Decellularized Scaffolds of Nopal (Opuntia Ficus-indica) for Bioengineering in Regenerative Dentistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nopal Scaffold Synthesis and Decellularization
2.2. Nopal Scaffold Characterization
2.3. Nopal Scaffolds Degradation
2.4. Nopal Scaffolds tensile Strength
2.5. Cell Culture
2.6. Scaffold-Cell Interaction and Proliferation
2.7. COX-1 and COX-2 Cell Expression-Scaffold
2.8. Statistical Analysis
3. Results
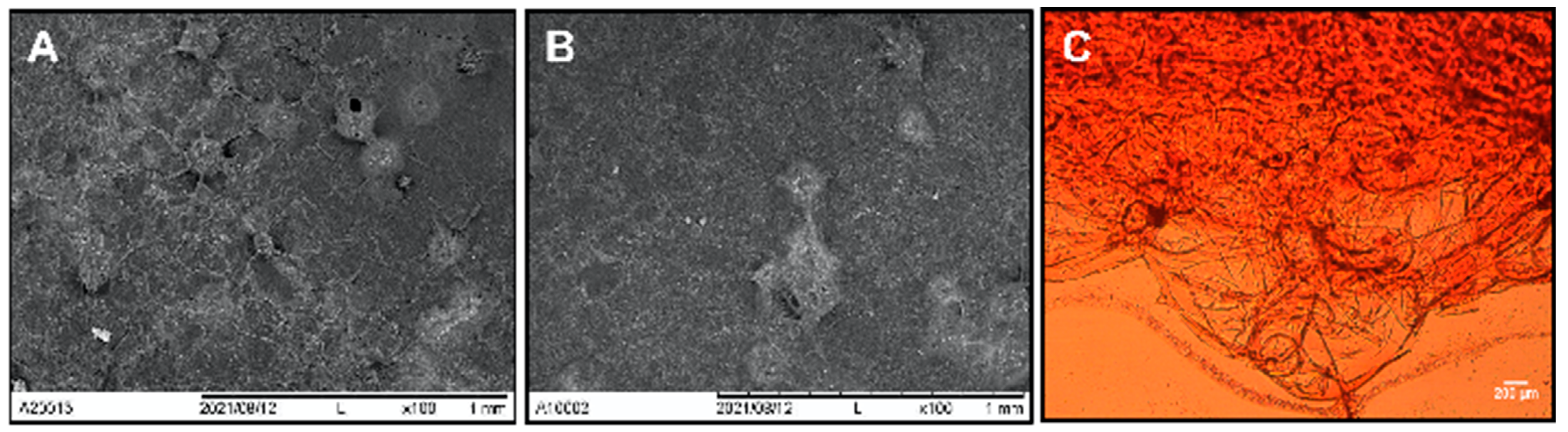
3.1. Nopal Scaffold Characterization
3.2. Nopal Scaffolds Degradation
3.3. Scaffold Tensile Strength
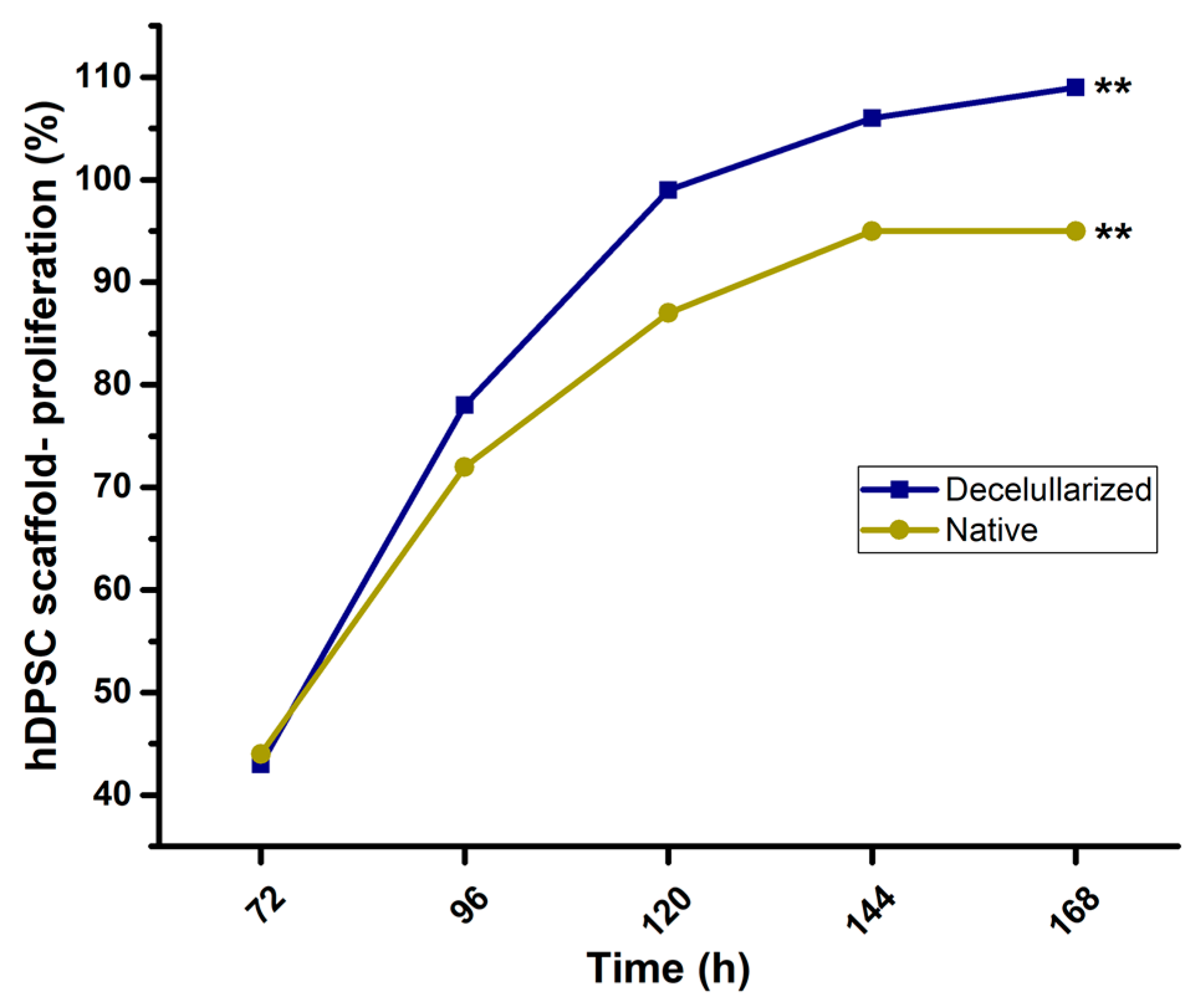
3.4. Cell-Scaffold Interaction and Proliferation
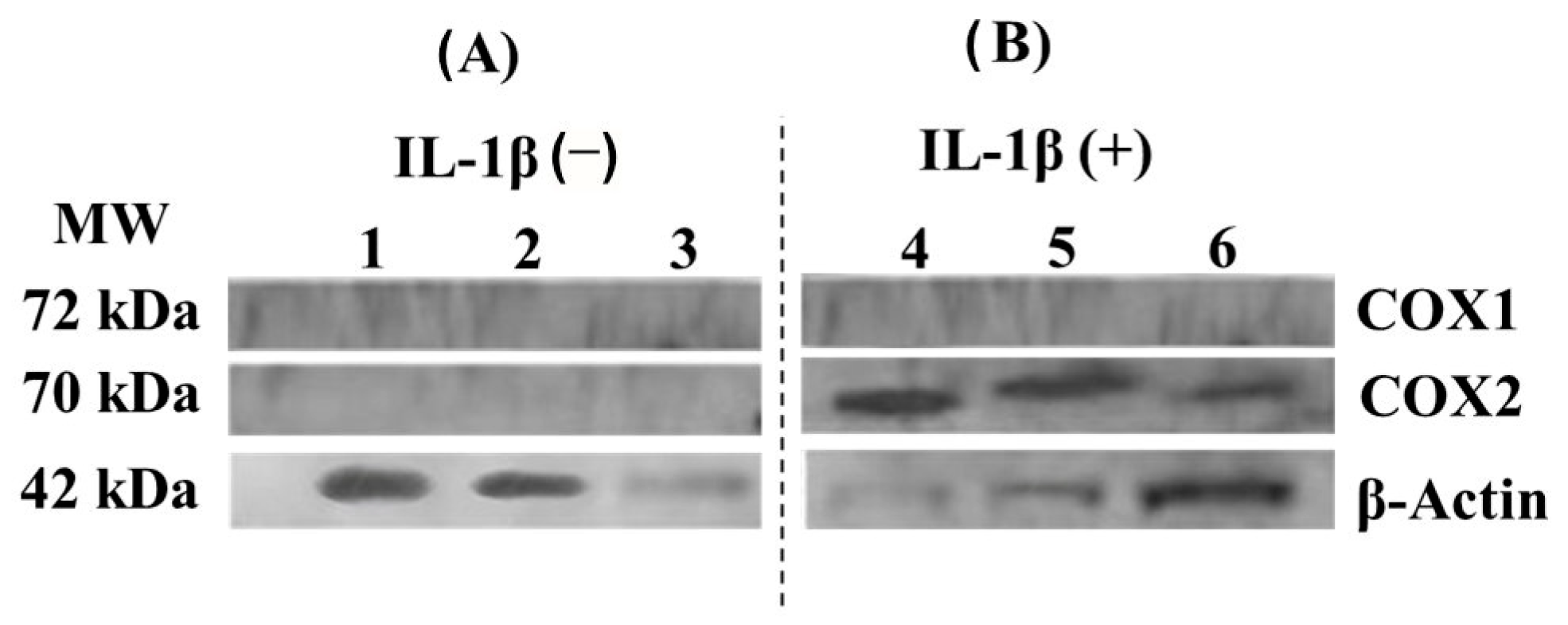
3.5. COX-1 and COX-2 Cell Expression-Scaffold
4. Discussion
4.1. Scaffold Decellularization
4.2. Nopal Scaffolds Degradation
4.3. Nopal Scaffold Tensile Strength
4.4. Cell-Scaffold Interaction and Proliferation
4.5. COX-1 and COX-2 Cell Expression-Scaffold
4.6. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stintzing, F.C.; Carle, R. Cactus Stems (Opuntia spp.): A Review on Their Chemistry, Technology, and Uses. Mol. Nutr. Food Res. 2006, 49, 175–194. [Google Scholar] [CrossRef]
- Maki-Díaz, G.; Peña-Valdivia, C.B.; García-Nava, R.; Arévalo-Galarza, M.L.; Calderón-Zavala, G.; Anaya-Rosales, S. Physical and Chemical Characteristics of Cactus Stems (Opuntia ficus-indica) for Exportation and Domestic Markets. Agrociencia 2015, 49, 31–51. Available online: https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1405-31952015000100003&lng=es&nrm=iso (accessed on 12 February 2023).
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Ghazali, F.A.; Syahrom, A.; Kamarul, T. The Effect of Tortuosity on Permeability of Porous Scaffold. Biomedicines 2023, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability 2023, 15, 823. [Google Scholar] [CrossRef]
- Stoppel, W.L.; Kaplan, D.L.; Black, L.D., 3rd. Electrical and Mechanical Stimulation of Cardiac Cells and Tissue Constructs. Adv. Drug Deliv. Rev. 2016, 96, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Blaudez, F.; Ivanovski, S.; Hamlet, S.; Vaquette, C. An Overview of Decellularisation Techniques of Native Tissues and Tissue Engineered Products for Bone, Ligament and Tendon Regeneration. Methods 2020, 171, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An Overview of Tissue and Whole Organ Decellularization Processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Modulevsky, D.J.; Lefebvre, C.; Haase, K.; Al-Rekabi, Z.; Pelling, A.E. Apple Derived Cellulose Scaffolds for 3D Mammalian Cell Culture. PLoS ONE 2014, 9, e97835. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, H.; Park, N.; Park, S.H.; Ju, J.H. Induced Osteogenesis in Plants Decellularized Scaffolds. Sci. Rep. 2019, 9, 20194. [Google Scholar] [CrossRef]
- Adamski, M.; Fontana, G.; Gershlak, J.R.; Gaudette, G.R.; Le, H.D.; Murphy, W.L. Two Methods for Decellularization of Plant Tissues for Tissue Engineering Applications. J. Vis. Exp. 2018, 135, 57586. [Google Scholar] [CrossRef]
- Contessi, N.N.; Toffoletto, N.; Farè, S.; Altomare, L. Plant Tissues as 3D Natural Scaffolds for Adipose, Bone and Tendon Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 723. [Google Scholar] [CrossRef] [PubMed]
- Chisci, G.; Fredianelli, L. Therapeutic Efficacy of Bromelain in Alveolar Ridge Preservation. Antibiotics 2022, 11, 1542. [Google Scholar] [CrossRef] [PubMed]
- Marin-Bustamante, M.Q.; Chanona-Pérez, J.J.; Güemes-Vera, M.; Cásarez-Santiago, R.; Perea-Flores, M.J.; Arzate-Vázquez, I.; Calderón-Domínguez, G. Production and Characterization of Cellulose Nanoparticles From Nopal Waste by Means of High Impact Milling. Procedia Eng. 2017, 200, 428–433. [Google Scholar] [CrossRef]
- Garcia-Contreras, R.; Chavez-Granados, P.A.; Jurado, C.A.; Aranda-Herrera, B.; Afrashtehfar, K.I.; Nurrohman, H. Natural Bioactive Epigallocatechin-Gallate Promote Bond Strength and Differentiation of Odontoblast-like Cells. Biomimetics 2023, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, J.; Harris, A.F.; Zenhausern, R.; Karsunsky, S.; Zenhausern, F. Plant-Based Scaffolds Modify Cellular Response to Drug and Radiation Exposure Compared to Standard Cell Culture Models. Front. Bioeng. Biotechnol. 2020, 8, 932. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Zhang, S.J.; Wang, L.Q.; Sheng, L.Y.; Zhou, Q.Z.; Xi, T.F. The relationship between microstructure and in vivo degradation of modified bacterial cellulose sponges. J. Mater. Chem. B 2015, 3, 9001–9010. [Google Scholar] [CrossRef]
- Modulevsky, D.J.; Cuerrier, C.M.; Pelling, A.E. Biocompatibility of Subcutaneously Implanted Plant-Derived Cellulose Biomaterials. PLoS ONE 2016, 11, e0157894. [Google Scholar] [CrossRef]
- Khoshgozaran-Abras, S.; Azizi, M.H.; Hamidy, Z.; Bagheripoor-Fallah, N. Mechanical, Physicochemical and Color Properties of Chitosan Based-Films as a Function of Aloe vera Gel Incorporation. Carbohydr. Polym. 2012, 87, 2058–2062. [Google Scholar] [CrossRef]
- Saibuatongbased, O.; Phisalaphong, M. Novo aloe vera–bacterial cellulose composite film from biosynthesis. Carbohydr. Polym. 2010, 79, 455–460. [Google Scholar] [CrossRef]
- Bhaarathy, V.; Venugopal, J.; Gandhimathi, C.; Ponpandian, N.; Mangalaraj, D.; Ramakrishna, S. Biologically Improved Nanofibrous Scaffolds for Cardiac Tissue Engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 268–277. [Google Scholar] [CrossRef]
- Bielli, A.; Bernardini, R.; Varvaras, D.; Rossi, P.; Di Blasi, G.; Petrella, G.; Buonomo, O.C.; Mattei, M.; Orlandi, A. Characterization of a New Decellularized Bovine Bericardial Biological Mesh: Structural and Mechanical properties. J. Mech. Behav. Biomed. Mater. 2019, 78, 420–426. [Google Scholar] [CrossRef]
- Gershlak, J.R.; Hernandez, S.; Fontana, G.; Perreault, L.R.; Hansen, K.J.; Larson, S.A.; Binder, B.Y.; Dolivo, D.M.; Yang, T.; Dominko, T.; et al. Crossing Kingdoms: Using decellularized Plants as Perfusable Tissue Engineering Scaffolds. Biomaterials 2017, 125, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Dugan, J.M.; Gough, J.E.; Eichhorn, S.J. Bacterial Cellulose Scaffolds and Cellulose Nanowhiskers for Tissue Engineering. Nanomedicine 2013, 8, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Saheli, M.; Sepantafar, M.; Pournasr, B.; Farzaneh, Z.; Vosough, M.; Piryaei, A.; Baharvand, H. Three-Dimensional Liver-Derived Extracellular Matrix Hydrogel Promotes Liver Organoids Function. J. Cell. Biochem. 2018, 119, 4320–4333. [Google Scholar] [CrossRef]
- Yi, P.; Xu, X.; Qiu, B.; Li, H. Impact of Chitosan Membrane Culture on the Expression of Pro- and Anti-Inflammatory Cytokines in Mesenchymal Stem Cells. Exp. Ther. Med. 2020, 20, 3695–3702. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Hartono, R.; Supriyono, T.; Santoso, G.; Sugiharto, S.; Permana, M.S. Polycrystalline Diamond as a Potential Material for the Hard-on-Hard Bearing of Total Hip Prosthesis: Von Mises Stress Analysis. Biomedicines 2023, 11, 951. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Wibowo, D.B.; Kurdi, O.; Tauviqirrahman, M.; Jamari, J. Minimizing Risk of Failure from Ceramic-on-Ceramic Total Hip Prosthesis by Selecting Ceramic Materials Based on Tresca Stress. Sustainability 2022, 14, 13413. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Afif, I.Y.; Maula, M.I.; Winarni, T.I.; Tauviqirrahman, M.; Akbar, I.; Basri, H.; van der Heide, E.; Jamari, J. Tresca Stress Simulation of Metal-on-Metal Total Hip Arthroplasty during Normal Walking Activity. Materials 2021, 14, 7554. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Permana, M.S.; Winarni, T.I.; van der Heide, E. Adopted Walking Condition for Computational Simulation Approach on Bearing of Hip Joint Prosthesis: Review Over the Past 30 Years. Heliyon 2022, 8, e12050. [Google Scholar] [CrossRef]
- Tauviqirrahman, M.; Ammarullah, M.I.; Jamari, J.; Saputra, J.; Winarni, E.; Kurniawan, T.I.; Shiddiq, F.D.; van der Heide, E. Analysis of Contact Pressure in a 3D Model of Dual-Mobility Hip Joint Prosthesis Under a Gait Cycle. Sci. Rep. 2023, 13, 3564. [Google Scholar] [CrossRef] [PubMed]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; van der Heide, E. In Silico Contact Pressure of Metal-on-Metal Total Hip Implant with Different Materials Subjected to Gait Loading. Metals 2022, 12, 1241. [Google Scholar] [CrossRef]



| Scaffold | Tensile Strength (MPa) | t-Student Test |
|---|---|---|
| Native nopal scaffold | 12.5 ± 1 | p = 0.0748 |
| Decellularized nopal scaffold | 11.8 ± 0.5 | |
| MPa = Megapascals |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamudio-Ceja, R.B.; Garcia-Contreras, R.; Chavez-Granados, P.A.; Aranda-Herrera, B.; Alvarado-Garnica, H.; Jurado, C.A.; Fischer, N.G. Decellularized Scaffolds of Nopal (Opuntia Ficus-indica) for Bioengineering in Regenerative Dentistry. J. Funct. Biomater. 2023, 14, 252. https://doi.org/10.3390/jfb14050252
Zamudio-Ceja RB, Garcia-Contreras R, Chavez-Granados PA, Aranda-Herrera B, Alvarado-Garnica H, Jurado CA, Fischer NG. Decellularized Scaffolds of Nopal (Opuntia Ficus-indica) for Bioengineering in Regenerative Dentistry. Journal of Functional Biomaterials. 2023; 14(5):252. https://doi.org/10.3390/jfb14050252
Chicago/Turabian StyleZamudio-Ceja, Ruth Betsabe, Rene Garcia-Contreras, Patricia Alejandra Chavez-Granados, Benjamin Aranda-Herrera, Hugo Alvarado-Garnica, Carlos A. Jurado, and Nicholas G. Fischer. 2023. "Decellularized Scaffolds of Nopal (Opuntia Ficus-indica) for Bioengineering in Regenerative Dentistry" Journal of Functional Biomaterials 14, no. 5: 252. https://doi.org/10.3390/jfb14050252
APA StyleZamudio-Ceja, R. B., Garcia-Contreras, R., Chavez-Granados, P. A., Aranda-Herrera, B., Alvarado-Garnica, H., Jurado, C. A., & Fischer, N. G. (2023). Decellularized Scaffolds of Nopal (Opuntia Ficus-indica) for Bioengineering in Regenerative Dentistry. Journal of Functional Biomaterials, 14(5), 252. https://doi.org/10.3390/jfb14050252











