Microparticles of Sericin-Dextran Conjugate for Improving the Solubility of Antiviral Drug
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials
2.2. Measurements
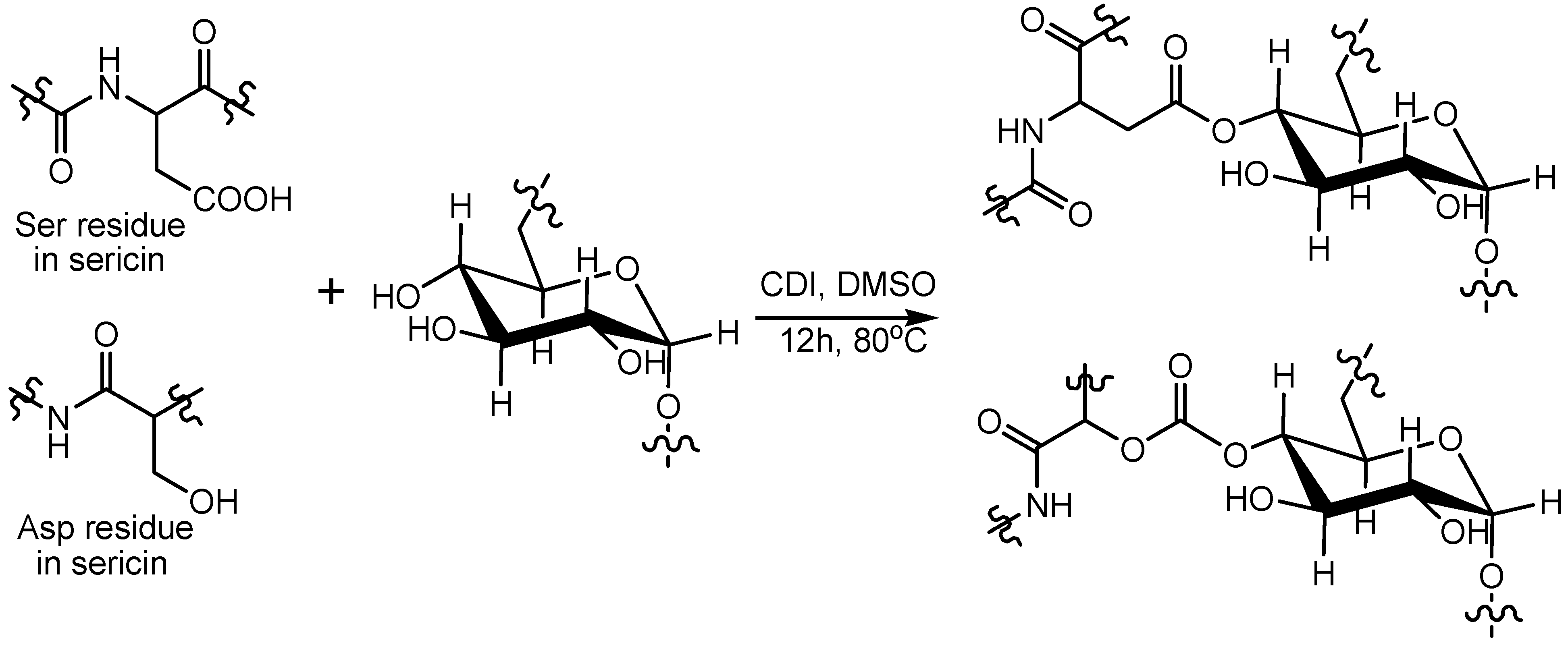
2.3. Preparation of Sericin-Dextran Conjugate
2.4. Microparticles Preparation
2.5. In Vitro Release
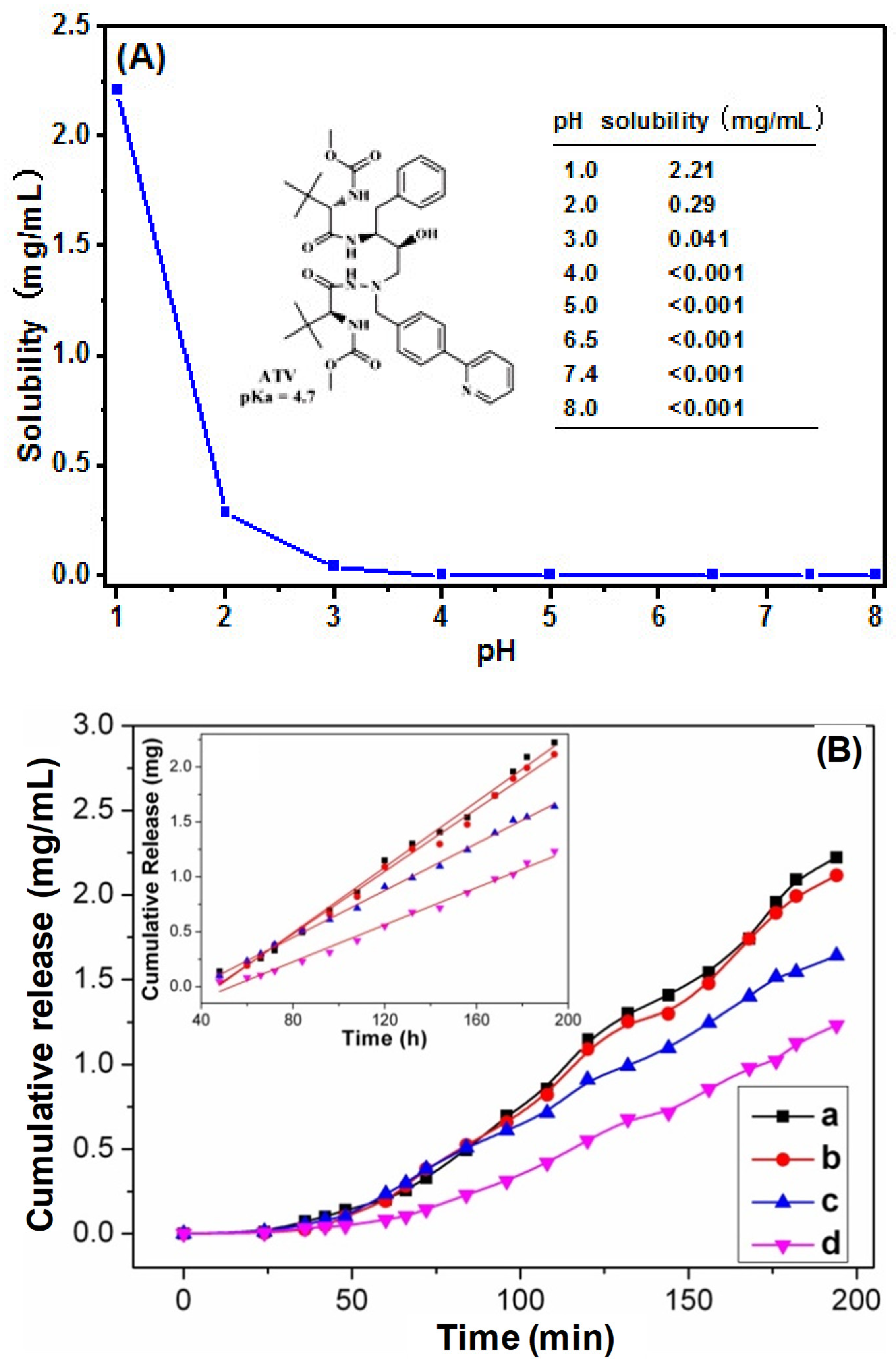
2.6. Solubility Study
3. Results and Discussion
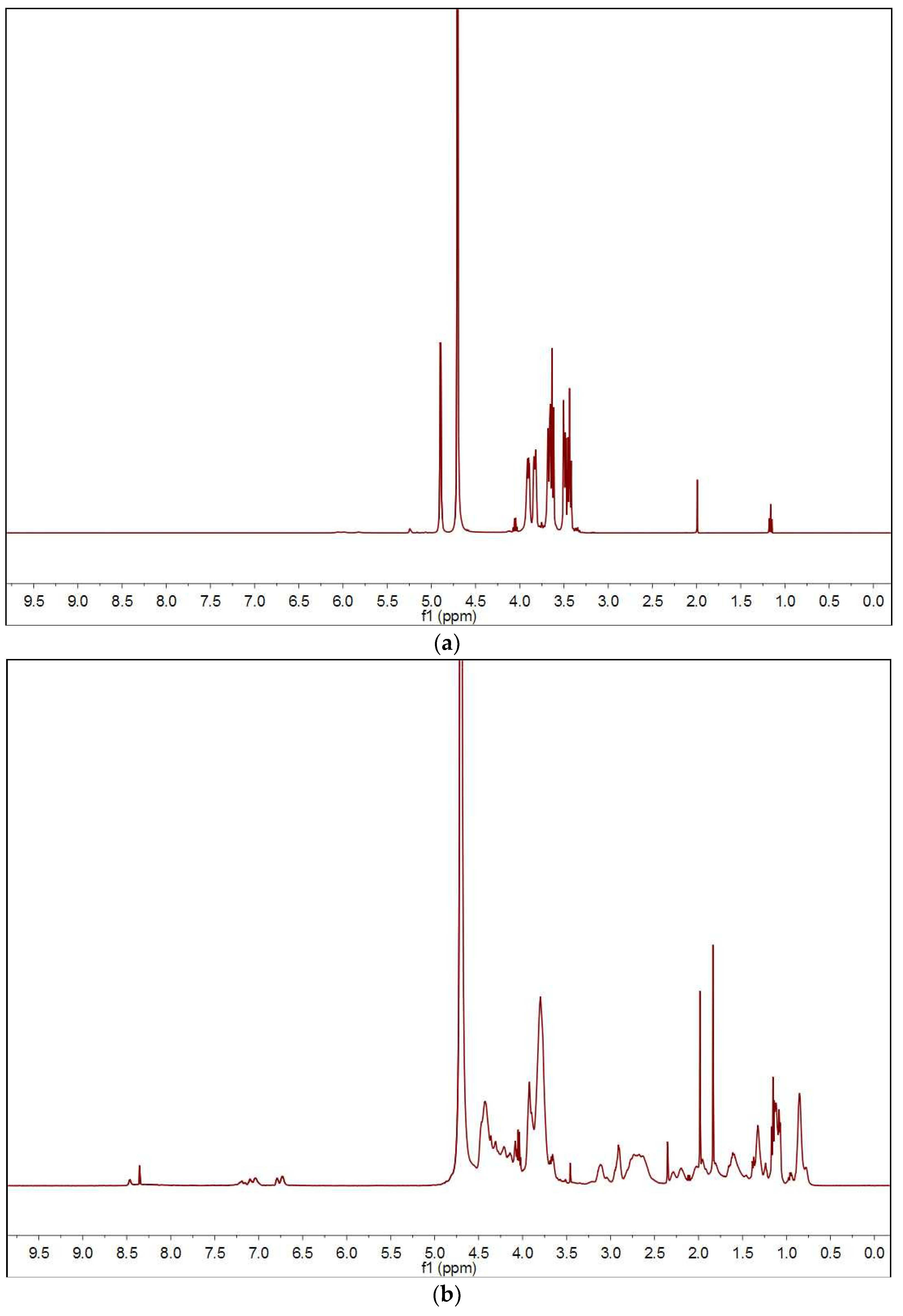
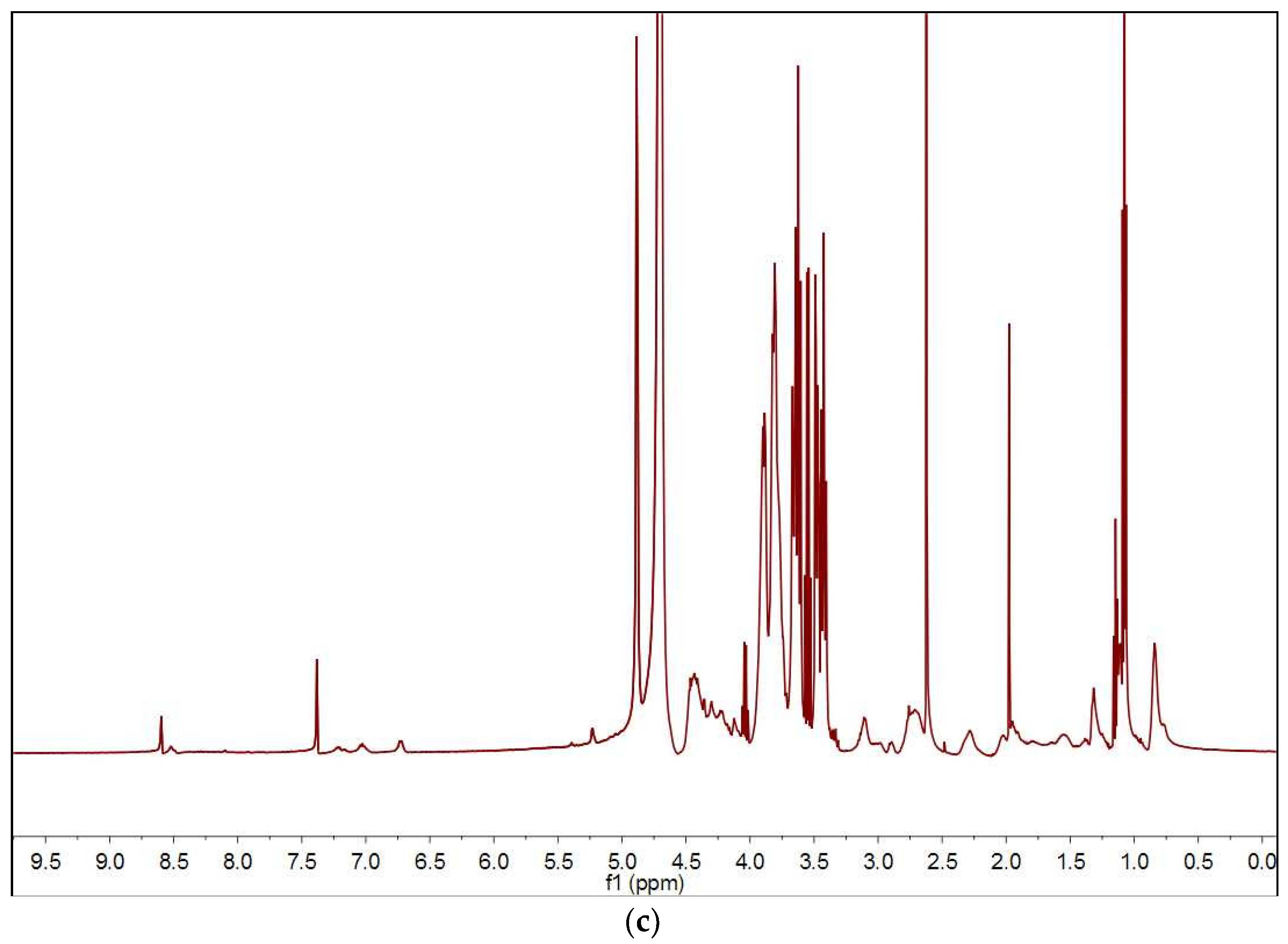
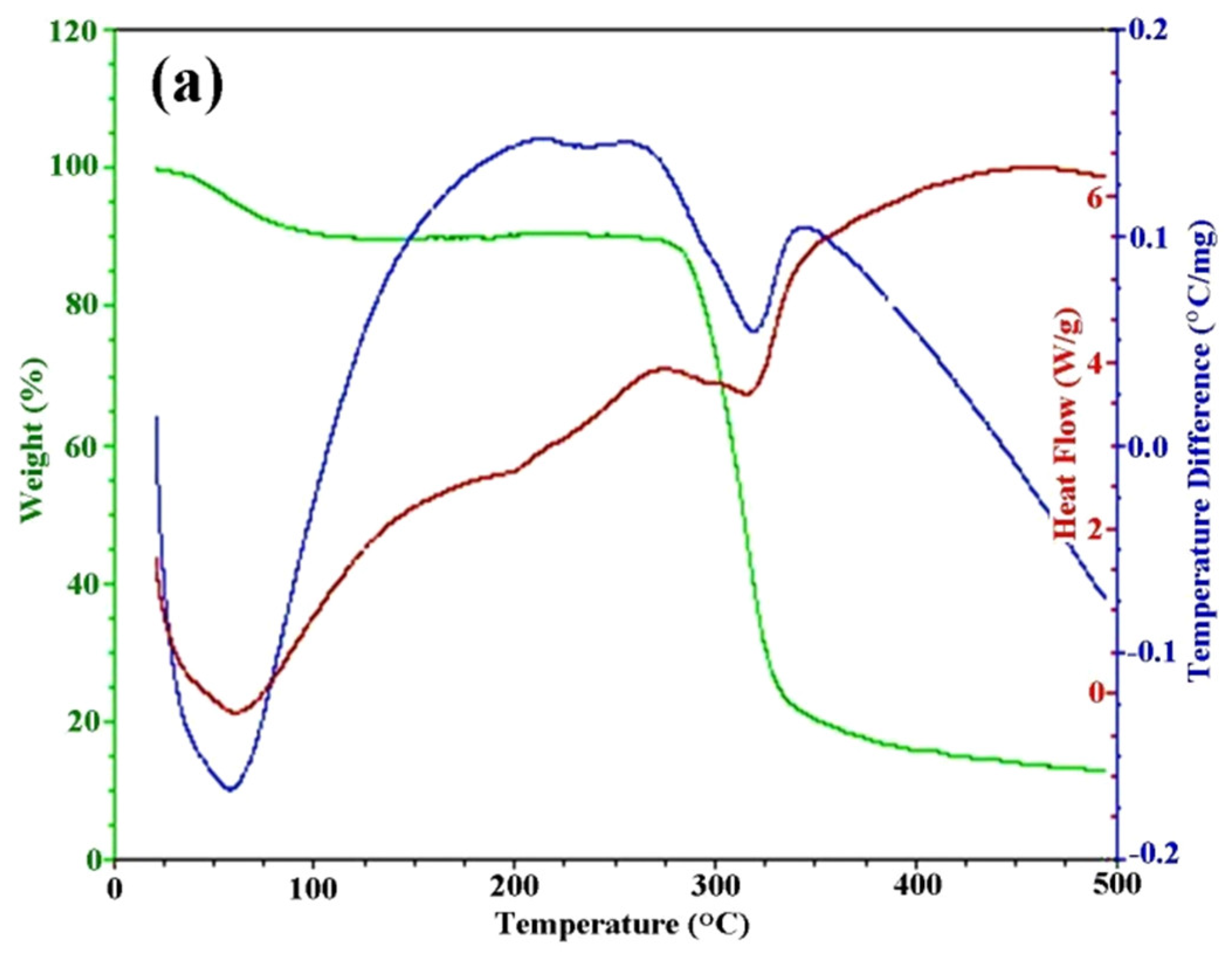
3.1. Preparation of Sericin-Dextran Conjugate
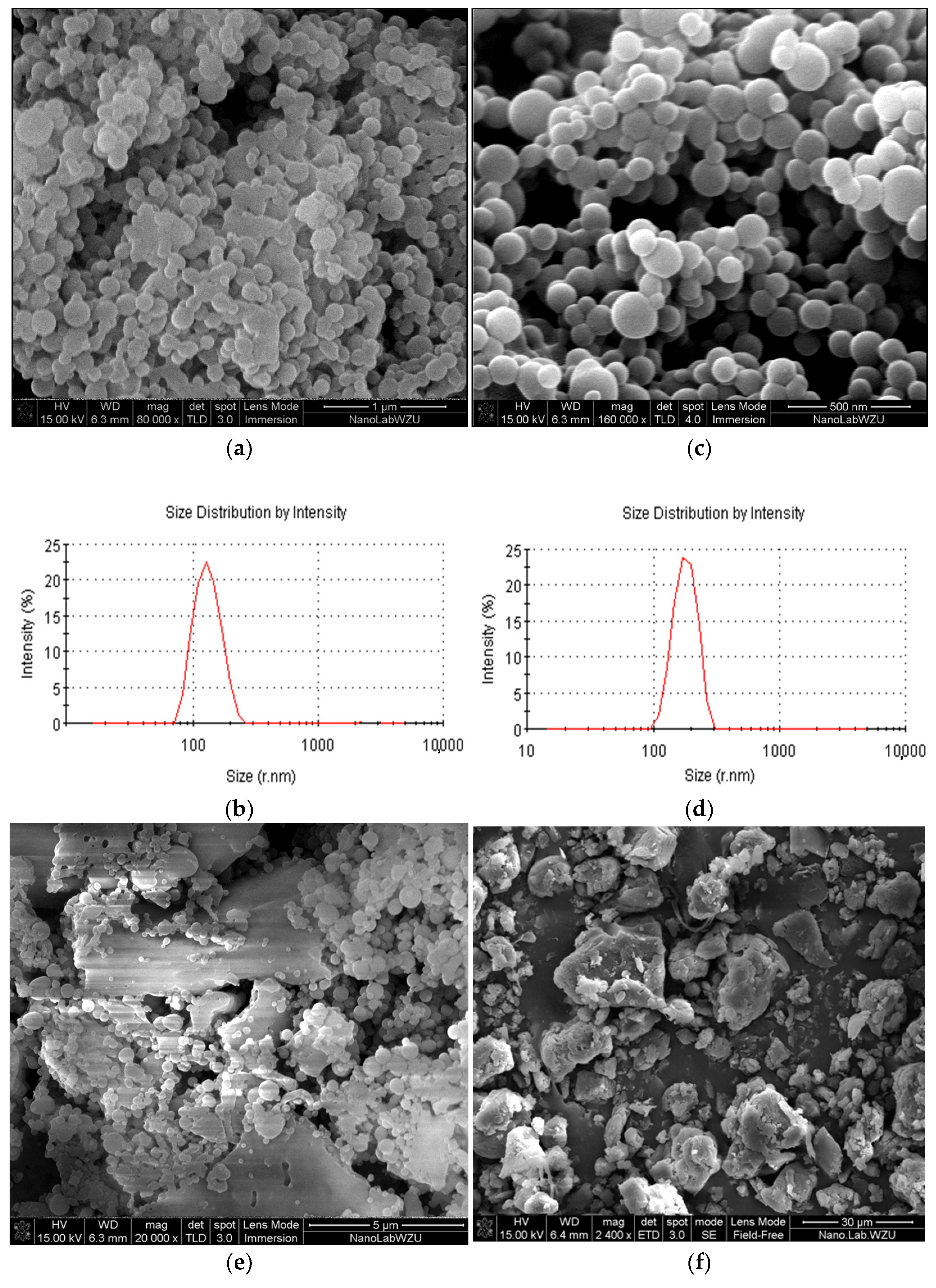
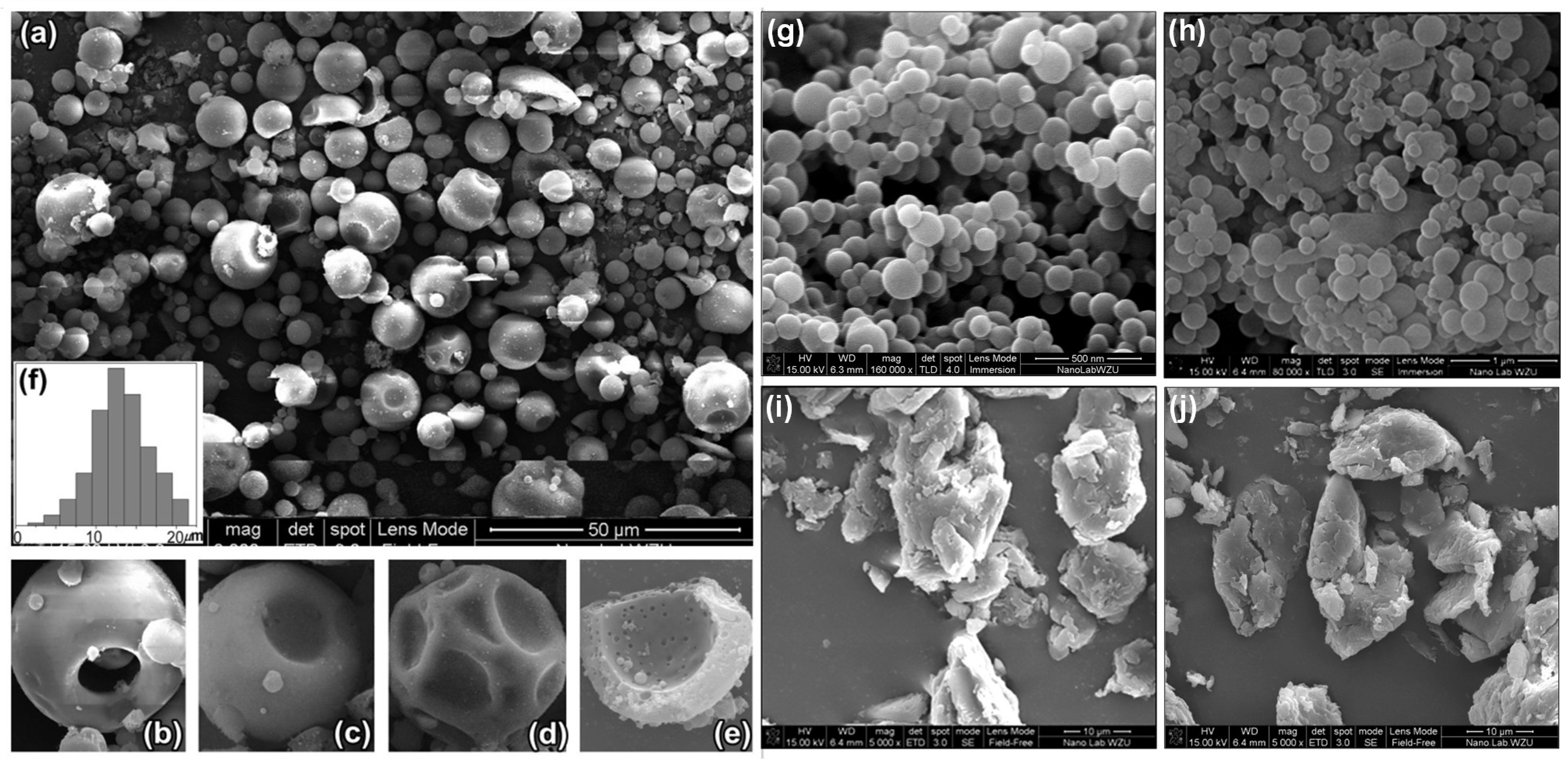
3.2. Effect of Concentration on Microspheres’ Forming
3.3. Effect of Solvents on Microparticles
3.4. Solubility Study
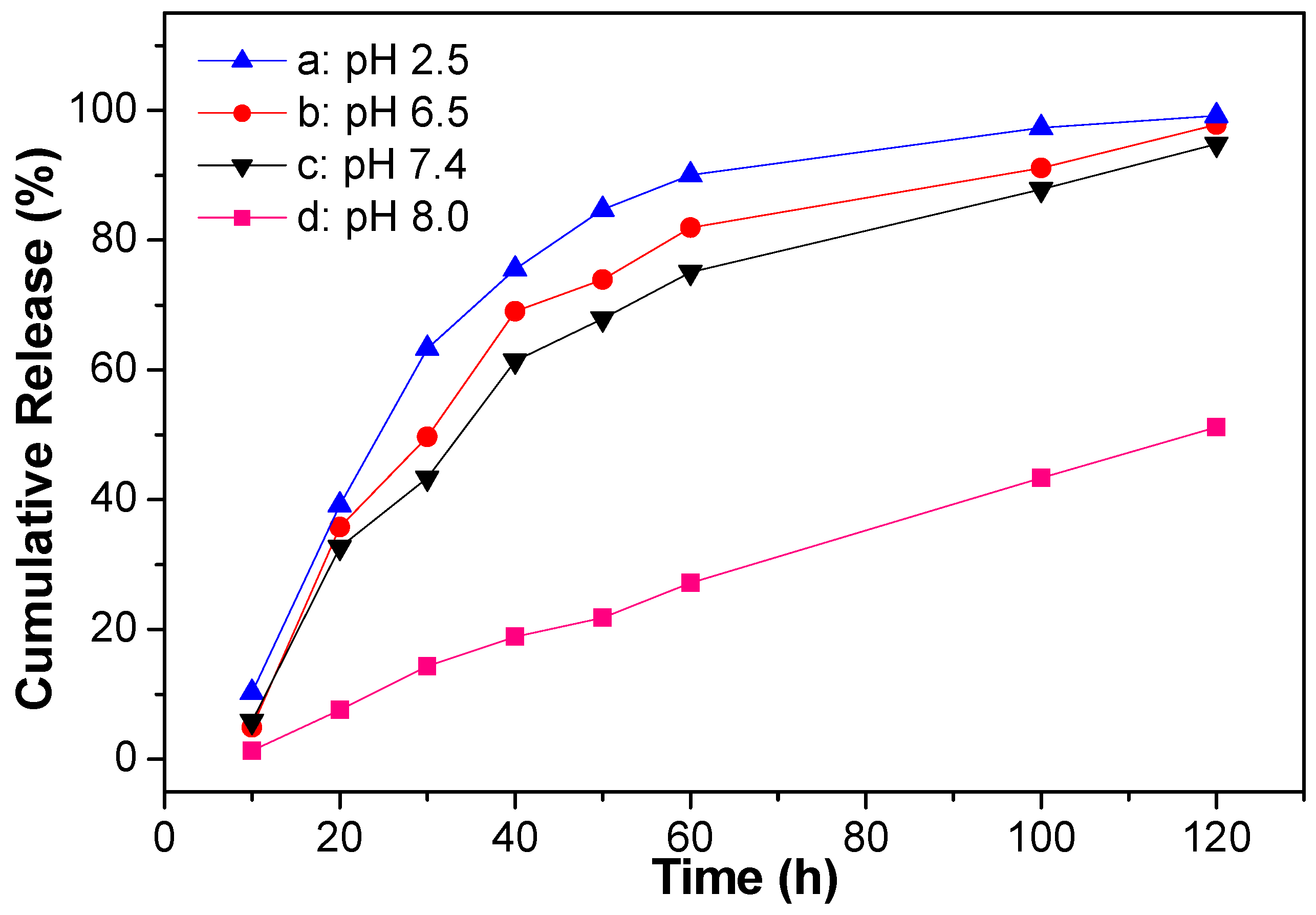
3.5. In Vitro Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khwaza, V.; Buyana, B.; Nqoro, X.; Peter, S.; Mbese, Z.; Feketshane, Z.; Alven, S.; Aderibigbe, B.A. Chapter 19—Strategies for delivery of antiviral agents. In Viral Infections and Antiviral Therapies; Academic Press: Cambridge, MA, USA, 2023; pp. 407–492. [Google Scholar]
- Debotton, N.; Garsiani, S.; Cohen, Y.; Dahan, A. Enabling oral delivery of antiviral drugs: Double emulsion carriers to improve the intestinal absorption of zanamivir. Int. J. Pharm. 2022, 629, 122392. [Google Scholar] [CrossRef] [PubMed]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Franklyne, J.S.; Mukherjee, A.; Chandrasekaran, N. Nanoemulsions: The rising star of antiviral therapeutics and nanodelivery system-current status and prospects. Curr. Opin. Colloid Interface Sci. 2021, 54, 101458. [Google Scholar] [CrossRef]
- Maus, A.; Strait, L.; Zhu, D. Microparticles as delivery vehicles for antiviral therapeutic drugs. Eng. Regen. 2021, 2, 31–46. [Google Scholar]
- Meziadi, A.; Zuberi, N.; de Haan, H.W.; Gauthier, M.A. Overcoming PEG-protein mutual repulsion to improve the efficiency of PEGylation. Biomacromolecules 2022, 23, 4948–4956. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, H.; Wu, J.; Wang, Z.; Sun, H.; Yuan, J.; Chen, S. A novel zwitterionic copolymer with a short poly(methyl acrylic acid) block for improving both conjugation and separation efficiency of a protein without losing its bioactivity. J. Mater. Chem. B 2013, 1, 2482–2488. [Google Scholar] [CrossRef]
- Sun, J.W.; Liu, X.Y.; Guo, J.W.; Zhao, W.G.; Gao, W.P. Pyridine-2,6-dicarboxaldehyde-enabled N-terminal in situ growth of polymer-interferon alpha conjugates with significantly improved pharmacokinetics and in vivo bioactivity. ACS Appl. Mater. Interfaces 2021, 13, 88–96. [Google Scholar] [CrossRef]
- Huynh, V.; Ifraimov, N.; Wylie, R.G. Modulating the thermoresponse of polymer-protein conjugates with hydrogels for controlled release. Polymers 2021, 16, 2772. [Google Scholar] [CrossRef]
- Hu, D.; Li, T.; Liang, W.; Wang, Y.; Feng, M.; Sun, J. Silk sericin as building blocks of bioactive materials for advanced therapeutics. J. Control Release 2023, 353, 303–316. [Google Scholar] [CrossRef]
- Li, Y.; Wu, T.; Zhang, G.; Fang, A.; Li, Y.; Wang, S.; Yan, H.; Liang, P.; Lian, J.; Zhang, Y. A native sericin wound dressing spun directly from silkworms enhances wound healing. Colloids Surf. B Biointerfaces 2023, 225, 113228. [Google Scholar] [CrossRef]
- Yang, C.; Yao, L.; Zhang, L. Silk sericin-based biomaterials shine in food and pharmaceutical industries. Smart Mater. Med. 2023, 4, 447–459. [Google Scholar] [CrossRef]
- Kundu, S.C.; Dash, B.C.; Dash, R.; Kaplan, D.L. Natural protective glue protein, sericin bioengineered by silkworms: Potential for biomedical and biotechnological applications. Prog. Polym. Sci. 2008, 33, 998–1012. [Google Scholar] [CrossRef]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric microparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef]
- Liu, J.; Shi, L.; Deng, Y.; Zou, M.; Cai, B.; Song, Y.; Wang, Z. Silk sericin-based materials for biomedical applications. Biomaterials 2022, 287, 121638. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; He, H.; Cai, R.; Tao, G.; Yang, M.; Zuo, H.; Umar, A.; Wang, Y. Cross-linking of dialdehyde carboxymethyl cellulose with silk sericin to reinforce sericin film for potential biomedical application. Carbohydr. Polym. 2019, 212, 403–411. [Google Scholar] [CrossRef] [PubMed]
- El-Samad, L.M.; Hassan, M.A.; Basha, A.A.; El-Ashram, S.; Radwan, E.H.; Aziz, K.K.A.; Tamer, T.M.; Augustyniak, M.; Wakil, A.E. Carboxymethyl cellulose/sericin-based hydrogels with intrinsic antibacterial, antioxidant, and anti-inflammatory properties promote re-epithelization of diabetic wounds in rats. Int. J. Pharm. 2022, 629, 122328. [Google Scholar] [CrossRef] [PubMed]
- Behnke, M.; Klemm, P.; Dahlke, P.; Shkodra, B.; Beringer-Siemers, B.; Czaplewska, J.A.; Stumpf, S.; Jordan, P.M.; Schubert, S.; Hoeppener, S.; et al. Ethoxy acetalated dextran microparticles for drug delivery: A comparative study of formulation methods. Int. J. Pharm. 2023, 5, 100173. [Google Scholar]
- Singh, G.; Pai, R.S. Optimized self-nanoemulsifying drug delivery system of atazanavir with enhanced oral bioavailability: In vitro/in vivo characterization. Expert Opin. Drug Deliv. 2014, 11, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Berlin, M.; Ruff, A.; Kesisoglou, F.; Xu, W.; Wang, M.H.; Dressman, J.B. Advances and challenges in PBPK modeling-Analysis of factors contributing to the oral absorption of atazanavir, a poorly soluble weak base. Eur. J. Pharm. Biopharm. 2015, 93, 267–280. [Google Scholar] [CrossRef]
- Wondraczek, H.; Elschner, T.; Heinze, T. Synthesis of highly functionalized dextran alkyl carbonates showing nanosphere formation. Carbohydr. Polym. 2011, 83, 1112–1118. [Google Scholar] [CrossRef]
- Zhang, X.; Tsukada, M.; Morikawa, H.; Aojima, K.; Zhang, G.; Miura, M. Production of silk sericin/silk fibroin blend nanofibers. Nanoscale Res. Lett. 2011, 6, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.Y.; Moon, J.Y.; Lee, Y.W.; Lee, K.G.; Yeo, J.H.; Kweon, H.Y.; Kim, K.H.; Cho, C.S. Preparation of self-assembled silk sericin microparticles. Int. J. Biol. Macromol. 2003, 32, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhu, C.; Sun, W.; Xu, Y.; Xiao, X.; Zheng, H.; Wu, H.; Liu, C. Plasma-initiated polymerization of chitosan-based CS-g-P(AM-DMDAAC) flocculant for the enhanced flocculation of low-algal-turbidity water. Carbohydr. Polym. 2017, 164, 222–232. [Google Scholar] [CrossRef]
- Ha, S.W.; Park, Y.H.; Hudson, S.M. Dissolution of bombyx mori silk fibroin in the calcium nitrate tetrahydrate-methanol system and aspects of wet spinning of fibroin solution. Biomacromolecules 2003, 4, 488–496. [Google Scholar] [CrossRef]
- Alamdarnejad, G.; Sharif, A.; Taranejoo, S.; Janmaleki, M.; Kalaee, M.R.; Dadgar, M.; Khakpour, M. Synthesis and characterization of thiolate carboxymethyl chitosan-graft-cyclodextrin microparticles as a drug delivery vehicle for albendazole. J. Mater. Sci. 2013, 24, 1939–1949. [Google Scholar]
- Yooyod, M.; Ross, G.M.; Limpeanchob, N.; Suphrom, N.; Mahasaranon, S.; Ross, S. Investigation of silk sericin conformational structure for fabrication into porous scaffolds with poly(vinyl alcohol) for skin tissue reconstruction. Eur. Polym. J. 2016, 81, 43–52. [Google Scholar] [CrossRef]
- Teramoto, H.; Kakazu, A.; Asakura, T. Native structure and degradation pattern of silk sericin studied by 13C NMR spectroscopy. Macromolecules 2006, 39, 6–8. [Google Scholar] [CrossRef]
- Andrabi, S.M.; Majumder, S.; Gupta, K.C.; Kumar, A. Dextran based amphiphilic nano-hybrid hydrogel system incorporated with curcumin and cerium oxide microparticles for wound healing. Colloids Surf. B Biointerfaces 2020, 195, 111263. [Google Scholar] [CrossRef]
- Das, M.; Joshi, A.; Devkar, R.; Seshadri, S.; Thakore, S. Tumor homing dextran and curcumin derived amphiphilic functional polymer self-assembling to tubustecan nanoarchitectures: A strategy of adorning the golden spice (curcumin) for taming the red devil (Dox). J. Drug Deliv. Sci. Technol. 2022, 76, 103666. [Google Scholar] [CrossRef]
- Zhou, C.; Gao, W.; Lu, G.; Ding, J.; Wu, X.; Huang, X.; Chen, J.; Liu, M.; Jiang, J.; Wu, H. Preparation, characterization and in vitro release of microparticles based on dextran-osuvastatin conjugate. Carbohydr. Polym. 2013, 96, 156–162. [Google Scholar] [CrossRef]
- Liu, H.L.; Wang, D.; Yang, X.L. Preparation of polymer@titania raspberry-like core-corona composite via heterocoagulated self-assembly based on hydrogen-bonding interaction. Colloid Surf. A 2012, 397, 48–58. [Google Scholar] [CrossRef]
- Shahab, S.; Sheikhi, M.; Alnajjar, R.; Saud, S.A.; Khancheuski, M.; Strogova, A. DFT investigation of atazanavir as potential inhibitor for 2019-nCoV coronavirus M protease. J. Mol. Struct. 2021, 1228, 129461. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Feng, X.; Li, X.; Liu, M.; Gao, W.; Miao, Q.; Wu, H. Microparticles of Sericin-Dextran Conjugate for Improving the Solubility of Antiviral Drug. J. Funct. Biomater. 2023, 14, 292. https://doi.org/10.3390/jfb14060292
Chen S, Feng X, Li X, Liu M, Gao W, Miao Q, Wu H. Microparticles of Sericin-Dextran Conjugate for Improving the Solubility of Antiviral Drug. Journal of Functional Biomaterials. 2023; 14(6):292. https://doi.org/10.3390/jfb14060292
Chicago/Turabian StyleChen, Shuqi, Xiaolong Feng, Xinwei Li, Miaochang Liu, Wenxia Gao, Qian Miao, and Huayue Wu. 2023. "Microparticles of Sericin-Dextran Conjugate for Improving the Solubility of Antiviral Drug" Journal of Functional Biomaterials 14, no. 6: 292. https://doi.org/10.3390/jfb14060292




