Experimental Composite Resin with Myristyltrimethylammonium Bromide (MYTAB) and Alpha-Tricalcium Phosphate (α-TCP): Antibacterial and Remineralizing Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Formulation of Experimental Resin
2.2. Degree of Conversion (DC)
2.3. Knoop Microhardness and Softening in Solvent
2.4. Flexural Strength
2.5. Cytotoxicity
2.6. Antibacterial Activity
2.7. Mineral Deposition
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferracane, J.L. Resin Composite--State of the Art. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Pallesen, U.; van Dijken, J.W.V. A Randomized Controlled 30 Years Follow up of Three Conventional Resin Composites in Class II Restorations. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2015, 31, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Demarco, F.F.; Cenci, M.S.; Montagner, A.F.; de Lima, V.P.; Correa, M.B.; Moraes, R.R.; Opdam, N.J.M. Longevity of Composite Restorations Is Definitely Not Only about Materials. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2023, 39, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Opdam, N.J.M.; van de Sande, F.H.; Bronkhorst, E.; Cenci, M.S.; Bottenberg, P.; Pallesen, U.; Gaengler, P.; Lindberg, A.; Huysmans, M.C.D.N.J.M.; van Dijken, J.W. Longevity of Posterior Composite Restorations: A Systematic Review and Meta-Analysis. J. Dent. Res. 2014, 93, 943–949. [Google Scholar] [CrossRef]
- Imazato, S. Antibacterial Properties of Resin Composites and Dentin Bonding Systems. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2003, 19, 449–457. [Google Scholar] [CrossRef]
- Chen, L.; Shen, H.; Suh, B.I. Antibacterial Dental Restorative Materials: A State-of-the-Art Review. Am. J. Dent. 2012, 25, 337–346. [Google Scholar]
- Huang, Q.; Huang, S.; Liang, X.; Qin, W.; Liu, F.; Lin, Z.; He, J. The Antibacterial, Cytotoxic, and Flexural Properties of a Composite Resin Containing a Quaternary Ammonium Monomer. J. Prosthet. Dent. 2018, 120, 609–616. [Google Scholar] [CrossRef]
- Vidal, M.L.; Rego, G.F.; Viana, G.M.; Cabral, L.M.; Souza, J.P.B.; Silikas, N.; Schneider, L.F.; Cavalcante, L.M. Physical and Chemical Properties of Model Composites Containing Quaternary Ammonium Methacrylates. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2018, 34, 143–151. [Google Scholar] [CrossRef]
- de Souza Balbinot, G.; Marcon, N.; Sauro, S.; Luxan, S.A.; Collares, F.M. Alkyl Trimethyl Ammonium Bromide for the Formulation of Antibacterial Orthodontic Resins. Clin. Oral Investig. 2022, 26, 7011–7019. [Google Scholar] [CrossRef]
- Garcia, I.M.; Leitune, V.C.B.; Arthur, R.A.; Nunes, J.; Visioli, F.; Giovarruscio, M.; Sauro, S.; Collares, F.M. Chemical, Mechanical and Biological Properties of an Adhesive Resin with Alkyl Trimethyl Ammonium Bromide-Loaded Halloysite Nanotubes. J. Adhes. Dent. 2020, 22, 399–407. [Google Scholar] [CrossRef]
- Garcia, I.M.; Rodrigues, S.B.; de Souza Balbinot, G.; Visioli, F.; Leitune, V.C.B.; Collares, F.M. Quaternary Ammonium Compound as Antimicrobial Agent in Resin-Based Sealants. Clin. Oral Investig. 2019, 24, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Hu, Y.; Huang, F.; Xiao, Y. Quaternary Ammonium Compounds in Dental Restorative Materials. Dent. Mater. J. 2018, 37, 183–191. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y. Structure–Activity Relationship of Cationic Surfactants as Antimicrobial Agents. Curr. Opin. Colloid Interface Sci. 2020, 45, 28–43. [Google Scholar] [CrossRef]
- Quan, A.; McGeachie, A.B.; Keating, D.J.; van Dam, E.M.; Rusak, J.; Chau, N.; Malladi, C.S.; Chen, C.; McCluskey, A.; Cousin, M.A.; et al. Myristyl Trimethyl Ammonium Bromide and Octadecyl Trimethyl Ammonium Bromide Are Surface-Active Small Molecule Dynamin Inhibitors That Block Endocytosis Mediated by Dynamin I or Dynamin II. Mol. Pharmacol. 2007, 72, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Joondan, N.; Caumul, P.; Jackson, G.; Jhaumeer Laulloo, S. Novel Quaternary Ammonium Compounds Derived from Aromatic and Cyclic Amino Acids: Synthesis, Physicochemical Studies and Biological Evaluation. Chem. Phys. Lipids 2021, 235, 105051. [Google Scholar] [CrossRef]
- Mena Silva, P.A.; Garcia, I.M.; Nunes, J.; Visioli, F.; Castelo Branco Leitune, V.; Melo, M.A.; Collares, F.M. Myristyltrimethylammonium Bromide (MYTAB) as a Cationic Surface Agent to Inhibit Streptococcus Mutans Grown over Dental Resins: An In Vitro Study. J. Funct. Biomater. 2020, 11, 9. [Google Scholar] [CrossRef]
- Giardino, L.; Andrade, F.B.D.; Beltrami, R. Antimicrobial Effect and Surface Tension of Some Chelating Solutions with Added Surfactants. Braz. Dent. J. 2016, 27, 584–588. [Google Scholar] [CrossRef]
- Schwendicke, F.; Splieth, C.H.; Bottenberg, P.; Breschi, L.; Campus, G.; Doméjean, S.; Ekstrand, K.; Giacaman, R.A.; Haak, R.; Hannig, M.; et al. How to Intervene in the Caries Process in Adults: Proximal and Secondary Caries? An EFCD-ORCA-DGZ Expert Delphi Consensus Statement. Clin. Oral Investig. 2020, 24, 3315–3321. [Google Scholar] [CrossRef]
- Diercke, K.; Lussi, A.; Kersten, T.; Seemann, R. Isolated Development of Inner (Wall) Caries like Lesions in a Bacterial-Based in Vitro Model. Clin. Oral Investig. 2009, 13, 439–444. [Google Scholar] [CrossRef]
- Braga, R.R. Calcium Phosphates as Ion-Releasing Fillers in Restorative Resin-Based Materials. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2018, 35, 3–14. [Google Scholar] [CrossRef]
- Sauro, S.; Spagnuolo, G.; Del Giudice, C.; Neto, D.M.A.; Fechine, P.B.A.; Chen, X.; Rengo, S.; Chen, X.; Feitosa, V.P. Chemical, structural and cytotoxicity characterisation of experimental fluoride-doped calcium phosphates as promising remineralising materials for dental applications. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2023, 39, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Skallevold, H.E.; Rokaya, D.; Khurshid, Z.; Zafar, M.S. Bioactive Glass Applications in Dentistry. Int. J. Mol. Sci. 2019, 20, 5960. [Google Scholar] [CrossRef] [PubMed]
- de Souza Balbinot, G.; Collares, F.M.; Herpich, T.L.; Visioli, F.; Samuel, S.M.W.; Leitune, V.C.B. Niobium Containing Bioactive Glasses as Remineralizing Filler for Adhesive Resins. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2020, 36, 221–228. [Google Scholar] [CrossRef]
- Al-Eesa, N.A.; Karpukhina, N.; Hill, R.G.; Johal, A.; Wong, F.S.L. Bioactive Glass Composite for Orthodontic Adhesives—Formation and Characterisation of Apatites Using MAS-NMR and SEM. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2019, 35, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Chiari, M.D.S.; Rodrigues, M.C.; Xavier, T.A.; de Souza, E.M.N.; Arana-Chavez, V.E.; Braga, R.R. Mechanical Properties and Ion Release from Bioactive Restorative Composites Containing Glass Fillers and Calcium Phosphate Nano-Structured Particles. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2015, 31, 726–733. [Google Scholar] [CrossRef]
- Langhorst, S.E.; O’Donnell, J.N.R.; Skrtic, D. In Vitro Remineralization of Enamel by Polymeric Amorphous Calcium Phosphate Composite: Quantitative Microradiographic Study. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2009, 25, 884–891. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphates in Dentistry. J. Mater. Sci. Mater. Med. 2013, 24, 1335–1363. [Google Scholar] [CrossRef]
- Collares, F.M.; Leitune, V.C.B.; Portella, F.F.; Santos, P.D.; de Souza Balbinot, G.; Dos Santos, L.A.; Parolo, C.C.F.; Samuel, S.M.W. Methacrylate-Based Root Canal Sealer Containing Chlorexidine and α-Tricalcium Phosphate. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 106, 1439–1443. [Google Scholar] [CrossRef]
- Garcia, I.M.; Leitune, V.C.B.; Samuel, S.M.W.; Collares, F.M. Influence of Different Calcium Phosphates on an Experimental Adhesive Resin. J. Adhes. Dent. 2017, 19, 379–384. [Google Scholar] [CrossRef]
- Altmann, A.S.P.; Collares, F.M.; Ogliari, F.A.; Samuel, S.M.W. Effect of Methacrylated-Based Antibacterial Monomer on Orthodontic Adhesive System Properties. Am. J. Orthod. Dentofac. Orthop. Off. Publ. Am. Assoc. Orthod. Its Const. Soc. Am. Board Orthod. 2015, 147, S82–S87. [Google Scholar] [CrossRef]
- Thürmer, M.B.; Diehl, C.E.; Brum, F.J.B.; dos Santos, L.A. Development of Dual-Setting Calcium Phosphate Cement Using Absorbable Polymer. Artif. Organs 2013, 37, 992–997. [Google Scholar] [CrossRef] [PubMed]
- de Souza Balbinot, G.; Leitune, V.C.B.; Ogliari, F.A.; Collares, F.M. Niobium Silicate Particles as Bioactive Fillers for Composite Resins. Dent. Mater. 2020, 36, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Collares, F.M.; Portella, F.F.; Leitune, V.C.B.; Samuel, S.M.W. Discrepancies in Degree of Conversion Measurements by FTIR. Braz. Oral Res. 2014, 28, 9–15. [Google Scholar] [CrossRef]
- ISO 4049:2019—Dentistry—Polymer-Based Restorative Materials. Available online: https://webstore.ansi.org/Standards/ISO/ISO40492019?gclid=Cj0KCQjws_r0BRCwARIsAMxfDRgnrHyZLvC22FOre1HjCFS_BdHL1HYzkXlqkmm3O40M5kHkeLZv3zoaApfUEALw_wcB (accessed on 21 April 2020).
- Yap, A.U.J.; Teoh, S.H. Comparison of Flexural Properties of Composite Restoratives Using the ISO and Mini-Flexural Tests. J. Oral Rehabil. 2003, 30, 171–177. [Google Scholar] [CrossRef]
- Tongtaksin, A.; Leevailoj, C. Battery Charge Affects the Stability of Light Intensity from Light-Emitting Diode Light-Curing Units. Oper. Dent. 2017, 42, 497–504. [Google Scholar] [CrossRef]
- da Silva, E.M.; Poskus, L.T.; Guimarães, J.G.A. Influence of Light-Polymerization Modes on the Degree of Conversion and Mechanical Properties of Resin Composites: A Comparative Analysis Between a Hybrid and a Nanofilled Composite. Oper. Dent. 2008, 33, 287–293. [Google Scholar] [CrossRef]
- Ferracane, J.L.; Greener, E.H. The Effect of Resin Formulation on the Degree of Conversion and Mechanical Properties of Dental Restorative Resins. J. Biomed. Mater. Res. 1986, 20, 121–131. [Google Scholar] [CrossRef]
- Durner, J.; Obermaier, J.; Draenert, M.; Ilie, N. Correlation of the Degree of Conversion with the Amount of Elutable Substances in Nano-Hybrid Dental Composites. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2012, 28, 1146–1153. [Google Scholar] [CrossRef]
- Furche, S.; Hickel, R.; Reichl, F.X.; van Landuyt, K.; Shehata, M.; Durner, J. Quantification of Elutable Substances from Methacrylate Based Sealers and Their Cytotoxicity Effect on with Human Gingival Fibroblasts. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2013, 29, 618–625. [Google Scholar] [CrossRef]
- Schiroky, P.R.; Leitune, V.C.B.; Garcia, I.M.; Ogliari, F.A.; Samuel, S.M.W.; Collares, F.M. Triazine Compound as Copolymerized Antibacterial Agent in Adhesive Resins. Braz. Dent. J. 2017, 28, 196–200. [Google Scholar] [CrossRef]
- Beigi Burujeny, S.; Atai, M.; Yeganeh, H. Assessments of Antibacterial and Physico-Mechanical Properties for Dental Materials with Chemically Anchored Quaternary Ammonium Moieties: Thiol-Ene-Methacrylate vs. Conventional Methacrylate System. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2015, 31, 244–261. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.F.J.; Moraes, R.R.; Cavalcante, L.M.; Sinhoreti, M.A.C.; Correr-Sobrinho, L.; Consani, S. Cross-Link Density Evaluation through Softening Tests: Effect of Ethanol Concentration. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2008, 24, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Matinlinna, J.P.; Lung, C.Y.K.; Tsoi, J.K.H. Silane Adhesion Mechanism in Dental Applications and Surface Treatments: A Review. Dent. Mater. 2018, 34, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Nagamune, H.; Maeda, T.; Ohkura, K.; Yamamoto, K.; Nakajima, M.; Kourai, H. Evaluation of the Cytotoxic Effects of Bis-Quaternary Ammonium Antimicrobial Reagents on Human Cells. Toxicol. Vitro 2000, 14, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Luo, X.; Mao, X.; Wen, D.; Zhang, H.; Wang, J. Inflammatory and Immune-Related Factor Caspase 1 Contributes to the Development of Oral Lichen Planus. Arch. Oral Biol. 2021, 131, 105244. [Google Scholar] [CrossRef]
- Franca, C.M.; de Souza Balbinot, G.; Cunha, D.; Saboia, V.D.; Ferracane, J.; Bertassoni, L.E. In-Vitro Models of Biocompatibility Testing for Restorative Dental Materials: From 2D Cultures to Organs on-a-Chip. Acta Biomater. 2022, 150, 58–66. [Google Scholar] [CrossRef]
- Nedeljkovic, I.; Teughels, W.; De Munck, J.; Van Meerbeek, B.; Van Landuyt, K.L. Is Secondary Caries with Composites a Material-Based Problem? Dent. Mater. Off. Publ. Acad. Dent. Mater. 2015, 31, e247–e277. [Google Scholar] [CrossRef]
- Vallittu, P.K.; Boccaccini, A.R.; Hupa, L.; Watts, D.C. Bioactive Dental Materials-Do They Exist and What Does Bioactivity Mean? Dent. Mater. Off. Publ. Acad. Dent. Mater. 2018, 34, 693–694. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Sun, L.; Weir, M.D.; Antonucci, J.M.; Takagi, S.; Chow, L.C.; Peltz, M. Nano DCPA-Whisker Composites with High Strength and Ca and PO(4) Release. J. Dent. Res. 2006, 85, 722–727. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Bioceramics of Calcium Orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef]

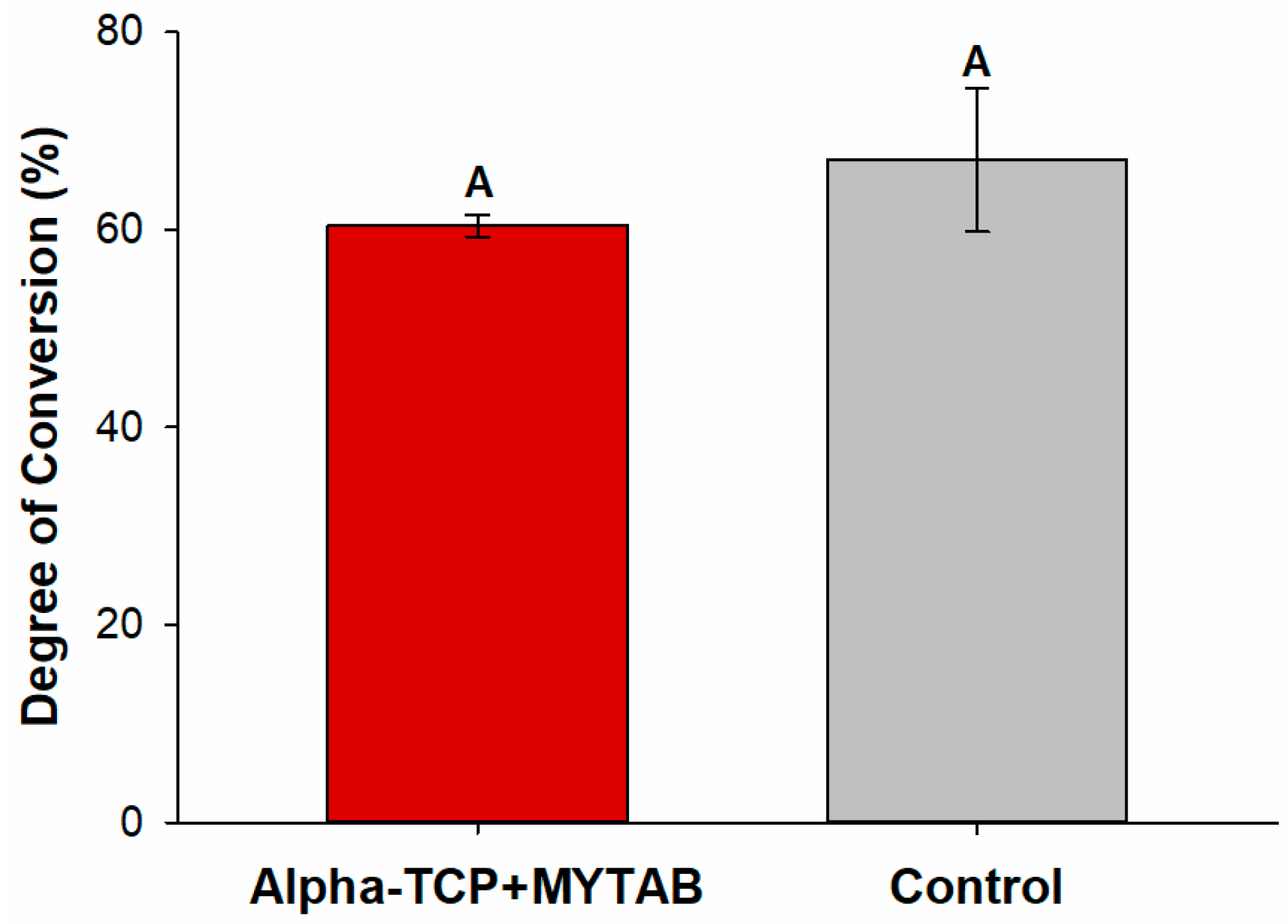
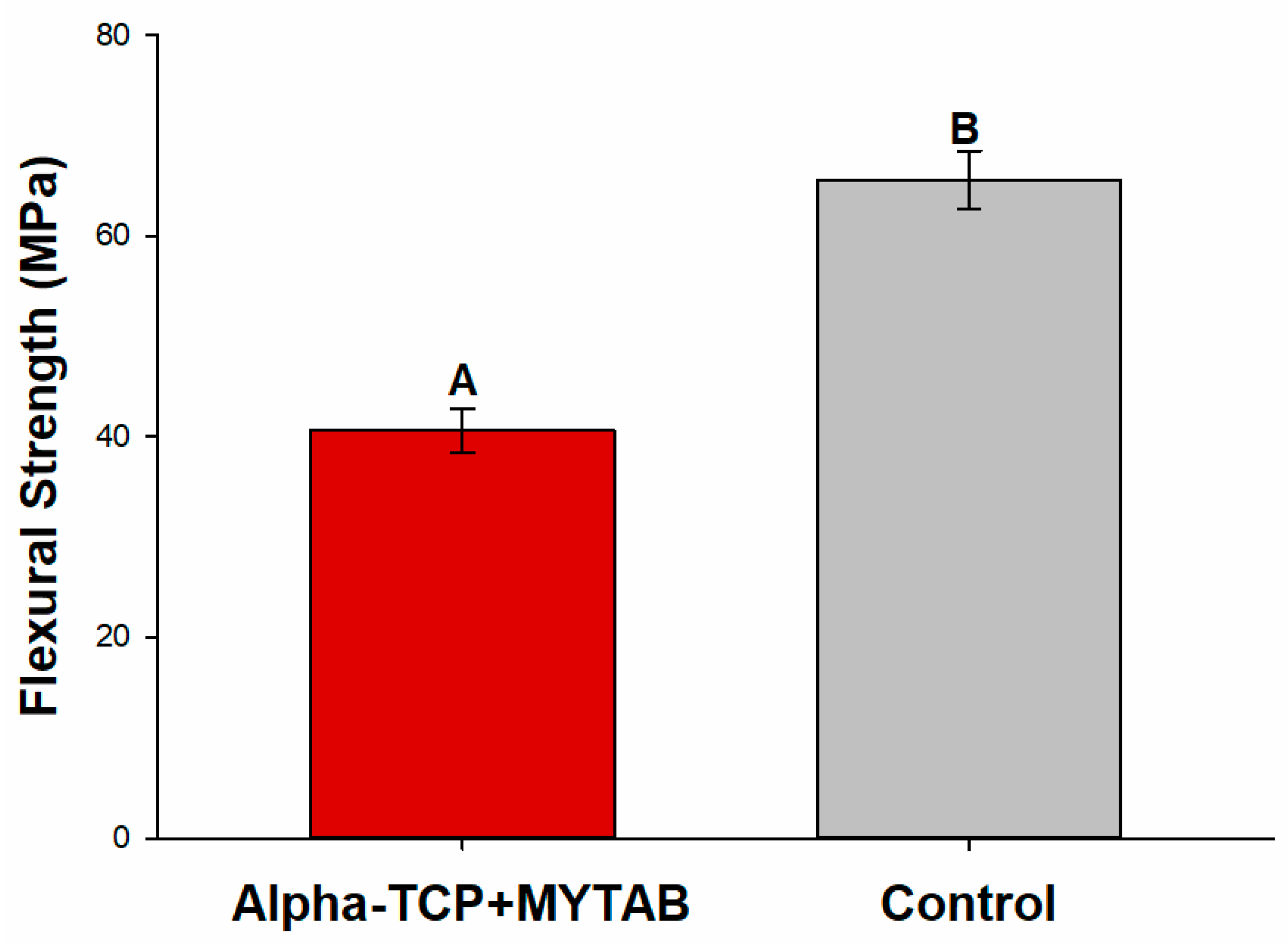
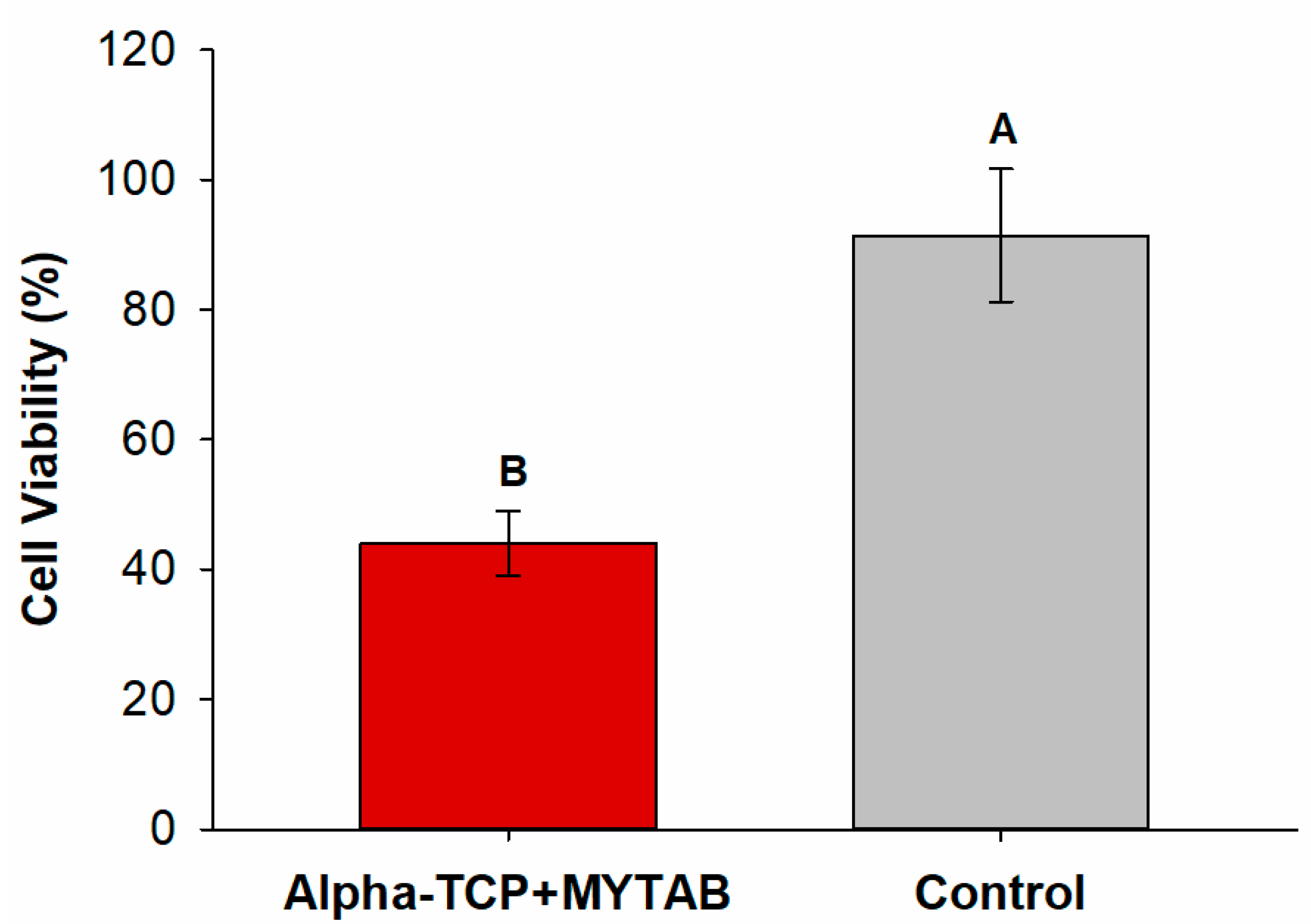
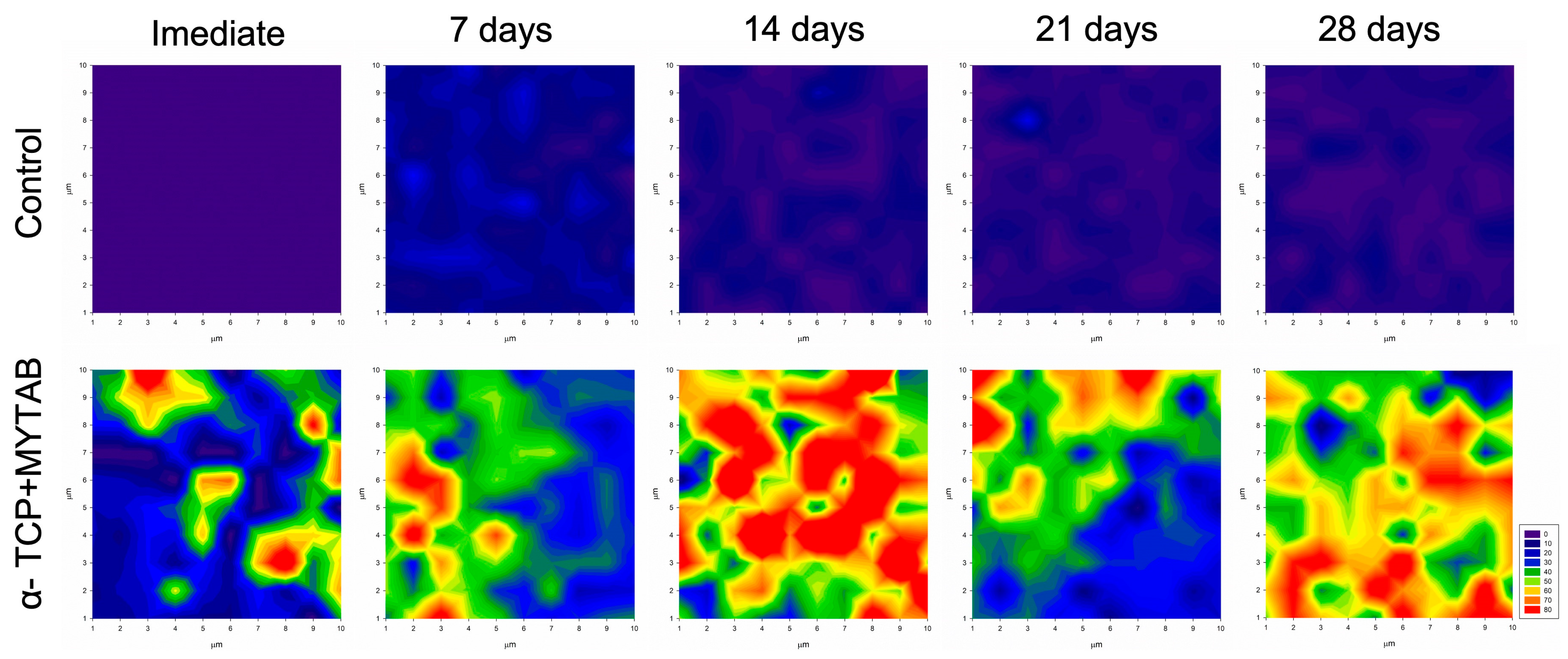
| KHN1 | KHN2 | %ΔKHN | |
|---|---|---|---|
| α-TCP/MYTAB | 80.49 (±7.11) Aa | 59.92 (±5.10) b | 25.49 (±2.14) B |
| Control | 73.28 (±4.98) Aa | 64.33 (±4.20) b | 12.17 (±3.11) A |
| Antibacterial Activity (log CFU/ML) | ||
|---|---|---|
| Biofilm | Planktonic | |
| α-TCP/MYTAB | 0.00 (±0.00) B | 1.28(±2.22) A |
| Control | 6.90 (±0.44) A | 5.87(±0.87) B |
| Negative Control | - | 6.85 (±0.90) B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pontons-Melo, J.C.; Balbinot, G.d.S.; Sauro, S.; Collares, F.M. Experimental Composite Resin with Myristyltrimethylammonium Bromide (MYTAB) and Alpha-Tricalcium Phosphate (α-TCP): Antibacterial and Remineralizing Effect. J. Funct. Biomater. 2023, 14, 303. https://doi.org/10.3390/jfb14060303
Pontons-Melo JC, Balbinot GdS, Sauro S, Collares FM. Experimental Composite Resin with Myristyltrimethylammonium Bromide (MYTAB) and Alpha-Tricalcium Phosphate (α-TCP): Antibacterial and Remineralizing Effect. Journal of Functional Biomaterials. 2023; 14(6):303. https://doi.org/10.3390/jfb14060303
Chicago/Turabian StylePontons-Melo, Juan Carlos, Gabriela de Souza Balbinot, Salvatore Sauro, and Fabrício Mezzomo Collares. 2023. "Experimental Composite Resin with Myristyltrimethylammonium Bromide (MYTAB) and Alpha-Tricalcium Phosphate (α-TCP): Antibacterial and Remineralizing Effect" Journal of Functional Biomaterials 14, no. 6: 303. https://doi.org/10.3390/jfb14060303
APA StylePontons-Melo, J. C., Balbinot, G. d. S., Sauro, S., & Collares, F. M. (2023). Experimental Composite Resin with Myristyltrimethylammonium Bromide (MYTAB) and Alpha-Tricalcium Phosphate (α-TCP): Antibacterial and Remineralizing Effect. Journal of Functional Biomaterials, 14(6), 303. https://doi.org/10.3390/jfb14060303










