Hypochlorous Acid-Activated UCNPs-LMB/VQIVYK Multifunctional Nanosystem for Alzheimer’s Disease Treatment
Abstract
1. Introduction
2. Materials and Methods
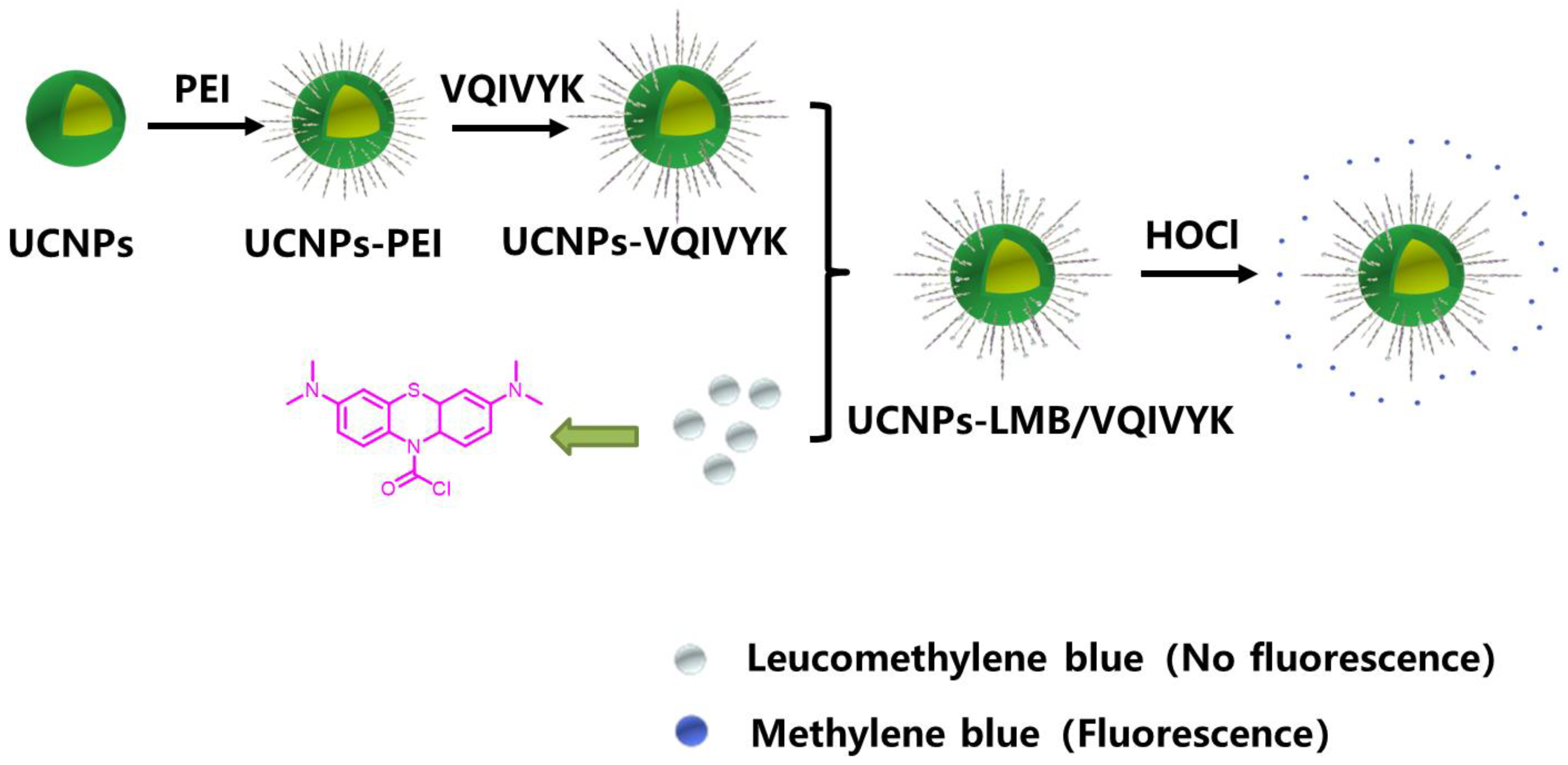
2.1. Synthesis of UCNPs-LMB/VQIVYK
2.2. ThT Fluorescence Assay
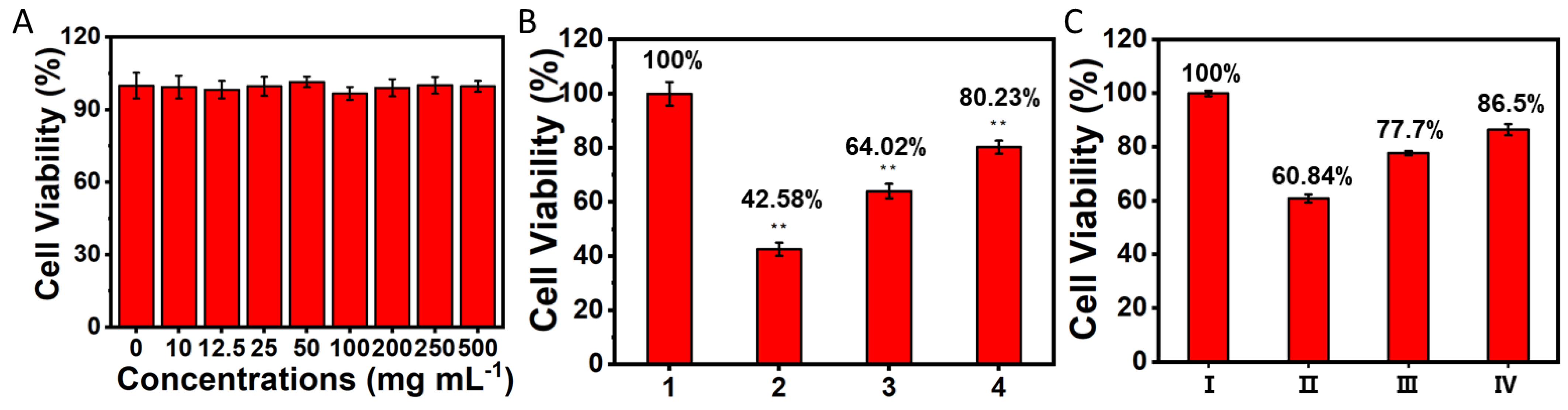
2.3. Cytotoxicity Assay
3. Results
3.1. Synthesis and Characterization of the UCNPs-LMB/VQIVYK Nanosystems
3.2. Drug Loading and Releasing Properties
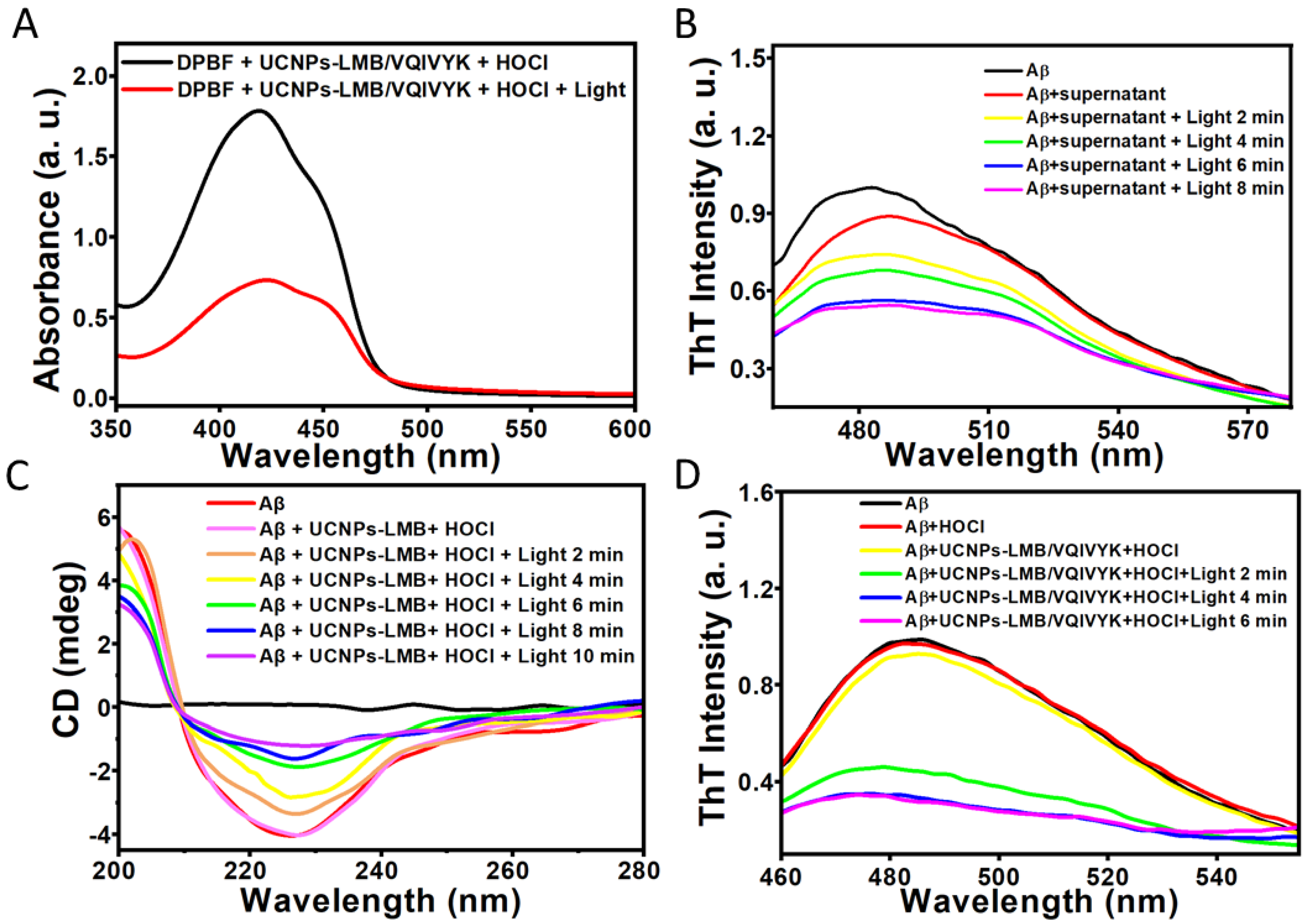
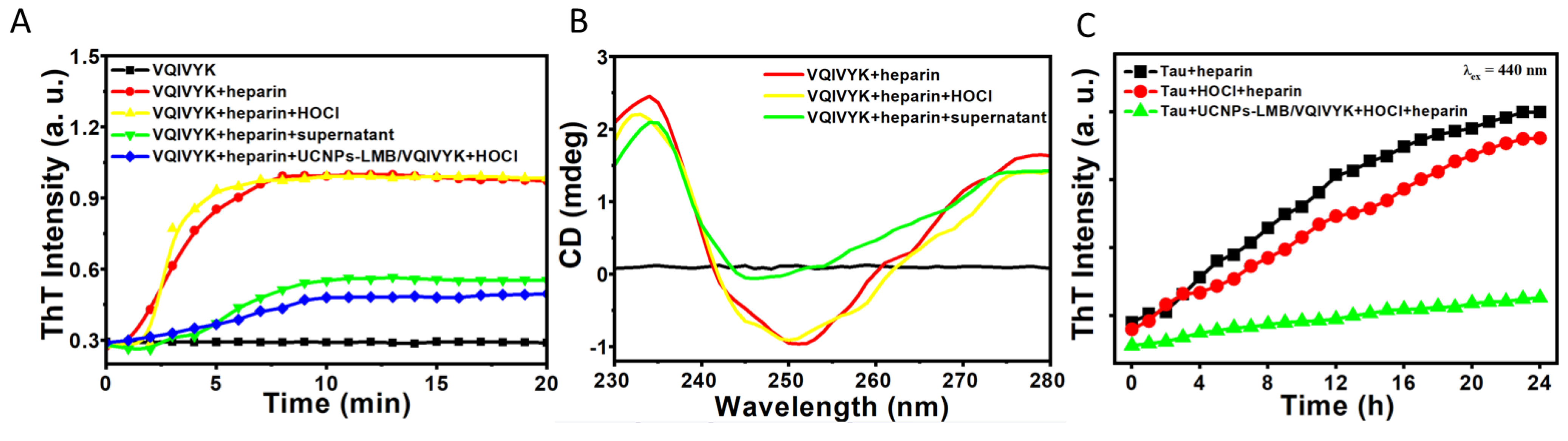
3.3. Photodynamic Effect of UCNPs-LMB/VQIVYK on Suppressing Aβ42 Aggregation
3.4. UCNPs-LMB/VQIVYK Restrained Tau Protein Aggregation
3.5. Release of MB in Cells
3.6. UCNPs-LMB/VQIVYK Reduced the Cytotoxicity of Aβ42/Tau
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mattson, M.P. Pathways towards and away from Alzheimer’s disease. Nature 2004, 430, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; de Leon, M.J.; Zetterberg, H. Alzheimer’s disease. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.R.; Chi, W.J.; Shi, W.J.; Zhang, L.; Yan, J.W. An in situ-triggered and chemi-excited photooxygenation system for Aβ aggregates. Chem. Eng. J. 2023, 456, 140998. [Google Scholar] [CrossRef]
- He, Z.; Guo, J.L.; McBride, J.D.; Narasimhan, S.; Kim, H.; Changolkar, L.; Zhang, B.; Gathagan, R.J.; Yue, C.; Dengler, C.; et al. Amyloid-beta plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat. Med. 2018, 24, 29–38. [Google Scholar] [CrossRef]
- McCoy, D.E.; Feo, T.; Harvey, T.A.; Prum, R.O. Structural absorption by barbule microstructures of super black bird of paradise feathers. Nat. Commun. 2018, 9, 1. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, J.; Wang, F.; Wang, Y.; Lu, L.; Feng, C.; Xu, Z.; Zhang, W. Selective amyloid beta oligomer assay based on abasic site-containing molecular beacon and enzyme-free amplification. Biosens Bioelectron 2016, 78, 206–212. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353. [Google Scholar] [CrossRef]
- Hamley, I.W. The amyloid beta peptide: A chemist’s perspective. Role in Alzheimer’s and fibrillization. Chem. Rev. 2012, 112, 5147–5192. [Google Scholar] [CrossRef]
- Jeganathan, S.; von Bergen, M.; Mandelkow, E.-M.; Mandelkow, E. The Natively Unfolded Character of Tau and Its Aggregation to Alzheimer-like Paired Helical Filaments. Biochemistry 2008, 47, 10526–10539. [Google Scholar] [CrossRef] [PubMed]
- Jara, C.; Aranguiz, A.; Cerpa, W.; Tapia-Rojas, C.; Quintanilla, R.A. Genetic ablation of tau improves mitochondrial function and cognitive abilities in the hippocampus. Redox Biol. 2018, 18, 279–294. [Google Scholar] [CrossRef]
- Du, Z.; Gao, N.; Wang, X.; Ren, J.; Qu, X. Near-Infrared Switchable Fullerene-Based Synergy Therapy for Alzheimer’s Disease. Small 2018, 14, e1801852. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Guo, Z.; Yuan, W.; Kong, M.; Liu, Y.; Liu, Y.; Gao, Y.; Feng, W.; Wang, F.; Zhou, J.; et al. High-sensitivity imaging of time-domain near-infrared light transducer. Nat. Photonics 2019, 13, 525–531. [Google Scholar] [CrossRef]
- Kuang, Y.; Xu, J.; Wang, C.; Li, T.; Gai, S.; He, F.; Yang, P.; Lin, J. Fine-Tuning Ho-Based Red-Upconversion Luminescence by Altering NaHoF4 Core Size and NaYbF4 Shell Thickness. Chem. Mater. 2019, 31, 7898–7909. [Google Scholar] [CrossRef]
- Chen, W.; Ouyang, J.; Liu, H.; Chen, M.; Zeng, K.; Sheng, J.; Liu, Z.; Han, Y.; Wang, L.; Li, J.; et al. Black Phosphorus Nanosheet-Based Drug Delivery System for Synergistic Photodynamic/Photothermal/Chemotherapy of Cancer. Adv. Mater. 2017, 29, 1603864. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Zangabad, P.S.; Mehdizadeh, F.; Malekzad, H.; Ghasemi, A.; Bahrami, S.; Zare, H.; Moghoofei, M.; Hekmatmanesh, A.; Hamblin, M.R. Nanocaged platforms: Modification, drug delivery and nanotoxicity. Opening synthetic cages to release the tiger. Nanoscale 2017, 9, 1356–1392. [Google Scholar] [CrossRef] [PubMed]
- Gamelin, D.R.; Güdel, H.U. Design of Luminescent Inorganic Materials: New Photophysical Processes Studied by Optical Spectroscopy. Acc. Chem. Res. 2000, 33, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion nanoparticles: Design, nanochemistry, and applications in theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Bunzli, J.C. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef] [PubMed]
- Gai, S.; Li, C.; Yang, P.; Lin, J. Recent progress in rare earth micro/nanocrystals: Soft chemical synthesis, luminescent properties, and biomedical applications. Chem. Rev. 2014, 114, 2343–2389. [Google Scholar] [CrossRef]
- Gargas, D.J.; Chan, E.M.; Ostrowski, A.D.; Aloni, S.; Altoe, M.V.; Barnard, E.S.; Sanii, B.; Urban, J.J.; Milliron, D.J.; Cohen, B.E.; et al. Engineering bright sub-10-nm upconverting nanocrystals for single-molecule imaging. Nat. Nanotechnol. 2014, 9, 300–305. [Google Scholar] [CrossRef]
- Haase, M.; Schafer, H. Upconverting nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 5808–5829. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zheng, W.; Zhou, S.; Tu, D.; Chen, Z.; Zhu, H.; Li, R.; Ma, E.; Huang, M.; Chen, X. Lanthanide-doped LiLuF(4) upconversion nanoprobes for the detection of disease biomarkers. Angew. Chem. Int. Ed. 2014, 53, 1252–1257. [Google Scholar] [CrossRef]
- Liu, C.; Hou, Y.; Gao, M. Are rare-earth nanoparticles suitable for in vivo applications? Adv. Mater. 2014, 26, 6922–6932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Deng, R.; Tian, J.; Zong, Y.; Jin, D.; Liu, X. Multicolor barcoding in a single upconversion crystal. J. Am. Chem. Soc. 2014, 136, 4893–4896. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Z.; Li, F. Upconversion nanophosphors for small-animal imaging. Chem. Soc. Rev. 2012, 41, 1323–1349. [Google Scholar] [CrossRef]
- Zhu, D.; Liu, F.; Ma, L.; Liu, D.; Wang, Z. Nanoparticle-based systems for T(1)-weighted magnetic resonance imaging contrast agents. Int. J. Mol. Sci. 2013, 14, 10591–10607. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.; Zhang, J.; Bu, W.; Xing, H.; Han, F.; Xiao, Q.; Yao, Z.; Chen, F.; He, Q.; Liu, J.; et al. Dual-Targeting Upconversion Nanoprobes across the Blood–Brain Barrier for Magnetic Resonance/Fluorescence Imaging of Intracranial Glioblastoma. ACS Nano 2014, 8, 1231–1242. [Google Scholar] [CrossRef]
- Allison, R.; Moghissi, K.; Downie, G.; Dixon, K. Photodynamic therapy (PDT) for lung cancer. Photodiagnosis Photodyn. Ther. 2011, 8, 231–239. [Google Scholar] [CrossRef]
- Chang, M.; Wang, M.; Chen, Y.; Shu, M.; Zhao, Y.; Ding, B.; Hou, Z.; Lin, J. Self-assembled CeVO4/Ag nanohybrid as photoconversion agents with enhanced solar-driven photocatalysis and NIR-responsive photothermal/photodynamic synergistic therapy performance. Nanoscale 2019, 11, 10129–10136. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Mackey, M.A.; Huang, X.; Kang, B.; El-Sayed, M.A. Beating cancer in multiple ways using nanogold. Chem. Soc. Rev. 2011, 40, 3391–3404. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Y.; Niu, G.; Lan, M.; Jia, Q.; Liang, Q. Near-Infrared Organic Dye-Based Nanoagent for the Photothermal Therapy of Cancer. ACS Appl. Mater. Interfaces 2016, 8, 29899–29905. [Google Scholar] [CrossRef] [PubMed]
- Nishita, M.; Park, S.Y.; Nishio, T.; Kamizaki, K.; Wang, Z.; Tamada, K.; Takumi, T.; Hashimoto, R.; Otani, H.; Pazour, G.J.; et al. Ror2 signaling regulates Golgi structure and transport through IFT20 for tumor invasiveness. Sci. Rep. 2017, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Tardivo, J.P.; Del Giglio, A.; de Oliveira, C.S.; Gabrielli, D.S.; Junqueira, H.C.; Tada, D.B.; Severino, D.; de Fátima Turchiello, R.; Baptista, M.S. Methylene blue in photodynamic therapy: From basic mechanisms to clinical applications. Photodiagnosis Photodyn. Ther. 2005, 2, 175–191. [Google Scholar] [CrossRef]
- Wainwright, M.; McLean, A. Rational design of phenothiazinium derivatives and photoantimicrobial drug discovery. Dyes Pigments 2017, 136, 590–600. [Google Scholar] [CrossRef]
- Wischik, C.M.; Edwards, P.C.; Lai, R.Y.; Roth, M.; Harrington, C.R. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc. Natl. Acad. Sci. USA 1996, 93, 11213. [Google Scholar] [CrossRef] [PubMed]
- Belostozky, A.; Richman, M.; Lisniansky, E.; Tovchygrechko, A.; Chill, J.H.; Rahimipour, S. Inhibition of tau-derived hexapeptide aggregation and toxicity by a self-assembled cyclic d,l-alpha-peptide conformational inhibitor. Chem. Commun. (Camb) 2018, 54, 5980–5983. [Google Scholar] [CrossRef]
- Goux, W.J.; Kopplin, L.; Nguyen, A.D.; Leak, K.; Rutkofsky, M.; Shanmuganandam, V.D.; Sharma, D.; Inouye, H.; Kirschner, D.A. The formation of straight and twisted filaments from short tau peptides. J. Biol. Chem. 2004, 279, 26868–26875. [Google Scholar] [CrossRef]
- Sourav, S.; Thimmaiah, G. Unambiguous Detection of Elevated Levels of Hypochlorous Acid in Double Transgenic AD Mouse Brain. ACS Chem. Neurosci. 2019, 10, 4847–4853. [Google Scholar] [CrossRef]
- Wang, M.; Chang, M.Y.; Li, C.X.; Chen, Q.; Hou, Z.Y.; Xing, B.G.; Lin, J. Tumor-Microenvironment-Activated Reactive Oxygen Species Amplifier for Enzymatic Cascade Cancer Starvation/Chemodynamic /Immunotherapy. Adv. Mater. 2022, 34, 2106010. [Google Scholar] [CrossRef]
- Hou, Z.; Zhang, Y.; Deng, K.; Chen, Y.; Li, X.; Deng, X.; Cheng, Z.; Lian, H.; Li, C.; Lin, J. UV-Emitting Upconversion-Based TiO2 Photosensitizing Nanoplatform: Near-Infrared Light Mediated in Vivo Photodynamic Therapy via Mitochondria-Involved Apoptosis Pathway. ACS Nano 2015, 9, 2584–2599. [Google Scholar] [CrossRef]
- Teng, B.; Han, Y.; Zhang, X.; Xiao, H.; Yu, C.; Li, H.; Cheng, Z.; Jin, D.; Wong, K.L.; Ma, P.; et al. Phenanthriplatin(iv) conjugated multifunctional up-converting nanoparticles for drug delivery and biomedical imaging. J. Mater. Chem. B 2018, 6, 5059–5068. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, C.; Zhao, A.; Li, M.; Ren, J.; Qu, X. New insights in amyloid beta interactions with human telomerase. J. Am. Chem. Soc. 2015, 137, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Gu, Y.-J.; Man, C.W.-Y.; Cheng, J.; Xu, Z.; Zhang, Y.; Wang, H.; Lee, V.H.-Y.; Cheng, S.H.; Wong, W.-T. Polymer-Coated NaYF4:Yb3+, Er3+ Upconversion Nanoparticles for Charge-Dependent Cellular Imaging. ACS Nano 2011, 5, 7838–7847. [Google Scholar] [CrossRef]
- Guller, A.E.; Nadort, A.; Generalova, A.N.; Khaydukov, E.V.; Nechaev, A.V.; Kornienko, I.A.; Petersen, E.V.; Liang, L.; Shekhter, A.B.; Qian, Y.; et al. Rational Surface Design of Upconversion Nanoparticles with Polyethylenimine Coating for Biomedical Applications: Better Safe than Brighter? ACS Biomater. Sci. Eng. 2018, 4, 3143–3153. [Google Scholar] [CrossRef]
- Brambilla, D.; Le Droumaguet, B.; Nicolas, J.; Hashemi, S.H.; Wu, L.P.; Moghimi, S.M.; Couvreur, P.; Andrieux, K. Nanotechnologies for Alzheimer’s disease: Diagnosis, therapy, and safety issues. Nanomedicine 2011, 7, 521–540. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, M.; Liu, G.; Geng, J.; Wang, J.; Ren, J.; Zhao, C.; Qu, X. Metallosupramolecular complex targeting an α/β discordant stretch of amyloid β peptide. Chem. Sci. 2012, 3, 3145–3153. [Google Scholar] [CrossRef]
- von Bergen, M.; Friedhoff, P.; Biernat, J.; Heberle, J.; Mandelkow, E.M.; Mandelkow, E. Assembly of τ protein into Alzheimer paired helical filaments depends on a local sequence motif (306VQIVYK311) forming β structure. Proc. Natl. Acad. Sci. USA 2000, 97, 5129. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Hirata, A.; Tainaka, K.; Morii, T.; Konno, T. Charge-Pairing Mechanism of Phosphorylation Effect upon Amyloid Fibrillation of Human Tau Core Peptide. Biochemistry 2008, 47, 11847–11857. [Google Scholar] [CrossRef]
- Chemerovski-Glikman, M.; Frenkel-Pinter, M.; Mdah, R.; Abu-Mokh, A.; Gazit, E.; Segal, D. Inhibition of the Aggregation and Toxicity of the Minimal Amyloidogenic Fragment of Tau by Its Pro-Substituted Analogues. Chemistry 2017, 23, 9618–9624. [Google Scholar] [CrossRef]
- Frenkel-Pinter, M.; Richman, M.; Belostozky, A.; Abu-Mokh, A.; Gazit, E.; Rahimipour, S.; Segal, D. Selective Inhibition of Aggregation and Toxicity of a Tau-Derived Peptide using Its Glycosylated Analogues. Chemistry 2016, 22, 5945–5952. [Google Scholar] [CrossRef]
- KrishnaKumar, V.G.; Paul, A.; Gazit, E.; Segal, D. Mechanistic insights into remodeled Tau-derived PHF6 peptide fibrils by Naphthoquinone-Tryptophan hybrids. Sci. Rep. 2018, 8, 71. [Google Scholar] [CrossRef]
- Wang, C.K.; Northfield, S.E.; Huang, Y.H.; Ramos, M.C.; Craik, D.J. Inhibition of tau aggregation using a naturally-occurring cyclic peptide scaffold. Eur. J. Med. Chem. 2016, 109, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.; Ahmad, M.I.; Javed, H.; Naseem, S. D-ribose and pathogenesis of Alzheimer’s disease. Mol. Biol. Rep. 2020, 47, 2289–2299. [Google Scholar] [CrossRef]
- Golde, T. The Pathogenesis of Alzheimer’s Disease and the Role of Aβ42. CNS Spectr. 2014, 12, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Barani, M.; Sabir, F.; Rahdar, A.; Kyzas, G.Z. Nanomaterials for the treatment and diagnosis of Alzheimer’s disease: An overview. NanoImpact 2020, 20, 100251. [Google Scholar] [CrossRef]
- Qiao, L.Y.; Shen, Y.; Zhang, S.Y.; Wang, M.; Lv, G.L.; Dou, Q.Q.; Li, C.X. H2O2-responsive multifunctional nanocomposite for the inhibition of amyloid-β and Tau aggregation in Alzheimer’s disease. BMEMat 2023, 1, e12011. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, L.; Shen, Y.; Li, G.; Lv, G.; Li, C. Hypochlorous Acid-Activated UCNPs-LMB/VQIVYK Multifunctional Nanosystem for Alzheimer’s Disease Treatment. J. Funct. Biomater. 2023, 14, 207. https://doi.org/10.3390/jfb14040207
Qiao L, Shen Y, Li G, Lv G, Li C. Hypochlorous Acid-Activated UCNPs-LMB/VQIVYK Multifunctional Nanosystem for Alzheimer’s Disease Treatment. Journal of Functional Biomaterials. 2023; 14(4):207. https://doi.org/10.3390/jfb14040207
Chicago/Turabian StyleQiao, Luying, Yang Shen, Guangzhi Li, Guanglei Lv, and Chunxia Li. 2023. "Hypochlorous Acid-Activated UCNPs-LMB/VQIVYK Multifunctional Nanosystem for Alzheimer’s Disease Treatment" Journal of Functional Biomaterials 14, no. 4: 207. https://doi.org/10.3390/jfb14040207
APA StyleQiao, L., Shen, Y., Li, G., Lv, G., & Li, C. (2023). Hypochlorous Acid-Activated UCNPs-LMB/VQIVYK Multifunctional Nanosystem for Alzheimer’s Disease Treatment. Journal of Functional Biomaterials, 14(4), 207. https://doi.org/10.3390/jfb14040207





