Effect of Intracoronal Sealing Biomaterials on the Histological Outcome of Endodontic Revitalisation in Immature Sheep Teeth—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Isolation
2.2. Sample Preparation
2.3. qRT-PCR Analysis
2.4. Animal Model
2.5. Randomization and Blinding
2.6. General Anaesthesia
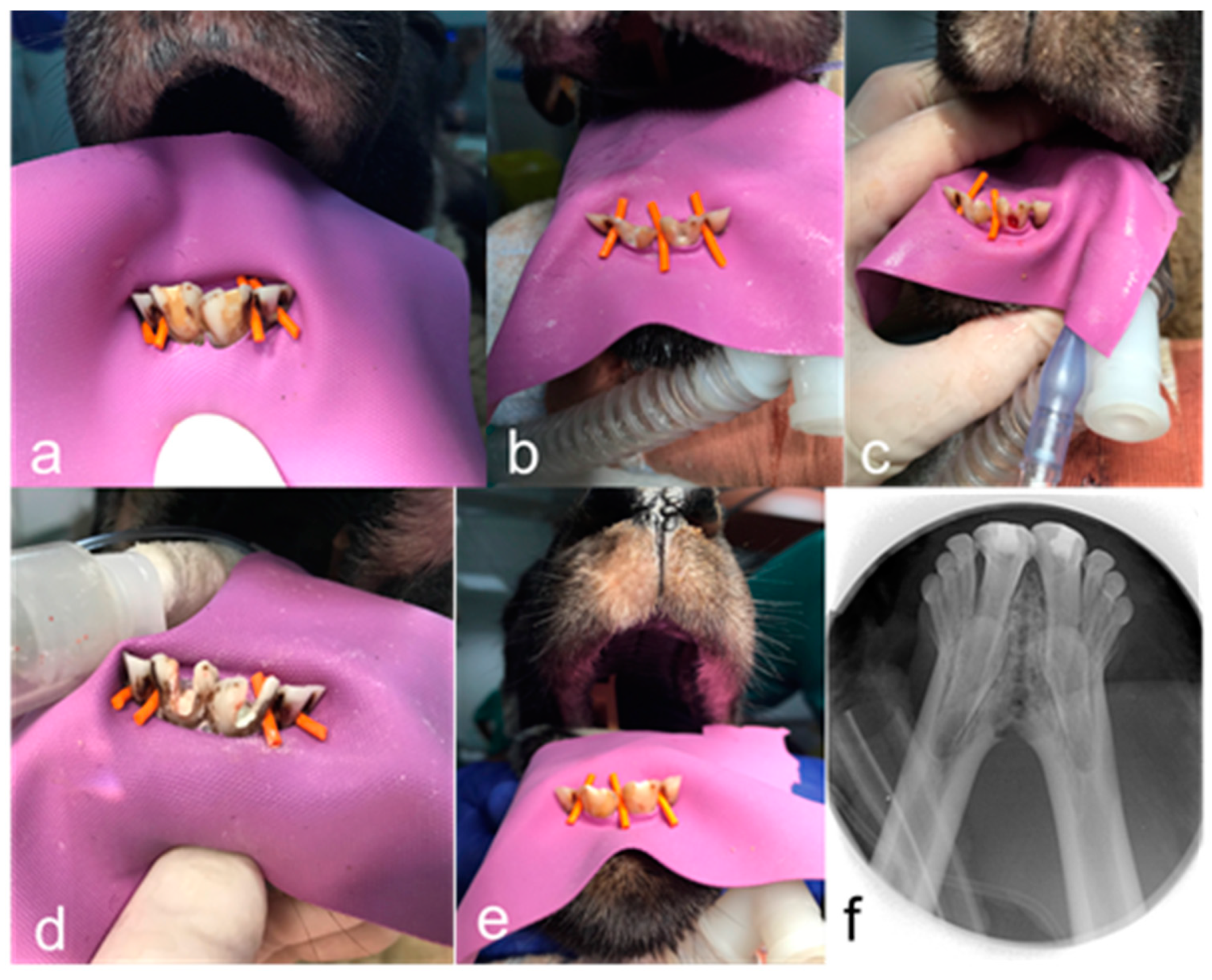
2.7. Intervention
2.8. Phase 1
2.9. Phase 2
2.10. Phase 3
2.11. Radiographic Analysis
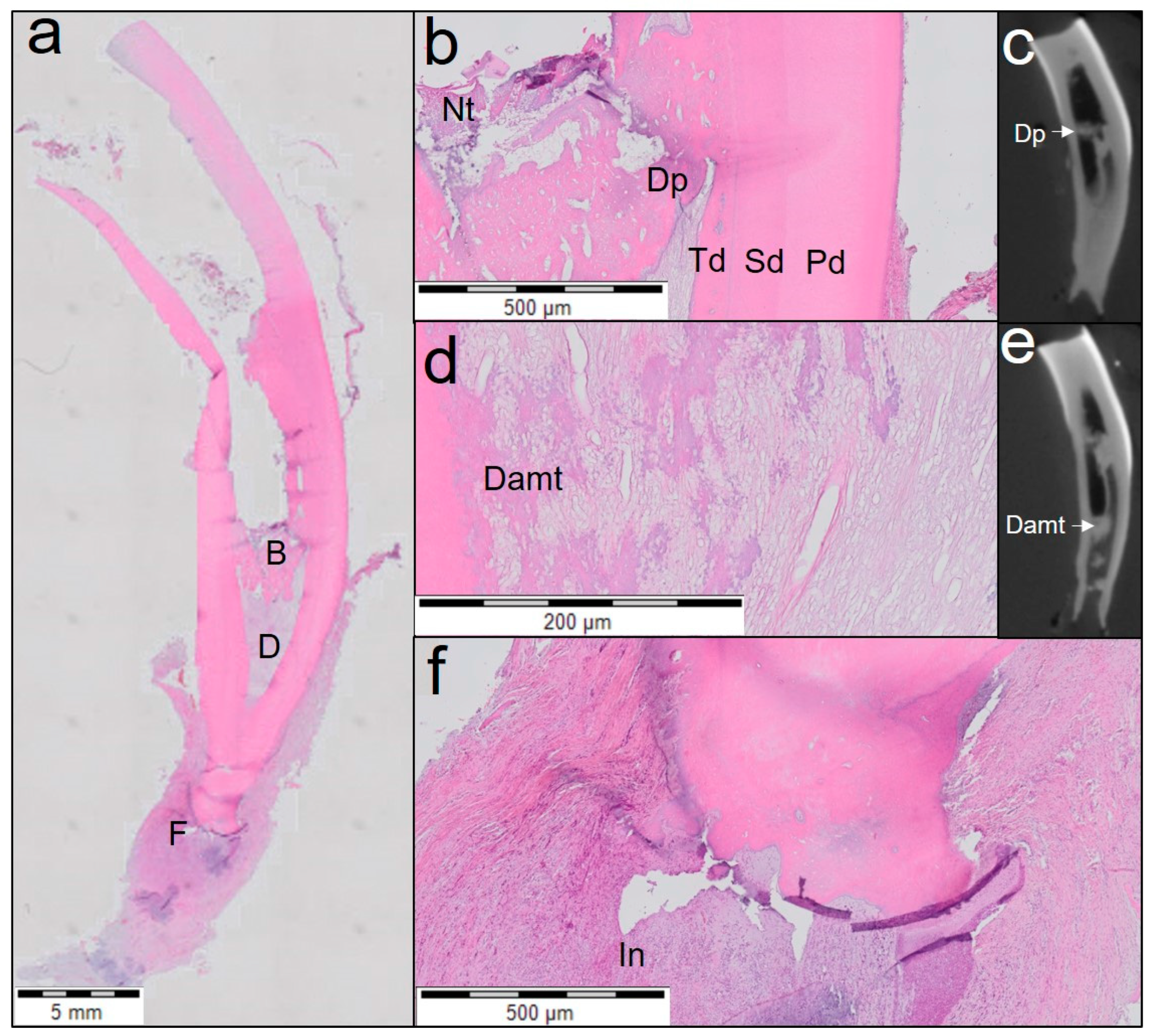
2.12. Histological Analysis
- Extent of inflammation was scored from 0 to 4 as follows: score 0, absent—absence of inflammatory cells; score 1, mild—small number of scattered inflammatory cells; score 2, moderate—some foci of inflammatory cells; score 3, severe—intense infiltration with inflammatory cells and altered tissue architecture; and score 4: necrosis: amorphous clumps of tissue remnants.
- Presence or absence of tissue with cellularity and vascularity inside the pulp space was scored from 0 to 3 as follows: score 0—no tissue in-growth into the canal space; score 1—evidence of tissue in-growth into the apical third of the canal; score 2—evidence of tissue in-growth extending to the middle third of the canal; and score 3—evidence of tissue in-growth extending to the cervical third of the canal.
- For area of tissue with cellularity and vascularity, only soft tissue with the presence of cells and blood vessels were measured.
- Length of odontoblast lining attached to the dentinal wall was measured on both sides of the root for each tooth.
- Number of blood vessels in each section was counted.
- Area of blood vessels expressed as percentage of vascularity was calculated as the ratio of area of blood vessels to that of the area of tissue with cellularity and vascularity within each histological section.
- The area of empty root canal space was measured as the area inside the root canal where neither soft nor hard tissue structures were present.
2.13. Statistical Analysis
3. Results
3.1. Gene Expression
3.2. Qualitative Analysis of Revitalisation Therapy
3.3. Quantitative Analysis of Revitalisation Therapy
4. Discussion
4.1. Limitations of the Present Study
4.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, L.H.; Wang, J.; Yin, S.; Zhu, W.T.; Zhou, G.D.; Cao, Y.L.; Cen, L. Regeneration of dentin-pulp-like tissue using an injectable tissue engineering technique. Rsc. Adv. 2015, 5, 59723–59737. [Google Scholar] [CrossRef]
- Harlamb, S.C. Management of incompletely developed teeth requiring root canal treatment. Aust. Dent. J. 2016, 61 (Suppl. S1), 95–106. [Google Scholar] [CrossRef] [PubMed]
- Kahler, B.; Rossi-Fedele, G.; Chugal, N.; Lin, L.M. An Evidence-based Review of the Efficacy of Treatment Approaches for Immature Permanent Teeth with Pulp Necrosis. J. Endodont. 2017, 43, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Altaii, M.; Richards, L.; Rossi-Fedele, G. Histological assessment of regenerative endodontic treatment in animal studies with different scaffolds: A systematic review. Dent. Traumatol. 2017, 33, 235–244. [Google Scholar] [CrossRef]
- Nosrat, A.; Kolahdouzan, A.; Hosseini, F.; Mehrizi, E.A.; Verma, P.; Torabinejad, M. Histologic Outcomes of Uninfected Human Immature Teeth Treated with Regenerative Endodontics: 2 Case Reports. J. Endodont. 2015, 41, 1725–1729. [Google Scholar] [CrossRef]
- Albuquerque, M.T.; Valera, M.C.; Nakashima, M.; Nor, J.E.; Bottino, M.C. Tissue-engineering-based strategies for regenerative endodontics. J. Dent. Res. 2014, 93, 1222–1231. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Lolato, A.; Bucchi, C.; Taschieri, S.; Weinstein, R.L. Autologous Platelet Concentrates for Pulp and Dentin Regeneration: A Literature Review of Animal Studies. J. Endodont. 2016, 42, 250–257. [Google Scholar] [CrossRef]
- Gomes-Filho, J.E.; Duarte, P.C.; Ervolino, E.; Mogami Bomfim, S.R.; Xavier Abimussi, C.J.; Mota da Silva Santos, L.; Lodi, C.S.; Penha De Oliveira, S.H.; Dezan, E., Jr.; Cintra, L.T. Histologic characterization of engineered tissues in the canal space of closed-apex teeth with apical periodontitis. J. Endodont. 2013, 39, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.S.; Chen, Y.M.; Zhou, R.H.; Huang, X.J.; Cai, Z.Y. Histologic and Immunohistochemical Findings of a Human Immature Permanent Tooth with Apical Periodontitis after Regenerative Endodontic Treatment. J. Endodont. 2015, 41, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Nosrat, A.; Kolahdouzan, A.; Khatibi, A.H.; Verma, P.; Jamshidi, D.; Nevins, A.J.; Torabinejad, M. Clinical, Radiographic, and Histologic Outcome of Regenerative Endodontic Treatment in Human Teeth Using a Novel Collagen-hydroxyapatite Scaffold. J. Endodont. 2019, 45, 136–143. [Google Scholar] [CrossRef]
- Hilkens, P.; Meschi, N.; Lambrechts, P.; Bronckaers, A.; Lambrichts, I. Dental Stem Cells in Pulp Regeneration: Near Future or Long Road Ahead? Stem. Cells. Dev. 2015, 24, 1610–1622. [Google Scholar] [CrossRef]
- Yang, J.W.; Zhang, Y.F.; Sun, Z.Y.; Song, G.T.; Chen, Z. Dental pulp tissue engineering with bFGF-incorporated silk fibroin scaffolds. J. Biomater. Appl. 2015, 30, 221–229. [Google Scholar] [CrossRef]
- Chisini, L.A.; Conde, M.C.; Alcazar, J.C.; Silva, A.F.; Nor, J.E.; Tarquinio, S.B.; Demarco, F.F. Immunohistochemical Expression of TGF-beta1 and Osteonectin in engineered and Ca(OH)2-repaired human pulp tissues. Braz. Oral. Res. 2016, 30, e93. [Google Scholar] [CrossRef]
- Zhu, L.; Dissanayaka, W.L.; Zhang, C. Dental pulp stem cells overexpressing stromal-derived factor-1alpha and vascular endothelial growth factor in dental pulp regeneration. Clin. Oral Investig. 2019, 23, 2497–2509. [Google Scholar] [CrossRef]
- Hayashi, Y.; Murakami, M.; Kawamura, R.; Ishizaka, R.; Fukuta, O.; Nakashima, M. CXCL14 and MCP1 are potent trophic factors associated with cell migration and angiogenesis leading to higher regenerative potential of dental pulp side population cells. Stem. Cell. Res. 2015, 6, 111. [Google Scholar] [CrossRef]
- Yoo, Y.J.; Lee, W.; Cho, Y.A.; Park, J.C.; Shon, W.J.; Baek, S.H. Effect of conditioned medium from preameloblasts on regenerative cellular differentiation of the immature teeth with necrotic pulp and apical periodontitis. J. Endodont. 2014, 40, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Altaii, M.; Cathro, P.; Broberg, M.; Richards, L. Endodontic regeneration and tooth revitalization in immature infected sheep teeth. Int. Endod. J. 2017, 50, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, E.; Rajasekharan, S.; Chitturi, R.T.; Martens, L.; De Coster, P. Gene Expression Profiling and Molecular Signaling of Dental Pulp Cells in Response to Tricalcium Silicate Cements: A Systematic Review. J. Endodont. 2015, 41, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, E.; Rajasekharan, S.; Chitturi, R.T.; Declercq, H.; Martens, L.; De Coster, P. Gene Expression Profiling and Molecular Signaling of Various Cells in Response to Tricalcium Silicate Cements: A Systematic Review. J. Endodont. 2016, 42, 1713–1725. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.K.M.; Desmeules, M.; Cielecki, M.; Dabbagh, B.; dos Santos, B.F. Longitudinal Cohort Study of Regenerative Endodontic Treatment for Immature Necrotic Permanent Teeth. J. Endodont. 2017, 43, 395–400. [Google Scholar] [CrossRef]
- Estefan, B.S.; El Batouty, K.M.; Nagy, M.M.; Diogenes, A. Influence of Age and Apical Diameter on the Success of Endodontic Regeneration Procedures. J. Endodont. 2016, 42, 1620–1625. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T. Dental pulp and dentin tissue engineering and regeneration: Advancement and challenge. Front. Biosci. 2011, 3, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Bezgin, T.; Yilmaz, A.D.; Celik, B.N.; Kolsuz, M.E.; Sonmez, H. Efficacy of Platelet-rich Plasma as a Scaffold in Regenerative Endodontic Treatment. J. Endodont. 2015, 41, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Alexander, A.; Vandati, S.A.; Grandhi, A.; Baylink, D.; Shabahang, S. Effect of Residual Dental Pulp Tissue on Regeneration of Dentin-pulp Complex: An In Vivo Investigation. J. Endodont. 2018, 44, 1796–1801. [Google Scholar] [CrossRef]
- Verma, P.; Nosrat, A.; Kim, J.R.; Price, J.B.; Wang, P.; Bair, E.; Xu, H.H.; Fouad, A.F. Effect of Residual Bacteria on the Outcome of Pulp Regeneration In Vivo. J. Dent. Res. 2017, 96, 100–106. [Google Scholar] [CrossRef]
- Zarei, M.; Jafarian, A.H.; Harandi, A.; Javidi, M.; Gharechahi, M. Evaluation of the expression of VIII factor and VEGF in the regeneration of non-vital teeth in dogs using propolis. Iran. J. Basic Med. Sci. 2017, 20, 172–177. [Google Scholar] [CrossRef]
- Fouad, A.F. Microbial Factors and Antimicrobial Strategies in Dental Pulp Regeneration. J. Endodont. 2017, 43, S46–S50. [Google Scholar] [CrossRef]
- Diogenes, A.R.; Ruparel, N.B.; Teixeira, F.B.; Hargreaves, K.M. Translational science in disinfection for regenerative endodontics. J. Endodont. 2014, 40, S52–S57. [Google Scholar] [CrossRef]
- Nagata, J.Y.; Gomes, B.P.F.D.; Lima, T.F.R.; Murakami, L.S.; de Faria, D.E.; Campos, G.R.; de Souza, F.J.; Soares, A.D. Traumatized Immature Teeth Treated with 2 Protocols of Pulp Revascularization. J. Endodont. 2014, 40, 606–612. [Google Scholar] [CrossRef]
- Zhai, Q.; Dong, Z.; Wang, W.; Li, B.; Jin, Y. Dental stem cell and dental tissue regeneration. Front. Med. 2019, 13, 152–159. [Google Scholar] [CrossRef]
- Karamzadeh, R.; Eslaminejad, M.B.; Aflatoonian, R. Isolation, characterization and comparative differentiation of human dental pulp stem cells derived from permanent teeth by using two different methods. J. Vis. Exp. 2012, 69, e4372. [Google Scholar] [CrossRef]
- Drennan, M.B.; Govindarajan, S.; Verheugen, E.; Coquet, J.M.; Staal, J.; McGuire, C.; Taghon, T.; Leclercq, G.; Beyaert, R.; van Loo, G.; et al. NKT sublineage specification and survival requires the ubiquitin-modifying enzyme TNFAIP3/A20. J. Exp. Med. 2016, 213, 1973–1981. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Krastl, G.; Simon, S.; Van Gorp, G.; Meschi, N.; Vahedi, B.; Lambrechts, P. European Society of Endodontology position statement: Revitalization procedures. Int. Endod. J. 2016, 49, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Bolcaen, J.; Descamps, B.; Deblaere, K.; Boterberg, T.; Hallaert, G.; Van den Broecke, C.; Decrock, E.; Vral, A.; Leybaert, L.; Vanhove, C.; et al. MRI-guided 3D conformal arc micro-irradiation of a F98 glioblastoma rat model using the Small Animal Radiation Research Platform (SARRP). J. Neurooncol. 2014, 120, 257–266. [Google Scholar] [CrossRef]
- Tawfik, H.; Abu-Seida, A.M.; Hashem, A.A.; Nagy, M.M. Regenerative potential following revascularization of immature permanent teeth with necrotic pulps. Int. Endod. J. 2013, 46, 910–922. [Google Scholar] [CrossRef]
- Fahmy, S.H.; Hassanien, E.E.S.; Nagy, M.M.; El Batouty, K.M.; Mekhemar, M.; Fawzy El Sayed, K.; Hassanein, E.H.; Wiltfang, J.; Dorfer, C. Investigation of the regenerative potential of necrotic mature teeth following different revascularisation protocols. Aust. Endod. J. 2017, 43, 73–82. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using Effect Size-or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Kahler, B.; Chugal, N.; Lin, L.M. Alkaline Materials and Regenerative Endodontics: A Review. Materials 2017, 10, 1389. [Google Scholar] [CrossRef]
- Rajasekharan, S.; Vercruysse, C.; Martens, L.; Verbeeck, R. Effect of Exposed Surface Area, Volume and Environmental pH on the Calcium Ion Release of Three Commercially Available Tricalcium Silicate Based Dental Cements. Materials 2018, 11, 123. [Google Scholar] [CrossRef]
- Rajasekharan, S.; Martens, L.C.; Cauwels, R.G.; Verbeeck, R.M. Biodentine material characteristics and clinical applications: A review of the literature. Eur. Arch. Paediatr. Dent. 2014, 15, 147–158. [Google Scholar] [CrossRef]
- Kaushik, S.N.; Kim, B.; Walma, A.M.; Choi, S.C.; Wu, H.; Mao, J.J.; Jun, H.W.; Cheon, K. Biomimetic microenvironments for regenerative endodontics. Biomater. Res. 2016, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Altaii, M.; Broberg, M.; Cathro, P.; Richards, L. Standardisation of sheep model for endodontic regeneration/revitalisation research. Arch. Oral Biol. 2016, 65, 87–94. [Google Scholar] [CrossRef]
- Ruangsawasdi, N.; Zehnder, M.; Patcas, R.; Ghayor, C.; Weber, F.E. Regenerative Dentistry: Animal Model for Regenerative Endodontology. Transfus. Med. Hemoth. 2016, 43, 359–364. [Google Scholar] [CrossRef]
- Lin, L.M.; Shimizu, E.; Gibbs, J.L.; Loghin, S.; Ricucci, D. Histologic and Histobacteriologic Observations of Failed Revascularization/Revitalization Therapy: A Case Report. J. Endodont. 2014, 40, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Lenherr, P.; Allgayer, N.; Weiger, R.; Filippi, A.; Attin, T.; Krastl, G. Tooth discoloration induced by endodontic materials: A laboratory study. Int. Endod. J. 2012, 45, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Berkhoff, J.A.; Chen, P.B.; Teixeira, F.B.; Diogenes, A. Evaluation of triple antibiotic paste removal by different irrigation procedures. J. Endodont. 2014, 40, 1172–1177. [Google Scholar] [CrossRef]
- Graham, L.; Cooper, P.R.; Cassidy, N.; Nor, J.E.; Sloan, A.J.; Smith, A.J. The effect of calcium hydroxide on solubilisation of bio-active dentine matrix components. Biomaterials 2006, 27, 2865–2873. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, H.; Peng, C. Clinical and Radiographic Assessment of the Efficacy of a Collagen Membrane in Regenerative Endodontics: A Randomized, Controlled Clinical Trial. J. Endodont. 2017, 43, 1465–1471. [Google Scholar] [CrossRef]
- Palma, P.J.; Ramos, J.C.; Martins, J.B.; Diogenes, A.; Figueiredo, M.H.; Ferreira, P.; Viegas, C.; Santos, J.M. Histologic Evaluation of Regenerative Endodontic Procedures with the Use of Chitosan Scaffolds in Immature Dog Teeth with Apical Periodontitis. J. Endodont. 2017, 43, 1279–1287. [Google Scholar] [CrossRef]
- Neves, V.C.; Babb, R.; Chandrasekaran, D.; Sharpe, P.T. Promotion of natural tooth repair by small molecule GSK3 antagonists. Sci. Rep. 2017, 7, 39654. [Google Scholar] [CrossRef]
- Mjor, I.A.; Tronstad, L. The healing of experimentally induced pulpitis. Oral Surg. Oral Med. Oral Pathol. 1974, 38, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Mjor, I.A. Pulp-dentin biology in restorative dentistry. Part 7: The exposed pulp. Quintessence. Int. 2002, 33, 113–135. [Google Scholar] [PubMed]
- Galler, K.M. Clinical procedures for revitalization: Current knowledge and considerations. Int. Endod. J. 2016, 49, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Zhou, J.; Solomon, C.; Zheng, Y.; Suzuki, T.; Chen, M.; Song, S.; Jiang, N.; Cho, S.; Mao, J.J. Effects of growth factors on dental stem/progenitor cells. Dent. Clin. N. Am. 2012, 56, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Washio, A.; Kitamura, C.; Morotomi, T.; Terashita, M.; Nishihara, T. Possible Involvement of Smad Signaling Pathways in Induction of Odontoblastic Properties in KN-3 Cells by Bone Morphogenetic Protein-2: A Growth Factor to Induce Dentin Regeneration. Int. J. Dent. 2012, 2012, 258469. [Google Scholar] [CrossRef]
- Abd-Elmeguid, A.; Abdeldayem, M.; Kline, L.W.; Moqbel, R.; Vliagoftis, H.; Yu, D.C. Osteocalcin expression in pulp inflammation. J. Endod. 2013, 39, 865–872. [Google Scholar] [CrossRef]
- Ishizaka, R.; Hayashi, Y.; Iohara, K.; Sugiyama, M.; Murakami, M.; Yamamoto, T.; Fukuta, O.; Nakashima, M. Stimulation of angiogenesis, neurogenesis and regeneration by side population cells from dental pulp. Biomaterials 2013, 34, 1888–1897. [Google Scholar] [CrossRef]
- Iohara, K.; Zheng, L.; Wake, H.; Ito, M.; Nabekura, J.; Wakita, H.; Nakamura, H.; Into, T.; Matsushita, K.; Nakashima, M. A novel stem cell source for vasculogenesis in ischemia: Subfraction of side population cells from dental pulp. Stem. Cells 2008, 26, 2408–2418. [Google Scholar] [CrossRef]
- Nakashima, M.; Iohara, K.; Sugiyama, M. Human dental pulp stem cells with highly angiogenic and neurogenic potential for possible use in pulp regeneration. Cytokine Growth Factor Rev. 2009, 20, 435–440. [Google Scholar] [CrossRef]
- Huang, G.T.; Garcia-Godoy, F. Missing Concepts in De Novo Pulp Regeneration. J. Dent. Res. 2014, 93, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Meschi, N.; Hilkens, P.; Lambrichts, I.; Van den Eynde, K.; Mavridou, A.; Strijbos, O.; De Ketelaere, M.; Van Gorp, G.; Lambrechts, P. Regenerative endodontic procedure of an infected immature permanent human tooth: An immunohistological study. Clin. Oral Investig. 2016, 20, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.N.; Jiang, S.; Chen, Q.Y.; Ye, Y.Y.; Chen, J.J.; Heng, B.C.; Jiang, Q.L.; Wu, B.L.; Ding, Z.H.; Zhang, C.F. Systemically Transplanted Bone Marrow-derived Cells Contribute to Dental Pulp Regeneration in a Chimeric Mouse Model. J. Endodont. 2016, 42, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Faras, H.; Corr, R.; Wright, K.R.; Shabahang, S. Histologic Examinations of Teeth Treated with 2 Scaffolds: A Pilot Animal Investigation. J. Endodont. 2014, 40, 515–520. [Google Scholar] [CrossRef]
- Mullane, E.M.; Dong, Z.; Sedgley, C.M.; Hu, J.C.; Botero, T.M.; Holland, G.R.; Nor, J.E. Effects of VEGF and FGF2 on the revascularization of severed human dental pulps. J. Dent. Res. 2008, 87, 1144–1148. [Google Scholar] [CrossRef]
- Choung, H.W.; Lee, J.H.; Lee, D.S.; Choung, P.H.; Park, J.C. The role of preameloblast-conditioned medium in dental pulp regeneration. J. Mol. Histol. 2013, 44, 715–721. [Google Scholar] [CrossRef]
- Huang, G.T.; Yamaza, T.; Shea, L.D.; Djouad, F.; Kuhn, N.Z.; Tuan, R.S.; Shi, S. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng. Part A. 2010, 16, 605–615. [Google Scholar] [CrossRef]
- Nie, X.; Tian, W.; Zhang, Y.; Chen, X.; Dong, R.; Jiang, M.; Chen, F.; Jin, Y. Induction of transforming growth factor-beta 1 on dentine pulp cells in different culture patterns. Cell Biol. Int. 2006, 30, 295–300. [Google Scholar] [CrossRef]
- Duncan, H.F.; Cooper, P.R.; Smith, A.J. Dissecting dentine-pulp injury and wound healing responses: Consequences for regenerative endodontics. Int. Endod. J. 2019, 52, 261–266. [Google Scholar] [CrossRef]
- Laurent, P.; Camps, J.; About, I. Biodentine(TM) induces TGF-beta1 release from human pulp cells and early dental pulp mineralization. Int. Endod. J. 2012, 45, 439–448. [Google Scholar] [CrossRef]
- Yu, C.; Abbott, P.V. An overview of the dental pulp: Its functions and responses to injury. Aust. Dent. J. 2007, 52, S4–S16. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.W.; Lee, S.Y.; Ann, H.J.; Kum, K.Y.; Kim, E.C. Effects of calcium silicate endodontic cements on biocompatibility and mineralization-inducing potentials in human dental pulp cells. J. Endod. 2014, 40, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Song, Y.S.; Min, K.S.; Kim, S.H.; Koh, J.T.; Lee, B.N.; Chang, H.S.; Hwang, I.N.; Oh, W.M.; Hwang, Y.C. Evaluation of reparative dentin formation of ProRoot MTA, Biodentine and BioAggregate using micro-CT and immunohistochemistry. Restor. Dent. Endod. 2016, 41, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Rajasekharan, S.; Martens, L.C.; Vandenbulcke, J.; Jacquet, W.; Bottenberg, P.; Cauwels, R.G. Efficacy of three different pulpotomy agents in primary molars: A randomized control trial. Int. Endod. J. 2017, 50, 215–228. [Google Scholar] [CrossRef]
- Cardoso, M.; Dos Anjos Pires, M.; Correlo, V.; Reis, R.; Paulo, M.; Viegas, C. Biodentine for Furcation Perforation Repair: An Animal Study with Histological, Radiographic and Micro-Computed Tomographic Assessment. Iran. Endod. J. 2018, 13, 323–330. [Google Scholar] [CrossRef]
- Noetzel, J.; Ozer, K.; Reisshauer, B.H.; Anil, A.; Rossler, R.; Neumann, K.; Kielbassa, A.M. Tissue responses to an experimental calcium phosphate cement and mineral trioxide aggregate as materials for furcation perforation repair: A histological study in dogs. Clin. Oral Investig. 2006, 10, 77–83. [Google Scholar] [CrossRef]
- Aktemur Turker, S.; Uzunoglu, E.; Deniz Sungur, D.; Tek, V. Fracture Resistance of Teeth with Simulated Perforating Internal Resorption Cavities Repaired with Different Calcium Silicate-based Cements and Backfilling Materials. J. Endod. 2018, 44, 860–863. [Google Scholar] [CrossRef]
- Rajasekharan, S.; Martens, L.C.; Cauwels, R.; Anthonappa, R.P.; Verbeeck, R.M.H. Biodentine material characteristics and clinical applications: A 3 year literature review and update. Eur. Arch. Paediatr. Dent. 2018, 19, 1–22. [Google Scholar] [CrossRef]
- Nowicka, A.; Wilk, G.; Lipski, M.; Kolecki, J.; Buczkowska-Radlinska, J. Tomographic Evaluation of Reparative Dentin Formation after Direct Pulp Capping with Ca(OH)2, MTA, Biodentine, and Dentin Bonding System in Human Teeth. J. Endod. 2015, 41, 1234–1240. [Google Scholar] [CrossRef]
- De Rossi, A.; Silva, L.A.; Gaton-Hernandez, P.; Sousa-Neto, M.D.; Nelson-Filho, P.; Silva, R.A.; de Queiroz, A.M. Comparison of pulpal responses to pulpotomy and pulp capping with biodentine and mineral trioxide aggregate in dogs. J. Endod. 2014, 40, 1362–1369. [Google Scholar] [CrossRef]
- Shayegan, A.; Jurysta, C.; Atash, R.; Petein, M.; Abbeele, A.V. Biodentine used as a pulp-capping agent in primary pig teeth. Pediatr. Dent. 2012, 34, e202–e208. [Google Scholar] [PubMed]
- Feng, X.; Feng, G.; Xing, J.; Shen, B.; Li, L.; Tan, W.; Xu, Y.; Liu, S.; Liu, H.; Jiang, J.; et al. TNF-alpha triggers osteogenic differentiation of human dental pulp stem cells via the NF-kappaB signalling pathway. Cell Biol. Int. 2013, 37, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, C.L.; Li, R.M.; Hui, T.Q.; Su, Y.Y.; Yuan, Q.; Zhou, X.D.; Ye, L. Wnt5a promotes inflammatory responses via nuclear factor kappaB (NF-kappaB) and mitogen-activated protein kinase (MAPK) pathways in human dental pulp cells. J. Biol. Chem. 2014, 289, 21028–21039. [Google Scholar] [CrossRef] [PubMed]




| Target Gene | Primer | |
|---|---|---|
| Forward | Reverse | |
| GAPDH | CTACCAGTGCAAAGAGCCCA | TGGTCATCAACCCTTCCACG |
| ACTB | CTTCGCGGGCGACGAT | CCACATAGGAATCCTTCTGACC |
| B2M | ACTTAGAGGTGGGGAGCAGA | GCCCTTTACACTGTGAGCC |
| EIF4b | GTGCGTTTACCACGTGAACC | CGTGCATCCTGGTCTGACTT |
| RPH3a | CTGGTCCGAGTTTTCTCCGC | TTCTTTATCATTTGATTGAAGGGGC |
| TGF-β1 | AGGGCTACCATGCCAACTTC | GACACAGAGATCCGCAGTCC |
| BMP2 | AGTCCTGATGAGCATGAGCC | CTCACCTATCTGTATACTGC |
| BGLAP | CTCACACTCCTCGCCCTAT | TCTCTTCACTACCTCGCTGC |
| VEGFA | ATGCGGATCAAACCTCACCA | CACCAACGTACACGCTCCAG |
| WNT5a | AAGCAGACGTTTCGGCTACA | TTTCCAACGTCCATCAGCGA |
| MMP1 | CCCAGCGACTCTAGAAACACA | CTGCTTGACCCTCAGAGACC |
| TNF-α | GTGACAAGCCTGTAGCCCAT | CTCTGATGGCACCACCAACT |
| SMAD6 | AAAACCGTCACGTACTCGCT | GGTCGTACACCGCATAGAGG |
| Biodentine | ProRoot WMTA | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tooth 1 | Tooth 2 | Tooth 3 | Tooth 1 | Tooth 2 | Tooth 3 | Tooth 4 | ||||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| Extent of inflammation (0–4) | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 3 | 0 | 0 | 0 | 3 | 0 |
| Presence or absence of tissue with cellularity and vascularity inside the pulp space (0–3) | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Area of tissue with cellularity and vascularity (mm2) | 13.14 | 1.6 | 11.78 | 1.47 | 0 | 0 | 0 | 0 | 0 | 0 | 0.23 | 0.12 | 0 | 0 |
| Length of odontoblast lining (mm) | 13.4 | 1.29 | 11.27 | 1.43 | 0 | 0 | 0 | 0 | 0 | 0 | 1.41 | 0.72 | 0 | 0 |
| Number of blood vessels (n) | 64.33 | 19.33 | 176.33 | 19.62 | 0 | 0 | 0 | 0 | 0 | 0 | 45 | 16.77 | 0 | 0 |
| Area of blood vessels expressed as percentage of vascularity (%) | 7.45 | 1.85 | 10.03 | 0.99 | 0 | 0 | 0 | 0 | 0 | 0 | 3.76 | 0.16 | 0 | 0 |
| Area of empty root canal space (mm2) | 1.36 | 0.51 | 1.15 | 0.38 | 23.70 | 1.08 | 11.69 | 0.37 | 12.91 | 2.71 | 11.03 | 0.71 | 22.28 | 2.32 |
| Area of mineralized tissue (%) | 0.33 | 0.01 | 0.26 | 0.01 | 0.28 | 0.02 | 0.27 | 0.01 | 0.08 | 0.01 | 0.38 | 0.02 | 0.31 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rathinam, E.; Rajasekharan, S.; Declercq, H.; Vanhove, C.; De Coster, P.; Martens, L. Effect of Intracoronal Sealing Biomaterials on the Histological Outcome of Endodontic Revitalisation in Immature Sheep Teeth—A Pilot Study. J. Funct. Biomater. 2023, 14, 214. https://doi.org/10.3390/jfb14040214
Rathinam E, Rajasekharan S, Declercq H, Vanhove C, De Coster P, Martens L. Effect of Intracoronal Sealing Biomaterials on the Histological Outcome of Endodontic Revitalisation in Immature Sheep Teeth—A Pilot Study. Journal of Functional Biomaterials. 2023; 14(4):214. https://doi.org/10.3390/jfb14040214
Chicago/Turabian StyleRathinam, Elanagai, Sivaprakash Rajasekharan, Heidi Declercq, Christian Vanhove, Peter De Coster, and Luc Martens. 2023. "Effect of Intracoronal Sealing Biomaterials on the Histological Outcome of Endodontic Revitalisation in Immature Sheep Teeth—A Pilot Study" Journal of Functional Biomaterials 14, no. 4: 214. https://doi.org/10.3390/jfb14040214
APA StyleRathinam, E., Rajasekharan, S., Declercq, H., Vanhove, C., De Coster, P., & Martens, L. (2023). Effect of Intracoronal Sealing Biomaterials on the Histological Outcome of Endodontic Revitalisation in Immature Sheep Teeth—A Pilot Study. Journal of Functional Biomaterials, 14(4), 214. https://doi.org/10.3390/jfb14040214







