Green Synthesis of Zinc Oxide Nanoparticles Using an Aqueous Extract of Punica granatum for Antimicrobial and Catalytic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biogenic Synthesis of ZnO-NPs Using Punica granatum
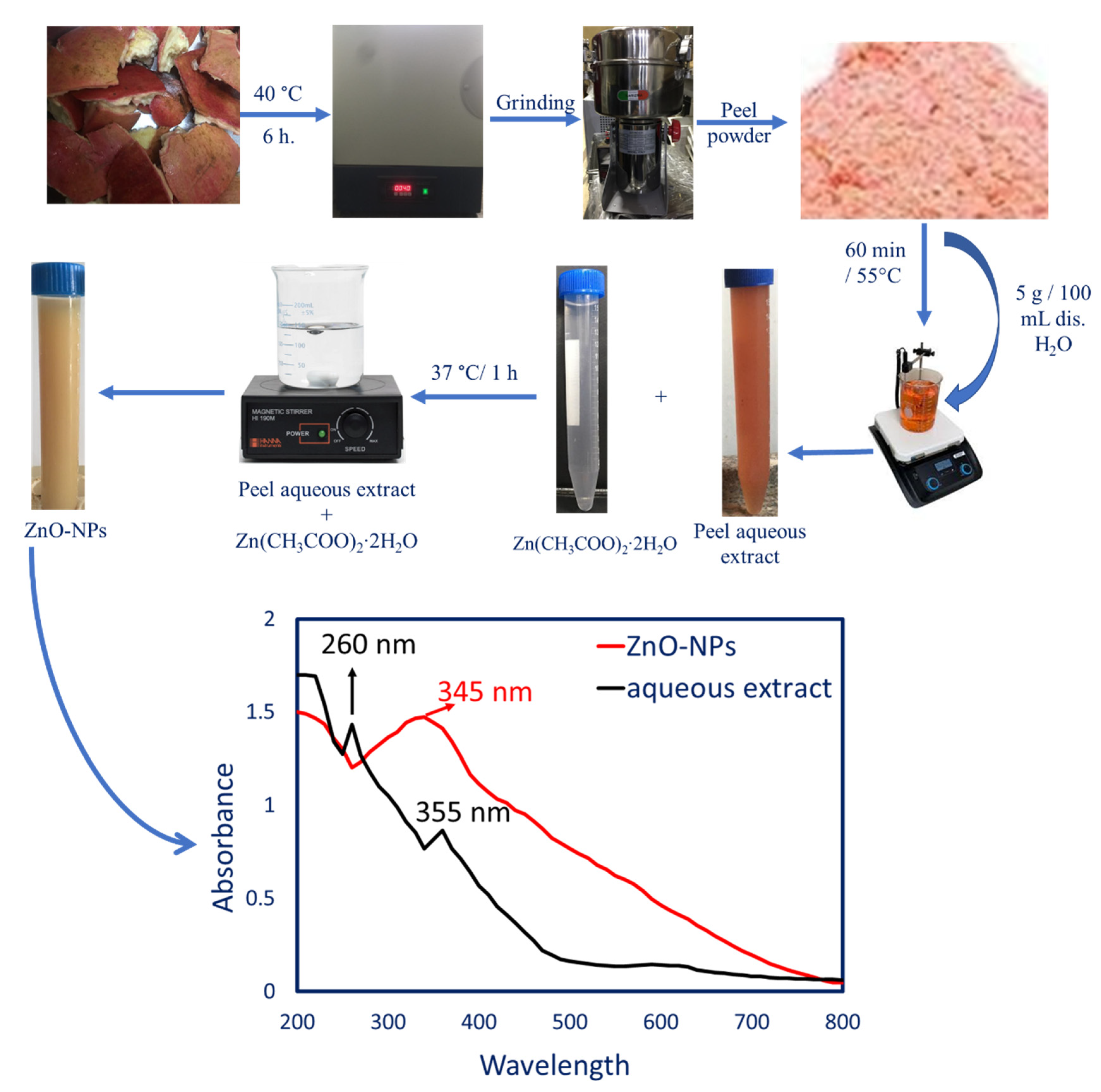
2.2.1. Prepared Peel Aqueous Extract
2.2.2. Peel Aqueous Extract-Based Biogenic Synthesis of ZnO-NPs
2.3. Characterizations
2.4. Antimicrobial Activity
2.5. Catalytic Activity against Methylene Blue (MB) Dye
2.6. Statistical Analysis
3. Results and Discussion
3.1. Punica Granatum-Based Green Synthesis of ZnO-NPs
3.2. Characterizations
3.2.1. Color Change and UV-Vis Spectroscopy
3.2.2. X-ray Diffraction
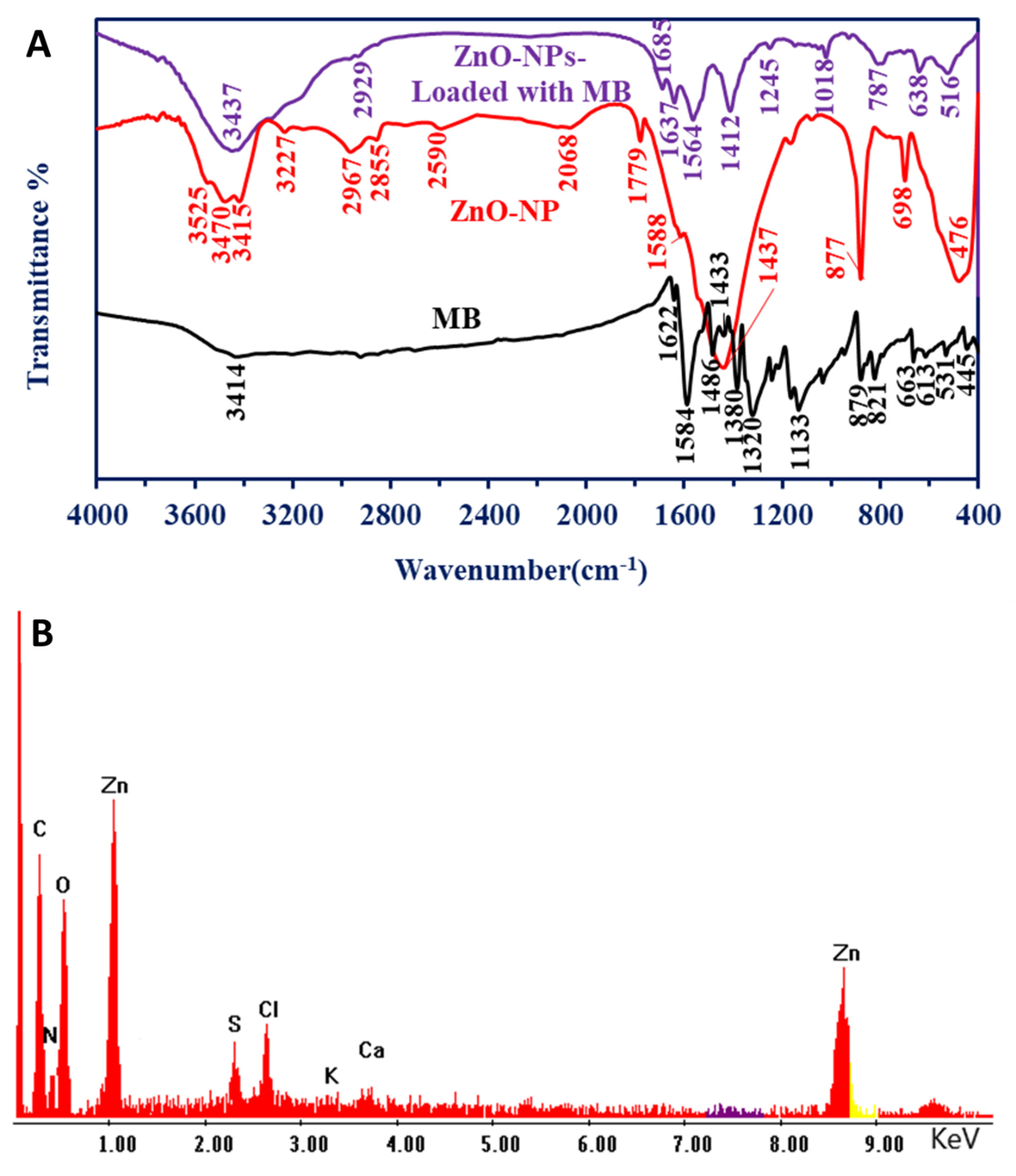
3.2.3. Fourier Transform Infrared (FT-IR)
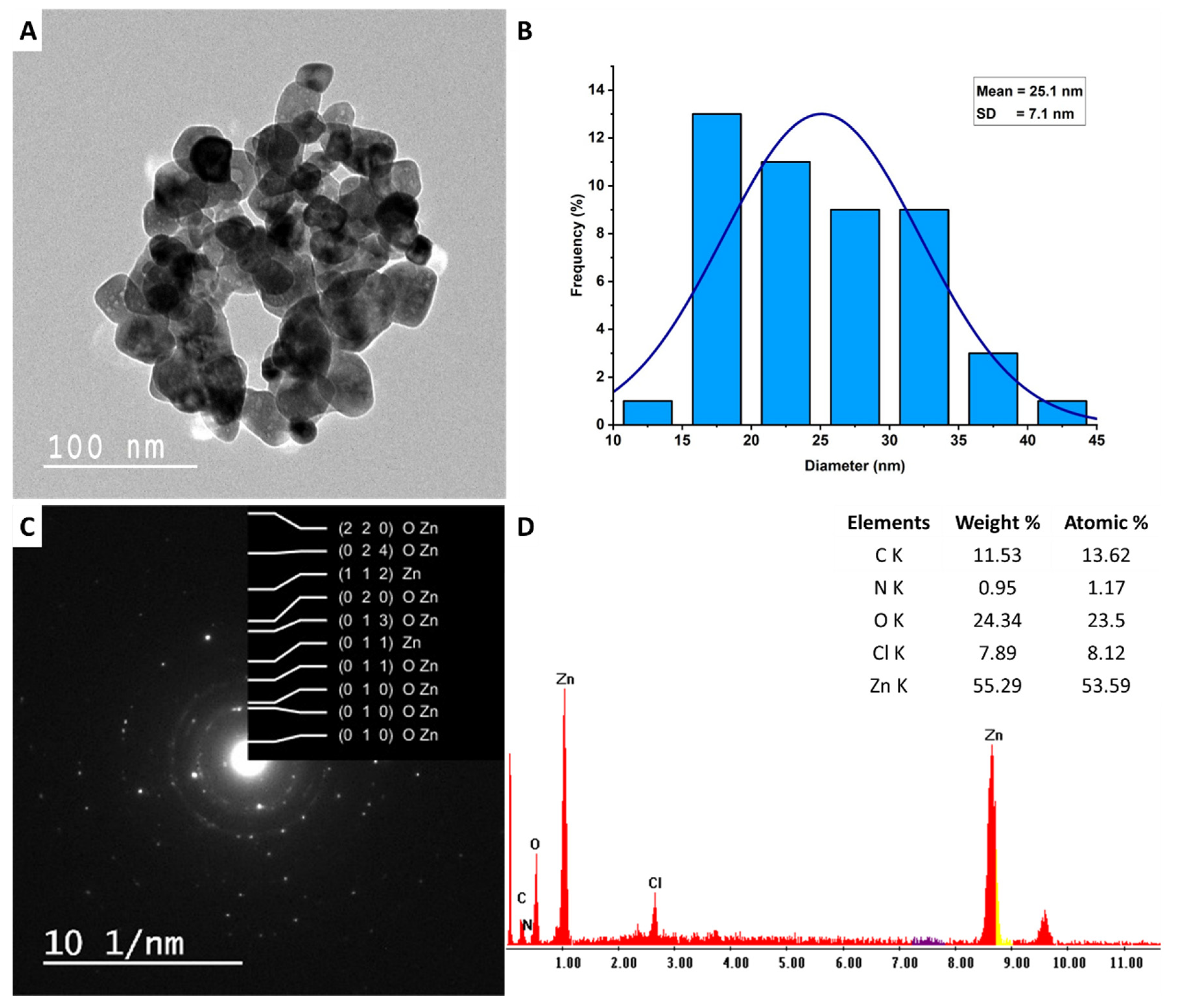
3.2.4. TEM, SAED, and EDX
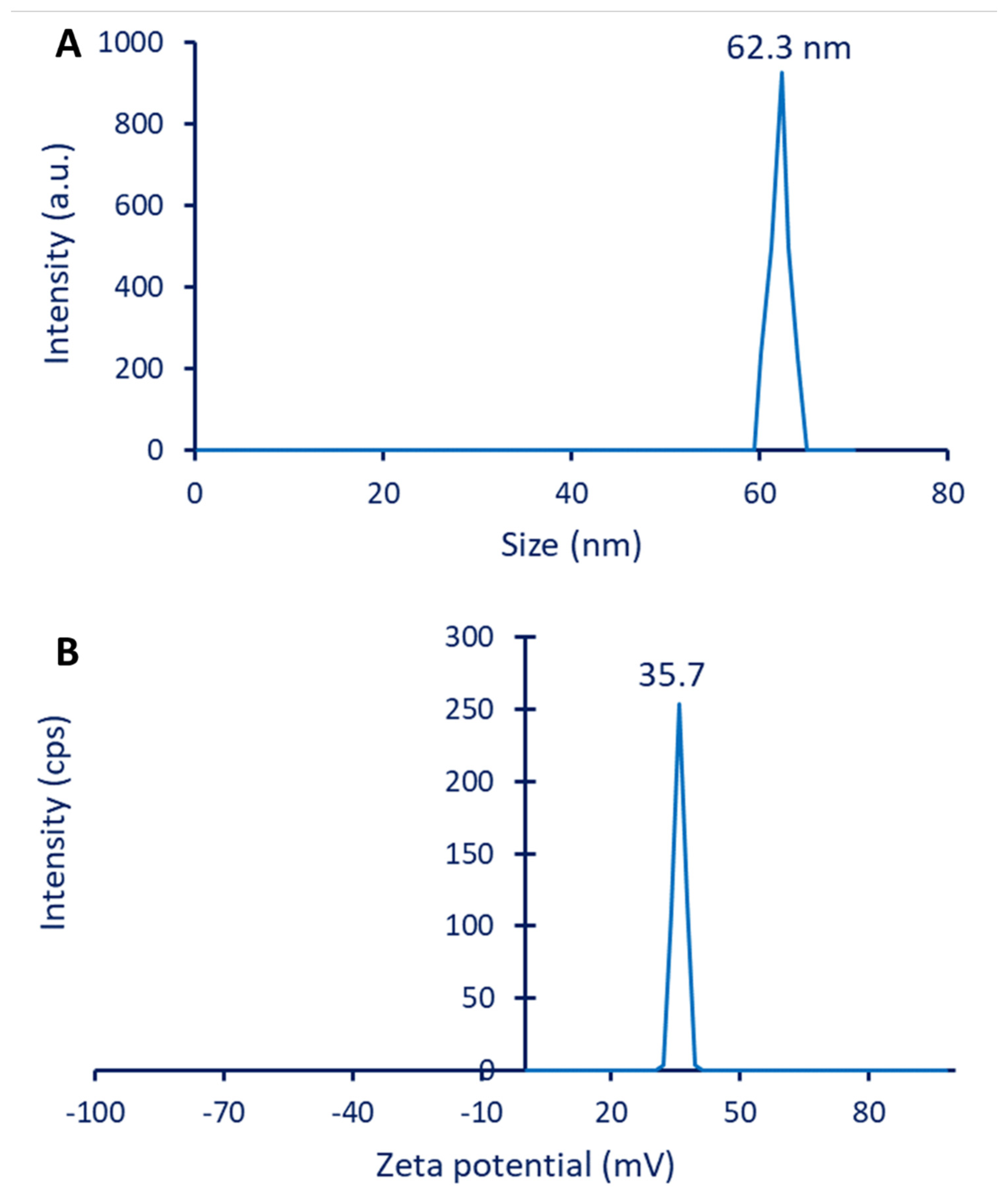
3.2.5. DLS and Zeta Potential
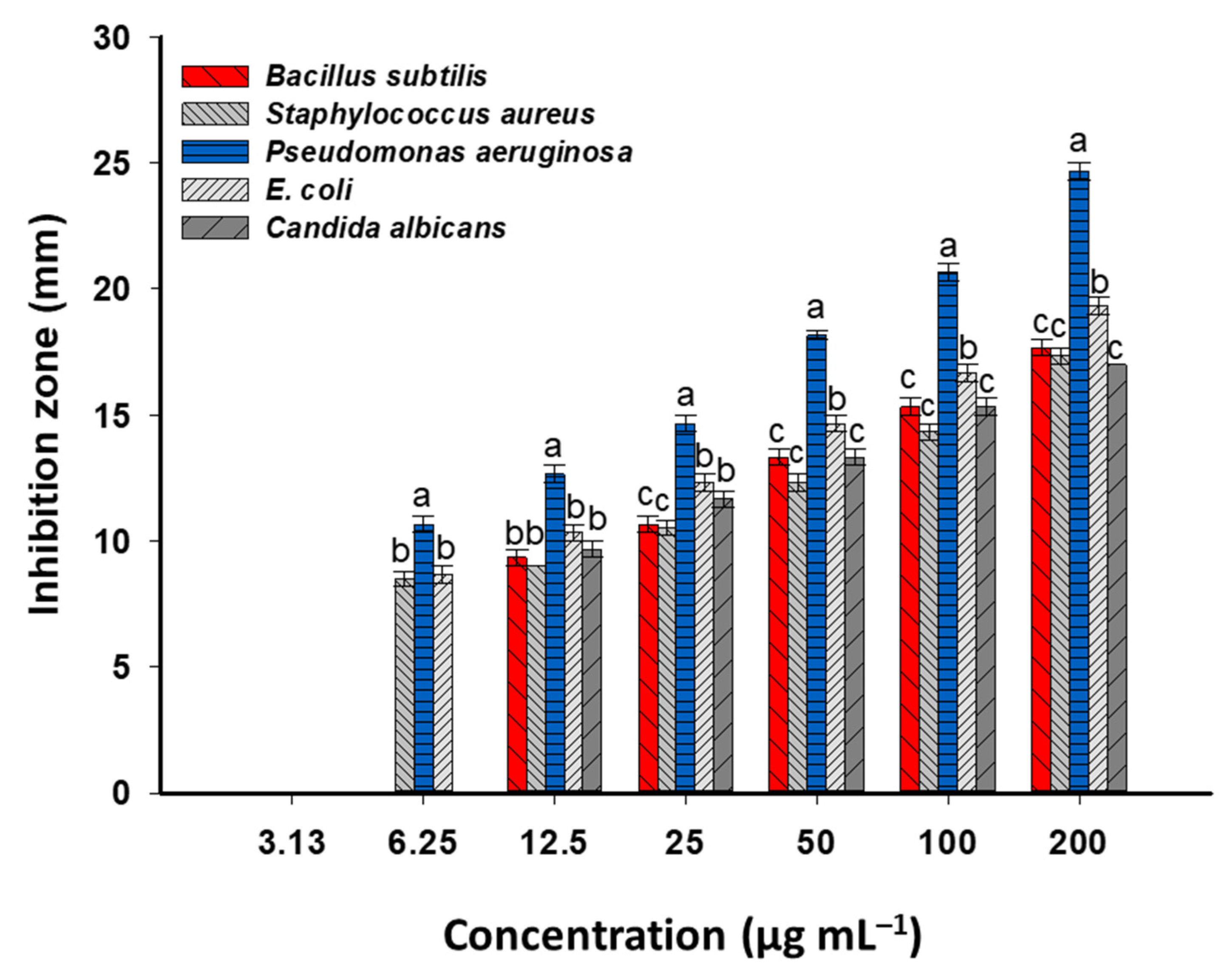
3.3. Antimicrobial Activity
3.4. Catalytic Activity
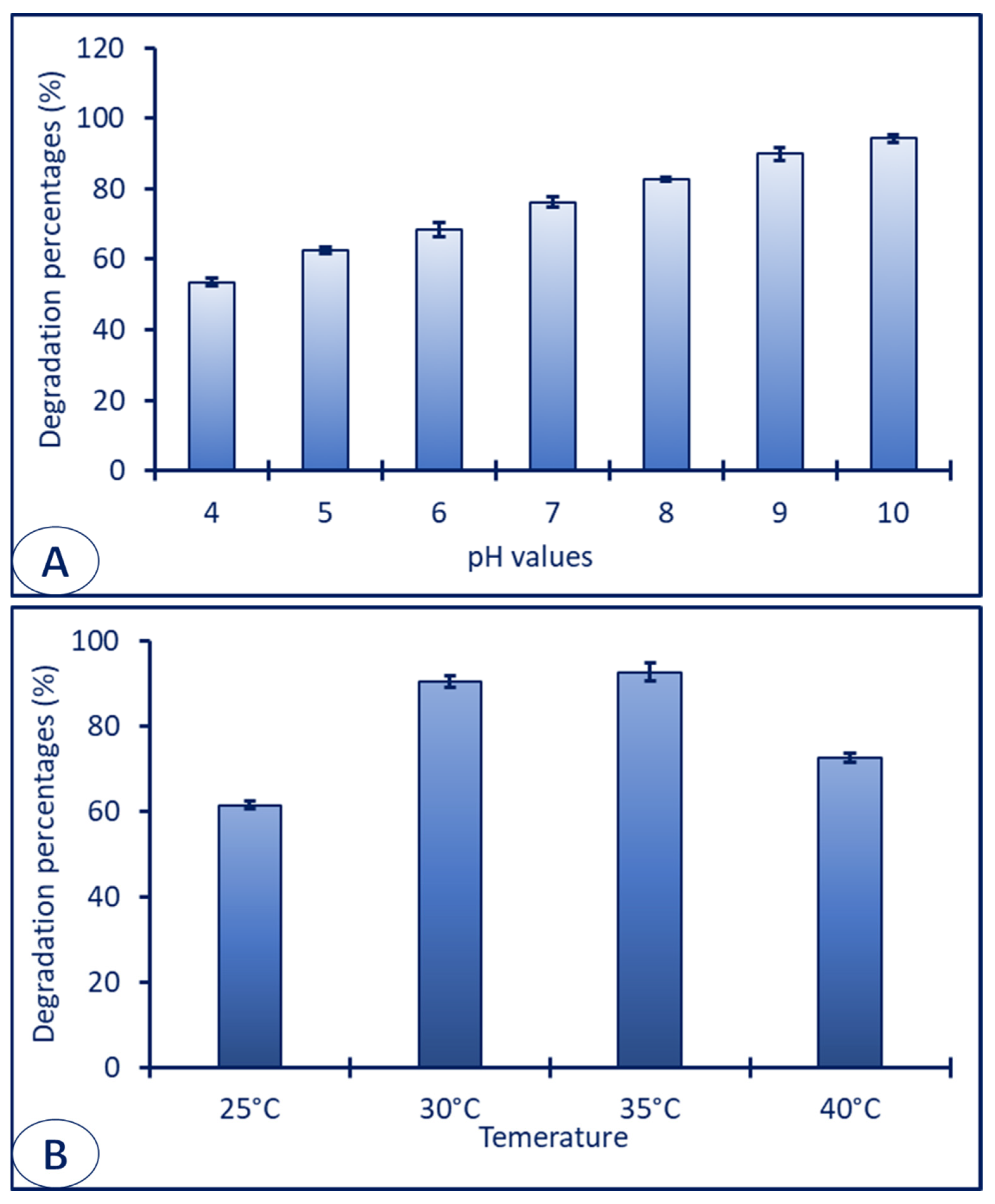
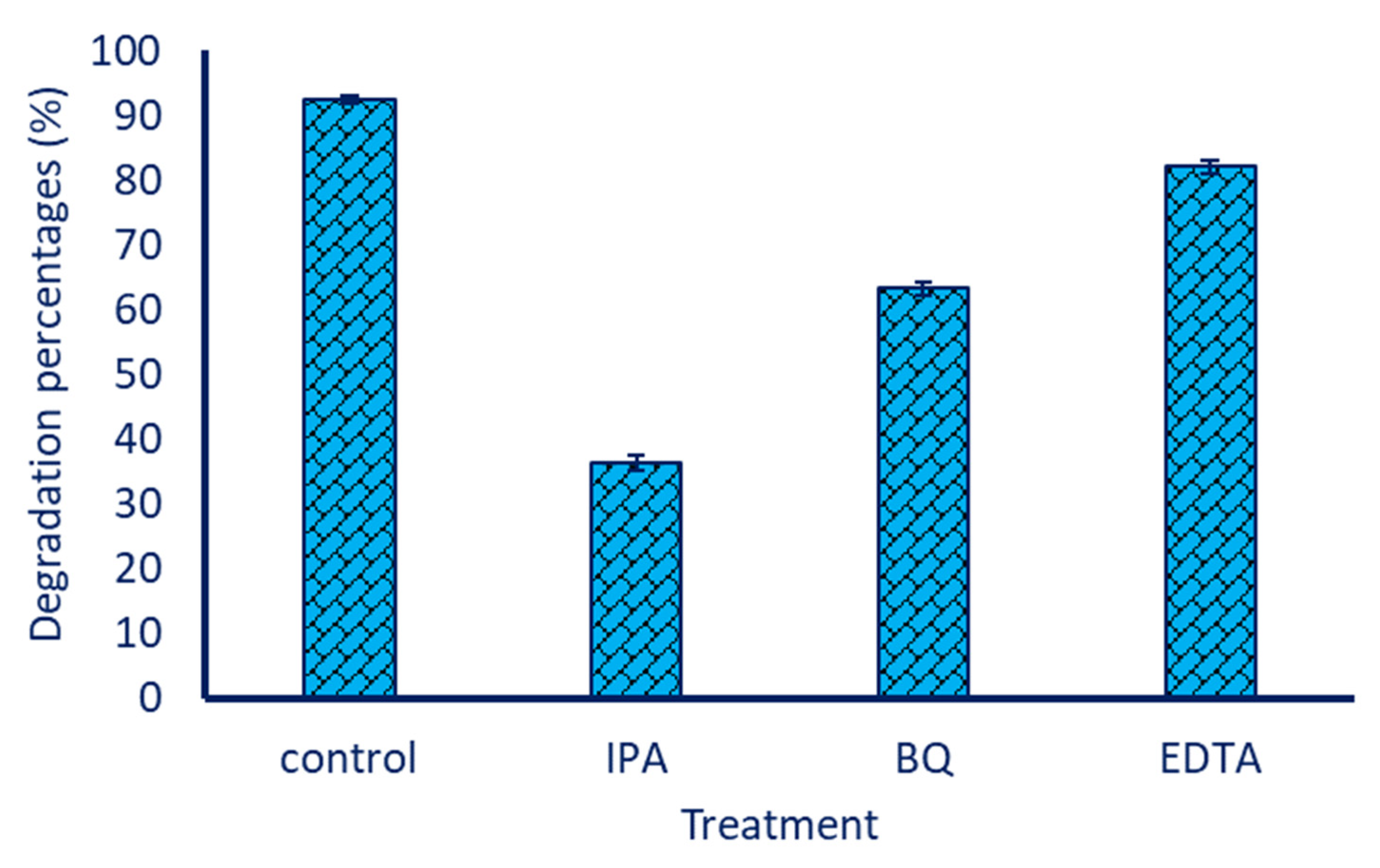
3.5. Sorption of Dye on the Surface of ZnO-NPs
3.6. Reusability of Nano-Catalyst
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fouda, A.; Eid, A.M.; Guibal, E.; Hamza, M.F.; Hassan, S.E.; Alkhalifah, D.H.M.; El-Hossary, D. Green Synthesis of Gold Nanoparticles by Aqueous Extract of Zingiber officinale: Characterization and Insight into Antimicrobial, Antioxidant, and In Vitro Cytotoxic Activities. Appl. Sci. 2022, 12, 12879. [Google Scholar] [CrossRef]
- Danish, M.S.; Estrella, L.L.; Alemaida, I.M.A.; Lisin, A.; Moiseev, N.; Ahmadi, M.; Nazari, M.; Wali, M.; Zaheb, H.; Senjyu, T. Photocatalytic Applications of Metal Oxides for Sustainable Environmental Remediation. Metals 2021, 11, 80. [Google Scholar] [CrossRef]
- Rafeeq, H.; Hussain, A.; Ambreen, A.; Zill-e-Huma; Waqas, M.; Bilal, M.; Iqbal, H.M.N. Functionalized Nanoparticles and Their Environmental Remediation Potential: A Review. J. Nanostructure Chem. 2022, 12, 1007–1031. [Google Scholar] [CrossRef]
- Shaheen, T.I.; Fouda, A.; Salem, S.S. Integration of Cotton Fabrics with Biosynthesized CuO Nanoparticles for Bactericidal Activity in the Terms of Their Cytotoxicity Assessment. Ind. Eng. Chem. Res. 2021, 60, 1553–1563. [Google Scholar] [CrossRef]
- Mahlaule-Glory, L.M.; Hintsho-Mbita, N.C. Green Derived Zinc Oxide (ZnO) for the Degradation of Dyes from Wastewater and Their Antimicrobial Activity: A Review. Catalysts 2022, 12, 833. [Google Scholar] [CrossRef]
- Raha, S.; Ahmaruzzaman, M. ZnO Nanostructured Materials and Their Potential Applications: Progress, Challenges and Perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef]
- Soliman, A.M.; Abdel-Latif, W.; Shehata, I.H.; Fouda, A.; Abdo, A.M.; Ahmed, Y.M. Green Approach to Overcome the Resistance Pattern of Candida spp. Using Biosynthesized Silver Nanoparticles Fabricated by Penicillium chrysogenum F9. Biol. Trace Elem. Res. 2021, 199, 800–811. [Google Scholar] [CrossRef]
- Al Jabri, H.; Saleem, M.H.; Rizwan, M.; Hussain, I.; Usman, K.; Alsafran, M. Zinc Oxide Nanoparticles and Their Biosynthesis: Overview. Life 2022, 12, 594. [Google Scholar] [CrossRef]
- Hamza, M.F.; Hamad, N.A.; Hamad, D.M.; Khalafalla, M.S.; Abdel-Rahman, A.A.H.; Zeid, I.F.; Wei, Y.; Hessien, M.M.; Fouda, A.; Salem, W.M. Synthesis of Eco-Friendly Biopolymer, Alginate-Chitosan Composite to Adsorb the Heavy Metals, Cd(II) and Pb(II) from Contaminated Effluents. Materials 2021, 14, 2189. [Google Scholar] [CrossRef]
- Fouda, A.; Awad, M.A.; Eid, A.M.; Saied, E.; Barghoth, M.G.; Hamza, M.F.; Awad, M.F.; Abdelbary, S.; Hassan, S.E. An Eco-Friendly Approach to the Control of Pathogenic Microbes and Anopheles stephensi Malarial Vector Using Magnesium Oxide Nanoparticles (Mg-NPs) Fabricated by Penicillium chrysogenum. Int. J. Mol. Sci. 2021, 22, 5096. [Google Scholar] [CrossRef]
- Ifeanyichukwu, U.L.; Fayemi, O.E.; Ateba, C.N. Green Synthesis of Zinc Oxide Nanoparticles from Pomegranate (Punica granatum) Extracts and Characterization of Their Antibacterial Activity. Molecules 2020, 25, 4521. [Google Scholar] [CrossRef]
- Selim, Y.A.; Azb, M.A.; Ragab, I.; Abd El-Azim, M.H.M. Green Synthesis of Zinc Oxide Nanoparticles Using Aqueous Extract of Deverra tortuosa and Their Cytotoxic Activities. Sci. Rep. 2020, 10, 3445. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, T.; Raza, A.; Zafar, M.; Afsheen, S.; Kebaili, I.; Alrobei, H. Plant-Mediated Green Synthesis of Zinc Oxide Nanoparticles for Novel Application to Enhance the Shelf Life of Tomatoes. Appl. Nanosci. 2022, 12, 179–191. [Google Scholar] [CrossRef]
- Fouda, A.; Abdel-Nasser, M.; Khalil, A.M.A.; Hassan, S.E.-D.; Abdel-Maksoud, G. Investigate the Role of Fungal Communities Associated with a Historical Manuscript From the 17th Century in Biodegradation. npj Mater. Degrad. 2022, 6, 88. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Shi, Y.; Song, H.; Yu, C. Antibiotic-Free Antibacterial Strategies Enabled by Nanomaterials: Progress and Perspectives. Adv. Mater. 2020, 32, 1904106. [Google Scholar] [CrossRef]
- Mubeen, B.; Ansar, A.N.; Rasool, R.; Ullah, I.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Alzarea, S.I.; Nadeem, M.S.; Kazmi, I. Nanotechnology as a Novel Approach in Combating Microbes Providing an Alternative to Antibiotics. Antibiotics 2021, 10, 1473. [Google Scholar] [CrossRef]
- Mohamed, A.E.; Elgammal, W.E.; Eid, A.M.; Dawaba, A.M.; Ibrahim, A.G.; Fouda, A.; Hassan, S.M. Synthesis and Characterization of New Functionalized Chitosan and its Antimicrobial and In-Vitro Release Behavior from Topical Gel. Int. J. Biol. Macromol. 2022, 207, 242–253. [Google Scholar] [CrossRef]
- Kim, I.; Viswanathan, K.; Kasi, G.; Thanakkasaranee, S.; Sadeghi, K.; Seo, J. ZnO Nanostructures in Active Antibacterial Food Packaging: Preparation Methods, Antimicrobial Mechanisms, Safety Issues, Future Prospects, and Challenges. Food Rev. Int. 2022, 38, 537–565. [Google Scholar] [CrossRef] [Green Version]
- Jain, D.; Shivani; Bhojiya, A.A.; Singh, H.; Daima, H.K.; Singh, M.; Mohanty, S.R.; Stephen, B.J.; Singh, A. Microbial Fabrication of Zinc Oxide Nanoparticles and Evaluation of Their Antimicrobial and Photocatalytic Properties. Front. Chem. 2020, 8, 778. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Abdel-Rahman, M.A.; Farag, M.M.S.; Shehal-deen, A.; Mohamed, A.A.; Alsharif, S.M.; Saied, E.; Moghanim, S.A.; Azab, M.S. Catalytic Degradation of Wastewater from the Textile and Tannery Industries by Green Synthesized Hematite (α-Fe2O3) and Magnesium Oxide (MgO) Nanoparticles. Curr. Res. Biotechnol. 2021, 3, 29–41. [Google Scholar] [CrossRef]
- Saied, E.; Fouda, A.; Alemam, A.M.; Sultan, M.H.; Barghoth, M.G.; Radwan, A.A.; Desouky, S.G.; Azab, I.H.E.; Nahhas, N.E.; Hassan, S.E. Evaluate the Toxicity of Pyrethroid Insecticide Cypermethrin before and after Biodegradation by Lysinibacillus cresolivuorans Strain HIS7. Plants 2021, 10, 1903. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.F.; Abdellah, W.M.; Zaki, D.I.; Wei, Y.; Althumayri, K.; Brostow, W.; Hamad, N.A. A Phosphonic Functionalized Biopolymer for the Sorption of Lanthanum (III) and Application in the Recovery of Rare Earth Elements. Sustainability 2023, 15, 2843. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Hamza, M.F.; Guibal, E.; Wei, Y.; Ning, S. Synthesis, characterization, and evaluation of thiocarbazide-functionalized maleic-based polymer for thorium (IV) removal from aqueous solutions. Chem. Eng. J. 2023, 464, 142638. [Google Scholar] [CrossRef]
- Dodoo-Arhin, D.; Asiedu, T.; Agyei-Tuffour, B.; Nyankson, E.; Obada, D.; Mwabora, J.M. Photocatalytic Degradation of Rhodamine Dyes using Zinc Oxide Nanoparticles. Mater. Today Proc. 2021, 38, 809–815. [Google Scholar] [CrossRef]
- Mijinyawa, A.H.; Durga, G.; Mishra, A. A Sustainable Process for Adsorptive Removal of Methylene Blue onto a Food Grade Mucilage: Kinetics, Thermodynamics, and Equilibrium Evaluation. Int. J. Phytoremediation 2019, 21, 1122–1129. [Google Scholar] [CrossRef]
- Hamza, M.F.; Abdel-Rahman, A.A.; Negm, A.S.; Hamad, D.M.; Khalafalla, M.S.; Fouda, A.; Wei, Y.; Amer, H.H.; Alotaibi, S.H.; Goda, A.E. Grafting of Thiazole Derivative on Chitosan Magnetite Nanoparticles for Cadmium Removal-Application for Groundwater Treatment. Polymers 2022, 14, 1240. [Google Scholar] [CrossRef]
- Gusain, R.; Gupta, K.; Joshi, P.; Khatri, O.P. Adsorptive Removal and Photocatalytic Degradation of Organic Pollutants Using Metal Oxides and Their Composites: A Comprehensive Review. Adv. Colloid Interface Sci. 2019, 272, 102009. [Google Scholar] [CrossRef]
- Batterjee, M.G.; Nabi, A.; Kamli, M.R.; Alzahrani, K.A.; Danish, E.Y.; Malik, M.A. Green Hydrothermal Synthesis of Zinc Oxide Nanoparticles for UV-Light-Induced Photocatalytic Degradation of Ciprofloxacin Antibiotic in an Aqueous Environment. Catalysts 2022, 12, 1347. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Hamza, M.F.; Guibal, E.; Althumayri, K.; Vincent, T.; Yin, X.; Wei, Y.; Li, W. New Process for the Sulfonation of Algal/PEI Biosorbent for Enhancing Sr(II) Removal from Aqueous Solutions—Application to Seawater. Molecules 2022, 27, 7128. [Google Scholar] [CrossRef]
- Ahmed, A.; Usman, M.; Yu, B.; Ding, X.; Peng, Q.; Shen, Y.; Cong, H. Efficient Photocatalytic Degradation of Toxic Alizarin Yellow R Dye from Industrial Wastewater Using Biosynthesized Fe Nanoparticle and Study of Factors Affecting the Degradation Rate. J. Photochem. Photobiol. B Biol. 2020, 202, 111682. [Google Scholar] [CrossRef]
- Fouda, A.; Eid, A.M.; Abdelkareem, A.; Said, H.A.; El-Belely, E.F.; Alkhalifah, D.H.M.; Alshallash, K.S.; Hassan, S.E. Phyco-Synthesized Zinc Oxide Nanoparticles Using Marine Macroalgae, Ulva fasciata Delile, Characterization, Antibacterial Activity, Photocatalysis, and Tanning Wastewater Treatment. Catalysts 2022, 12, 756. [Google Scholar] [CrossRef]
- Saied, E.; Eid, A.M.; Hassan, S.E.; Salem, S.S.; Radwan, A.A.; Halawa, M.; Saleh, F.M.; Saad, H.A.; Saied, E.M.; Fouda, A. The Catalytic Activity of Biosynthesized Magnesium Oxide Nanoparticles (MgO-NPs) for Inhibiting the Growth of Pathogenic Microbes, Tanning Effluent Treatment, and Chromium Ion Removal. Catalysts 2021, 11, 821. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Eid, A.M.; Awad, M.A.; Althumayri, K.; Badr, N.F.; Hamza, M.F. Endophytic Bacterial Strain, Brevibacillus brevis-Mediated Green Synthesis of Copper Oxide Nanoparticles, Characterization, Antifungal, InVitro Cytotoxicity, and Larvicidal Activity. Green Process. Synth. 2022, 11, 931–950. [Google Scholar] [CrossRef]
- Hamza, M.F.; Guibal, E.; Wei, Y.; Fouda, A. Magnetic amino-sulfonic dual sorbent for uranyl sorption from aqueous solutions–Influence of light irradiation on sorption properties. Chem. Eng. J. 2023, 456, 141099. [Google Scholar] [CrossRef]
- Hamza, M.F.; Guibal, E.; Althumayri, K.; Wei, Y.; Eid, A.M.; Fouda, A. Poly-Condensation of N-(2-acetamido)-2-Aminoethanesulfonic Acid with Formaldehyde for the Synthesis of a Highly Efficient Sorbent for Cs (I). Chem. Eng. J. 2023, 454, 140155. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Ashokkumar, T. Plant-Mediated Biosynthesis of Metallic Nanoparticles: A Review of Literature, Factors Affecting Synthesis, Characterization Techniques and Applications. J. Environ. Chem. Eng. 2017, 5, 4866–4883. [Google Scholar] [CrossRef]
- Barzinjy, A.A.; Hamad, S.M.; Esmaeel, M.M.; Aydın, S.K.; Hussain, F.H.S. Biosynthesis and Characterisation of Zinc Oxide Nanoparticles from Punica granatum (pomegranate) Juice Extract and its Application in Thin Films Preparation by Spin-Coating Method. Micro Nano Lett. 2020, 15, 415–420. [Google Scholar] [CrossRef]
- Romani, A.; Campo, M.; Pinelli, P. HPLC/DAD/ESI-MS Analyses and Anti-radical Activity of Hydrolyzable Tannins from Different Vegetal Species. Food Chemistry 2012, 130, 214–221. [Google Scholar] [CrossRef]
- El-Belely, E.F.; Farag, M.M.S.; Said, H.A.; Amin, A.S.; Azab, E.; Gobouri, A.A.; Fouda, A. Green Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) Using Arthrospira platensis (Class: Cyanophyceae) and Evaluation of their Biomedical Activities. Nanomaterials 2021, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Marica, I.; Nekvapil, F.; Ștefan, M.; Farcău, C.; Falamaș, A. Zinc Oxide Nanostructures for Fluorescence and Raman Signal Enhancement: A Review. Beilstein J. Nanotechnol. 2022, 13, 472–490. [Google Scholar] [CrossRef]
- Salem, S.S.; El-Belely, E.F.; Niedbała, G.; Alnoman, M.M.; Hassan, S.E.; Eid, A.M.; Shaheen, T.I.; Elkelish, A.; Fouda, A. Bactericidal and In-Vitro Cytotoxic Efficacy of Silver Nanoparticles (Ag-NPs) Fabricated by Endophytic Actinomycetes and Their Use as Coating for the Textile Fabrics. Nanomaterials 2020, 10, 2082. [Google Scholar] [CrossRef]
- Templeton, A.C.; Pietron, J.J.; Murray, R.W.; Mulvaney, P. Solvent Refractive Index and Core Charge Influences on the Surface Plasmon Absorbance of Alkanethiolate Monolayer-Protected Gold Clusters. J. Phys. Chem. B 2000, 104, 564–570. [Google Scholar] [CrossRef]
- Singh, K.; Singh, J.; Rawat, M. Green Synthesis of Zinc Oxide Nanoparticles Using Punica granatum Leaf Extract and its Application Towards Photocatalytic Degradation of Coomassie Brilliant Blue R-250 Dye. SN Appl. Sci. 2019, 1, 624. [Google Scholar] [CrossRef] [Green Version]
- Fouda, A.; Awad, M.A.; Al-Faifi, Z.E.; Gad, M.E.; Al-Khalaf, A.A.; Yahya, R.; Hamza, M.F. Aspergillus flavus-Mediated Green Synthesis of Silver Nanoparticles and Evaluation of Their Antibacterial, Anti-Candida, Acaricides, and Photocatalytic Activities. Catalysts 2022, 12, 462. [Google Scholar] [CrossRef]
- Hamza, M.F.; Wei, Y.; Althumayri, K.; Fouda, A.; Hamad, N.A. Synthesis and Characterization of Functionalized Chitosan Nanoparticles with Pyrimidine Derivative for Enhancing Ion Sorption and Application for Removal of Contaminants. Materials 2022, 15, 4676. [Google Scholar] [CrossRef]
- Zahra, M.H.; Hamza, M.F.; El-Habibi, G.; Abdel-Rahman, A.A.H.; Mira, H.I.; Wei, Y.; Alotaibi, S.H.; Amer, H.H.; Goda, A.E.S.; Hamad, N.A. Synthesis of a Novel Adsorbent Based on Chitosan Magnetite Nanoparticles for the High Sorption of Cr (VI) Ions: A Study of Photocatalysis and Recovery on Tannery Effluents. Catalysts 2022, 12, 678. [Google Scholar] [CrossRef]
- Hamza, M.F.; Wei, Y.; Khalafalla, M.S.; Abed, N.S.; Fouda, A.; Elwakeel, K.Z.; Guibal, E.; Hamad, N.A. U(VI) and Th(IV) Recovery Using Silica Beads Functionalized With Urea- or Thiourea-Based Polymers—Application to Ore Leachate. Sci. Total Environ. 2022, 821, 153184. [Google Scholar] [CrossRef]
- Hamza, M.F.; Lu, S.; Salih, K.A.M.; Mira, H.; Dhmees, A.S.; Fujita, T.; Wei, Y.; Vincent, T.; Guibal, E. As(V) Sorption from Aqueous Solutions Using Quaternized Algal/Polyethyleneimine Composite Beads. Sci. Total Environ. 2020, 719, 137396. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach, In Encyclopedia of Analytical Chemistry, Applications, Theory and Instrumentation. Infrared Spectroscopy in Analysis of Polymer Crystallinity; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Mohamed, A.A.; Fouda, A.; Abdel-Rahman, M.A.; Hassan, S.E.-D.; El-Gamal, M.S.; Salem, S.S.; Shaheen, T.I. Fungal Strain Impacts the Shape, Bioactivity and Multifunctional Properties of Green Synthesized Zinc Oxide Nanoparticles. Biocatal. Agric. Biotechnol. 2019, 19, 101103. [Google Scholar] [CrossRef]
- Rafique, M.; Sohaib, M.; Tahir, R.; Bilal Tahir, M.; Rizwan, M. Plant-Mediated Green Synthesis of Zinc Oxide Nanoparticles Using Peel Extract of Citrus reticulate for Boosting Seed Germination of Brassica nigra Seeds. J. Nanosci. Nanotechnol. 2021, 21, 3573–3579. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, K.; Thakur, N.; Chauhan, S.; Chauhan, M.S. The Effect of Shape and Size of ZnO Nanoparticles on Their Antimicrobial and Photocatalytic Activities: A Green Approach. Bull. Mater. Sci. 2019, 43, 20. [Google Scholar] [CrossRef]
- Mirhosseini, F.; Amiri, M.; Daneshkazemi, A.; Zandi, H.; Javadi, Z.S. Antimicrobial Effect of Different Sizes of Nano Zinc Oxide on Oral Microorganisms. Front. Dent. 2019, 16, 105–112. [Google Scholar] [CrossRef]
- Álvarez-Chimal, R.; García-Pérez, V.I.; Álvarez-Pérez, M.A.; Tavera-Hernández, R.; Reyes-Carmona, L.; Martínez-Hernández, M.; Arenas-Alatorre, J.Á. Influence of the Particle Size on the Antibacterial Activity of Green Synthesized Zinc Oxide Nanoparticles Using Dysphania ambrosioides Extract, Supported by Molecular Docking Analysis. Arab. J. Chem. 2022, 15, 103804. [Google Scholar] [CrossRef]
- Klinger, M.; Jäger, A. Crystallographic Tool Box (CrysTBox): Automated Tools for Transmission Electron Microscopists and Crystallographers. J. Appl. Crystallogr. 2015, 48, 2012–2018. [Google Scholar] [CrossRef] [Green Version]
- Matos, R.S.; Attah-Baah, J.M.; Monteiro, M.D.S.; Costa, B.F.O.; Mâcedo, M.A.; Silva Junior, R.S.; da Fonseca Filho, H.D.; Oliveira, R.M.P.B.; Ferreira, N.S. Effect of the Amapá-latex Chelating Agent Contents on the Microstructure and Photocatalytic Properties of ZnO Nanoparticles. J. Mater. Res. Technol. 2023, 22, 2673–2689. [Google Scholar] [CrossRef]
- Al-Kordy, H.M.H.; Sabry, S.A.; Mabrouk, M.E.M. Statistical Optimization of Experimental Parameters for Extracellular Synthesis of Zinc Oxide Nanoparticles by a Novel Haloalaliphilic Alkalibacillus sp.W7. Sci. Rep. 2021, 11, 10924. [Google Scholar] [CrossRef]
- Hassan, S.E.; Fouda, A.; Saied, E.; Farag, M.M.S.; Eid, A.M.; Barghoth, M.G.; Awad, M.A.; Hamza, M.F.; Awad, M.F. Rhizopus oryzae-Mediated Green Synthesis of Magnesium Oxide Nanoparticles (MgO-NPs): A Promising Tool for Antimicrobial, Mosquitocidal Action, and Tanning Effluent Treatment. J. Fungi 2021, 7, 372. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection Limits of DLS and UV-Vis Spectroscopy in Characterization of Polydisperse Nanoparticles Colloids. J. Nanomater. 2013, 2013, 313081. [Google Scholar] [CrossRef] [Green Version]
- Eid, A.M.; Fouda, A.; Hassan, S.E.; Hamza, M.F.; Alharbi, N.K.; Elkelish, A.; Alharthi, A.; Salem, W.M. Plant-Based Copper Oxide Nanoparticles; Biosynthesis, Characterization, Antibacterial Activity, Tanning Wastewater Treatment, and Heavy Metals Sorption. Catalysts 2023, 13, 348. [Google Scholar] [CrossRef]
- Abdelbaky, A.S.; Abd El-Mageed, T.A.; Babalghith, A.O.; Selim, S.; Mohamed, A.M.H.A. Green Synthesis and Characterization of ZnO Nanoparticles Using Pelargonium odoratissimum (L.) Aqueous Leaf Extract and Their Antioxidant, Antibacterial and Anti-inflammatory Activities. Antioxidants 2022, 11, 1444. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaszuba, M.; Corbett, J.; Watson, F.M.; Jones, A. High-Concentration Zeta Potential Measurements Using Light-Scattering Techniques. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 4439–4451. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, S. DLS and Zeta Potential–What They are and What They are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M. Green Synthesis of Pd Nanoparticles Mediated by Euphorbia thymifolia L. Leaf Extract: Catalytic Activity for Cyanation of Aryl Iodides Under Ligand-Free Conditions. J. Colloid Interface Sci. 2016, 469, 191–195. [Google Scholar] [CrossRef]
- Babayevska, N.; Przysiecka, Ł.; Iatsunskyi, I.; Nowaczyk, G.; Jarek, M.; Janiszewska, E.; Jurga, S. ZnO Size and Shape Effect on Antibacterial Activity and Cytotoxicity Profile. Sci. Rep. 2022, 12, 8148. [Google Scholar] [CrossRef]
- Fouda, A.; Eid, A.M.; Abdel-Rahman, M.A.; EL-Belely, E.F.; Awad, M.A.; Hassan, S.E.-D.; AL-Faifi, Z.E.; Hamza, M.F. Enhanced Antimicrobial, Cytotoxicity, Larvicidal, and Repellence Activities of Brown Algae, Cystoseira crinita-Mediated Green Synthesis of Magnesium Oxide Nanoparticles. Front. Bioeng. Biotechnol. 2022, 10, 849921. [Google Scholar] [CrossRef]
- Umavathi, S.; Mahboob, S.; Govindarajan, M.; Al-Ghanim, K.A.; Ahmed, Z.; Virik, P.; Al-Mulhm, N.; Subash, M.; Gopinath, K.; Kavitha, C. Green Synthesis of ZnO Nanoparticles for Antimicrobial and Vegetative Growth Applications: A Novel Approach for Advancing Efficient High Quality Health Care to Human Wellbeing. Saudi J. Biol. Sci. 2021, 28, 1808–1815. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Eid, A.M.; Abdel-Rahman, M.A.; Hamza, M.F. Light Enhanced the Antimicrobial, Anticancer, and Catalytic Activities of Selenium Nanoparticles Fabricated by Endophytic Fungal Strain, Penicillium crustosum EP-1. Sci. Rep. 2022, 12, 11834. [Google Scholar] [CrossRef]
- Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. Green Synthesis of Zinc Oxide Nanoparticles Using Hibiscus subdariffa Leaf Extract: Effect of Temperature on Synthesis, Anti-bacterial Activity and Anti-diabetic Activity. RSC Adv. 2015, 5, 4993–5003. [Google Scholar] [CrossRef]
- Dörr, T.; Moynihan, P.J.; Mayer, C. Editorial: Bacterial Cell Wall Structure and Dynamics. Front. Microbiol. 2019, 10, 2051. [Google Scholar] [CrossRef] [Green Version]
- Hamza, M.F.; Hamad, D.M.; Hamad, N.A.; Abdel-Rahman, A.A.H.; Fouda, A.; Wei, Y.; Guibal, E.; El-Etrawy, A.-A.S. Functionalization of Magnetic Chitosan Microparticles for High-Performance Removal of Chromate from Aqueous Solutions and Tannery Effluent. Chem. Eng. J. 2022, 428, 131775. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.; Abdo, A.M.; El-Gamal, M.S. Antimicrobial, Antioxidant and Larvicidal Activities of Spherical Silver Nanoparticles Synthesized by Endophytic Streptomyces spp. Biol. Trace Elem. Res. 2020, 195, 707–724. [Google Scholar] [CrossRef]
- Abd-Elaziz, A.M.; Aly, H.M.; Saleh, N.M.; Fouad, S.A.; Ismail, A.A.; Fouda, A. Synthesis and Characterization of the Novel Pyrimidine’s Derivatives, as a Promising Tool for Antimicrobial Agent and In-Vitro Cytotoxicity. J. Iran. Chem. Soc. 2022, 19, 2279–2296. [Google Scholar] [CrossRef]
- Lv, Q.Z.; Yan, L.; Jiang, Y.Y. The Synthesis, Regulation, and Functions of Sterols in Candida albicans: Well-known but Still Lots to Learn. Virulence 2016, 7, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Jalal, M.; Ansari, M.A.; Ali, S.G.; Khan, H.M.; Rehman, S. Anticandidal Activity of Bioinspired ZnO NPs: Effect on Growth, Cell Morphology and Key Virulence Attributes of Candida Species. Artif. Cells Nanomed. Biotechnol. 2018, 46, 912–925. [Google Scholar] [CrossRef] [Green Version]
- Fouda, A.; El-Din Hassan, S.; Salem, S.S.; Shaheen, T.I. In-Vitro Cytotoxicity, Antibacterial, and UV Protection Properties of the Biosynthesized Zinc Oxide Nanoparticles for Medical Textile Applications. Microb. Pathog. 2018, 125, 252–261. [Google Scholar] [CrossRef]
- Abdo, A.M.; Fouda, A.; Eid, A.M.; Fahmy, N.M.; Elsayed, A.M.; Khalil, A.M.A.; Alzahrani, O.M.; Ahmed, A.F.; Soliman, A.M. Green Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) by Pseudomonas aeruginosa and Their Activity Against Pathogenic Microbes and Common House Mosquito, Culex pipiens. Materials 2021, 14, 6983. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced Bioactivity of ZnO Nanoparticles-an Antimicrobial Study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef] [PubMed]
- Ramani, M.; Ponnusamy, S.; Muthamizhchelvan, C. From Zinc Oxide Nanoparticles to Microflowers: A Study of Growth Kinetics and Biocidal Activity. Mater. Sci. Eng. C 2012, 32, 2381–2389. [Google Scholar] [CrossRef]
- Song, W.; Zhang, J.; Guo, J.; Zhang, J.; Ding, F.; Li, L.; Sun, Z. Role of the Dissolved Zinc Ion and Reactive Oxygen Species in Cytotoxicity of ZnO Nanoparticles. Toxicol. Lett. 2010, 199, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Hassan, S.E.-D.; Saied, E.; Hamza, M.F. Photocatalytic Degradation of Real Textile and Tannery Effluent Using Biosynthesized Magnesium Oxide Nanoparticles (MgO-NPs), Heavy Metal Adsorption, Phytotoxicity, and Antimicrobial Activity. J. Environ. Chem. Eng. 2021, 9, 105346. [Google Scholar] [CrossRef]
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.-A. The Contribution of Zinc Ions to the Antimicrobial Activity of Zinc Oxide. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 263–274. [Google Scholar] [CrossRef]
- Peng, X.; Palma, S.; Fisher, N.S.; Wong, S.S. Effect of Morphology of ZnO Nanostructures on Their Toxicity to Marine Algae. Aquat. Toxicol. 2011, 102, 186–196. [Google Scholar] [CrossRef]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial Activity and Mechanism of Action of Zinc Oxide Nanoparticles Against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [Green Version]
- Vinayagam, R.; Selvaraj, R.; Arivalagan, P.; Varadavenkatesan, T. Synthesis, Characterization and Photocatalytic Dye Degradation Capability of Calliandra haematocephala-Mediated Zinc Oxide Nanoflowers. J. Photochem. Photobiol. B Biol. 2020, 203, 111760. [Google Scholar] [CrossRef]
- Hamza, M.F.; Mira, H.; Khalafalla, M.S.; Wang, J.; Wei, Y.; Yin, X.; Ning, S.; Althumayri, K.; Fouda, A. Photocatalytic Performance of Functionalized Biopolymer for Neodymium (III) Sorption and the Recovery from Leachate Solution. Catalysts 2023, 13, 672. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Saied, E.; Azab, M.S. An Eco-Friendly Approach to Textile and Tannery Wastewater Treatment Using Maghemite Nanoparticles (γ-Fe2O3-NPs) fabricated by Penicillium expansum strain (K-w). J. Environ. Chem. Eng. 2021, 9, 104693. [Google Scholar] [CrossRef]
- Kahsay, M.H. Synthesis and Sharacterization of ZnO Nanoparticles Using Aqueous Extract of Becium grandiflorum for Antimicrobial Activity and Adsorption of Methylene Blue. Appl. Water Sci. 2021, 11, 45. [Google Scholar] [CrossRef]
- Witzel, C.; Gegenfurtner, K. Memory Color. In Encyclopedia of Color Science and Technology; Shamey, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–7. [Google Scholar]
- Chanu, L.A.; Singh, W.J.; Singh, K.J.; Devi, K.N. Effect of Operational Parameters on the Photocatalytic Degradation of Methylene Blue Dye Solution Using Manganese Doped ZnO Nanoparticles. Results Phys. 2019, 12, 1230–1237. [Google Scholar] [CrossRef]
- Ertaş, M.; Acemioğlu, B.; Alma, M.H.; Usta, M. Removal of Methylene Blue from Aqueous Solution Using Cotton Stalk, Cotton Waste and Cotton Dust. J. Hazard. Mater. 2010, 183, 421–427. [Google Scholar] [CrossRef]
- Hamza, M.F.; Alotaibi, S.H.; Wei, Y.; Mashaal, N.M. High-Performance Hydrogel Based on Modified Chitosan for Removal of Heavy Metal Ions in Borehole: A Case Study from the Bahariya Oasis, Egypt. Catalysts 2022, 12, 721. [Google Scholar] [CrossRef]
- Hamza, M.F.; Fouda, A.; Wei, Y.; El Aassy, I.E.; Alotaibi, S.H.; Guibal, E.; Mashaal, N.M. Functionalized Biobased Composite for Metal Decontamination—Insight on Uranium and Application to Water Samples Collected from Wells in Mining Areas (Sinai, Egypt). Chem. Eng. J. 2022, 431, 133967. [Google Scholar] [CrossRef]
- Saad Algarni, T.; Abduh, N.A.Y.; Al Kahtani, A.; Aouissi, A. Photocatalytic Degradation of Some Dyes Under Solar Light Irradiation Using ZnO Nanoparticles Synthesized from Rosmarinus officinalis Extract. Green Chem. Lett. Rev. 2022, 15, 460–473. [Google Scholar] [CrossRef]
- Fouda, A.; Salem, S.S.; Wassel, A.R.; Hamza, M.F.; Shaheen, T.I. Optimization of Green Biosynthesized Visible Light Active CuO/ZnO Nano-Photocatalysts for the Degradation of Organic Methylene Blue Dye. Heliyon 2020, 6, e04896. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fouda, A.; Saied, E.; Eid, A.M.; Kouadri, F.; Alemam, A.M.; Hamza, M.F.; Alharbi, M.; Elkelish, A.; Hassan, S.E.-D. Green Synthesis of Zinc Oxide Nanoparticles Using an Aqueous Extract of Punica granatum for Antimicrobial and Catalytic Activity. J. Funct. Biomater. 2023, 14, 205. https://doi.org/10.3390/jfb14040205
Fouda A, Saied E, Eid AM, Kouadri F, Alemam AM, Hamza MF, Alharbi M, Elkelish A, Hassan SE-D. Green Synthesis of Zinc Oxide Nanoparticles Using an Aqueous Extract of Punica granatum for Antimicrobial and Catalytic Activity. Journal of Functional Biomaterials. 2023; 14(4):205. https://doi.org/10.3390/jfb14040205
Chicago/Turabian StyleFouda, Amr, Ebrahim Saied, Ahmed M. Eid, Fayza Kouadri, Ahmed M. Alemam, Mohammed F. Hamza, Maha Alharbi, Amr Elkelish, and Saad El-Din Hassan. 2023. "Green Synthesis of Zinc Oxide Nanoparticles Using an Aqueous Extract of Punica granatum for Antimicrobial and Catalytic Activity" Journal of Functional Biomaterials 14, no. 4: 205. https://doi.org/10.3390/jfb14040205
APA StyleFouda, A., Saied, E., Eid, A. M., Kouadri, F., Alemam, A. M., Hamza, M. F., Alharbi, M., Elkelish, A., & Hassan, S. E. -D. (2023). Green Synthesis of Zinc Oxide Nanoparticles Using an Aqueous Extract of Punica granatum for Antimicrobial and Catalytic Activity. Journal of Functional Biomaterials, 14(4), 205. https://doi.org/10.3390/jfb14040205









