Fabrication of Novel Pre-Polymerized BisGMA/Silica Nanocomposites: Physio-Mechanical Considerations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
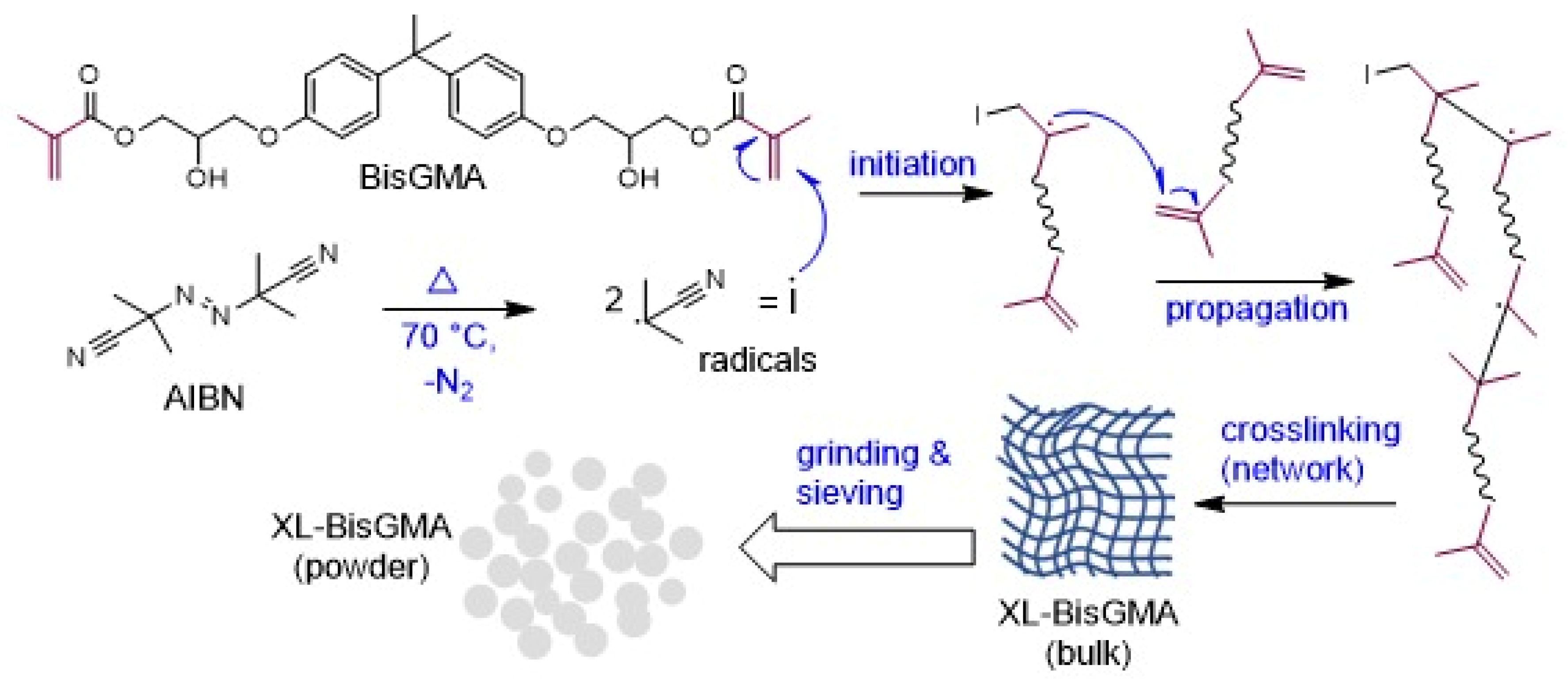
2.2. Preparation of Prepolymerized BisGMA (XL-BisGMA)
2.3. Synthesis of Silanized Silica
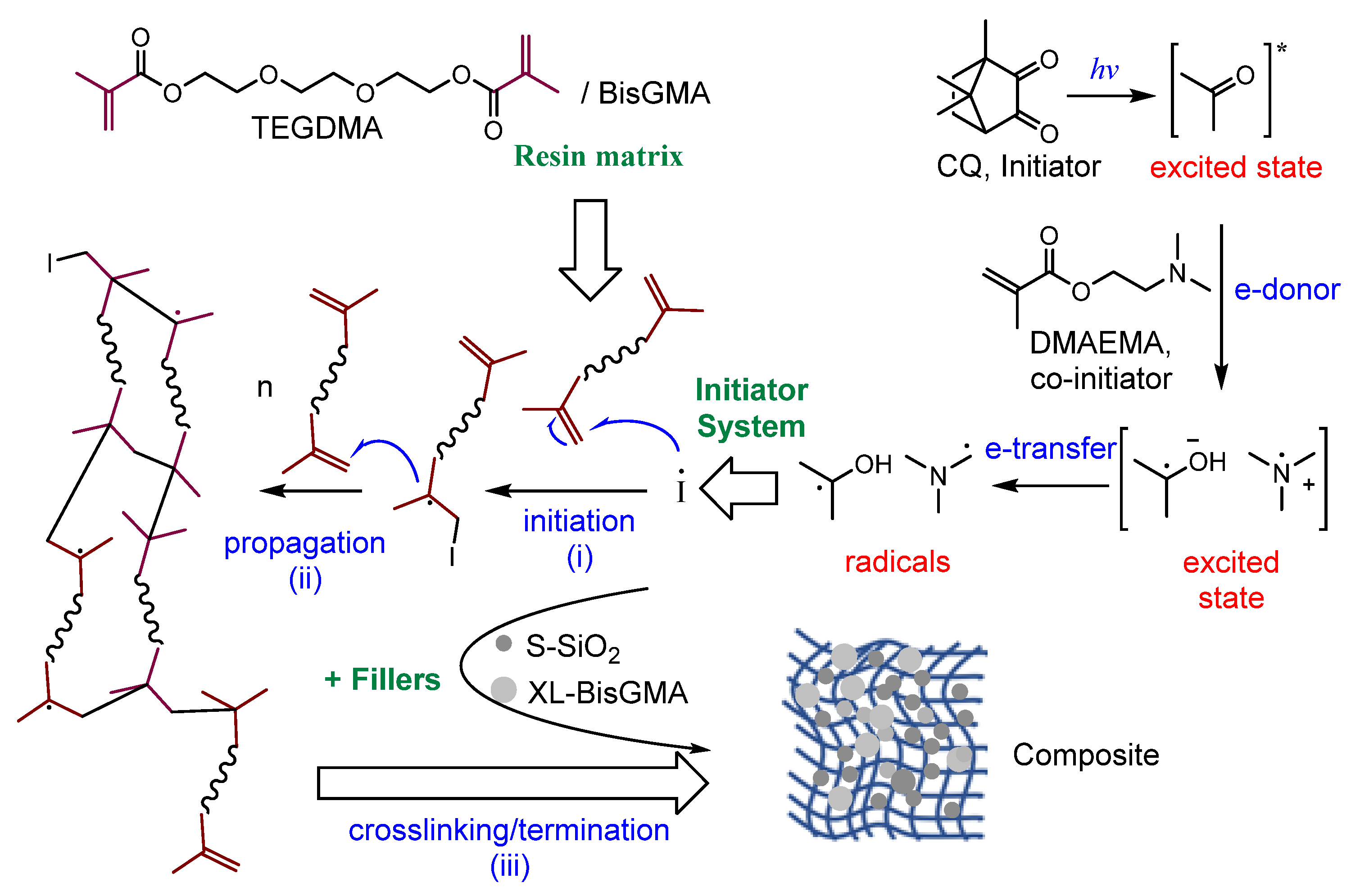
2.4. Dental Composites Preparation
2.5. Characterization
2.6. Statistical Analysis
3. Results
3.1. Rheological Properties
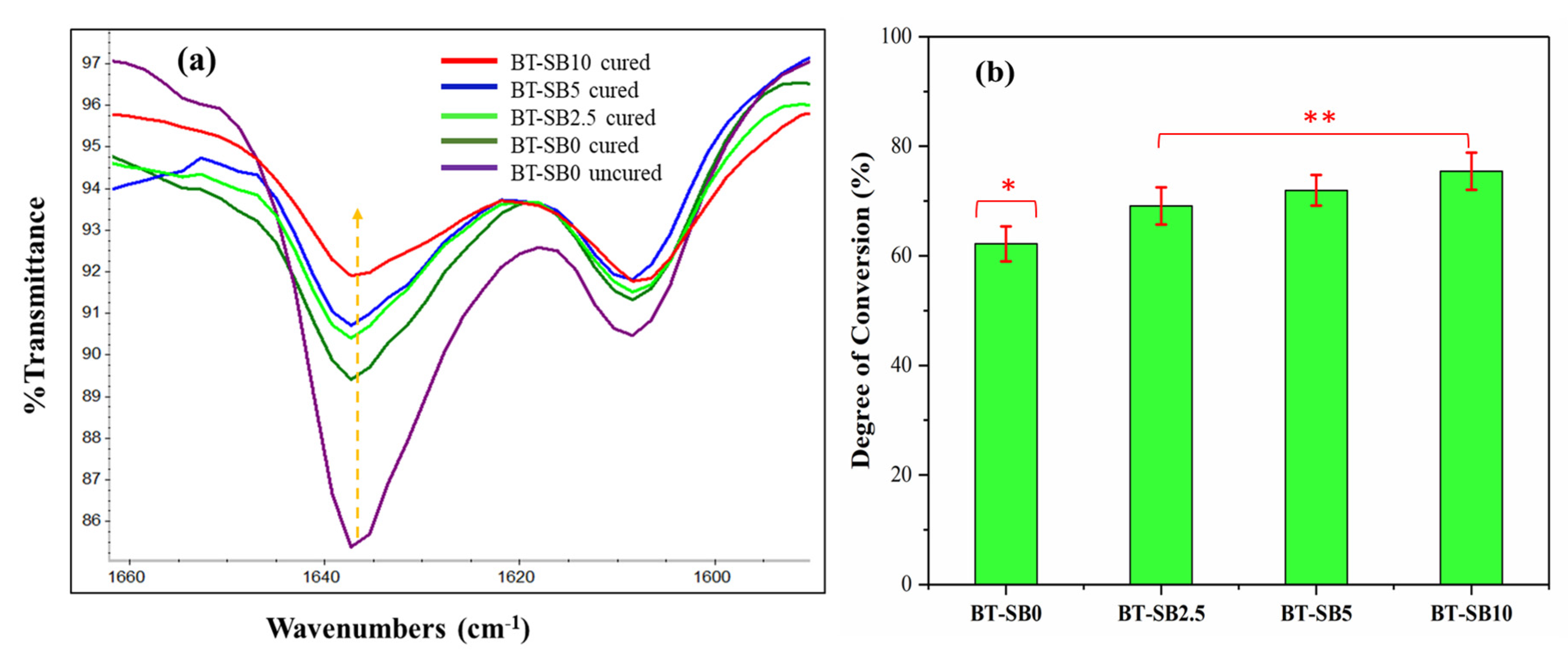
3.2. FTIR Analysis and DC
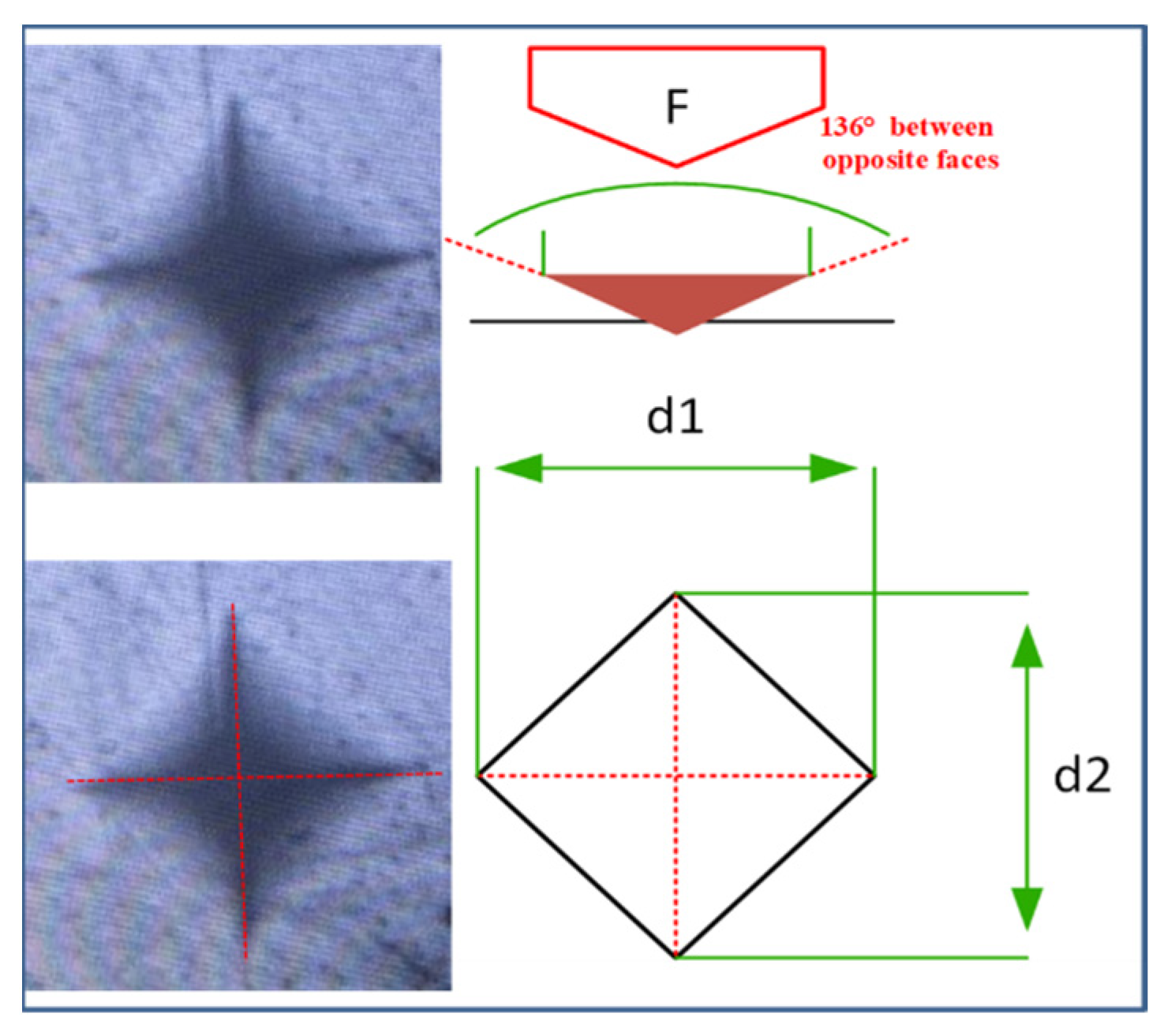
3.3. Microhardness
3.4. Thermal Gravimetric Analysis (TGA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sachdeva, S.; Kapoor, P.; Tamrakar, A.; Noor, R. Nano-composite dental resins: An overview. Ann. Dent. Spec. 2015, 3, 52–55. [Google Scholar]
- Gopikrishna, V. Sturdevant’s Art & Science of Operative Dentistry-E-Book: A South Asian Edition; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Montagner, A.F.; Sande, F.H.V.D.; Müller, C.; Cenci, M.S.; Susin, A.H. Survival, reasons for failure and clinical characteristics of anterior/posterior composites: 8-year findings. Braz. Dent. J. 2018, 29, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Elfakhri, F.; Alkahtani, R.; Li, C.; Khaliq, J. Influence of filler characteristics on the performance of dental composites: A comprehensive review. Ceram. Int. 2022, 48, 27280–27294. [Google Scholar] [CrossRef]
- Tian, M.; Gao, Y.; Liu, Y.; Liao, Y.; Hedin, N.E.; Fong, H. Fabrication and evaluation of Bis-GMA/TEGDMA dental resins/composites containing nano fibrillar silicate. Dent. Mater. 2008, 24, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Sun, Y.; Xie, W.; Liu, Y.; Song, X. Development of novel dental nanocomposites reinforced with polyhedral oligomeric silsesquioxane (POSS). Dent. Mater. 2010, 26, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhang, F.; Xie, H.; Gu, N. Nanoparticle-reinforced resin-based dental composites. J. Dent. 2008, 36, 450–455. [Google Scholar] [CrossRef]
- Chen, M.-H.; Chen, C.-R.; Hsu, S.-H.; Sun, S.-P.; Su, W.-F. Low shrinkage light curable nanocomposite for dental restorative material. Dent. Mater. 2006, 22, 138–145. [Google Scholar] [CrossRef]
- Fong, H. Electrospun nylon 6 nanofiber reinforced BIS-GMA/TEGDMA dental restorative composite resins. Polymer 2004, 45, 2427–2432. [Google Scholar] [CrossRef]
- Hosseinalipour, M.; Javadpour, J.; Rezaie, H.; Dadras, T.; Hayati, A.N. Investigation of mechanical properties of experimental Bis-GMA/TEGDMA dental composite resins containing various mass fractions of silica nanoparticles. J. Prosthodont. Implant Esthet. Reconstr. Dent. 2010, 19, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.P.; Li, S.; Mukherjee, I.; Guo, Y.; Patel, A.C.; Baran, G.; Wei, Y. Mechanical properties of experimental dental composites containing a combination of mesoporous and nonporous spherical silica as fillers. Dent. Mater. 2009, 25, 296–301. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Huang, C.-K.; Lin, S.-P.; Han, J.-L.; Hsieh, K.-H.; Lin, C.-P. Low-shrinkage visible-light-curable urethane-modified epoxy acrylate/SiO2 composites as dental restorative materials. Compos. Sci. Technol. 2008, 68, 2811–2817. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.M.; Chrószcz, M.W.; Chladek, G. Novel urethane-dimethacrylate monomers and compositions for use as matrices in dental restorative materials. Int. J. Mol. Sci. 2020, 21, 2644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Davy, K.; Kalachandra, S.; Pandain, M.; Braden, M. Relationship between composite matrix molecular structure and properties. Biomaterials 1998, 19, 2007–2014. [Google Scholar] [CrossRef]
- Lastumäki, T.M.; Lassila, L.V.; Vallittu, P.K. The semi-interpenetrating polymer network matrix of fiber-reinforced composite and its effect on the surface adhesive properties. J. Mater. Sci. Mater. Med. 2003, 14, 803–809. [Google Scholar] [CrossRef]
- Al-Odayni, A.-B.; Alfotawi, R.; Khan, R.; Saeed, W.S.; Al-Kahtani, A.; Aouak, T.; Alrahlah, A. Synthesis of chemically modified BisGMA analog with low viscosity and potential physical and biological properties for dental resin composite. Dent. Mater. 2019, 35, 1532–1544. [Google Scholar] [CrossRef]
- Alrahlah, A.; Al-Odayni, A.-B.; Al-Mutairi, H.F.; Almousa, B.M.; Alsubaie, F.S.; Khan, R.; Saeed, W.S. A Low-Viscosity BisGMA Derivative for Resin Composites: Synthesis, Characterization, and Evaluation of Its Rheological Properties. Materials 2021, 14, 338. [Google Scholar] [CrossRef]
- Yoshida, K.; Greener, E. Effects of two amine reducing agents on the degree of conversion and physical properties of an unfilled light-cured resin. Dent. Mater. 1993, 9, 246–251. [Google Scholar] [CrossRef]
- Stansbury, J.W. Dimethacrylate network formation and polymer property evolution as determined by the selection of monomers and curing conditions. Dent. Mater. 2012, 28, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Tarle, Z. Degree of conversion. In Dental Composite Materials for Direct Restorations; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 63–85. [Google Scholar]
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef]
- Klapdohr, S.; Moszner, N. New inorganic components for dental filling composites. Mon. Für Chem./Chem. Mon. 2005, 136, 21–45. [Google Scholar] [CrossRef]
- Asmussen, S.; Vallo, C. Characterization of light-cured dimethacrylate resins modified with silsesquioxanes. J. Mater. Sci. 2011, 46, 2308–2317. [Google Scholar] [CrossRef]
- Mucci, V.; Pérez, J.; Vallo, C.I. Preparation and characterization of light-cured methacrylate/montmorillonite nanocomposites. Polym. Int. 2011, 60, 247–254. [Google Scholar] [CrossRef]
- Chen, M.-H. Update on dental nanocomposites. J. Dent. Res. 2010, 89, 549–560. [Google Scholar] [CrossRef]
- Mucci, V.L.; Arenas, G.F.; Pérez, C.J.; Vallo, C.I. Prepolymerized organic–inorganic hybrid nanoparticles as fillers for light-cured methacrylate monomers. J. Mater. Sci. 2012, 47, 2951–2959. [Google Scholar] [CrossRef]
- Habib, E.; Wang, R.; Zhu, X. Monodisperse silica-filled composite restoratives mechanical and light transmission properties. Dent. Mater. 2017, 33, 280–287. [Google Scholar] [CrossRef]
- Alrahlah, A.; Al-Odayni, A.-B.; Saeed, W.S.; Abduh, N.A.Y.; Khan, R.; Alshabib, A.; Almajhdi, F.F.N.; Alodeni, R.M.; De Vera, M.A.T. Influence of Eugenol and Its Novel Methacrylated Derivative on the Polymerization Degree of Resin-Based Composites. Polymers 2023, 15, 1124. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Y.; Mao, Y.; Chen, K.; Cao, Z.; Qi, D. Controllable synthesis of raspberry-like PS–SiO2 nanocomposite particles via Pickering emulsion polymerization. RSC Adv. 2018, 8, 3910–3918. [Google Scholar] [CrossRef] [Green Version]
- Suk, J.W.; Piner, R.D.; An, J.; Ruoff, R.S. Mechanical properties of monolayer graphene oxide. ACS Nano 2010, 4, 6557–6564. [Google Scholar] [CrossRef]
- Alrahlah, A.; Khan, R.; Al-Odayni, A.-B.; Saeed, W.S.; Bautista, L.S.; Vohra, F. Evaluation of Synergic Potential of rGO/SiO2 as Hybrid Filler for BisGMA/TEGDMA Dental Composites. Polymers 2020, 12, 3025. [Google Scholar] [CrossRef]
- Seemann, R.; Flury, S.; Pfefferkorn, F.; Lussi, A.; Noack, M.J. Restorative dentistry and restorative materials over the next 20 years: A Delphi survey. Dent. Mater. 2014, 30, 442–448. [Google Scholar] [CrossRef]
- Odian, G. Principles of Polymerization; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Habib, E.; Wang, R.; Zhu, X.X. Correlation of resin viscosity and monomer conversion to filler particle size in dental composites. Dent. Mater. 2018, 34, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.; Kawano, Y.; Pfeifer, C.; Stansbury, J.W.; Braga, R.R. Influence of BisGMA, TEGDMA, and BisEMA contents on viscosity, conversion, and flexural strength of experimental resins and composites. Eur. J. Oral Sci. 2009, 117, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Halvorson, R.H.; Erickson, R.L.; Davidson, C.L. The effect of filler and silane content on conversion of resin-based composite. Dent. Mater. 2003, 19, 327–333. [Google Scholar] [CrossRef]
- Baroudi, K.; Rodrigues, J.C. Flowable Resin Composites: A Systematic Review and Clinical Considerations. J. Clin. Diagn. Res. JCDR 2015, 9, Ze18–Ze24. [Google Scholar] [CrossRef]
- Peutzfeldt, A. Resin composites in dentistry: The monomer systems. Eur. J. Oral Sci. 1997, 105, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Amirouche-Korichi, A.; Mouzali, M.; Watts, D.C. Effects of monomer ratios and highly radiopaque fillers on degree of conversion and shrinkage-strain of dental resin composites. Dent. Mater. 2009, 25, 1411–1418. [Google Scholar] [CrossRef]
- Danesh Kazemi, A.; Johar, N. Comparison of Different Bleaching Treatments Effect on Micro Hardness of Four Different Aged Composites. Jentashapir J. Health Res. 2016, 7, e39039. [Google Scholar] [CrossRef] [Green Version]
- AlQahtani, M. The effect of a 10% carbamide peroxide bleaching agent on the microhardness of four types of direct resin-based restorative materials. Oper. Dent. 2013, 38, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Yap, A.U.; Wattanapayungkul, P. Effects of in-office tooth whiteners on hardness of tooth-colored restoratives. Oper. Dent. 2002, 27, 137–141. [Google Scholar]
- Lombardini, M.; Chiesa, M.; Scribante, A.; Colombo, M.; Poggio, C. Influence of polymerization time and depth of cure of resin composites determined by Vickers hardness. Dent. Res. J. 2012, 9, 735. [Google Scholar]
- Colombo, M.; Gallo, S.; Poggio, C.; Ricaldone, V.; Arciola, C.R.; Scribante, A. New resin-based bulk-fill composites: In vitro evaluation of micro-hardness and depth of cure as infection risk indexes. Materials 2020, 13, 1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferracane, J.L. Correlation between hardness and degree of conversion during the setting reaction of unfilled dental restorative resins. Dent. Mater. 1985, 1, 11–14. [Google Scholar] [CrossRef]
- Chung, K.-H.; Greener, E.H. Correlation between degree of conversion, filler concentration and mechanical properties of posterior composite resins. J. Oral Rehabil. 1990, 17, 487–494. [Google Scholar] [CrossRef]
- Knobloch, L.A.; Kerby, R.E.; Clelland, N.; Lee, J. Hardness and degree of conversion of posterior packable composites. Oper. Dent. 2004, 29, 642–649. [Google Scholar]
- Herrera-González, A.M.; Cuevas-Suárez, C.E. Evaluation of a biobased polycarbonate interpenetrated network in a dental resin composite. J. Mech. Behav. Biomed. Mater. 2023, 143, 105876. [Google Scholar] [CrossRef]
- Blackham, J.T.; Vandewalle, K.S.; Lien, W. Properties of hybrid resin composite systems containing prepolymerized filler particles. Oper. Dent. 2009, 34, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Alrahlah, A. Diametral Tensile Strength, Flexural Strength, and Surface Microhardness of Bioactive Bulk Fill Restorative. J. Contemp. Dent. Pract. 2018, 19, 13–19. [Google Scholar] [CrossRef]
- Macan, J.; Brnardić, I.; Orlić, S.; Ivanković, H.; Ivanković, M. Thermal degradation of epoxy–silica organic–inorganic hybrid materials. Polym. Degrad. Stab. 2006, 91, 122–127. [Google Scholar] [CrossRef]
- Grassie, N.; Guy, M.I.; Tennent, N.H. Degradation of epoxy polymers: Part 4—Thermal degradation of bisphenol-A diglycidyl ether cured with ethylene diamine. Polym. Degrad. Stab. 1986, 14, 125–137. [Google Scholar] [CrossRef]
- Kinoshita, M.; Nemoto, T.; Souda, T.; Takeda, K. Semi-quantitative analysis of thermal degradation in polyphenylene-ether. Polym. Degrad. Stab. 2000, 68, 437–443. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, S.; Ryu, J.; Budhathoki, M.; Liang, G.; Guo, Z. In situ stabilized carbon nanofiber (CNF) reinforced epoxy nanocomposites. J. Mater. Chem. 2010, 20, 4937–4948. [Google Scholar] [CrossRef]




| Composite | Filler (wt.%) | Matrix (wt.%) | Initiator System (wt.%) | ||||
|---|---|---|---|---|---|---|---|
| No. | Code | Synthesized Silica | XL-BisGMA Powder | BisGMA | TEGDMA | Initiator (CQ) | Co-Initiator (DMAEMA) |
| 1 | BT-SB0 | 50 | 0 | 24.5 | 24.5 | 0.2 | 0.8 |
| 2 | BT-SB2.5 | 47.5 | 2.5 | 24.5 | 24.5 | 0.2 | 0.8 |
| 3 | BT-SB5 | 45 | 5 | 24.5 | 24.5 | 0.2 | 0.8 |
| 4 | BT-SB10 | 40 | 10 | 24.5 | 24.5 | 0.2 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrahlah, A.; Khan, R.; Al-Odayni, A.-B.; Saeed, W.S.; Bautista, L.S.; Haider, S.; De Vera, M.A.T.; Alshabib, A. Fabrication of Novel Pre-Polymerized BisGMA/Silica Nanocomposites: Physio-Mechanical Considerations. J. Funct. Biomater. 2023, 14, 323. https://doi.org/10.3390/jfb14060323
Alrahlah A, Khan R, Al-Odayni A-B, Saeed WS, Bautista LS, Haider S, De Vera MAT, Alshabib A. Fabrication of Novel Pre-Polymerized BisGMA/Silica Nanocomposites: Physio-Mechanical Considerations. Journal of Functional Biomaterials. 2023; 14(6):323. https://doi.org/10.3390/jfb14060323
Chicago/Turabian StyleAlrahlah, Ali, Rawaiz Khan, Abdel-Basit Al-Odayni, Waseem Sharaf Saeed, Leonel S. Bautista, Sajjad Haider, Merry Angelyn Tan De Vera, and Abdulrahman Alshabib. 2023. "Fabrication of Novel Pre-Polymerized BisGMA/Silica Nanocomposites: Physio-Mechanical Considerations" Journal of Functional Biomaterials 14, no. 6: 323. https://doi.org/10.3390/jfb14060323
APA StyleAlrahlah, A., Khan, R., Al-Odayni, A.-B., Saeed, W. S., Bautista, L. S., Haider, S., De Vera, M. A. T., & Alshabib, A. (2023). Fabrication of Novel Pre-Polymerized BisGMA/Silica Nanocomposites: Physio-Mechanical Considerations. Journal of Functional Biomaterials, 14(6), 323. https://doi.org/10.3390/jfb14060323










