Antifungal Activity of Juglans-regia-Mediated Silver Nanoparticles (AgNPs) against Aspergillus-ochraceus-Induced Toxicity in In Vitro and In Vivo Settings
Abstract
1. Introduction
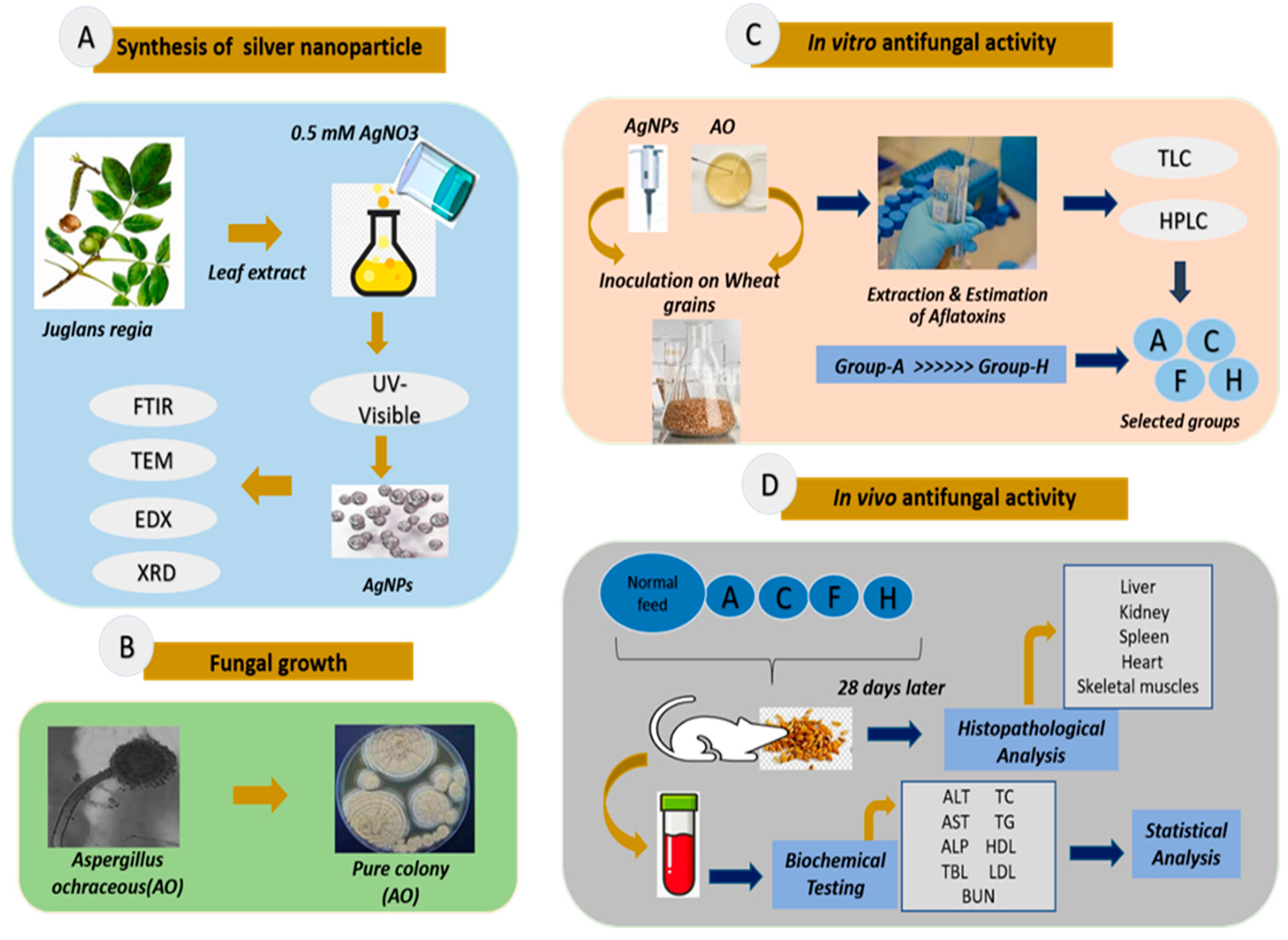
2. Materials and Methods
2.1. Preparation of J. regia Leaf Extract
2.2. Synthesis of Silver Nanoparticles Using Leaf Extract of J. regia
2.3. Phytochemical Analysis of J. regia Leaf Extract
2.3.1. Estimation of Total Phenolic and Flavonoid Content
2.3.2. 2,2-Diphenyl-1-picrylhydrazyl (DPPH)
2.4. Characterization of J. regia-Mediated Silver Nanoparticles
2.5. Collection and Growth of Fungus
2.6. In Vitro Antifungal Activity of Silver Nanoparticles
2.6.1. Collection and Sampling of Wheat for In Vitro Antifungal Activity
2.6.2. Extraction and Estimation of Toxins
2.7. In Vivo Antifungal Activity of Silver Nanoparticles
2.7.1. Study Design
2.7.2. Biochemical Testing
2.7.3. Histopathological Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Determination of Phytochemical Content
3.2. Synthesis of J. regia-Mediated AgNPs
3.3. Characterization of J. regia-Mediated Silver Nanoparticles
3.3.1. UV-Visible Spectroscopy
3.3.2. Transmission Electron Microscopy (TEM) and Energy-Dispersive X-ray (EDX) Spectroscopy
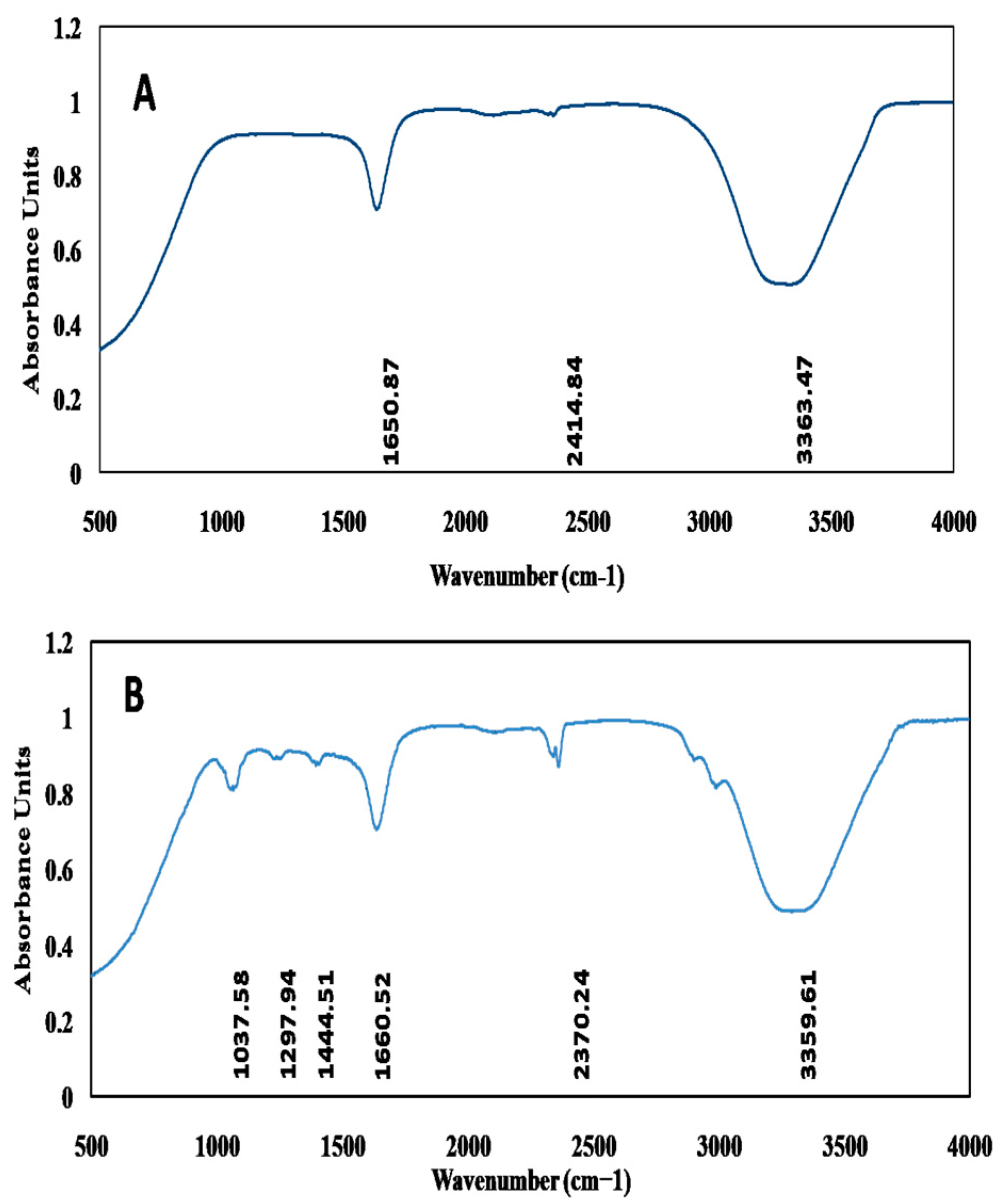
3.3.3. Fourier-Transform Infrared Spectroscopy (FT-IR)
3.3.4. X-ray Diffraction Analysis (XRD)
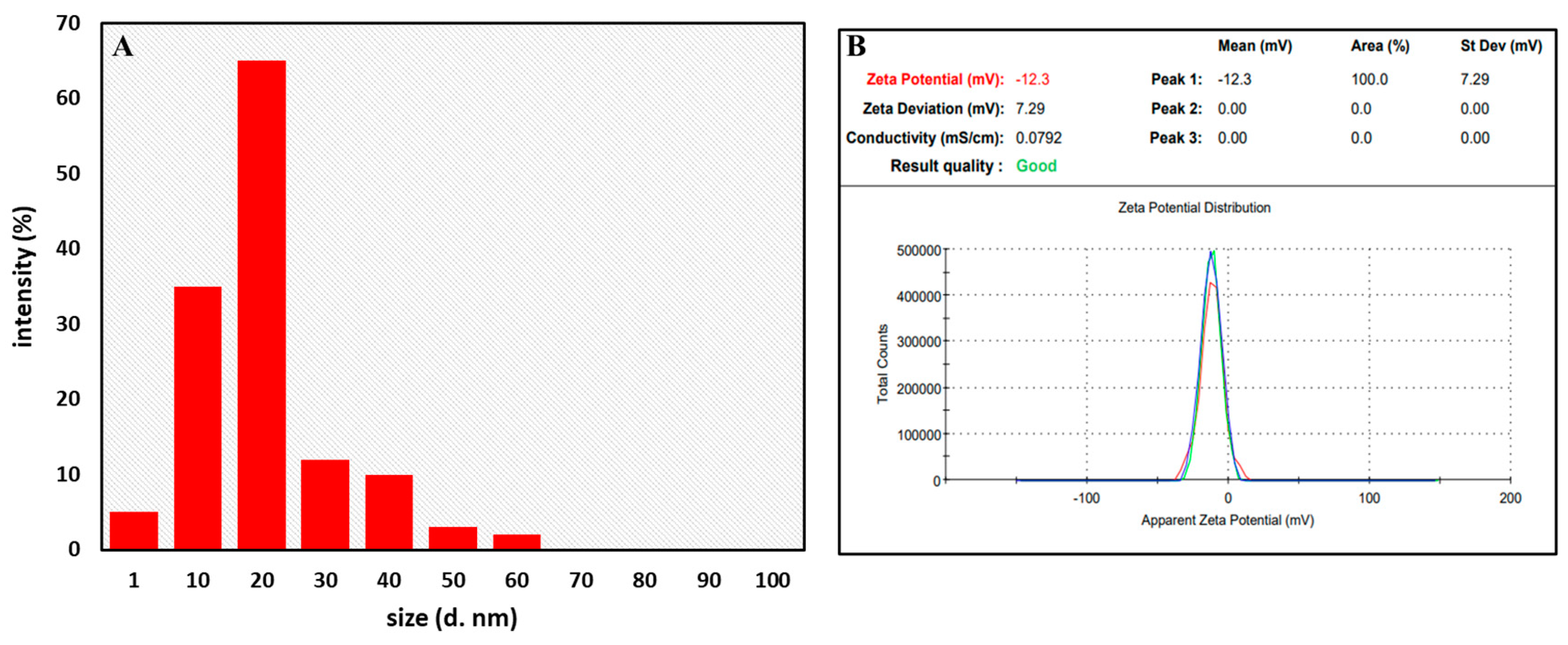
3.3.5. Dynamic Light Scattering (DLS) and Zeta Potential Analysis
3.4. In Vitro Antifungal Activity of AgNPs
3.5. In Vivo Antifungal Activity of AgNPs
3.5.1. Effect on Body Weight, Feed Consumption, and Feed Conversion Ratio in Albino Rats
3.5.2. Effects on Hematological Parameters
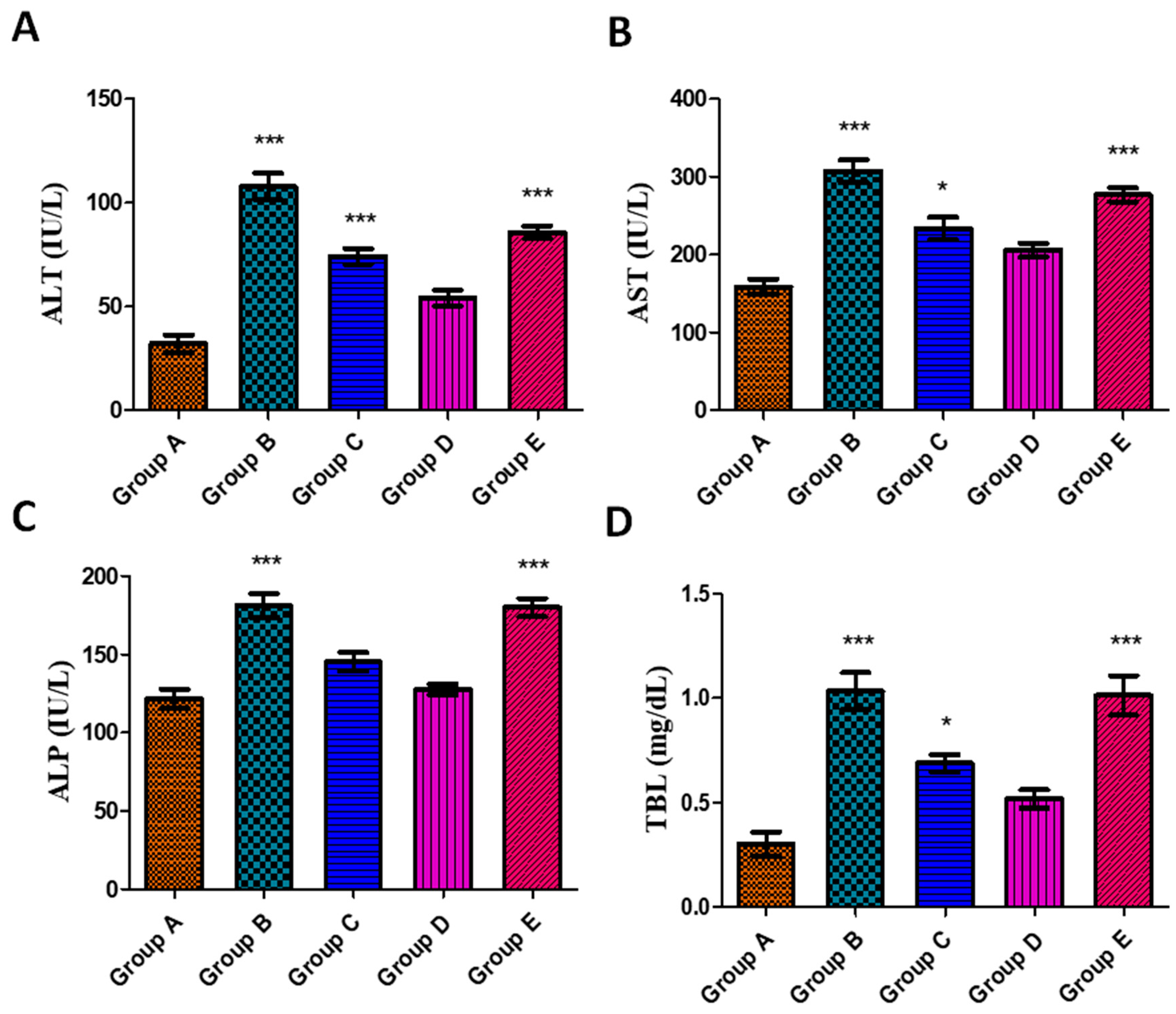
3.5.3. Effects on Liver Function Parameters
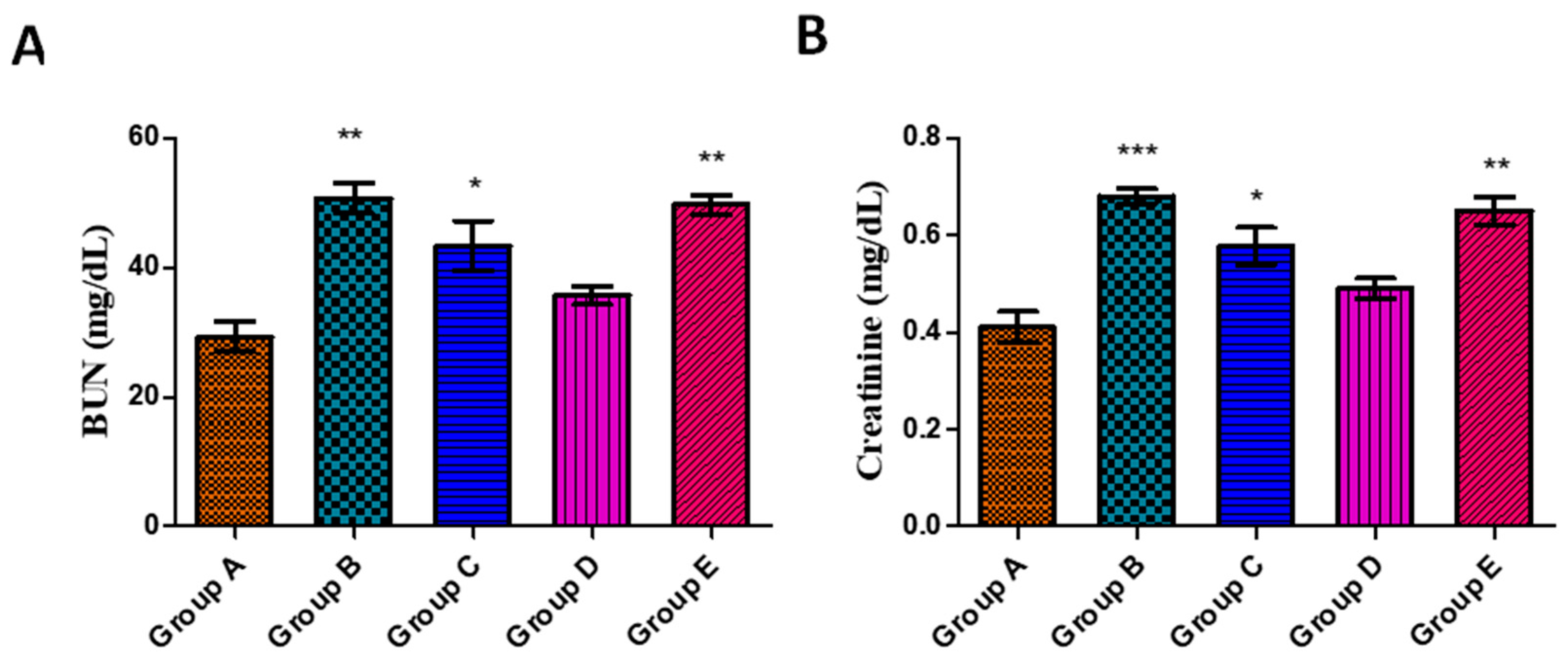
3.5.4. Effects on Kidney Function Parameters
3.5.5. Effects on Serum Lipid Profile
3.6. Histopathology Studies of Organs
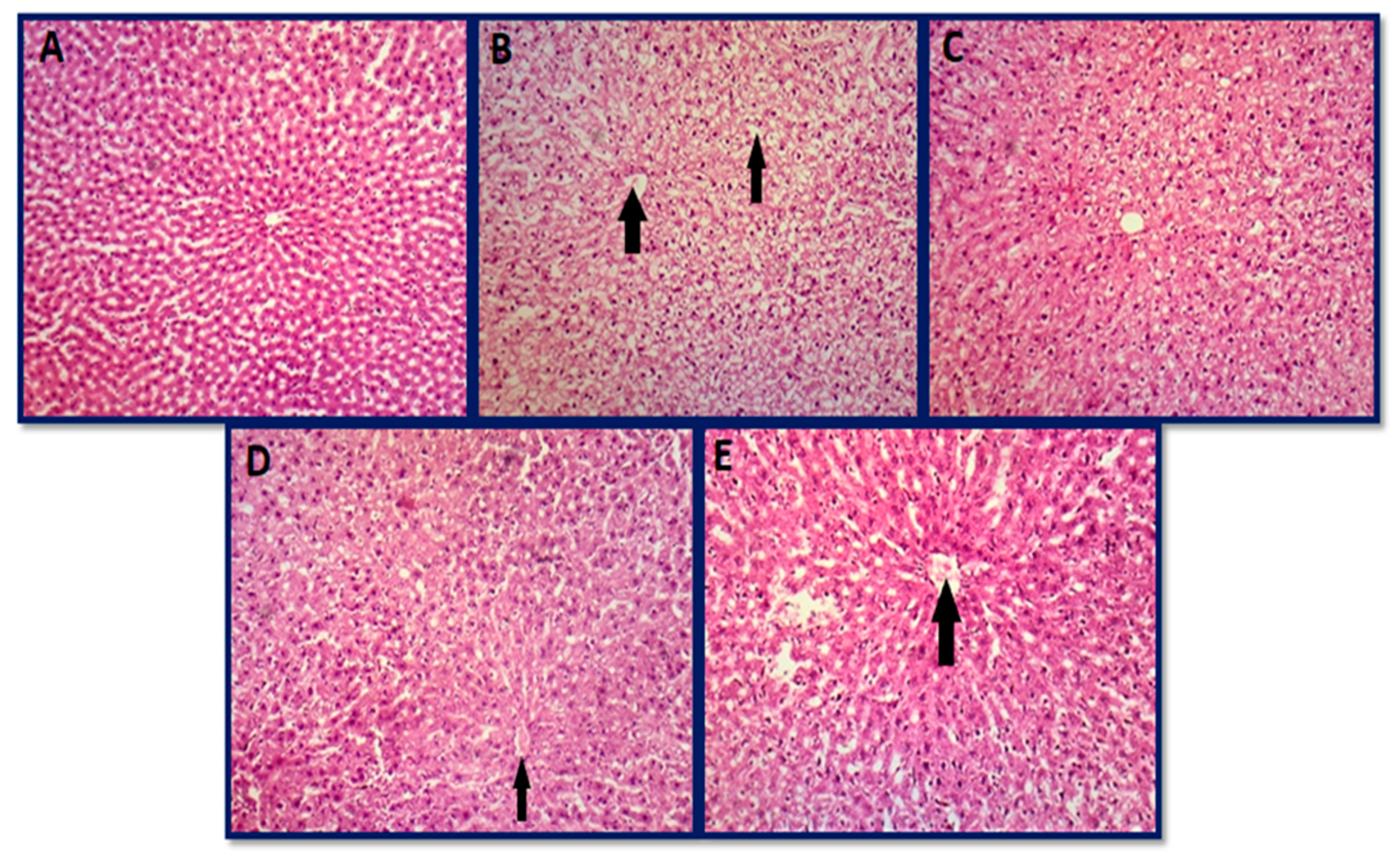
3.6.1. Histopathology of Liver
3.6.2. Histopathology of Kidney
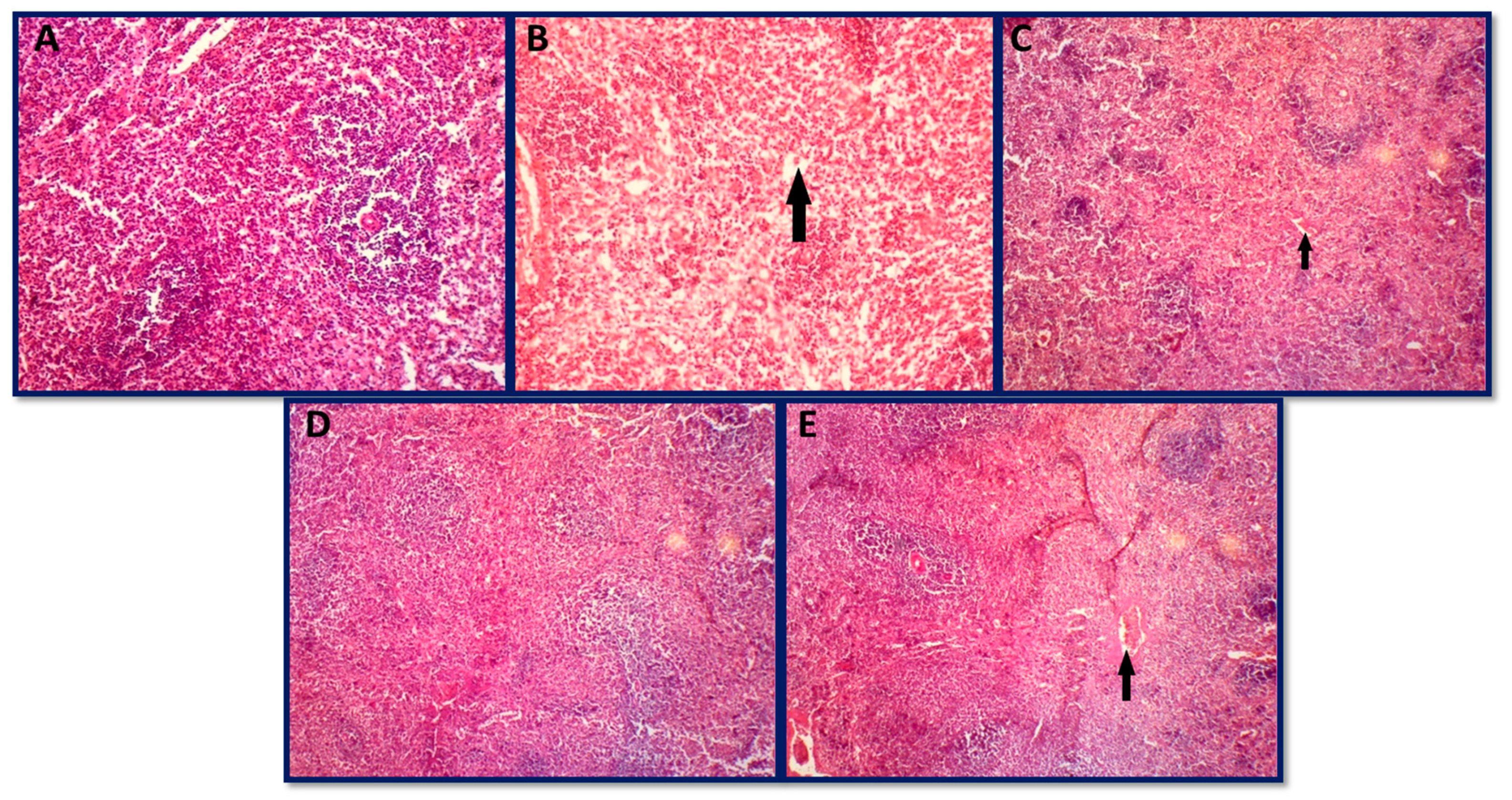
3.6.3. Histopathology of Spleen
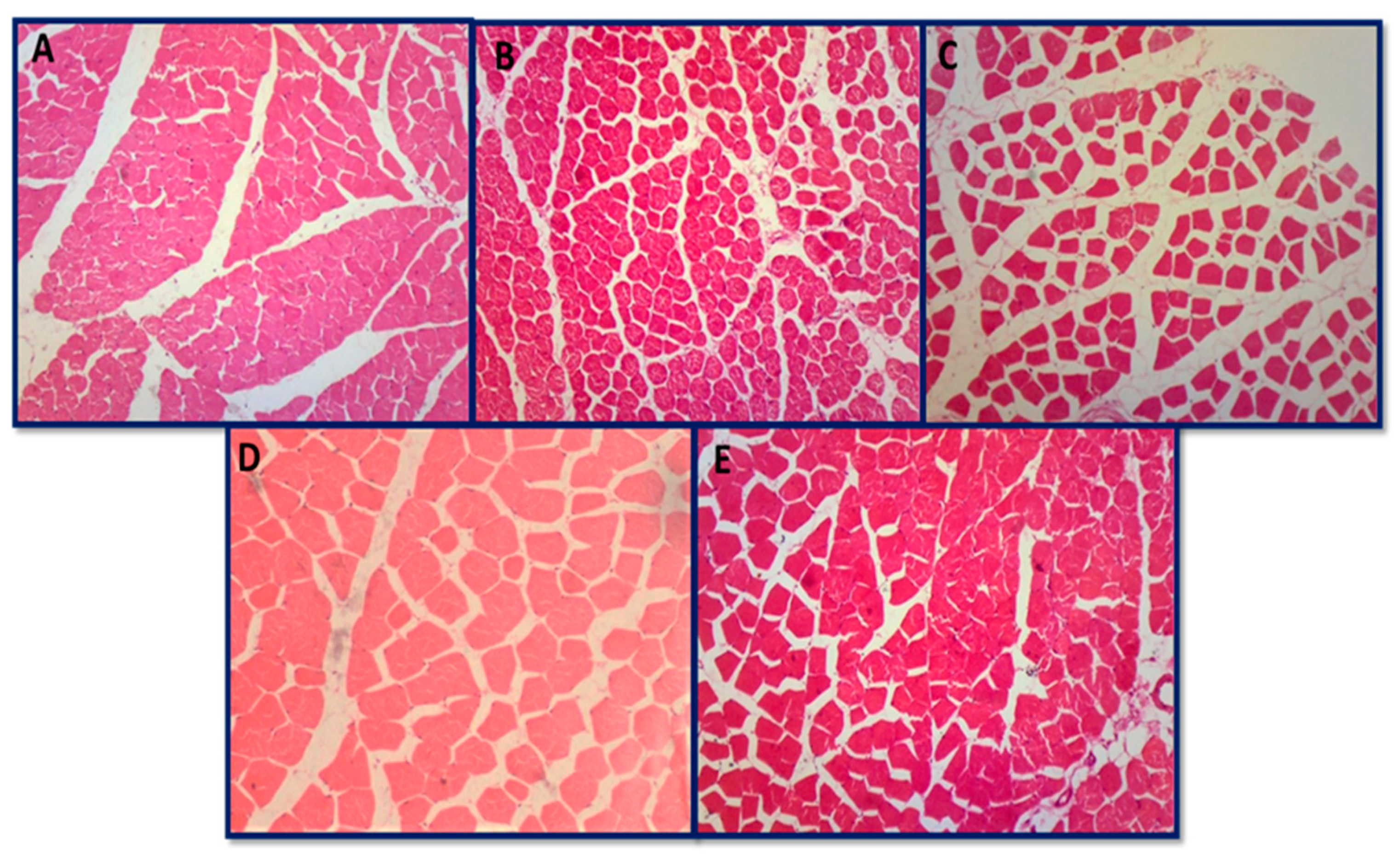
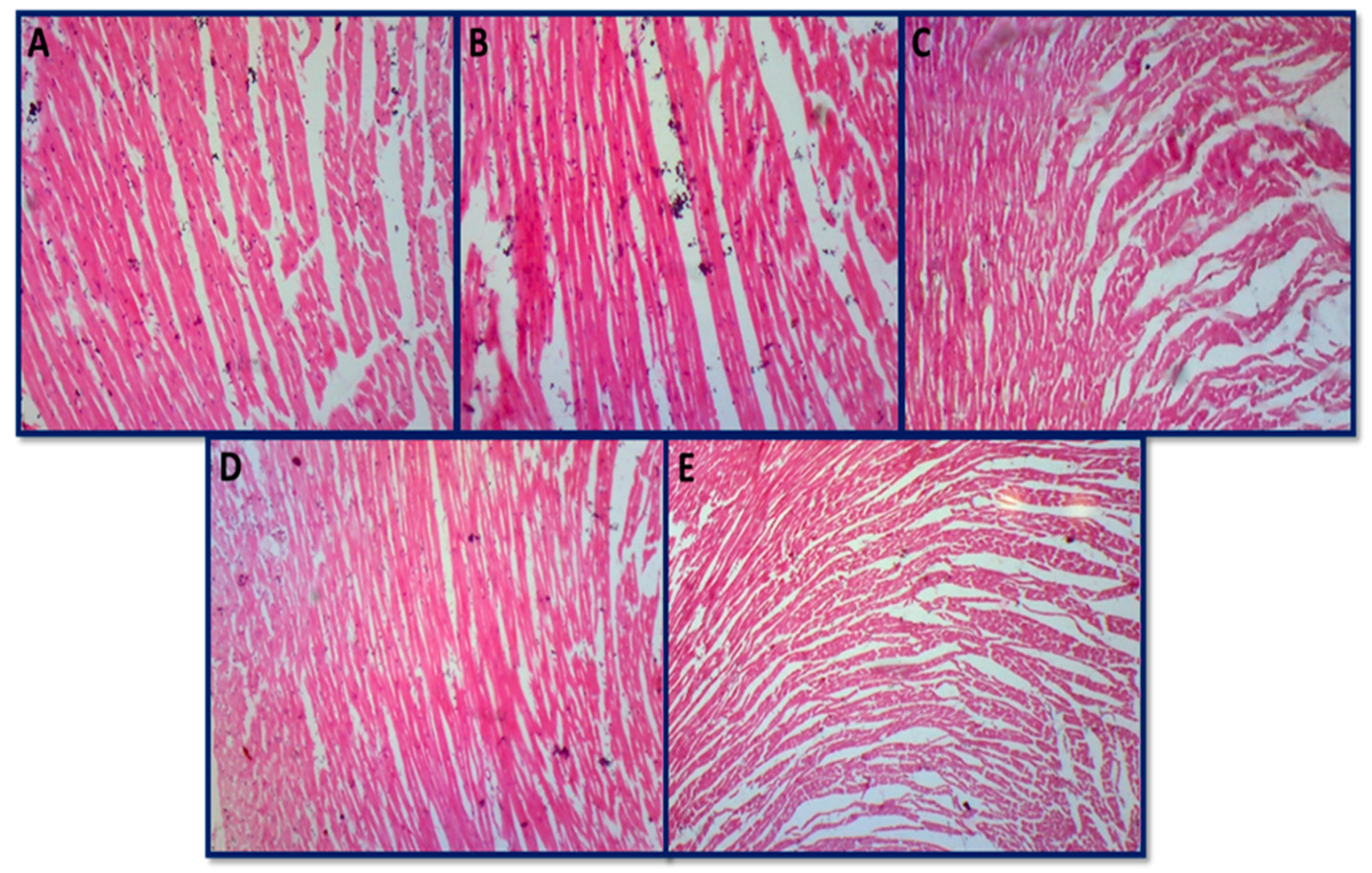
3.6.4. Histopathology of Skeletal Muscle and Heart
4. General Mechanism of Antifungal Activity by AgNPs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reddy, K.; Salleh, B.; Saad, B.; Abbas, H.; Abel, C.; Shier, W. An overview of mycotoxin contamination in foods and its implications for human health. Toxin Rev. 2010, 29, 3–26. [Google Scholar] [CrossRef]
- Beuchat, L.R. Food and Beverage Mycology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1987. [Google Scholar]
- Büchi, G.; Rae, I.D. The Structure and Chemistry of the Aflatoxins. In Aflatoxin; Elsevier: Amsterdam, The Netherlands, 1969; pp. 55–75. [Google Scholar]
- Ellis, W.; Smith, J.; Simpson, B.; Oldham, J.; Scott, P.M. Aflatoxins in food: Occurrence, biosynthesis, effects on organisms, detection, and methods of control. Crit. Rev. Food Sci. Nutr. 1991, 30, 403–439. [Google Scholar] [CrossRef] [PubMed]
- Klvana, M.; Bren, U. Aflatoxin B1–formamidopyrimidine DNA adducts: Relationships between structures, free energies, and melting temperatures. Molecules 2019, 24, 150. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghasham, A.; Ata, H.S.; El-Deep, S.; Meki, A.-R.; Shehada, S. Study of protective effect of date and Nigella sativa on aflatoxin B1 toxicity. Int. J. Health Sci. 2008, 2, 26. [Google Scholar]
- Mahato, D.K.; Lee, K.E.; Kamle, M.; Devi, S.; Dewangan, K.N.; Kumar, P.; Kang, S.G. Aflatoxins in food and feed: An overview on prevalence, detection and control strategies. Front. Microbiol. 2019, 10, 2266. [Google Scholar] [CrossRef] [PubMed]
- Abu-El-Zahab, H.; Baiomy, A.; Saad, D. Studies on detoxification of aflatoxins contaminated rabbits’ rations treated with clay and ammonia. Life Sci. J. 2012, 9, 2713–2721. [Google Scholar]
- Ajayi, I.A.; Raji, A.A.; Ogunkunle, E.O. Green synthesis of silver nanoparticles from seed extracts of Cyperus esculentus and Butyrospermum paradoxum. J. Pharm. Biol. Sci. 2015, 10, 76–90. [Google Scholar]
- Kalaiselvan, V.; Rajasekaran, A. Biosynthesis of silver nanoparticles from Aspergillus niger and evaluation of its wound healing activity in experimental rat model. Int. J. Pharm. Tech. Res. 2009, 4, 1523–1529. [Google Scholar]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Yamanaka, M.; Hara, K.; Kudo, J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl. Environ. Microbiol. 2005, 71, 7589–7593. [Google Scholar] [CrossRef]
- Dhoondia, Z.H.; Chakraborty, H. Lactobacillus mediated synthesis of silver oxide nanoparticles. Nanomater. Nanotechnol. 2012, 2, 15. [Google Scholar] [CrossRef]
- Cho, K.-H.; Park, J.-E.; Osaka, T.; Park, S.-G. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim. Acta 2005, 51, 956–960. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346. [Google Scholar] [CrossRef] [PubMed]
- Maynard, A.D. Nanotechnology: Assessing the risks. Nano Today 2006, 1, 22–33. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.S.; Cho, H.S.; Rha, D.S.; Kim, J.M.; Park, J.D.; Choi, B.S.; Lim, R.; Chang, H.K.; Chung, Y.H.; et al. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal. Toxicol. 2008, 20, 575–583. [Google Scholar] [CrossRef]
- Jo, Y.-K.; Kim, B.H.; Jung, G. Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Dis. 2009, 93, 1037–1043. [Google Scholar] [CrossRef]
- Baran, M.F.; Keskin, C.; Baran, A.; Hatipoğlu, A.; Yildiztekin, M.; Küçükaydin, S.; Kurt, K.; Hoşgören, H.; Sarker, M.M.R.; Sufianov, A.; et al. Green Synthesis of Silver Nanoparticles from Allium cepa L. Peel Extract, Their Antioxidant, Antipathogenic, and Anticholinesterase Activity. Molecules 2023, 28, 2310. [Google Scholar] [CrossRef]
- Giri, A.K.; Jena, B.; Biswal, B.; Pradhan, A.K.; Arakha, M.; Acharya, S.; Acharya, L. Green synthesis and characterization of silver nanoparticles using Eugenia roxburghii, D.C. extract and activity against biofilm-producing bacteria. Sci. Rep. 2022, 12, 8383. [Google Scholar] [CrossRef]
- Alahmad, A.; Al-Zereini, W.A.; Hijazin, T.J.; Al-Madanat, O.Y.; Alghoraibi, I.; Al-Qaralleh, O.; Al-Qaraleh, S.; Feldhoff, A.; Walter, J.-G.; Scheper, T. Green synthesis of silver nanoparticles using Hypericum perforatum L. aqueous extract with the evaluation of its antibacterial activity against clinical and food pathogens. Pharmaceutics 2022, 14, 1104. [Google Scholar] [CrossRef]
- Naseer, M.; Aslam, U.; Khalid, B.; Chen, B. Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci. Rep. 2020, 10, 9055. [Google Scholar] [CrossRef]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Valentão, P.; Andrade, P.B.; Ferreira, I.C.; Ferreres, F.; Bento, A.; Seabra, R.; Estevinho, L. Walnut (Juglans regia L.) leaves: Phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem. Toxicol. 2007, 45, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liao, L.; Moore, J.; Wu, T.; Wang, Z. Antioxidant phenolic compounds from walnut kernels (Juglans regia L.). Food Chem. 2009, 113, 160–165. [Google Scholar] [CrossRef]
- Chaleshtori, R.S.; Chaleshtori, F.S.; Rafieian, M. Biological characterization of Iranian walnut (Juglans regia) leaves. Turk. J. Biol. 2011, 35, 635–639. [Google Scholar] [CrossRef]
- Taha, N.A.; Al-wadaan, M.A. Utility and importance of walnut, Juglans regia Linn: A. Afr. J. Microbiol. Res. 2011, 5, 5796–5805. [Google Scholar]
- Croitoru, A.; Ficai, D.; Craciun, L.; Ficai, A.; Andronescu, E. Evaluation and exploitation of bioactive compounds of walnut, Juglans regia. Curr. Pharm. Des. 2019, 25, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Sharma, V. A Scientific Update on Juglans regia Linn. Asian J. Pharm. Res. Dev. 2020, 8, 166–175. [Google Scholar] [CrossRef]
- Bhat, A.A.; Shakeel, A.; Rafiq, S.; Farooq, I.; Malik, A.Q.; Alghuthami, M.E.; Alharthi, S.; Qanash, H.; Alharthy, S.A. Juglans regia Linn.: A Natural Repository of Vital Phytochemical and Pharmacological Compounds. Life 2023, 13, 380. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Singh, A.; Patnaik, R.S.; Bhardwaj, S. Juglans Regia L: A Review of its Traditional Uses Phytochemistry and Therapeutic Applications. J. Pharm. Negat. Results 2023, 14, 11–16. [Google Scholar]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; van der Heide, E. In silico contact pressure of metal-on-metal total hip implant with different materials subjected to gait loading. Metals 2022, 12, 1241. [Google Scholar] [CrossRef]
- Ravichandran, S.; Paluri, V.; Kumar, G.; Loganathan, K.; Kokati Venkata, B.R. A novel approach for the biosynthesis of silver oxide nanoparticles using aqueous leaf extract of Callistemon lanceolatus (Myrtaceae) and their therapeutic potential. J. Exp. Nanosci. 2016, 11, 445–458. [Google Scholar] [CrossRef]
- Velioglu, Y.; Mazza, G.; Gao, L.; Oomah, B. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar]
- Ahmed, M.; Fatima, H.; Qasim, M.; Gul, B. Polarity directed optimization of phytochemical and in vitro biological potential of an indigenous folklore: Quercus dilatata Lindl. ex Royle. BMC Complement. Altern. Med. 2017, 17, 386. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Renouard, S.; Drouet, S.; Blondeau, J.-P.; Hano, C. A critical cross-species comparison of pollen from Nelumbo nucifera Gaertn. vs. Nymphaea lotus L. for authentication of Thai medicinal herbal tea. Plants 2020, 9, 921. [Google Scholar] [PubMed]
- Fakhari, S.; Jamzad, M.; Kabiri Fard, H. Green synthesis of zinc oxide nanoparticles: A comparison. Green Chem. Lett. Rev. 2019, 12, 19–24. [Google Scholar] [CrossRef]
- Sohail, M.F.; Rehman, M.; Hussain, S.Z.; Huma, Z.-E.; Shahnaz, G.; Qureshi, O.S.; Khalid, Q.; Mirza, S.; Hussain, I.; Webster, T.J. Green synthesis of zinc oxide nanoparticles by Neem extract as multi-facet therapeutic agents. J. Drug Deliv. Sci. Technol. 2020, 59, 101911. [Google Scholar] [CrossRef]
- Trenk, H.L.; Butz, M.E.; Chu, F.S. Production of ochratoxins in different cereal products by Aspergillus ochraceus. Appl. Microbiol. 1971, 21, 1032–1035. [Google Scholar] [CrossRef]
- Bayman, P.; Baker, J.L.; Doster, M.A.; Michailides, T.J.; Mahoney, N.E. Ochratoxin production by the Aspergillus ochraceus group and Aspergillus alliaceus. Appl. Environ. Microbiol. 2002, 68, 2326–2329. [Google Scholar] [CrossRef]
- Bancroft, J.; Cook, H. Manual of Histological Techniques and Their Diagnostic Application. Churchill Livingstone; Longman Singapore Publishers (Pte) Ltd.: Singapore, 1994. [Google Scholar]
- Zargari, A. Medicinal Plants; Tehran University of Medical Sciences: Tehran, Iran, 1997. [Google Scholar]
- Mohammadi, J.; Mirzaie, A.; Azizi, A.; Roozbehi, A.; Delaviz, H. The effects of hydroalcoholic extract of Juglans regia leaf on histological changes of Langerhans islet in diabetic rats model. ISMJ 2012, 15, 293–302. [Google Scholar]
- Shah, T.I.; Ganesh, N.; Akthar, S. Preliminary phytochemical evaluation and antibacterial potential of different leaf extracts of Juglana Regia: A ubiquitous dry fruit from Kashmir-India. Pharm. Sci. Rev. Res. 2013, 19, 93–96. [Google Scholar]
- Panth, N.; Paudel, K.R.; Karki, R. Phytochemical profile and biological activity of Juglans regia. J. Integr. Med. 2016, 14, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Delaviz, H.; Mohammadi, J.; Ghalamfarsa, G.; Mohammadi, B.; Farhadi, N. A review study on phytochemistry and pharmacology applications of Juglans regia plant. Pharmacogn. Rev. 2017, 11, 145. [Google Scholar] [PubMed]
- Nabavi, S.F.; Ebrahimzadeh, M.A.; Nabavi, S.M.; Mahmoudi, M.; Rad, S.K. Biological activities of Juglans regia flowers. Rev. Bras. Farmacogn. 2011, 21, 465–470. [Google Scholar] [CrossRef]
- Oliveira, I.; Sousa, A.; Ferreira, I.C.; Bento, A.; Estevinho, L.; Pereira, J.A. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem. Toxicol. 2008, 46, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, C.; Zhao, S.; Ge, F.; Liu, D. Effect of solvents on the antioxidant activity of walnut (Juglans regia L.) shell extracts. J. Food Nutr. Res. 2014, 2, 621–626. [Google Scholar] [CrossRef]
- Shah, U.N.; Mir, J.I.; Ahmed, N.; Jan, S.; Fazili, K.M. Bioefficacy potential of different genotypes of walnut Juglans regia L. J. Food Sci. Technol. 2018, 55, 605–618. [Google Scholar] [CrossRef]
- Christopoulos, M.V.; Tsantili, E. Participation of phenylalanine ammonia-lyase (PAL) in increased phenolic compounds in fresh cold stressed walnut (Juglans regia L.) kernels. Postharvest Biol. Technol. 2015, 104, 17–25. [Google Scholar] [CrossRef]
- Abbasi, Z.; Feizi, S.; Taghipour, E.; Ghadam, P. Green synthesis of silver nanoparticles using aqueous extract of dried Juglans regia green husk and examination of its biological properties. Green Process. Synth. 2017, 6, 477–485. [Google Scholar] [CrossRef]
- Mittal, A.K.; Bhaumik, J.; Kumar, S.; Banerjee, U.C. Biosynthesis of silver nanoparticles: Elucidation of prospective mechanism and therapeutic potential. J. Colloid Interface Sci. 2014, 415, 39–47. [Google Scholar] [CrossRef]
- Nabikhan, A.; Kandasamy, K.; Raj, A.; Alikunhi, N.M. Synthesis of antimicrobial silver nanoparticles by callus and leaf extracts from saltmarsh plant, Sesuvium portulacastrum L. Colloids Surf. B Biointerfaces 2010, 79, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Duh, P.-D.; Tu, Y.-Y.; Yen, G.-C. Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat). LWT-Food Sci. Technol. 1999, 32, 269–277. [Google Scholar] [CrossRef]
- Slatnar, A.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Solar, A. HPLC-MSn identification and quantification of phenolic compounds in hazelnut kernels, oil and bagasse pellets. Food Res. Int. 2014, 64, 783–789. [Google Scholar] [CrossRef]
- Song, J.Y.; Kim, B.S. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst. Eng. 2009, 32, 79–84. [Google Scholar] [CrossRef]
- Nazeruddin, G.; Prasad, N.; Waghmare, S.; Garadkar, K.; Mulla, I. Extracellular biosynthesis of silver nanoparticle using Azadirachta indica leaf extract and its anti-microbial activity. J. Alloys Compd. 2014, 583, 272–277. [Google Scholar] [CrossRef]
- Gecer, E.N.; Erenler, R.; Temiz, C.; Genc, N.; Yildiz, I. Green synthesis of silver nanoparticles from Echinacea purpurea (L.) Moench with antioxidant profile. Part. Sci. Technol. 2022, 40, 50–57. [Google Scholar] [CrossRef]
- Qu, D.; Sun, W.; Chen, Y.; Zhou, J.; Liu, C. Synthesis and in vitro antineoplastic evaluation of silver nanoparticles mediated by Agrimoniae herba extract. Int. J. Nanomed. 2014, 9, 1871. [Google Scholar]
- Żurek, N.; Pycia, K.; Pawłowska, A.; Potocki, L.; Kapusta, I.T. Chemical Profiling, Bioactive Properties, and Anticancer and Antimicrobial Potential of Juglans regia L. Leaves. Molecules 2023, 28, 1989. [Google Scholar] [CrossRef]
- Kamiński, P.; Tyśkiewicz, K.; Fekner, Z.; Gruba, M.; Kobus, Z. The Influence of Subcritical Water Extraction Parameters on the Chemical Composition and Antioxidant Activity of Walnut (Juglans regia L.) Bark Extracts. Appl. Sci. 2022, 12, 12490. [Google Scholar] [CrossRef]
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.; De, S.P.; Misra, A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf. A Physicochem. Eng. Asp. 2009, 339, 134–139. [Google Scholar] [CrossRef]
- Rao, K.J.; Paria, S. Green synthesis of silver nanoparticles from aqueous Aegle marmelos leaf extract. Mater. Res. Bull. 2013, 48, 628–634. [Google Scholar]
- Ahmad, T. Reviewing the tannic acid mediated synthesis of metal nanoparticles. J. Nanotechnol. 2014, 2014, 954206. [Google Scholar] [CrossRef]
- Anjum, S.; Abbasi, B.H. Thidiazuron-enhanced biosynthesis and antimicrobial efficacy of silver nanoparticles via improving phytochemical reducing potential in callus culture of Linum usitatissimum L. Int. J. Nanomed. 2016, 11, 715. [Google Scholar]
- Kumar, R.; Roopan, S.M.; Prabhakarn, A.; Khanna, V.G.; Chakroborty, S. Agricultural waste Annona squamosa peel extract: Biosynthesis of silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 90, 173–176. [Google Scholar] [CrossRef]
- Mie, R.; Samsudin, M.W.; Din, L.B.; Ahmad, A.; Ibrahim, N.; Adnan, S.N.A. Synthesis of silver nanoparticles with antibacterial activity using the lichen Parmotrema praesorediosum. Int. J. Nanomed. 2014, 9, 121. [Google Scholar] [CrossRef]
- Arokiyaraj, S.; Arasu, M.V.; Vincent, S.; Prakash, N.U.; Choi, S.H.; Oh, Y.-K.; Choi, K.C.; Kim, K.H. Rapid green synthesis of silver nanoparticles from Chrysanthemum indicum L. and its antibacterial and cytotoxic effects: An in vitro study. Int. J. Nanomed. 2014, 9, 379. [Google Scholar] [CrossRef] [PubMed]
- Hawar, S.N.; Al-Shmgani, H.S.; Al-Kubaisi, Z.A.; Sulaiman, G.M.; Dewir, Y.H.; Rikisahedew, J.J. Green synthesis of silver nanoparticles from Alhagi graecorum leaf extract and evaluation of their cytotoxicity and antifungal activity. J. Nanomater. 2022, 2022, 1058119. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Al-Farhan, B.S.; Abou El-ezz, D.; Abd–El Sayed, M.; Zikry, M.M.; Abu-Dief, A.M. Green biogenic synthesis of silver nanoparticles using aqueous extract of moringa oleifera: Access to a powerful antimicrobial, anticancer, pesticidal and catalytic agents. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1422–1435. [Google Scholar] [CrossRef]
- Naik, J.R.; David, M. Green synthesis of silver nanoparticles using Caesalpinia bonducella leaf extract: Characterization and evaluation of in vitro anti-inflammatory and anti-cancer activities. Inorg. Nano-Met. Chem. 2022, 1–11. [Google Scholar] [CrossRef]
- Deivanathan, S.K.; Prakash, J.T.J. Bio-synthesis of silver nanoparticles using leaf extract of Rhaphidophora pertusa and its characterization, antimicrobial, antioxidant and cytotoxicity activities. Res. Chem. Intermed. 2023, 49, 423–439. [Google Scholar] [CrossRef]
- Devanesan, S.; AlSalhi, M.S. Green synthesis of silver nanoparticles using the flower extract of Abelmoschus esculentus for cytotoxicity and antimicrobial studies. Int. J. Nanomed. 2021, 16, 3343. [Google Scholar] [CrossRef] [PubMed]
- Jabir, M.S.; Saleh, Y.M.; Sulaiman, G.M.; Yaseen, N.Y.; Sahib, U.I.; Dewir, Y.H.; Alwahibi, M.; Soliman, D. Green synthesis of silver nanoparticles using Annona muricata extract as an inducer of apoptosis in cancer cells and inhibitor for NLRP3 inflammasome via enhanced autophagy. Nanomaterials 2021, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Suriyakala, G.; Sathiyaraj, S.; Devanesan, S.; AlSalhi, M.S.; Rajasekar, A.; Maruthamuthu, M.K.; Babujanarthanam, R. Phytosynthesis of silver nanoparticles from Jatropha integerrima Jacq. flower extract and their possible applications as antibacterial and antioxidant agent. Saudi J. Biol. Sci. 2022, 29, 680–688. [Google Scholar] [CrossRef]
- Pei, J.; Fu, B.; Jiang, L.; Sun, T. Biosynthesis, characterization, and anticancer effect of plant-mediated silver nanoparticles using Coptis chinensis. Int. J. Nanomed. 2019, 14, 1969. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Hoque, S.M.; Hossain, K.F.B.; Siddique, M.A.B.; Uddin, M.K.; Rahman, M.M. Green synthesis of silver nanoparticles using Hibiscus sabdariffa leaf extract and its cytotoxicity assay. Inorg. Nano-Met. Chem. 2022, 1–11. [Google Scholar] [CrossRef]
- Kapoor, S.; Sood, H.; Saxena, S.; Chaurasia, O.P. Green synthesis of silver nanoparticles using Rhodiola imbricata and Withania somnifera root extract and their potential catalytic, antioxidant, cytotoxic and growth-promoting activities. Bioprocess Biosyst. Eng. 2022, 45, 365–380. [Google Scholar] [CrossRef]
- Sengottaiyan, A.; Mythili, R.; Selvankumar, T.; Aravinthan, A.; Kamala-Kannan, S.; Manoharan, K.; Thiyagarajan, P.; Govarthanan, M.; Kim, J.-H. Green synthesis of silver nanoparticles using Solanum indicum L. and their antibacterial, splenocyte cytotoxic potentials. Res. Chem. Intermed. 2016, 42, 3095–3103. [Google Scholar] [CrossRef]
- Rizwana, H.; Alwhibi, M.S.; Al-Judaie, R.A.; Aldehaish, H.A.; Alsaggabi, N.S. Sunlight-mediated green synthesis of silver nanoparticles using the berries of Ribes rubrum (red currants): Characterisation and evaluation of their antifungal and antibacterial activities. Molecules 2022, 27, 2186. [Google Scholar] [CrossRef]
- Shameli, K.; Bin Ahmad, M.; Jaffar Al-Mulla, E.A.; Ibrahim, N.A.; Shabanzadeh, P.; Rustaiyan, A.; Abdollahi, Y.; Bagheri, S.; Abdolmohammadi, S.; Usman, M.S.; et al. Green biosynthesis of silver nanoparticles using Callicarpa maingayi stem bark extraction. Molecules 2012, 17, 8506–8517. [Google Scholar] [CrossRef]
- Gogoi, N.; Babu, P.J.; Mahanta, C.; Bora, U. Green synthesis and characterization of silver nanoparticles using alcoholic flower extract of Nyctanthes arbortristis and in vitro investigation of their antibacterial and cytotoxic activities. Mater. Sci. Eng. C 2015, 46, 463–469. [Google Scholar] [CrossRef]
- Tran, Q.H.; Le, A.-T. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 033001. [Google Scholar] [CrossRef]
- Nahar, K.; Rahaman, M.; Khan, G.; Islam, M.; Al-Reza, S.M. Green synthesis of silver nanoparticles from Citrus sinensis peel extract and its antibacterial potential. Asian J. Green Chem. 2021, 5, 135–150. [Google Scholar]
- Fouda, A.; Awad, M.A.; Al-Faifi, Z.E.; Gad, M.E.; Al-Khalaf, A.A.; Yahya, R.; Hamza, M.F. Aspergillus flavus-mediated green synthesis of silver nanoparticles and evaluation of their antibacterial, anti-candida, acaricides, and photocatalytic activities. Catalysts 2022, 12, 462. [Google Scholar] [CrossRef]
- Oves, M.; Rauf, M.A.; Aslam, M.; Qari, H.A.; Sonbol, H.; Ahmad, I.; Zaman, G.S.; Saeed, M. Green synthesis of silver nanoparticles by Conocarpus lancifolius plant extract and their antimicrobial and anticancer activities. Saudi J. Biol. Sci. 2022, 29, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Changez, M.; Anwar, M.F.; Al-Ghenaime, S.; Kapoor, S.; Al Balushi, R.; Chaudhuri, A. Synergic effect of aqueous extracts of Ocimum sanctum and Trigonella foenum-graecum L on the in situ green synthesis of silver nanoparticles and as a preventative agent against antibiotic-resistant food spoiling organisms. RSC Adv. 2022, 12, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Abbasi, B.H. Biomimetic synthesis of antimicrobial silver nanoparticles using in vitro-propagated plantlets of a medicinally important endangered species: Phlomis bracteosa. Int. J. Nanomed. 2016, 11, 1663. [Google Scholar]
- Carvalho, P.M.; Felício, M.R.; Santos, N.C.; Gonçalves, S.; Domingues, M.M. Application of light scattering techniques to nanoparticle characterization and development. Front. Chem. 2018, 6, 237. [Google Scholar] [CrossRef]
- Parvataneni, R. Biogenic synthesis and characterization of silver nanoparticles using aqueous leaf extract of Scoparia dulcis L. and assessment of their antimicrobial property. Drug Chem. Toxicol. 2020, 43, 307–321. [Google Scholar] [CrossRef]
- Heydari, R.; Rashidipour, M. Green synthesis of silver nanoparticles using extract of oak fruit hull (Jaft): Synthesis and in vitro cytotoxic effect on MCF-7 cells. Int. J. Breast Cancer 2015, 2015, 846743. [Google Scholar] [CrossRef]
- Hulikere, M.M.; Joshi, C.G. Characterization, antioxidant and antimicrobial activity of silver nanoparticles synthesized using marine endophytic fungus-Cladosporium cladosporioides. Process Biochem. 2019, 82, 199–204. [Google Scholar] [CrossRef]
- Patil, S.V.; Borase, H.P.; Patil, C.D.; Salunke, B.K. Biosynthesis of silver nanoparticles using latex from few euphorbian plants and their antimicrobial potential. Appl. Biochem. Biotechnol. 2012, 167, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Jebril, S.; Jenana, R.K.B.; Dridi, C. Green synthesis of silver nanoparticles using Melia azedarach leaf extract and their antifungal activities: In vitro and in vivo. Mater. Chem. Phys. 2020, 248, 122898. [Google Scholar] [CrossRef]
- Raja, S.; Ramesh, V.; Thivaharan, V. Green biosynthesis of silver nanoparticles using Calliandra haematocephala leaf extract, their antibacterial activity and hydrogen peroxide sensing capability. Arab. J. Chem. 2017, 10, 253–261. [Google Scholar] [CrossRef]
- Antony, J.J.; Sithika, M.A.A.; Joseph, T.A.; Suriyakalaa, U.; Sankarganesh, A.; Siva, D.; Kalaiselvi, S.; Achiraman, S. In vivo antitumor activity of biosynthesized silver nanoparticles using Ficus religiosa as a nanofactory in DAL induced mice model. Colloids Surf. B Biointerfaces 2013, 108, 185–190. [Google Scholar] [CrossRef]
- Ibrahim, E.; Zhang, M.; Zhang, Y.; Hossain, A.; Qiu, W.; Chen, Y.; Wang, Y.; Wu, W.; Sun, G.; Li, B. Green-synthesization of silver nanoparticles using endophytic bacteria isolated from garlic and its antifungal activity against wheat Fusarium head blight pathogen Fusarium graminearum. Nanomaterials 2020, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Bocate, K.P.; Reis, G.F.; de Souza, P.C.; Junior, A.G.O.; Durán, N.; Nakazato, G.; Furlaneto, M.C.; de Almeida, R.S.; Panagio, L.A. Antifungal activity of silver nanoparticles and simvastatin against toxigenic species of Aspergillus. Int. J. Food Microbiol. 2019, 291, 79–86. [Google Scholar] [CrossRef]
- Saleh, Z.A.; El-Garawany, G.A.; Assem, F.; El-Shibiny, S. Evaluation of the efficacy of whey protein to ameliorate the toxic effects of aflatoxins in rats. Int. Dairy J. 2007, 17, 854–859. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, M.Z.; Khan, A.; Javed, I.; Asi, M.R. Toxico-pathological effects in rats induced by concurrent exposure to aflatoxin and cypermethrin. Toxicon 2009, 53, 33–41. [Google Scholar] [CrossRef]
- Hassan, S.A.; Mujahid, H.; Ali, M.M.; Irshad, S.; Naseer, R.; Saeed, S.; Firyal, S.; Arooj, F. Synthesis, characterization and protective effect of green tea-mediated zinc oxide nanoparticles against ochratoxin A induced hepatotoxicity and nephrotoxicity in albino rats. Appl. Nanosci. 2021, 11, 2281–2289. [Google Scholar] [CrossRef]
- Witeska, M.; Jezierska, B.; Wolnicki, J. Respiratory and hematological response of tench, Tinca tinca (L.) to a short-term cadmium exposure. Aquac. Int. 2006, 14, 141–152. [Google Scholar] [CrossRef]
- Sampathkumar, P.; Kalavathy, S.; Anand, A.V.; Sangeetha, T.; Sujeetha, P. Impact of Tridax procumbens on Hematological Parameters in aflatoxin Induced Liver Toxicity in Albino Rats. Pharm. J. 2018, 10, 304–308. [Google Scholar] [CrossRef]
- Singh, C.; Prakash, C.; Mishra, P.; Tiwari, K.N.; Mishra, S.K.; More, R.S.; Kumar, V.; Singh, J. Hepatoprotective efficacy of Premna integrifolia L. leaves against aflatoxin B1-induced toxicity in mice. Toxicon 2019, 166, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Kececi, T.; Oguz, H.; Kurtoglu, V.; Demet, O. Effects of polyvinylpolypyrrolidone, synthetic zeolite and bentonite on serum biochemical and haematological characters of broiler chickens during aflatoxicosis. Br. Poult. Sci. 1998, 39, 452–458. [Google Scholar] [CrossRef]
- Oguz, H.; Kececi, T.; Birdane, Y.; Önder, F.; Kurtoglu, V. Effect of clinoptilolite on serum biochemical and haematological characters of broiler chickens during aflatoxicosis. Res. Vet. Sci. 2000, 69, 89–93. [Google Scholar] [CrossRef]
- Gholami-Ahangaran, M.; Zia-Jahromi, N. Effect of nanosilver on blood parameters in chickens having aflatoxicosis. Toxicol. Ind. Health 2014, 30, 192–196. [Google Scholar] [PubMed]
- Ozer, J.; Ratner, M.; Shaw, M.; Bailey, W.; Schomaker, S. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008, 245, 194–205. [Google Scholar] [CrossRef]
- Brinda, R.; Vijayanandraj, S.; Uma, D.; Malathi, D.; Paranidharan, V.; Velazhahan, R. Role of Adhatoda vasica (L.) Nees leaf extract in the prevention of aflatoxin-induced toxicity in Wistar rats. J. Sci. Food Agric. 2013, 93, 2743–2748. [Google Scholar] [CrossRef]
- Eftekhari, A.; Ahmadian, E.; Panahi-Azar, V.; Hosseini, H.; Tabibiazar, M.; Maleki Dizaj, S. Hepatoprotective and free radical scavenging actions of quercetin nanoparticles on aflatoxin B1-induced liver damage: In vitro/in vivo studies. Artif. Cells Nanomed. Biotechnol. 2018, 46, 411–420. [Google Scholar] [PubMed]
- Ismaiel, A.A.; El-Denshary, E.S.; El-Nekeety, A.A.; Al-Yamani, A.; Gad, S.; Hassan, N.S.; Abdel-Wahhab, M.A. Ameliorative effects of curcumin nanoparticles on hepatotoxicity induced by zearalenone mycotoxin. Glob. J. Pharmacol. 2015, 9, 234–245. [Google Scholar]
- Alamri, E.S.; El Rabey, H.A.; Alzahrani, O.R.; Almutairi, F.M.; Attia, E.S.; Bayomy, H.M.; Albalwi, R.A.; Rezk, S.M. Enhancement of the Protective Activity of Vanillic Acid against Tetrachloro-Carbon (CCl4) Hepatotoxicity in Male Rats by the Synthesis of Silver Nanoparticles (AgNPs). Molecules 2022, 27, 8308. [Google Scholar] [CrossRef]
- El-Mahalaway, A.M. Protective effect of curcumin against experimentally induced aflatoxicosis on the renal cortex of adult male albino rats: A histological and immunohisochemical study. Int. J. Clin. Exp. Pathol. 2015, 8, 6019. [Google Scholar] [PubMed]
- Abdel-Wahhab, M.A.; Salman, A.S.; Ibrahim, M.I.; El-Kady, A.A.; Abdel-Aziem, S.H.; Hassan, N.S.; Waly, A.I. Curcumin nanoparticles loaded hydrogels protects against aflatoxin B1-induced genotoxicity in rat liver. Food Chem. Toxicol. 2016, 94, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Biruntha, M.; Selvi, B.K.; Paul, J.A.J.; Sivakumar, P.; Rajamanikandan, S.; Prabhu, D. Evaluation of hepato and renal protective effect of synthesized nanoparticles using Tinospora cordifolia leaf extract. Mater. Lett. 2022, 312, 131642. [Google Scholar] [CrossRef]
- Edrington, T.; Kamps-Holtzapple, C.; Harvey, R.; Kubena, L.; Elissalde, M.; Rottinghaus, G. Acute hepatic and renal toxicity in lambs dosed with fumonisin-containing culture material. J. Anim. Sci. 1995, 73, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahhab, M.A.; Hassan, N.S.; El-Kady, A.A.; Khadrawy, Y.A.; El-Nekeety, A.A.; Mohamed, S.R.; Sharaf, H.A.; Mannaa, F.A. Red ginseng extract protects against aflatoxin B1 and fumonisins-induced hepatic pre-cancerous lesions in rats. Food Chem. Toxicol. 2010, 48, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Atef, H.A.; Mansour, M.K.; Ibrahim, E.M.; Sayed El-Ahl, R.M.; Al-Kalamawey, N.M.; El Kattan, Y.A.; Ali, M.A. Efficacy of zinc oxide nanoparticles and curcumin in amelioration the toxic effects in aflatoxicated rabbits. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 795–818. [Google Scholar] [CrossRef]
- El-Nekeety, A.A.; Mohamed, S.R.; Hathout, A.S.; Hassan, N.S.; Aly, S.E.; Abdel-Wahhab, M.A. Antioxidant properties of Thymus vulgaris oil against aflatoxin-induce oxidative stress in male rats. Toxicon 2011, 57, 984–991. [Google Scholar] [CrossRef]
- Meki, A.-R.M.; Abdel-Ghaffar, S.K.; El-Gibaly, I. Aflatoxin B1 induces apoptosis in rat liver: Protective effect of melatonin. Neuroendocrinol. Lett. 2001, 22, 417–426. [Google Scholar]
- Sharmila Banu, G.; Kumar, G.; Murugesan, A. Effect of ethanolic leaf extract of Trianthema portulacastrum L. on aflatoxin induced hepatic damage in rats. Indian J. Clin. Biochem. 2009, 24, 414–418. [Google Scholar] [CrossRef]
- Milićević, D.R.; Jurić, V.B.; Daković, A.; Jovanović, M.; Stefanović, S.; Petrović, Z.I. Mycotoxic porcine nephropathy and spontaneous occurrence of ochratoxin a residues in kidneys of slaughtered swine. Matica Srp. Proc. Nat. Sci. 2009, 81–90. [Google Scholar] [CrossRef]
- Morsy, F.A.; el Din, A.A.G.; Farrag, A.R.H.; Badawi, M.A.; Shaffie, N.M. Ochratoxin a toxic effect on rat kidneys and the potential protective effect of ginseng: Histopathologic, histochemical, and image analysis morphometric studies. Maced. J. Med. Sci. 2012, 5, 40–48. [Google Scholar] [CrossRef]
- Omar, N.A. Effect of some aflatoxins on a lymphatic organ (spleen) of male albino rats (histopathological study). Egypt. J. Hosp. Med. 2012, 48, 357–367. [Google Scholar] [CrossRef]
- Hinton, D.M.; Myers, M.J.; Raybourne, R.A.; Francke-Carroll, S.; Sotomayor, R.E.; Shaddock, J.; Warbritton, A.; Chou, M.W. Immunotoxicity of aflatoxin B1 in rats: Effects on lymphocytes and the inflammatory response in a chronic intermittent dosing study. Toxicol. Sci. 2003, 73, 362–377. [Google Scholar] [CrossRef] [PubMed]
- Gaylor, D.W.; Kadlubar, F.F.; Beland, F.A. Application of biomarkers to risk assessment. Environ. Health Perspect. 1992, 98, 139–141. [Google Scholar] [CrossRef]
- Kumar, R.; Balachandran, C. Histopathological changes in broiler chickens fed aflatoxin and cyclopiazonic acid. Vet. Arh. 2009, 79, 31–40. [Google Scholar]
- Peng, X.; Zhang, K.; Bai, S.; Ding, X.; Zeng, Q.; Yang, J.; Fang, J.; Chen, K. Histological lesions, cell cycle arrest, apoptosis and T cell subsets changes of spleen in chicken fed aflatoxin-contaminated corn. Int. J. Environ. Res. Public Health 2014, 11, 8567–8580. [Google Scholar] [CrossRef] [PubMed]
- Biró, K.; Solti, L.; Barna-Vetró, I.; Bagó, G.; Glávits, R.; Szabó, E.; Fink-Gremmels, J. Tissue distribution of ochratoxin A as determined by HPLC and ELISA and histopathological effects in chickens. Avian Pathol. 2002, 31, 141–148. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver nanoparticles: Mechanism of action and probable bio-application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.H.; Saied, E.; Amin, B.H.; Alotibi, F.O.; Al-Askar, A.A.; Arishi, A.A.; Elkady, F.M.; Elbahnasawy, M.A. Antifungal activity of biosynthesized silver nanoparticles (AgNPs) against aspergilli causing aspergillosis: Ultrastructure Study. J. Funct. Biomater. 2022, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Zahoor, I.; Baba, T.R.; Padder, S.A.; Bhat, Z.; Koul, A.M.; Jiang, L. Fabrication of silver nanoparticles against fungal pathogens. Front. Nanotechnol. 2021, 3, 679358. [Google Scholar] [CrossRef]
- Win, T.T.; Khan, S.; Fu, P. Fungus-(Alternaria sp.) mediated silver nanoparticles synthesis, characterization, and screening of antifungal activity against some phytopathogens. J. Nanotechnol. 2020, 2020, 8828878. [Google Scholar] [CrossRef]
- Xiang, S.; Ma, X.; Shi, H.; Ma, T.; Tian, C.; Chen, Y.; Chen, H.; Chen, X.; Luo, K.; Cai, L.; et al. Green synthesis of an alginate-coated silver nanoparticle shows high antifungal activity by enhancing its cell membrane penetrating ability. ACS Appl. Bio Mater. 2019, 2, 4087–4096. [Google Scholar] [CrossRef]
- Mirzajani, F.; Askari, H.; Hamzelou, S.; Farzaneh, M.; Ghassempour, A. Effect of silver nanoparticles on Oryza sativa L. and its rhizosphere bacteria. Ecotoxicol. Environ. Saf. 2013, 88, 48–54. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, L.; Xu, D.; Lu, Z. Involvement of ROS in nanosilver-caused suppression of aflatoxin production from Aspergillus flavus. RSC Adv. 2017, 7, 23021–23026. [Google Scholar] [CrossRef]
- Ameen, F.; Al-Homaidan, A.A.; Al-Sabri, A.; Almansob, A.; AlNAdhari, S. Anti-oxidant, anti-fungal and cytotoxic effects of silver nanoparticles synthesized using marine fungus Cladosporium halotolerans. Appl. Nanosci. 2023, 13, 623–631. [Google Scholar] [CrossRef]
- Ganash, M.; Abdel Ghany, T.; Omar, A. Morphological and biomolecules dynamics of phytopathogenic fungi under stress of silver nanoparticles. BioNanoScience 2018, 8, 566–573. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, G.; Zeng, G.; Guo, Z.; He, K.; Hu, L.; Wu, J.; Zhang, L.; Zhu, Y.; Song, Z. Toxicity mechanisms and synergies of silver nanoparticles in 2, 4-dichlorophenol degradation by Phanerochaete chrysosporium. J. Hazard. Mater. 2017, 321, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Roychoudhury, A. Go green to protect plants: Repurposing the antimicrobial activity of biosynthesized silver nanoparticles to combat phytopathogens. Nanotechnol. Environ. Eng. 2021, 6, 10. [Google Scholar] [CrossRef]





| Aflatoxin (ng/mL) | Group A | Group B | Group C | Group D | Group E | Group F | Group G | Group H |
|---|---|---|---|---|---|---|---|---|
| G1 (ng/mL) | 363 ± 3.06 | 333 ± 7.57 | 336 ± 11.5 | 319 ± 8.00 | 307 ± 7.64 | 175 ± 3.06 | 374 ± 4.04 | 401 ± 9.02 |
| B1 (ng/mL) | 9.74 ± 0.541 | 8.55 ± 0.415 | 5.68 ± 0.555 | 4.71 ± 0.536 | 1.56 ± 0.555 | Absent | Absent | Absent |
| G2 (ng/mL) | 54.4 ± 3.95 | 35.4 ± 4.44 | 32.7 ± 2.67 | 24.4 ± 3.50 | Absent | Absent | Absent | Absent |
| Group | Rat Weight (g) | Feed Consumed (g) | FCR |
|---|---|---|---|
| Group A (Control) | 650 ± 3.00 c | 720 ± 3.00 c | 1.10 ± 0.01 a,b |
| Group B (Toxin) | 627 ± 2.00 e | 642 ± 3.00 e | 1.02 ± 0.01 c |
| Group C (Toxin + 20 µg AgNPs) | 670 ± 2.00 b | 730 ± 1.00 b | 1.08 ± 0.02 b |
| Group D (Toxin + 50 µg AgNPs) | 692 ± 2.00 a | 770 ± 2.00 a | 1.11 ± 0.02 a |
| Group E (Toxin + 70 µg AgNPs) | 640 ± 2.00 d | 657 ± 2.00 d | 1.02 ± 0.01 c |
| Groups | RBCs (×1012/L) | WBCs (×109/L) | Hb (g/dL) | HCT (%) | MCV (fL) | MCH (g/dt) | Platelets (×109/L) |
| Group A (Control) | 7.9 ± 0.10 a | 13.4 ± 0.20 a | 13.1 ± 0.20 a | 45 ± 2.00 a | 61 ± 1.00 a | 22 ± 2.00 a | 565 ± 1.00 a |
| Group B (Toxin) | 6.3 ± 0.20 c,d | 2.5 ± 0.10 e | 9.6 ± 0.20 e | 34 ± 2.00 c | 55 ± 2.00 b | 18 ± 1.00 b | 200 ± 2.00 e |
| Group C (Toxin + 20 µg AgNPs) | 6.5 ± 0.20 c | 10.8 ± 0.10 c | 10.9 ± 0.20 c | 37 ± 1.00 b,c | 56 ± 2.00 b | 18 ± 2.00 b | 413 ± 1.00 c |
| Group D (Toxin + 50 µg AgNPs) | 7.2 ± 0.10 b | 11.2 ± 0.20 b | 11.9 ± 0.10 b | 40 ± 3.00 b | 58 ± 2.00 a,b | 20 ± 2.00 a,b | 522 ± 2.00 b |
| Group E (Toxin + 70 µg AgNPs) | 6.0 ± 0.20 d | 6.7 ± 0.20 d | 10.1 ± 0.30 d | 35 ± 2.00 c | 55 ± 2.00 b | 18 ± 1.00 b | 400 ± 3.00 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naqvi, S.I.Z.; Kausar, H.; Afzal, A.; Hashim, M.; Mujahid, H.; Javed, M.; Hano, C.; Anjum, S. Antifungal Activity of Juglans-regia-Mediated Silver Nanoparticles (AgNPs) against Aspergillus-ochraceus-Induced Toxicity in In Vitro and In Vivo Settings. J. Funct. Biomater. 2023, 14, 221. https://doi.org/10.3390/jfb14040221
Naqvi SIZ, Kausar H, Afzal A, Hashim M, Mujahid H, Javed M, Hano C, Anjum S. Antifungal Activity of Juglans-regia-Mediated Silver Nanoparticles (AgNPs) against Aspergillus-ochraceus-Induced Toxicity in In Vitro and In Vivo Settings. Journal of Functional Biomaterials. 2023; 14(4):221. https://doi.org/10.3390/jfb14040221
Chicago/Turabian StyleNaqvi, Syeda Itrat Zahra, Humera Kausar, Arooj Afzal, Mariam Hashim, Huma Mujahid, Maryam Javed, Christophe Hano, and Sumaira Anjum. 2023. "Antifungal Activity of Juglans-regia-Mediated Silver Nanoparticles (AgNPs) against Aspergillus-ochraceus-Induced Toxicity in In Vitro and In Vivo Settings" Journal of Functional Biomaterials 14, no. 4: 221. https://doi.org/10.3390/jfb14040221
APA StyleNaqvi, S. I. Z., Kausar, H., Afzal, A., Hashim, M., Mujahid, H., Javed, M., Hano, C., & Anjum, S. (2023). Antifungal Activity of Juglans-regia-Mediated Silver Nanoparticles (AgNPs) against Aspergillus-ochraceus-Induced Toxicity in In Vitro and In Vivo Settings. Journal of Functional Biomaterials, 14(4), 221. https://doi.org/10.3390/jfb14040221









