototyp. J. 2021, 27, 421–428. [Google Scholar] [CrossRef]
- Park, J.H.; Park, J.Y.; Nam, I.-C.; Hwang, S.-H.; Kim, C.-S.; Jung, J.W.; Jang, J.; Lee, H.; Choi, Y.; Park, S.H.; et al. Human turbinate mesenchymal stromal cell sheets with bellows graft for rapid tracheal epithelial regeneration. Acta Biomater. 2015, 25, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Lee, S.J.; Kim, H.Y.; Park, H.S.; Wang, Z.; Kim, H.J.; Yoo, J.J.; Chung, S.M.; Kim, H.S. 3D Printed polyurethane prosthesis for partial tracheal reconstruction: A pilot animal study. Biofabrication 2016, 8, 045015. [Google Scholar]
- Goldstein, T.A.; Smith, B.D.; Zeltsman, D.; Grande, D.; Smith, L.P. Introducing a 3-dimensionally printed, tissue-engineered graft for airway reconstruction: A pilot study. Otolaryngol. Head Neck Surg. 2015, 153, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Best, C.A.; Pepper, V.K.; Ohst, D.; Bodnyk, K.; Heuer, E.; Onwuka, E.A.; King, N.; Strouse, R.; Grischkan, J.; Breuer, C.K.; et al. Designing a tissue-engineered tracheal scaffold for preclinical evaluation. Int. J. Pediatr. Otorhinolaryngol. 2018, 104, 155–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maity, N.; Mansour, N.; Chakraborty, P.; Bychenko, D.; Gazit, E.; Cohn, D. A personalized multifunctional 3D printed shape memory-displaying, drug releasing tracheal stent. Adv. Funct. Mater. 2021, 31, 2108436. [Google Scholar]
- Macchiarini, P.; Jungebluth, P.; Go, T.; Asnaghi, M.A.; Rees, L.E.; Cogan, T.A.; Dodson, A.; Martorell, J.; Bellini, S.; Parnigotto, P.P.; et al. Clinical transplantation of a tissue-engineered airway. Lancet 2008, 372, 2023–2030. [Google Scholar] [CrossRef] [PubMed]
| Author | Fabrication Technology | Specific Fabrication Method | Material (Bio-Ink) | Target (Species) | Evaluation (Regeneration) |
|---|---|---|---|---|---|
| Zopf et al. (2013) [11] | 3D Printing | SLS | PCL | External splint (human) | Tracheomalacia |
| Morrison et al. (2015) [12] | 3D Printing | SLS | PCL/HA | External splint (human) | Tracheomalacia |
| Morrison et al. (2017) [13] | 3D Printing | FDM | PEKK | External splint (human) | Tracheomalacia |
| Les et al. (2019) [14] | 3D Printing | SLS | PCL/HA | External splint (human) | Tracheomalacia |
| Huang et al. (2016) [66] | 3D Printing | FDM | PCL | External splint (human) | Tracheomalacia |
| Zopf et al. (2014) [67] | 3D Printing | SLS | PCL/HA | External splint (porcine) | Tracheomalacia |
| Kaye et al. (2017) [68] | 3D Printing | FDM | PLA | External splint (porcine) | Tracheomalacia |
| Liu et al. (2021) [22] | 3D Printing | SLS | PCL | External splint (dog) | Epithelialization |
| Ott et al. (2016) [69] | 3D Printing | FDM + Electrospinning | PCL vs. PLGA | External splint (cell) | - |
| Liu et al. (2022) [70] | 3D Printing + decellularized graft | Vat | Biocompatible resin | External splint (mouse) | epithelialization |
| Chen et al. (2022) [71] | 3D Printing | FDM | PCL | Internal splint (rabbit) | Tracheal stenosis |
| She et al. (2021) [72] | 3D Printing | FDM + Coating | PCL/Collagen (Chondrocytes) | Circumferential graft (rabbit) | Cartilage formation |
| Hsieh et al. (2018) [73] | 3D Printing | FDM | PU (hMSCs) | Circumferential graft (mouse) | Cartilage formation |
| Park et al. (2021) [21] | 3D Bioprinting | FDM + Extrusion | PCL (hNCs, hNTSCs + Collagen) | Circumferential graft (mouse) | Cartilage formation |
| Lee et al. (2016) [74] | 3D Printing | FDM | PCL | Circumferential graft (rabbit) | Epithelialization |
| Bhora et al. (2017) [75] | 3D Printing | FDM | PCL | Circumferential graft (porcine) | Epithelialization |
| Park et al. (2018) [23] | 3D Printing | Vat (indirect) + Cell sheet | PCL/Silicone | Circumferential graft (rabbit) | Epithelialization |
| Park et al. (2018) [76] | 3D Printing | FDM | PCL | Circumferential graft (rabbit) | Epithelialization |
| Park et al. (2019) [18] | 3D Bioprinting | FDM + Extrusion | PCL (Chondrocytes, epithelial cells + Alginate) | Circumferential graft (rabbit) | Cartilage formation, epithelialization |
| Machino et al. (2019) [20] | 3D Bioprinting | Kenzan method | Spheroid | Circumferential graft (rat) | Cartilage formation, epithelialization, vascularization |
| Gao et al. (2017) [79] | 3D Printing | FDM | PCL (Chondrocytes) | Circumferential graft (rabbit) | Cartilage formation, epithelialization |
| Kim et al. (2020) [80] | 3D Printing | FDM + Electrospinning | PCL (iPSCs + Matrigel) | Circumferential graft (rabbit) | Cartilage formation, epithelialization |
| Park et al. (2018) [81] | 3D Printing | FDM | PCL | Circumferential graft (rabbit) | Cartilage formation, epithelialization |
| Zhang et al. (2021) [82] | 3D Printing | FDM | PCL (Chondrocytes + Matrigel) | Circumferential graft (rabbit) | Cartilage formation, epithelialization |
| Xia et al. (2019) [16] | 3D Printing | FDM | PCL (Chondrocytes + Collagen) | Circumferential graft (goat) | Cartilage formation, epithelialization, vascularization |
| Taniguchi et al. (2018) [19] | 3D Bioprinting | Kenzan method | Spheroid | Circumferential graft (rat) | Cartilage formation, vascularization |
| Gao et al. (2019) [83] | 3D Printing | FDM | PLLA (Chondrocytes + Matrigel) | Circumferential graft (rabbit) | Cartilage formation, vascularization |
| Frejo et al. (2022) [84] | 3D Printing | FDM + coating | PCL (Chondrocytes + Collagen/alginate) | Partial graft (rabbit) | Cartilage formation, vascularization |
| Pan et al. (2019) [77] | 3D Printing | FDM | PCL | Circumferential graft (rabbit) | Vascularization |
| Weber et al. (2021) [78] | 3D Printing | FDM | PCL/SIS-ECM | Circumferential graft (pig) | Vascularization |
| Ke et al. (2020) [85] | 3D Printing | FDM + Extrusion | PCL (hMSCs + Collagen/ hyaluronan) | Circumferential graft (cell) | Cartilage formation, muscle formation |
| Huo et al. (2022) [86] | 3D Bioprinting | Extrusion | (Chondrocytes, fibroblast + Decellularized hydrogels) | Circumferential graft (rabbit) | Cartilage formation, epithelialization, vascularization |
| Kang et al. (2019) [87] | 3D Printing | FDM + Electrospinning | TPU/PLA | Circumferential graft (rabbit) | - |
| Ahn et al. (2019) [88] | 3D Printing | FDM + Electrospinning | PCL/PU | Circumferential graft (cell) | - |
| Paunović et al. (2021) [89] | 3D Printing | Vat | p(DLLA-co-CL) | Circumferential graft (rabbit) | - |
| Kandi et al. (2021) [90] | 3D Printing | FDM | PCL/PU | Circumferential graft (cell) | - |
| Park et al. (2015) [91] | 3D Printing | Vat (indirect) | PCL | Segmental graft (rabbit) | Epithelialization |
| Jung et al. (2016) [92] | 3D Printing | FDM | PU | Segmental graft (rabbit) | Epithelialization |
| Goldstein et al. (2015) [93] | 3D Printing | FDM | PLA (Chondrocytes + Collagen) | Segmental graft (rabbit) | Cartilage formation, epithelialization |
| Kaye et al. (2019) [17] | 3D Bioprinting | FDM + Extrusion | PCL (Cartilage + Alginate/collagen) | Segmental graft (rabbit) | Cartilage formation, epithelialization |
| Rhemani et al. (2017) [15] | 3D Printing | FDM | PCL/ECM | Segmental graft (porcine) | Epithelialization, vascularization |
| Best et al. (2017) [94] | 3D Printing | FDM | PET/PU/PCL | Segmental graft (cell) | - |
| Maity et al. (2021) [95] | 3D Printing | Vat | PCL-PPG-PCL diacrylate | Segmental graft (cell) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-Y.; Lee, J.W. Current Status and Future Outlook of Additive Manufacturing Technologies for the Reconstruction of the Trachea. J. Funct. Biomater. 2023, 14, 196. https://doi.org/10.3390/jfb14040196
Lee H-Y, Lee JW. Current Status and Future Outlook of Additive Manufacturing Technologies for the Reconstruction of the Trachea. Journal of Functional Biomaterials. 2023; 14(4):196. https://doi.org/10.3390/jfb14040196
Chicago/Turabian StyleLee, Hwa-Yong, and Jin Woo Lee. 2023. "Current Status and Future Outlook of Additive Manufacturing Technologies for the Reconstruction of the Trachea" Journal of Functional Biomaterials 14, no. 4: 196. https://doi.org/10.3390/jfb14040196





