Cellular Alterations in Carbohydrate and Lipid Metabolism Due to Interactions with Nanomaterials
Abstract
1. Introduction
1.1. Nanomaterial Definition and Types of Classifications Regarding Their Composition
- -
- -
- Although the first classification of nanostructured materials (NSMs) was postulated according to their chemical composition and the dimensionality (shape) [11], the creation of novel nanostructures made it necessary to reformulate this classification [12] considering the electron movement along the dimensions [1,13].
- -
- Morphology is defined by flatness, sphericity, and aspect ratio [1,4]. The main phenomena of NSMs occur at the surface, meaning that the total surface should be considered in terms of toxicology [14]. The aspect ratio determines the internalization rate of a particle, and as the aspect ratio increases, toxicity increases as well [15]. Nanoparticles defined as having a high aspect ratio include nanotubes and nanowires, varying in shape and length. Meanwhile, nanoparticles defined as having a small aspect ratio include spheres, ovals, cubes, prisms, helixes, and pillars [4].
- -
- NSMs can be constituted by a single material or a mixture of various materials [4]. Most current NSMs are divided into four categories according to their material composition: carbon-based nanomaterials [8,9,16], inorganic-based nanomaterials (metal and metal oxide), organic-based nanomaterials [17], and composite-based nanomaterials [7,18].
- -
1.2. Nanomaterials and Their Classification Regarding Their Applications in Medicine
1.2.1. NPs as Drug Delivery Systems
1.2.2. NPs as Diagnosis Systems
2. Effect of NPs on Cell Metabolism and Toxicity
2.1. Implications in Carbohydrate Metabolism
2.1.1. NP Reprogramming of Carbohydrate Metabolism in Cancer
Beneficial Effect of NP Direct Reprogramming of Carbohydrate Metabolism for Cancer Treatment
Beneficial Effect of Indirect Reprogramming of Carbohydrate Metabolism for Cancer Treatment by Nanoparticle Alteration of Oxidative State
Undesired Effects on the Carbohydrate Metabolism in Cancer Studies due to NPs
2.1.2. NP Reprogramming of Carbohydrate Metabolism in Diabetes
Beneficial Applications of NPs as Antidiabetic Drugs
Undesired Effects of NPs Used in Food Industry on Carbohydrate Metabolism in Relation to Glycemia and Insulin Resistance
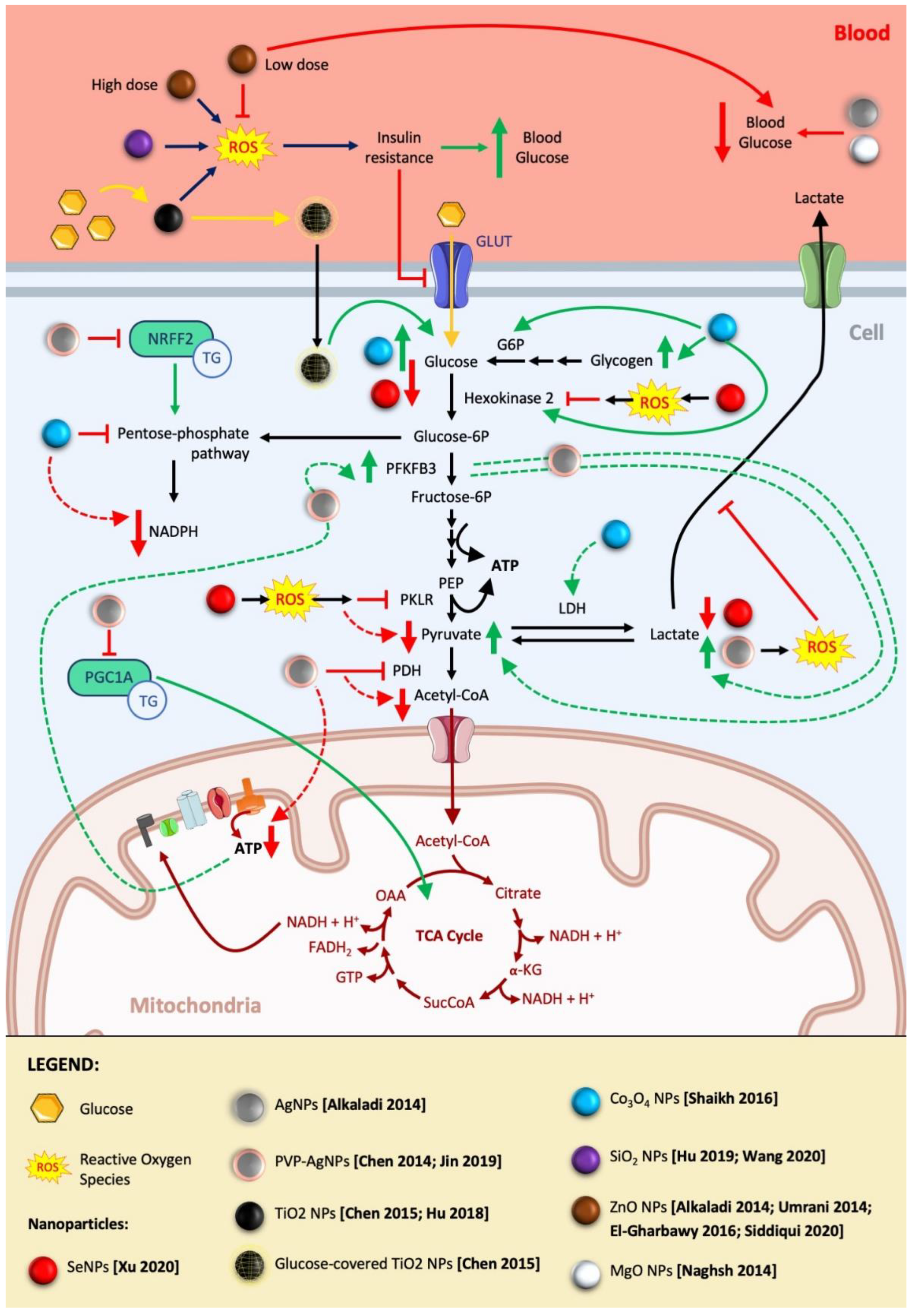
2.2. Implications in Lipid Metabolism
2.2.1. Therapeutic Use of NPs as Antiobesity Agents
Desired Antiobesity NP Effect by Inflammation Modulation
Antiobesity NP Effect by Adipocyte Differentiation and Maturation Modulation
2.2.2. Toxicity of NPs in Relation to Lipid Metabolism and Adipocyte Differentiation
Undesired Effects of Therapeutic NPs
Undesired Effects of Diagnostic NPs
Undesired Effects of NPs Used in Food and Personal Care Products

3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Chen, N.; Wang, H.; Huang, Q.; Li, J.; Yan, J.; He, D.; Fan, C.; Song, H. Long-Term Effects of Nanoparticles on Nutrition and Metabolism. Small 2014, 10, 3603–3611. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chang, X.; Chen, X.; Gu, Z.; Zhao, F.; Chai, Z.; Zhao, Y. Toxicity of inorganic nanomaterials in biomedical imaging. Biotechnol. Adv. 2014, 32, 727–743, Epub 2014 Jan 2. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR172. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I. Nanomaterial and Nanoparticle: Origin and Activity. In Nanoscience and Plant-Soil Systems. Soil Biology; Ghorbanpour, M., Manika, K., Varma, A., Eds.; Springer: Cham, Switzerland, 2017; Volume 48, p. 71. [Google Scholar]
- Griffin, S.; Masood, M.I.; Nasim, M.J.; Sarfraz, M.; Ebokaiwe, A.P.; Schäfer, K.-H.; Keck, C.M.; Jacob, C. Natural Nanoparticles: A Particular Matter Inspired by Nature. Antioxidants 2017, 7, 3. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, N.; Kim, T.; Kim, J.; Hyeon, T. Multifunctional Mesoporous Silica Nanocomposite Nanoparticles for Theranostic Applications. Accounts Chem. Res. 2011, 44, 893–902. [Google Scholar] [CrossRef]
- Jana, N.R.; Ray, S.C. Chapter 3—Graphene-Based Carbon Nanoparticles for Bioimaging Applications. In Applications of Graphene and Graphene-Oxide Based Nanomaterials Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2015; pp. 57–84. [Google Scholar] [CrossRef]
- Nazıroğlu, M.; Muhamad, S.; Pecze, L. Nanoparticles as potential clinical therapeutic agents in Alzheimer’s disease: Focus on selenium nanoparticles. Expert Rev. Clin. Pharmacol. 2017, 10, 773–782. [Google Scholar] [CrossRef]
- Shahbazi, M.-A.; Faghfouri, L.; Ferreira, M.P.A.; Figueiredo, P.; Maleki, H.; Sefat, F.; Hirvonen, J.; Santos, H.A. The versatile biomedical applications of bismuth-based nanoparticles and composites: Therapeutic, diagnostic, biosensing, and regenerative properties. Chem. Soc. Rev. 2020, 49, 1253–1321. [Google Scholar] [CrossRef]
- Gleiter, H. Nanostructured Materials: State of the Art and Perspectives. In Nanostructured Materials; Elsevier: Karlsruhe, Germany, 1995; Volume 6, Issues 1–4; pp. 3–14. [Google Scholar]
- Pokropivny, V.V.; Skorokhod, V.V. Classification of nanostructures by dimensionality and concept of surface forms engineering in nanomaterial science. Mater. Sci. Eng. C 2007, 27, 990. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Ramos, A.P.; Cruz, M.A.E.; Tovani, C.B.; Ciancaglini, P. Biomedical applications of nanotechnology. Biophys. Rev. 2017, 9, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Blandino, I.; Vanner, R.; Buzea, C. Toxicity of nanoparticles. Toxic. Build. Mater. 2012, 427–475. [Google Scholar] [CrossRef]
- Ray, S.C.; Jana, N.R. Chapter 5—Application of Carbon-Based Nanomaterials as Drug and Gene Delivery Carrier. In Carbon Nanomaterials for Biological and Medical Applications Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 163–203. [Google Scholar] [CrossRef]
- Kumar, R.; Lal, S. Synthesis of Organic Nanoparticles and their Applications in Drug Delivery and Food Nanotechnology: A Review. J. Nanomater. Mol. Nanotechnol. 2014, 3, 4. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, L. Composite nanoparticles for gene delivery. Adv. Genet. 2014, 88, 111–137. [Google Scholar] [CrossRef] [PubMed]
- Bantz, C.; Koshkina, O.; Lang, T.; Galla, H.-J.; Kirkpatrick, C.J.; Stauber, R.H.; Maskos, M. The surface properties of nanoparticles determine the agglomeration state and the size of the particles under physiological conditions. Beilstein J. Nanotechnol. 2014, 5, 1774–1786. [Google Scholar] [CrossRef] [PubMed]
- Zook, J.M.; MacCuspie, R.; Locascio, L.E.; Halter, M.D.; Elliott, J.T. Stable nanoparticle aggregates/agglomerates of different sizes and the effect of their size on hemolytic cytotoxicity. Nanotoxicology 2010, 5, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Anik, M.I.; Mahmud, N.; Al Masud, A.; Hasan, M. Gold nanoparticles (GNPs) in biomedical and clinical applications: A review. Nano Sel. 2021, 3, 792–828. [Google Scholar] [CrossRef]
- Wei, H.; Hu, Y.; Wang, J.; Gao, X.; Qian, X.; Tang, M. Superparamagnetic Iron Oxide Nanoparticles: Cytotoxicity, Metabolism, and Cellular Behavior in Biomedicine Applications. Int. J. Nanomed. 2021, 16, 6097–6113. [Google Scholar] [CrossRef]
- Saji, V.S. Supramolecular organic nanotubes for drug delivery. Mater. Today Adv. 2022, 14, 100239. [Google Scholar] [CrossRef]
- Othman, A.K.; El Kurdi, R.; Badran, A.; Mesmar, J.; Baydoun, E.; Patra, D. Liposome-based nanocapsules for the controlled release of dietary curcumin: PDDA and silica nanoparticle-coated DMPC liposomes enhance the fluorescence efficiency and anticancer activity of curcumin. RSC Adv. 2022, 12, 11282–11292. [Google Scholar] [CrossRef]
- Cheng, C.; Qiao, J.; Zhang, H.; Zhao, Z.; Qi, L. Polymer-capped gold nanoparticles as nanozymes with improved catalytic activity for the monitoring of serum ciprofloxacin. Analyst 2022, 147, 1509–1514. [Google Scholar] [CrossRef]
- Joy, R.; George, J.; John, F. Brief Outlook on Polymeric Nanoparticles, Micelles, Niosomes, Hydrogels and Liposomes: Preparative Methods and Action. Chem. Sel. 2022, 7, e202104045. [Google Scholar] [CrossRef]
- Baetke, S.C.; Lammers, T.; Kiessling, F. Applications of nanoparticles for diagnosis and therapy of cancer. Br. J. Radiol. 2015, 88, 20150207. [Google Scholar] [CrossRef] [PubMed]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Qi, J.; Gogoi, R.; Wong, J.; Mitragotri, S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J. Control. Release 2016, 238, 176–185. [Google Scholar] [CrossRef]
- Chen, L.; Xiao, S.; Zhu, H.; Wang, L.; Liang, H. Shape-dependent internalization kinetics of nanoparticles by membranes. Soft Matter 2016, 12, 2632–2641. [Google Scholar] [CrossRef]
- Zhang, B.; Feng, X.; Yin, H.; Ge, Z.; Wang, Y.; Chu, Z.; Raabova, H.; Vavra, J.; Cigler, P.; Liu, R.; et al. Anchored but not internalized: Shape dependent endocytosis of nanodiamond. Sci. Rep. 2017, 7, 46462. [Google Scholar] [CrossRef]
- Wang, W.; Gaus, K.; Tilley, R.D.; Gooding, J.J. The impact of nanoparticle shape on cellular internalisation and transport: What do the different analysis methods tell us? Mater. Horizons 2019, 6, 1538–1547. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Anjum, M.; Patel, K.K. Gemcitabine cationic polymeric nanoparticles against ovarian cancer: Formulation, characterization, and targeted drug delivery. Drug Deliv. 2022, 29, 1060–1074. [Google Scholar] [CrossRef]
- Nie, S. Understanding and overcoming major barriers in cancer nanomedicine. Nanomedicine 2010, 5, 523–528. [Google Scholar] [CrossRef]
- Kamaly, N.; Farokhzad, O.C.; Corbo, C. Nanoparticle protein corona evolution: From biological impact to biomarker discovery. Nanoscale 2022, 14, 1606–1620. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Beasock, D.; Fessler, A.; Szebeni, J.; Ljubimova, J.Y.; Afonin, K.A.; Dobrovolskaia, M.A. To PEGylate or not to PEGylate: Immunological properties of nanomedicine’s most popular component, polyethylene glycol and its alternatives. Adv. Drug Deliv. Rev. 2021, 180, 114079. [Google Scholar] [CrossRef]
- Betker, J.L.; Anchordoquy, T.J. The Use of Lactose as an Alternative Coating for Nanoparticles. J. Pharm. Sci. 2020, 109, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Jiang, J.; Jiang, L.; Zheng, P.; Wang, F.; Zhou, Y.; Chen, Z.; Li, M.; Lian, M.; Tang, S.; et al. Chitosan mediated solid lipid nanoparticles for enhanced liver delivery of zedoary turmeric oil in vivo. Int. J. Biol. Macromol. 2020, 149, 108–115. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Gao, H.; Yang, Z.; Zhang, S.; Cao, S.; Shen, S.; Pang, Z.; Jiang, X. Ligand modified nanoparticles increases cell uptake, alters endocytosis and elevates glioma distribution and internalization. Sci. Rep. 2013, 3, 2534. [Google Scholar] [CrossRef]
- Groneberg, D.; Giersig, M.; Welte, T.; Pison, U. Nanoparticle-Based Diagnosis and Therapy. Curr. Drug Targets 2006, 7, 643–648. [Google Scholar] [CrossRef]
- Encabo-Berzosa, M.M.; Sancho-Albero, M.; Sebastian, V.; Irusta, S.; Arruebo, M.; Santamaria, J.; Martin Duque, P. Polymer functionalized gold nanoparticles as nonviral gene delivery reagents. J. Gene Med. 2017, 19, e2964. [Google Scholar] [CrossRef]
- Agasti, S.S.; Rana, S.; Park, M.-H.; Kim, C.K.; You, C.-C.; Rotello, V.M. Nanoparticles for detection and diagnosis. Adv. Drug Deliv. Rev. 2010, 62, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Brun, E.; Roselli, C.S. Could nanoparticle corona characterization help for biological consequence prediction? Cancer Nanotechnol. 2014, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- NCBI. The Cell: A Molecular Approach, 2nd ed.; Cooper, G.M., Ed.; Sinauer Associates, EEUU: Sunderland, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK9879/ (accessed on 3 February 2022).
- Kaelin, W.; Thompson, C. Clues from cell metabolism. Nature 2010, 465, 562–564. [Google Scholar] [CrossRef]
- Nature. Cell Origins and Metabolism. Editor(s): Gary Coté, Mario De Tullio. Available online: https://www.nature.com/scitable/topic/cell-origins-and-metabolism-14122694/ (accessed on 19 May 2022).
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.; Newport, E.; Morten, K.J. The Warburg effect: 80 years on. Biochem. Soc. Trans. 2016, 44, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Z.; Xu, M.; Wang, X.; Liu, R.; Liu, Q.; Zhang, Z.; Xia, T.; Zhao, J.; Jiang, G.; et al. Nanosilver Incurs an Adaptive Shunt of Energy Metabolism Mode to Glycolysis in Tumor and Nontumor Cells. ACS Nano 2014, 8, 5813–5825. [Google Scholar] [CrossRef]
- Jin, L.; Zhou, Y. Crucial role of the pentose phosphate pathway in malignant tumors (Review). Oncol. Lett. 2019, 17, 4213–4221. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Q.; Luo, X.; Ning, X.; Luo, J.; Guo, J.; Liu, Q.; Ling, G.; Zhou, N. Selenium nanoparticles reduce glucose metabolism and promote apoptosis of glioma cells through reactive oxygen species-dependent manner. Neuroreport 2020, 31, 226–234. [Google Scholar] [CrossRef]
- Raj, A.; Shah, P.; Agrawal, N. Sedentary behavior and altered metabolic activity by AgNPs ingestion in Drosophila melanogaster. Sci. Rep. 2017, 7, 15617. [Google Scholar] [CrossRef]
- Lee, M.J.; Lee, S.J.; Yun, S.J.; Jang, J.-Y.; Kang, H.; Kim, K.; Choi, I.-H.; Park, S. Silver nanoparticles affect glucose metabolism in hepatoma cells through production of reactive oxygen species. Int. J. Nanomed. 2015, 11, 55–68. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-H.; Lee, J.H.; Quach, C.H.T.; Paik, J.-Y.; Oh, H.; Park, J.W.; Lee, E.J.; Moon, S.-H.; Lee, K.-H. Resveratrol Suppresses Cancer Cell Glucose Uptake by Targeting Reactive Oxygen Species–Mediated Hypoxia-Inducible Factor-1α Activation. J. Nucl. Med. 2013, 54, 2161–2167. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Li, X.; Lu, X.; Jiang, C.; Hu, Y.; Li, Q.; You, Y.; Fu, Z. Enhanced growth inhibition effect of Resveratrol incorporated into biodegradable nanoparticles against glioma cells is mediated by the induction of intracellular reactive oxygen species levels. Colloids Surf. B Biointerfaces 2009, 72, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, S.; Han, Y.; Guan, J.; Chung, S.; Wang, C.; Li, D. Poly(Ethylene Glycol)–Polylactide Micelles for Cancer Therapy. Front. Pharmacol. 2018, 9, 202. [Google Scholar] [CrossRef]
- Coimbra, M.; Isacchi, B.; van Bloois, L.; Torano, J.S.; Ket, A.; Wu, X.; Broere, F.; Metselaar, J.M.; Rijcken, C.J.; Storm, G.; et al. Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int. J. Pharm. 2011, 416, 433–442. [Google Scholar] [CrossRef]
- Neves, A.R.; Lúcio, M.; Martins, S.; Lima, J.L.C. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int. J. Nanomed. 2013, 8, 177–187. [Google Scholar]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef]
- Ong, W.K.; Jana, D.; Zhao, Y. A glucose-depleting silica nanosystem for increasing reactive oxygen species and scavenging glutathione in cancer therapy. Chem. Commun. 2019, 55, 13374–13377, Epub 2019 Oct 21. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Chakraborty, S.P.; Laha, D.; Baral, R.; Pramanik, P.; Roy, S. Surface-modified cobalt oxide nanoparticles: New opportunities for anti-cancer drug development. Cancer Nanotechnol. 2012, 3, 13–23. [Google Scholar] [CrossRef]
- Shaikh, S.M.; Desai, P.V. Effect of CoO nanoparticles on the carbohydrate metabolism of the brain of mice ‘‘Mus musculus”. J. Basic Appl. Zool. 2016, 77, 1–7. [Google Scholar] [CrossRef]
- Alkaladi, A.; Abdelazim, A.M.; Afifi, M. Antidiabetic Activity of Zinc Oxide and Silver Nanoparticles on Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2014, 15, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Naghsh, N.; Kazemi, S. Effect of Nano-magnesium Oxide on Glucose Concentration and Lipid Profile in Diabetic Laboratory Mice. Iran. J. Pharm. Sci. 2014, 10, 63–68. [Google Scholar]
- Umrani, R.D.; Paknikar, K.M. Zinc oxide nanoparticles show antidiabetic activity in streptozotocin-induced Type 1 and 2 diabetic rats. Nanomedicine 2014, 9, 89–104. [Google Scholar] [CrossRef]
- El-Gharbawy, R.M.; Emara, A.M.; Abu-Risha, S.E.-S. Zinc oxide nanoparticles and a standard antidiabetic drug restore the function and structure of beta cells in Type-2 diabetes. Biomed. Pharmacother. 2016, 84, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Rashid, M.O.; Uddin, G.; Robel, F.N.; Hossain, M.S.; Haque, A. Jakaria Biological efficacy of zinc oxide nanoparticles against diabetes: A preliminary study conducted in mice. Biosci. Rep. 2020, 40, BSR20193972. [Google Scholar] [CrossRef]
- Neumann, U.H.; Ho, J.S.; Chen, S.; Tam, Y.Y.C.; Cullis, P.R.; Kieffer, T.J. Lipid nanoparticle delivery of glucagon receptor siRNA improves glucose homeostasis in mouse models of diabetes. Mol. Metab. 2017, 6, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Matias, L.L.R.; Costa, R.O.A.; Passos, T.S.; Queiroz, J.L.C.; Serquiz, A.C.; Maciel, B.L.L.; Santos, P.P.A.; Camillo, C.S.; Gonçalves, C.; Amado, I.R.; et al. Tamarind Trypsin Inhibitor in Chitosan–Whey Protein Nanoparticles Reduces Fasting Blood Glucose Levels without Compromising Insulinemia: A Preclinical Study. Nutrients 2019, 11, 2770. [Google Scholar] [CrossRef]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Zhuo, L.; Chen, S.; Zhao, L.; Chen, T.; Li, Y.; Zhang, W.; Gao, X.; Li, P.; et al. Interaction of titanium dioxide nanoparticles with glucose on young rats after oral administration. Nanomedicine 2015, 11, 1633–1642. [Google Scholar] [CrossRef]
- Hu, H.; Li, L.; Guo, Q.; Zong, H.; Yan, Y.; Yin, Y.; Wang, Y.; Oh, Y.; Feng, Y.; Wu, Q.; et al. RNA sequencing analysis shows that titanium dioxide nanoparticles induce endoplasmic reticulum stress, which has a central role in mediating plasma glucose in mice. Nanotoxicology 2018, 12, 341–356. [Google Scholar] [CrossRef]
- Hu, H.; Li, L.; Guo, Q.; Jin, S.; Zhou, Y.; Oh, Y.; Feng, Y.; Wu, Q.; Gu, N. A mechanistic study to increase understanding of titanium dioxide nanoparticles-increased plasma glucose in mice. Food Chem. Toxicol. 2016, 95, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Kasaai, M.R. Nanosized Particles of Silica and Its Derivatives for Applications in Various Branches of Food and Nutrition Sectors. J. Nanotechnol. 2015, 2015, 852394. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules 2017, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Fan, X.; Guo, Q.; Wei, X.; Yang, D.; Zhang, B.; Liu, J.; Wu, Q.; Oh, Y.; Feng, Y.; et al. Silicon dioxide nanoparticles induce insulin resistance through endoplasmic reticulum stress and generation of reactive oxygen species. Part. Fibre Toxicol. 2019, 16, 41. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.H.; Seo, C.; Lee, D.Y.; Ji, M.; Manavalan, B.; Basith, S.; Chakkarapani, S.K.; Kang, S.H.; Lee, G.; Paik, M.J.; et al. Silica-coated magnetic nanoparticles induce glucose metabolic dysfunction in vitro via the generation of reactive oxygen species. Arch. Toxicol. 2019, 93, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-P.; Wang, Z.-J.; Zhao, R.; Lin, C.-X.; Sun, Q.-Y.; Yan, C.-P.; Zhou, X.; Cao, J.-M. Silica nanomaterials induce organ injuries by Ca2+-ROS-initiated disruption of the endothelial barrier and triggering intravascular coagulation. Part. Fibre Toxicol. 2020, 17, 21. [Google Scholar] [CrossRef]
- Hu, H.; Guo, Q.; Fan, X.; Wei, X.; Yang, D.; Zhang, B.; Liu, J.; Wu, Q.; Oh, Y.; Feng, Y.; et al. Molecular mechanisms underlying zinc oxide nanoparticle induced insulin resistance in mice. Nanotoxicology 2020, 14, 59–76. [Google Scholar] [CrossRef]
- Robin, J. Heyden (advisor). Chapter 24. Metabolism and Nutrition. 164 24.3 Lipid Metabolism. In Anatomy and Physiology; OpenStax. BCcampus Open Education: Victoria, BC, Canada, 2022; Available online: https://opentextbc.ca/anatomyandphysiology/front-matter/preface-2/ (accessed on 20 April 2022).
- Sibuyi, N.R.S.; Moabelo, K.L.; Meyer, M.; Onani, M.O.; Dube, A.; Madiehe, A.M. Nanotechnology advances towards development of targeted-treatment for obesity. J. Nanobiotechnol. 2019, 17, 122. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2011, 4, 1871–1880, Epub 2011 Nov 10. [Google Scholar] [CrossRef]
- Chen, H.; Dorrigan, A.; Saad, S.; Hare, D.J.; Cortie, M.B.; Valenzuela, S.M. In Vivo Study of Spherical Gold Nanoparticles: Inflammatory Effects and Distribution in Mice. PLoS ONE 2013, 8, e58208. [Google Scholar] [CrossRef]
- Chen, H.; Ng, J.P.M.; Tan, Y.; McGrath, K.; Bishop, D.P.; Oliver, B.; Chan, Y.L.; Cortie, M.B.; Milthorpe, B.K.; Valenzuela, S.M. Gold nanoparticles improve metabolic profile of mice fed a high-fat diet. J. Nanobiotechnol. 2018, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ng, J.P.M.; Bishop, D.P.; Milthorpe, B.K.; Valenzuela, S.M. Gold nanoparticles as cell regulators: Beneficial effects of gold nanoparticles on the metabolic profile of mice with pre-existing obesity. J. Nanobiotechnol. 2018, 16, 88. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Bari, A.; Ullah, R.; Mathanmohun, M.; Veeraraghavan, V.P.; Sun, Z. Gold nanoparticles synthesized with Smilax glabra rhizome modulates the anti-obesity parameters in high-fat diet and streptozotocin induced obese diabetes rat model. J. Photochem. Photobiol. B Biol. 2019, 201, 111643, Epub 2019 Oct 16. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.G.; Han, H.S.; Han, J.H.; Lee, H.; Nguyen, V.Q.; Lee, W.H.; Kwon, S.; Heo, S.; Yoon, J.; Shin, H.H.; et al. Self-assembled hyaluronic acid nanoparticles: Implications as a nanomedicine for treatment of type 2 diabetes. J. Control. Release 2018, 279, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Rho, J.G.; Han, H.S.; Kweon, S.; Nguyen, V.Q.; Park, J.H.; Kim, W. Self-assembled hyaluronic acid nanoparticle suppresses fat accumulation via CD44 in diet-induced obese mice. Carbohydr. Polym. 2020, 237, 116161. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, J.; Zhang, P.; Zhou, D.; Liu, F. Dynamics of Transcription Factors in Three Early Phases of Osteogenic, Adipogenic, and Chondrogenic Differentiation Determining the Fate of Bone Marrow Mesenchymal Stem Cells in Rats. Front. Cell Dev. Biol. 2021, 9, 768316. [Google Scholar] [CrossRef]
- Yi, C.; Liu, D.; Fong, C.-C.; Zhang, J.; Yang, M. Gold Nanoparticles Promote Osteogenic Differentiation of Mesenchymal Stem Cells through p38 MAPK Pathway. ACS Nano 2010, 4, 6439–6448. [Google Scholar] [CrossRef]
- Liu, D.; Yi, C.; Zhang, D.; Zhang, J.; Yang, M. Inhibition of Proliferation and Differentiation of Mesenchymal Stem Cells by Carboxylated Carbon Nanotubes. ACS Nano 2010, 4, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Dasuri, K.; Zhang, L.; Ebenezer, P.; Fernandez-Kim, S.O.; Bruce-Keller, A.J.; Szweda, L.I.; Keller, J.N. Proteasome alterations during adipose differentiation and aging: Links to impaired adipocyte differentiation and development of oxidative stress. Free Radic. Biol. Med. 2011, 51, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Przybytkowski, E.; Behrendt, M.; Dubois, D.; Maysinger, D. Nanoparticles can induce changes in the intracellular metabolism of lipids without compromising cellular viability. FEBS J. 2009, 276, 6204–6217. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yang, X.; Wu, Q. Photocatalytic Oxidation of Escherischia coli, Aspergillus niger, and Formaldehyde under Different Ultraviolet Irradiation Conditions. Environ. Sci. Technol. 2009, 43, 4606–4611. [Google Scholar] [CrossRef] [PubMed]
- Sohm, B.; Immel, F.; Bauda, P.; Pagnout, C. Insight into the primary mode of action of TiO2 nanoparticles on Escherichia coli in the dark. Proteomics 2015, 15, 98–113. [Google Scholar] [CrossRef]
- Chen, Z.; Han, S.; Zheng, P.; Zhou, D.; Zhou, S.; Jia, G. Effect of oral exposure to titanium dioxide nanoparticles on lipid metabolism in Sprague-Dawley rats. Nanoscale 2020, 12, 5973–5986. [Google Scholar] [CrossRef]
- Papazyan, R.; Sun, Z.; Kim, Y.H.; Titchenell, P.M.; Hill, D.A.; Lu, W.; Damle, M.; Wan, M.; Zhang, Y.; Briggs, E.R.; et al. Physiological Suppression of Lipotoxic Liver Damage by Complementary Actions of HDAC3 and SCAP/SREBP. Cell Metab. 2016, 24, 863–874. [Google Scholar] [CrossRef]
- Kumar, P.; Salve, R.; Gajbhiye, K.R.; Gajbhiye, V. Stimuli-Responsive Nanocarriers. Recent Advances in Tailor-Made Therapeutics: Chapter 1—An Overview of Stimuli-Responsive Nanocarriers: State of the Art; Virendra, G., Kavita, R.G., Seungpyo, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 978-0-12-824456-2. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Aggarwal, P.; Hall, J.B.; McNeil, S.E. Preclinical Studies To Understand Nanoparticle Interaction with the Immune System and Its Potential Effects on Nanoparticle Biodistribution. Mol. Pharm. 2008, 5, 487–495. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A. Pre-clinical immunotoxicity studies of nanotechnology-formulated drugs: Challenges, considerations and strategy. J. Control. Release 2015, 220, 571–583. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, Y.; Wargo, J.A.; Lang, F.F.; Kim, B.Y.S. Considerations for designing preclinical cancer immune nanomedicine studies. Nat. Nanotechnol. 2021, 16, 6–15. [Google Scholar] [CrossRef]
| Features | Categories | Description |
|---|---|---|
| Origin [4,5,6,7,8,9,10] | Natural | Erosion and dust storms, volcanic activity, forest fires, and from biogenic sources, e.g. shed skin and hair and o bioreductively formed deposits of elements in certain bacteria. |
| Synthetic | Pollutant: Simple combustion, food cooking, industrial manufacturing, combustion (in vehicle and airplane engines and for power generation). | |
| Intentionally produced: pesticides and fertilizers, cosmetic and personal care products, tires, clothing, water-repellent products, food additives and treatment and diagnosis in medicine. | ||
| Dimensionality (Electron movement) [1,11,12,13] | 0D | Quantum dots, nanoparticles arrays, core-shell nanoparticles, hollow cubes and nanospheres. |
| 1D | Nanowires, nanorods, nanotubes, nanobelts, nanoribbons, and hierarchical nanostructures. | |
| 2D | Junctions, branched structures, nanoprisms, nanoplates, nanosheets, nanowalls and nanodisks. | |
| 3D | Nanoballs(dendritic structures), nanocoils, nanocones, nanopillers and nanoflowers. | |
| Morphology [1,4,14,15] | Flatness, sphericity and aspect ratio | Implications: Internalization rate of a particle and toxicity. |
| High-aspect ratio: nanotubes and nanowires. | ||
| Low-aspect ratio: spherical, oval, cubic, prism, helical or pillar morphologies. | ||
| Composition (Single material or composite) [4,7,8,9,16,17,18] | Material-based categories | Carbon-based nanomaterials. |
| Inorganic-based nanomaterials(metal and metal oxide). | ||
| Organic-based nanomaterials | ||
| Composite-based nanomaterials. | ||
| Agglomeration state/Uniformity [4,19,20] | Disperses or agglomerated | The surface properties primarily determine the agglomeration state and uniformity of the particles and therefore their effective size, especially under physiological conditions. |
| Implications: cellular uptake, in vivo biodistribution, effective area of metal release and generation of reactive oxygen species generation(ROS),shape into a fractal and toxicity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Pardillos, A.; Martin-Duque, P. Cellular Alterations in Carbohydrate and Lipid Metabolism Due to Interactions with Nanomaterials. J. Funct. Biomater. 2023, 14, 274. https://doi.org/10.3390/jfb14050274
Martín-Pardillos A, Martin-Duque P. Cellular Alterations in Carbohydrate and Lipid Metabolism Due to Interactions with Nanomaterials. Journal of Functional Biomaterials. 2023; 14(5):274. https://doi.org/10.3390/jfb14050274
Chicago/Turabian StyleMartín-Pardillos, Ana, and Pilar Martin-Duque. 2023. "Cellular Alterations in Carbohydrate and Lipid Metabolism Due to Interactions with Nanomaterials" Journal of Functional Biomaterials 14, no. 5: 274. https://doi.org/10.3390/jfb14050274
APA StyleMartín-Pardillos, A., & Martin-Duque, P. (2023). Cellular Alterations in Carbohydrate and Lipid Metabolism Due to Interactions with Nanomaterials. Journal of Functional Biomaterials, 14(5), 274. https://doi.org/10.3390/jfb14050274






