Utilization of Stimuli-Responsive Biomaterials in the Formulation of Cancer Vaccines
Abstract
:1. Introduction
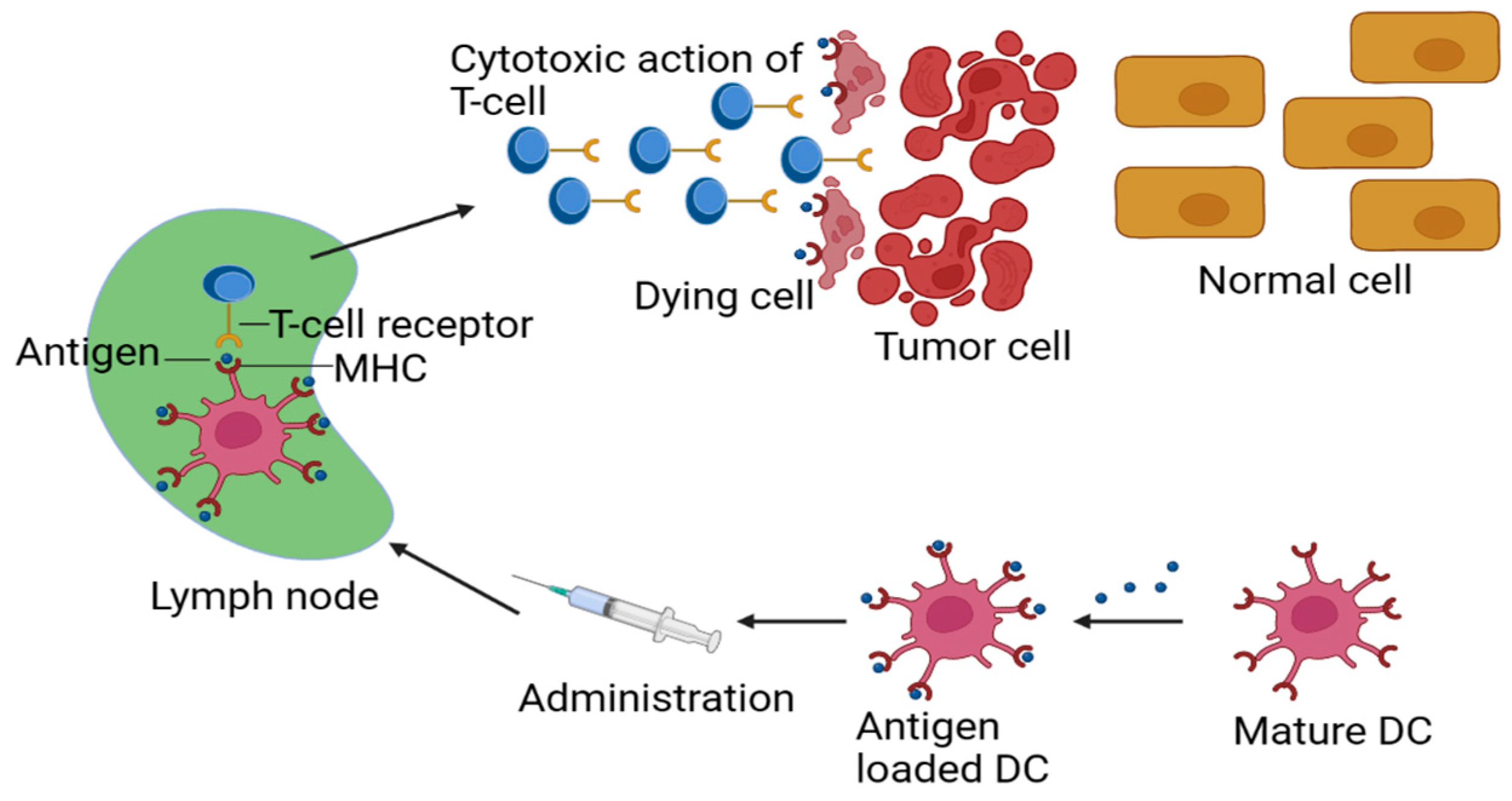
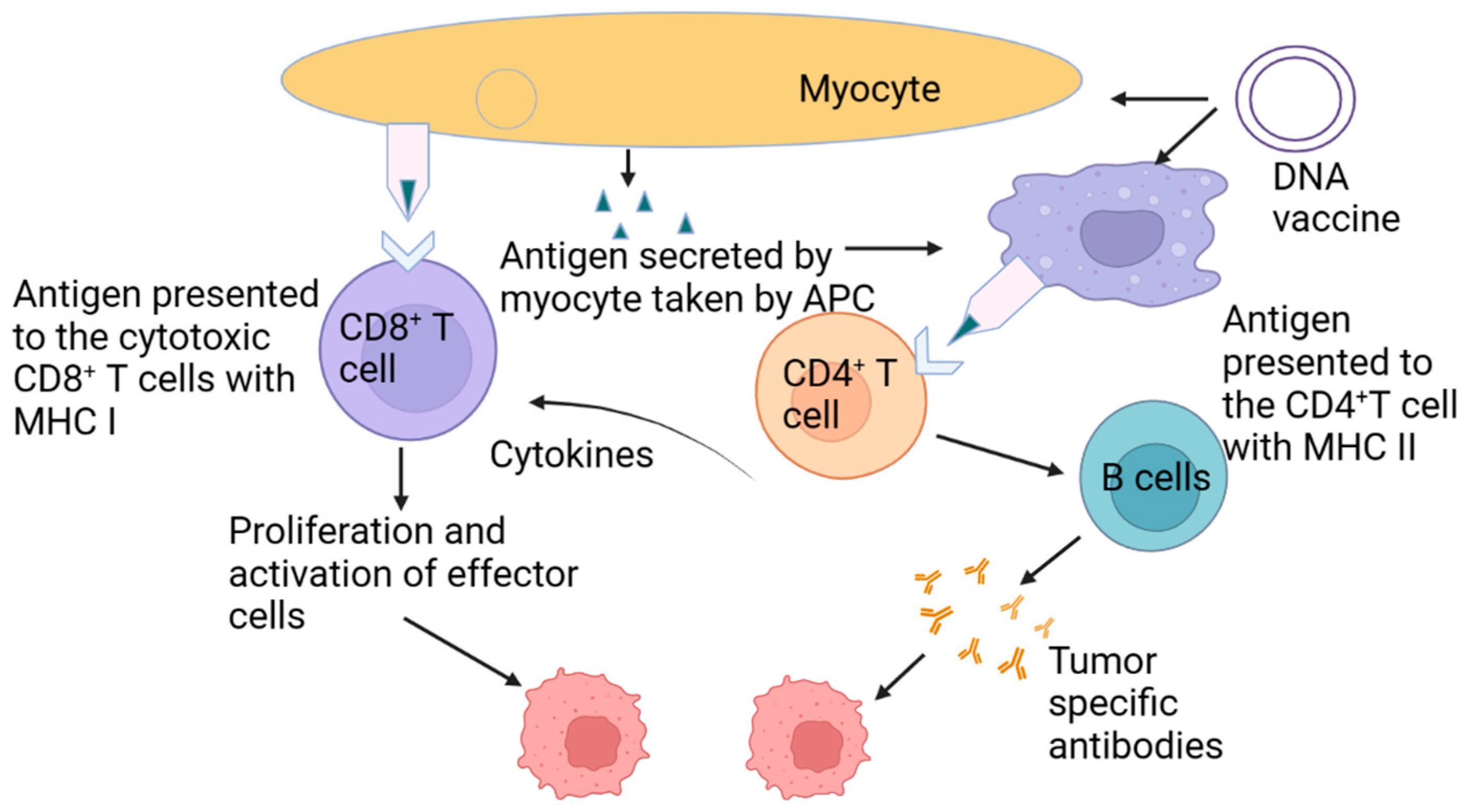
2. Designing an Effective Cancer Vaccine
3. Using Stimuli-Responsive Biomaterials to Manage the Distribution of the Cancer Vaccine
4. Responsive Biomaterials Improvement of LN and APC Targeting
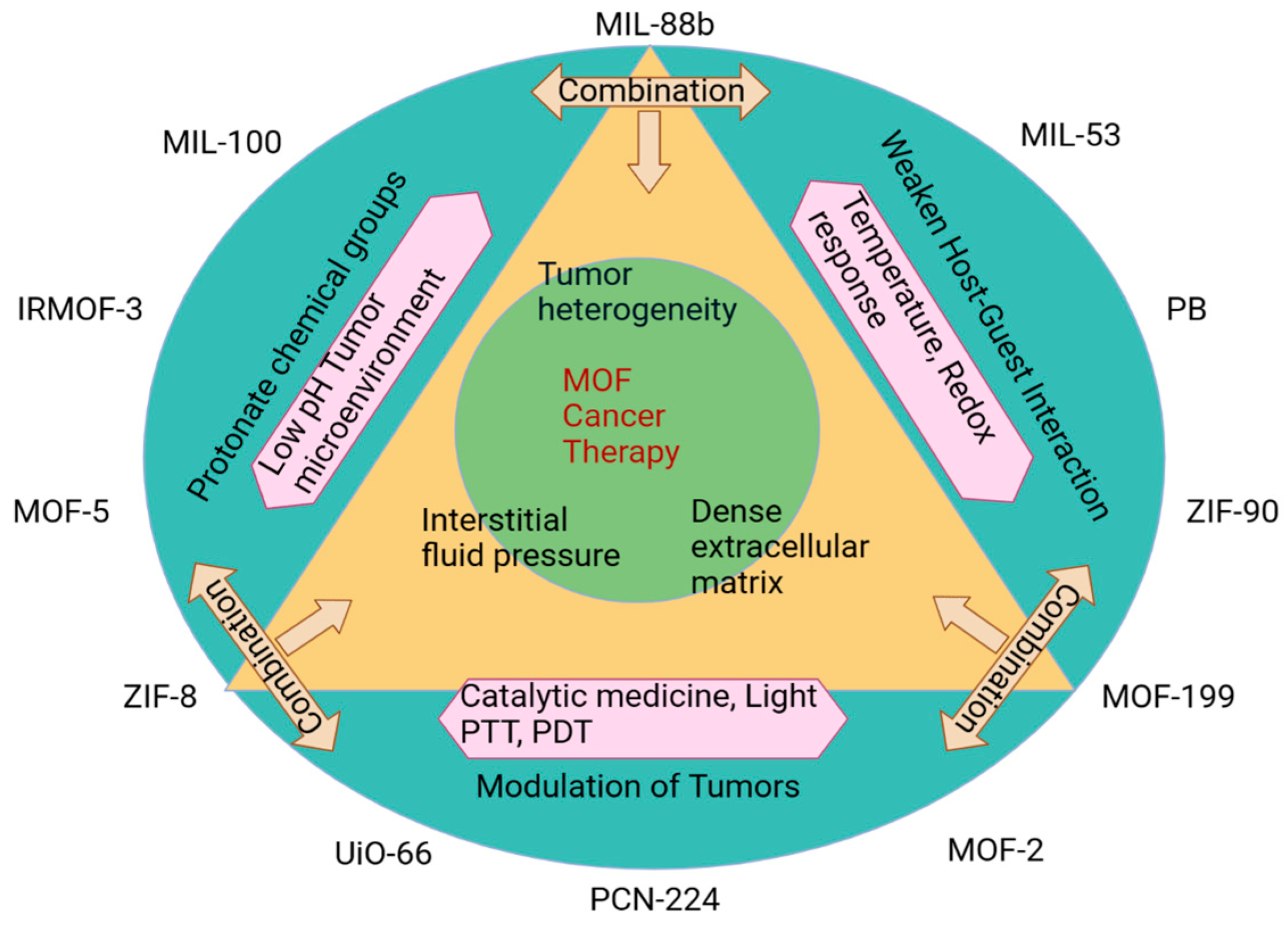
5. pH-Responsive Vaccine Delivery System
5.1. Acid-Labile Biomaterials with the Ability to Change pH
5.2. Acid-Triggered Phase Transition-Based pH-Responsive Biomaterials
5.3. pH-Responsive Biomaterials for “Proton Sponge” Effect
5.4. Other pH-Responsive Biomaterials
6. Redox-Responsive Vaccine Delivery System
7. Light-Responsive Vaccine Delivery System
8. Molecular Recognition-Responsive Materials
9. Magnetic Responsive Materials
10. Other Responsive Vaccine Delivery Systems
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melero, I.; Gaudernack, G.; Gerritsen, W.; Huber, C.; Parmiani, G.; Scholl, S.; Thatcher, N.; Wagstaff, J.; Zielinski, C.; Faulkner, I.; et al. Therapeutic vaccines for cancer: An overview of clinical trials. Nat. Rev. Clin. Oncol. 2014, 11, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Cheever, M.A.; Higano, C.S. PROVENGE (sipuleucel-T) in prostate cancer: The first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. 2011, 17, 3520–3526. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Türeci, Ö. Personalized vaccines for cancer immunotherapy. Science 2018, 359, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.Z.; Zhang, F.W.; Ni, Q.Q.; Niu, G.; Chen, X.Y. Efficient nanovaccine delivery in cancer immunotherapy. ACS Nano 2017, 11, 2387–2392. [Google Scholar] [CrossRef]
- Lu, Z.-R.; Qiao, P. Drug delivery in cancer therapy, Quo Vadis? Mol. Pharm. 2018, 15, 3603–3616. [Google Scholar] [CrossRef]
- Langer, R. Drug delivery and targeting. Nature 1998, 392, 5–10. [Google Scholar]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in biomaterials for drug delivery. Adv. Mater. 2018, 30, 1705328. [Google Scholar] [CrossRef]
- Luo, Z.C.; Wu, Q.J.; Yang, C.B.; Wang, H.M.; He, T.; Wang, Y.Z.; Wang, Z.Y.; Chen, H.; Li, X.Y.; Gong, C.Y.; et al. A powerful CD8+ T-cell stimulating D-tetra-peptide hydrogel as a very promising vaccine adjuvant. Adv. Mater. 2017, 29, 1601776. [Google Scholar] [CrossRef]
- Irvine, D.J.; Hanson, M.C.; Rakhra, K.; Tokatlian, T. Synthetic nanoparticles for vaccines and immunotherapy. Chem. Rev. 2015, 115, 11109–11146. [Google Scholar] [CrossRef]
- Mehta, N.K.; Moynihan, K.D.; Irvine, D.J. Engineering new approaches to cancer vaccines. Cancer Immunol. Res. 2015, 3, 836–843. [Google Scholar] [CrossRef]
- Guo, Y.G.; Lei, K.W.; Tang, L. Neoantigen vaccine delivery for personalized anticancer immunotherapy. Front. Immunol. 2018, 9, 1499. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Aimetti, A.A.; Langer, R.; Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2016, 2, 16075. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Nakayama, M.; Akimoto, J.; Okano, T. Polymeric micelles with stimuli-triggering systems for advanced cancer drug targeting. J. Drug Target. 2014, 22, 584–599. [Google Scholar] [CrossRef]
- Pardoll, D.M. Cancer vaccines. Nature 1998, 4, 525–531. [Google Scholar]
- van der Burg, S.H.; Arens, R.; Ossendorp, F.; van Hall, T.; Melief, C.J.M. Vaccines for established cancer: Overcoming the challenges posed by immune evasion. Nat. Rev. Cancer 2016, 16, 219–233. [Google Scholar] [CrossRef]
- Halle, S.; Halle, O.; Förster, R. Mechanisms and dynamics of T cell-mediated cytotoxicity in vivo. Trends Immunol. 2017, 38, 432–443. [Google Scholar] [CrossRef]
- Garrido, F.; Aptsiauri, N.; Doorduijn, E.M.; Garcia Lora, A.M.; van Hall, T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr. Opin. Immunol. 2016, 39, 44–51. [Google Scholar] [CrossRef]
- Neefjes, J.; Jongsma, M.L.M.; Paul, P.; Bakke, O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 2011, 11, 823–836. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Sahdev, P.; Ochyl, L.J.; Moon, J.J. Biomaterials for nanoparticle vaccine delivery systems. Pharm. Res. 2014, 31, 2563–2582. [Google Scholar] [CrossRef] [PubMed]
- Supersaxo, A.; Hein, W.R.; Steffen, H. Effect of molecular weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm. Res. 1990, 7, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Kaminskas, L.M.; Porter, C.J.H. Targeting the lymphatics using dendritic polymers (dendrimers). Adv. Drug Deliv. Rev. 2011, 63, 890–900. [Google Scholar] [CrossRef]
- Reddy, S.T.; Rehor, A.; Schmoekel, H.G.; Hubbell, J.A.; Swartz, M.A. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J. Control. Release 2006, 112, 26–34. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Wang, D.; Song, Q.L.; Wu, T.T.; Zhuang, X.T.; Bao, Y.L.; Kong, M.; Qi, Y.; Tan, S.W.; Zhang, Z.P. Erythrocyte membrane-enveloped polymeric nanoparticles as nanovaccine for induction of antitumor immunity against melanoma. ACS Nano 2015, 9, 6918–6933. [Google Scholar] [CrossRef]
- Wang, C.; Ye, Y.Q.; Hu, Q.Y.; Bellotti, A.; Gu, Z. Tailoring biomaterials for cancer immunotherapy: Emerging trends and future outlook. Adv. Mater. 2017, 29, 1606036. [Google Scholar] [CrossRef]
- Lynn, G.M.; Laga, R.; Darrah, P.A.; Ishizuka, A.S.; Balaci, A.J.; Dulcey, A.E.; Pechar, M.; Pola, R.; Gerner, M.Y.; Yamamoto, A.; et al. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat. Biotechnol. 2015, 33, 1201–1210. [Google Scholar] [CrossRef]
- Kwon, Y.J.; James, E.; Shastri, N.; Fréchet, J.M.J. In vivo targeting of dendritic cells for activation of cellular immunity using vaccine carriers based on pH-responsive microparticles. Proc. Natl. Acad. Sci. USA 2005, 102, 18264–18268. [Google Scholar] [CrossRef]
- Duan, F.; Feng, X.C.; Yang, X.J.; Sun, W.T.; Jin, Y.; Liu, H.F.; Ge, K.; Li, Z.H.; Zhang, J.C. A simple and powerful co-delivery system based on pH-responsive metal-organic frameworks for enhanced cancer immunotherapy. Biomaterials 2017, 122, 23–33. [Google Scholar] [CrossRef]
- Foster, S.; Duvall, C.L.; Crownover, E.F.; Hoffman, A.S.; Stayton, P.S. Intracellular delivery of a protein antigen with an endosomal-releasing polymer enhances CD8 T-cell production and prophylactic vaccine efficacy. Bioconjug. Chem. 2010, 21, 2205–2212. [Google Scholar] [CrossRef]
- Wilson, J.T.; Keller, S.; Manganiello, M.J.; Cheng, C.; Lee, C.C.; Opara, C.; Convertine, A.; Stayton, P.S. pHresponsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides. ACS Nano 2013, 7, 3912–3925. [Google Scholar] [CrossRef]
- Yuba, E.; Kono, Y.; Harada, A.; Yokoyama, S.; Arai, M.; Kubo, K.; Kono, K. The application of pH-sensitive polymer-lipids to antigen delivery for cancer immunotherapy. Biomaterials 2013, 34, 5711–5721. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, X.M.; Jia, J.L.; Zhang, W.F.; Yang, T.Y.; Wang, L.Y.; Ma, G.H. pH-responsive poly(D,L-lactic-coglycolic acid) nanoparticles with rapid antigen release behavior promote immune response. ACS Nano 2015, 9, 4925–4938. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Wang, H.; Wang, Z.H.; Cai, H.C.; Lu, Z.G.; Li, Y.; Du, M.J.; Huang, G.; Wang, C.S.; Chen, X.; et al. A STING-activating nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 2017, 12, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Morishita, M.; Takahashi, Y.; Nishikawa, M.; Ariizumi, R.; Takakura, Y. Enhanced class I tumor antigen presentation via cytosolic delivery of exosomal cargos by tumor-cell-derived exosomes displaying a pH-sensitive fusogenic peptide. Mol. Pharm. 2017, 14, 4079–4086. [Google Scholar] [CrossRef]
- Eby, J.K.; Dane, K.Y.; O’Neil, C.P.; Hirosue, S.; Swartz, M.A.; Hubbell, J.A. Polymer micelles with pyridyl disulfide-coupled antigen travel through lymphatics and show enhanced cellular responses following immunization. Acta Biomater. 2012, 8, 3210–3217. [Google Scholar] [CrossRef]
- Mochizuki, S.; Morishita, H.; Sakurai, K. Complex consisting of β-glucan and antigenic peptides with cleavage site for glutathione and aminopeptidases induces potent cytotoxic T lymphocytes. Bioconjug. Chem. 2017, 28, 2246–2253. [Google Scholar] [CrossRef]
- Kramer, K.; Shields, N.J.; Poppe, V.; Young, S.L.; Walker, G.F. Intracellular cleavable CpG oligodeoxynucleotideantigen conjugate enhances anti-tumor immunity. Mol. Ther. 2017, 25, 62–70. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Q.Q.; Wu, J.-P.; Kirk, T.B.; Xu, J.K.; Liu, Z.H.; Xue, W. Reduction-responsive codelivery system based on a metal–organic framework for eliciting potent cellular immune response. ACS Appl. Mater. Interfaces 2018, 10, 12463–12473. [Google Scholar] [CrossRef]
- Wang, K.; Wen, S.M.; He, L.H.; Li, A.; Li, Y.; Dong, H.Q.; Li, W.; Ren, T.B.; Shi, D.L.; Li, Y.Y. “Minimalist” nanovaccine constituted from near whole antigen for cancer immunotherapy. ACS Nano 2018, 12, 6398–6409. [Google Scholar] [CrossRef]
- Håkerud, M.; Waeckerle-Men, Y.; Selbo, P.K.; Kündig, T.M.; Høgset, A.; Johansen, P. Intradermal photosensitisation facilitates stimulation of MHC class-I restricted CD8 T-cell responses of co-administered antigen. J. Control. Release 2014, 174, 143–150. [Google Scholar] [CrossRef]
- Hjálmsdóttir, Á.; Bühler, C.; Vonwil, V.; Roveri, M.; Håkerud, M.; Wäckerle-Men, Y.; Gander, B.; Johansen, P. Cytosolic delivery of liposomal vaccines by means of the concomitant photosensitization of phagosomes. Mol. Pharma. 2016, 13, 320–329. [Google Scholar] [CrossRef]
- Zhang, C.N.; Zhang, J.; Shi, G.N.; Song, H.J.; Shi, S.B.; Zhang, X.Y.; Huang, P.S.; Wang, Z.H.; Wang, W.W.; Wang, C.; et al. A light responsive nanoparticle-based delivery system using pheophorbide A graft polyethylenimine for dendritic cell-based cancer immunotherapy. Mol. Pharm. 2017, 14, 1760–1770. [Google Scholar] [CrossRef]
- Cao, F.Q.; Yan, M.M.; Liu, Y.J.; Liu, L.X.; Ma, G.L. Photothermally controlled MHC class I restricted CD8+ T-cell responses elicited by hyaluronic acid decorated gold nanoparticles as a vaccine for cancer immunotherapy. Adv. Healthc. Mater. 2018, 7, 1701439. [Google Scholar] [CrossRef] [PubMed]
- Un, K.; Kawakami, S.; Suzuki, R.; Maruyama, K.; Yamashita, F.; Hashida, M. Suppression of melanoma growth and metastasis by DNA vaccination using an ultrasound-responsive and mannose-modified gene carrier. Mol. Pharma. 2011, 8, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Kawakami, S.; Kono, Y.; Un, K.; Higuchi, Y.; Maruyama, K.; Yamashita, F.; Hashida, M. Enhancement of the anti-tumor effect of DNA vaccination using an ultrasoundresponsive mannose-modified gene carrier in combination with doxorubicin-encapsulated PEGylated liposomes. Int. J. Pharm. 2014, 475, 401–407. [Google Scholar] [CrossRef]
- Reddy, S.T.; van der Vlies, A.J.; Simeoni, E.; Angeli, V.; Randolph, G.J.; O’Neil, C.P.; Lee, L.K.; Swartz, M.A.; Hubbell, J.A. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007, 25, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.P.; Moynihan, K.D.; Zheng, Y.R.; Szeto, G.L.; Li, A.V.; Huang, B.; van Egeren, D.S.; Park, C.; Irvine, D.J. Structure-based programming of lymph-node targeting in molecular vaccines. Nature 2014, 507, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Jewell, C.M.; Bustamante López, S.C.; Irvine, D.J. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc. Natl. Acad. Sci. USA 2011, 108, 15745–15750. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Lin, W.; Chen, S. Gene transfection in complex media using PCBMAEE-PCBMA copolymer with both hydrolytic and zwitterionic blocks. Biomaterials 2014, 35, 7909–7918. [Google Scholar] [CrossRef]
- Overly, C.C.; Lee, K.D.; Berthiaume, E.; Hollenbeck, P.J. Quantitative measurement of intraorganelle pH in the endosomal-lysosomal pathway in neurons by using ratiometric imaging with pyranine. Proc. Natl. Acad. Sci. USA 1995, 92, 3156–3160. [Google Scholar] [CrossRef] [PubMed]
- Murthy, N.; Thng, Y.X.; Schuck, S.; Xu, M.C.; Fréchet, J.M.J. A novel strategy for encapsulation and release of proteins: Hydrogels and microgels with acid-labile acetal cross-linkers. J. Am. Chem. Soc. 2002, 124, 12398–12399. [Google Scholar] [CrossRef] [PubMed]
- Murthy, N.; Xu, M.C.; Schuck, S.; Kunisawa, J.; Shastri, N.; Fréchet, J.M.J. A macromolecular delivery vehicle for protein-based vaccines: Acid-degradable protein-loaded microgels. Proc. Natl. Acad. Sci. USA 2003, 100, 4995–5000. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.A.; Beaudette, T.T.; Tseng, W.W.; Bachelder, E.M.; Mende, I.; Engleman, E.G.; Fréchet, J.M.J. T-cell activation by antigen-loaded pH-sensitive hydrogel particles in vivo: The effect of particle size. Bioconjug. Chem. 2009, 20, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Bachelder, E.M.; Beaudette, T.T.; Broaders, K.E.; Paramonov, S.E.; Dashe, J.; Fréchet, J.M.J. Acid-degradable polyurethane particles for protein-based vaccines: Biological evaluation and in vitro analysis of particle degradation products. Mol. Pharm. 2008, 5, 876–884. [Google Scholar] [CrossRef]
- Ruff, L.E.; Mahmoud, E.A.; Sankaranarayanan, J.; Morachis, J.M.; Katayama, C.D.; Corr, M.; Hedrick, S.M.; Almutairi, A. Antigen-loaded pH-sensitive hydrogel microparticles are taken up by dendritic cells with no requirement for targeting antibodies. Integr. Biol. 2013, 5, 195–203. [Google Scholar] [CrossRef]
- Nuhn, L.; Vanparijs, N.; De Beuckelaer, A.; Lybaert, L.; Verstraete, G.; Deswarte, K.; Lienenklaus, S.; Shukla, N.M.; Salyer, A.C.D.; Lambrecht, B.N.; et al. pH-degradable imidazoquinoline-ligated nanogels for lymph node-focused immune activation. Proc. Natl. Acad. Sci. USA 2016, 113, 8098–8103. [Google Scholar] [CrossRef]
- van Herck, S.; van Hoecke, L.; Louage, B.; Lybaert, L.; De Coen, R.; Kasmi, S.; Esser-Kahn, A.P.; David, S.A.; Nuhn, L.; Schepens, B.; et al. Transiently thermoresponsive acetal polymers for safe and effective administration of amphotericin B as a vaccine adjuvant. Bioconjug. Chem. 2018, 29, 748–760. [Google Scholar] [CrossRef]
- Magarian Blander, J.; Medzhitov, R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 2006, 440, 808–812. [Google Scholar] [CrossRef]
- Maier, K.; Wagner, E. Acid-labile traceless click linker for protein transduction. J. Am. Chem. Soc. 2012, 134, 10169–10173. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef] [PubMed]
- van der Maaden, K.; Varypataki, E.M.; Romeijn, S.; Ossendorp, F.; Jiskoot, W.; Bouwstra, J. Ovalbumin-coated pH-sensitive microneedle arrays effectively induce ovalbuminspecific antibody and T-cell responses in mice. Eur. J. Pharm. Biopharm. 2014, 88, 310–315. [Google Scholar] [CrossRef]
- Tirrell, D.A.; Takigawa, D.Y.; Seki, K. pH sensitization of phospholipid vesicles via complexation with synthetic poly(carboxylic acid). Ann. N. Y. Acad. Sci. 1985, 446, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Flanary, S.; Hoffman, A.S.; Stayton, P.S. Antigen delivery with poly(propylacrylic acid)conjugation enhances MHC-1 presentation and T-cell activation. Bioconjug. Chem. 2009, 20, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Wilson, J.T.; Patilea, G.I.; Kern, H.B.; Convertine, A.J.; Stayton, P.S. Neutral polymer micelle carriers with pH-responsive, endosome-releasing activity modulate antigen trafficking to enhance CD8+ T cell responses. J. Control. Release 2014, 191, 24–33. [Google Scholar] [CrossRef]
- Yuba, E.; Harada, A.; Sakanishi, Y.; Kono, K. Carboxylated hyperbranched poly(glycidol)s for preparation of pH-sensitive liposomes. J. Control. Release 2011, 149, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Arab, A.; Behravan, J.; Razazan, A.; Gholizadeh, Z.; Nikpoor, A.R.; Barati, N.; Mosaffa, F.; Badiee, A.; Jaafari, M.R. A nano-liposome vaccine carrying E75, a HER-2/neu-derived peptide, exhibits significant antitumour activity in mice. J. Drug Target 2018, 26, 365–372. [Google Scholar] [CrossRef]
- Yuba, E. Design of pH-sensitive polymer-modified liposomes for antigen delivery and their application in cancer immunotherapy. Polym. J. 2016, 48, 761–771. [Google Scholar] [CrossRef]
- Yuba, E.; Sakaguchi, N.; Kanda, Y.; Miyazaki, M.; Koiwai, K. pH-responsive micelle-based cytoplasmic delivery system for induction of cellular immunity. Vaccines 2017, 5, 41. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Ito, E.; Akita, H.; Oishi, M.; Nagasaki, Y.; Futaki, S.; Harashima, H. A pH-sensitive fusogenic peptide facilitates endosomal escape and greatly enhances the gene silencing of siRNA-containing nanoparticles in vitro and in vivo. J. Control. Release 2009, 139, 127–132. [Google Scholar] [CrossRef]
- Li, W.J.; Nicol, F.; Szoka, F.C. GALA: A designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Adv. Drug Deliv. Rev. 2004, 56, 967–985. [Google Scholar] [CrossRef]
- Moon, J.J.; Suh, H.; Bershteyn, A.; Stephan, M.T.; Liu, H.P.; Huang, B.; Sohail, M.; Luo, S.; Ho Um, S.; Khant, H.; et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat. Mater. 2011, 10, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.P.; Valente, M.; Dolen, Y.; Jäger, E.; ter Beest, M.; Zheng, L.Y.; Figdor, C.G.; Verdoes, M. Endolysosomalescape nanovaccines through adjuvant-induced tumor antigen assembly for enhanced effector CD8+ T cell activation. Small 2018, 14, 1703539. [Google Scholar] [CrossRef]
- Boussif, O.; Lezoualc’h, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.P.; Wang, Y.G.; Zhao, T.; Li, Y.; Su, L.C.; Wang, Z.H.; Huang, G.; Sumer, B.D.; Gao, J.M. Ultra-pH-sensitive nanoprobe library with broad pH tunability and fluorescence emissions. J. Am. Chem. Soc. 2014, 136, 11085–11092. [Google Scholar] [CrossRef] [PubMed]
- López-Mirabal, H.R.; Winther, J.R. Redox characteristics of the eukaryotic cytosol. Biochim. Biophys. Acta -Mol. Cell Res. 2008, 1783, 629–640. [Google Scholar] [CrossRef]
- Bearinger, J.P.; Terrettaz, S.; Michel, R.; Tirelli, N.; Vogel, H.; Textor, M.; Hubbell, J.A. Chemisorbed poly(propylene sulphide)-based copolymers resist biomolecular interactions. Nat. Mater. 2003, 2, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Sun, F.L.; Bourajjaj, M.; Chen, Y.N.; Pieters, E.H.; Chen, J.; van den Dikkenberg, J.B.; Lou, B.; Camps, M.G.M.; Ossendorp, F.; et al. Strong in vivo antitumor responses induced by an antigen immobilized in nanogels via reducible bonds. Nanoscale 2016, 8, 19592–19604. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.M.; Jones, D.P. Redox compartmentalization in eukaryotic cells. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 1273–1290. [Google Scholar] [CrossRef] [PubMed]
- Håkerud, M.; Selbo, P.K.; Waeckerle-Men, Y.; Contassot, E.; Dziunycz, P.; Kündig, T.M.; Høgset, A.; Johansen, P. Photosensitisation facilitates cross-priming of adjuvant-free protein vaccines and stimulation of tumour-suppressing CD8 T cells. J. Control. Release 2015, 198, 10–17. [Google Scholar] [CrossRef]
- Weissleder, R.; Ntziachristos, V. Shedding light onto live molecular targets. Nat. Med. 2003, 9, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhao, J.H.; Chu, C.C. Enhanced MHC-I antigen presentation from the delivery of ovalbumin by lightfacilitated biodegradable poly(ester amide)s nanoparticles. J. Mater. Chem. B 2018, 6, 1930–1942. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Jige, M.; Nakaminami, T.; Uragami, T. Tumor marker-responsive behavior of gels prepared by biomolecular imprinting. Proc. Natl. Acad. Sci. USA 2006, 103, 1190–1193. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Othman, S.F.; Curtis, E.T.; Gupta, B.K.; Jaggi, M.; Chauhan, S.C. Multi-functional magnetic nanoparticles for magnetic resonance imaging and cancer therapy. Biomaterials 2011, 32, 1890–1905. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.Y.; Katti, P.S.; Gu, Z. Enzyme-responsive nanomaterials for controlled drug delivery. Nanoscale 2014, 6, 12273–12286. [Google Scholar] [CrossRef] [PubMed]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Yu, J.C.; Bomba, H.N.; Zhu, Y.; Gu, Z. Mechanical force-triggered drug delivery. Chem. Rev. 2016, 116, 12536–12563. [Google Scholar] [CrossRef] [PubMed]

| Stimuli | Responsive Chemical Structure | Delivery System |
|---|---|---|
| Temperature-responsive biomaterials for improved LNa targeting | NIPAM | Immunization–polymer conjugate |
| Biomaterials that are responsive for improved cross-presentation Acidic surrounding (pH) | Acetal bond Coordination bond Carboxyl group NH4HCO3 Tertiary amine Fusogenic peptide | Cross-linked polymeric NPc MOFd |
| Reductive environment (redox) | Disulfide bond | NP polymer Conjugated vaccines Environmental reduction (redox) Sulfide bond MOF Adjuvant/Antigen NP |
| Light (Visf or NIRg) | TPCS2a PheoAi Gold NP | Conjugated antigen–polymer Inorganic NP polymer Polymeric nanoparticle |
| Ultrasound | Bubble lipoplex | Liposome |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.K.; Malviya, R.; Prajapati, B.; Singh, S.; Goyal, P. Utilization of Stimuli-Responsive Biomaterials in the Formulation of Cancer Vaccines. J. Funct. Biomater. 2023, 14, 247. https://doi.org/10.3390/jfb14050247
Singh AK, Malviya R, Prajapati B, Singh S, Goyal P. Utilization of Stimuli-Responsive Biomaterials in the Formulation of Cancer Vaccines. Journal of Functional Biomaterials. 2023; 14(5):247. https://doi.org/10.3390/jfb14050247
Chicago/Turabian StyleSingh, Arun Kumar, Rishabha Malviya, Bhupendra Prajapati, Sudarshan Singh, and Priyanshi Goyal. 2023. "Utilization of Stimuli-Responsive Biomaterials in the Formulation of Cancer Vaccines" Journal of Functional Biomaterials 14, no. 5: 247. https://doi.org/10.3390/jfb14050247






