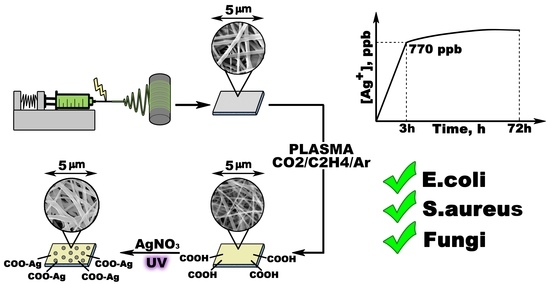
Self-Sanitizing Polycaprolactone Electrospun Nanofiber Membrane with Ag Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrospining of PCL
2.2. Plasma COOH Coating and Deposition of Ag NPs on the Surfaces of Fibers
2.3. Characterization
2.4. Stability Test
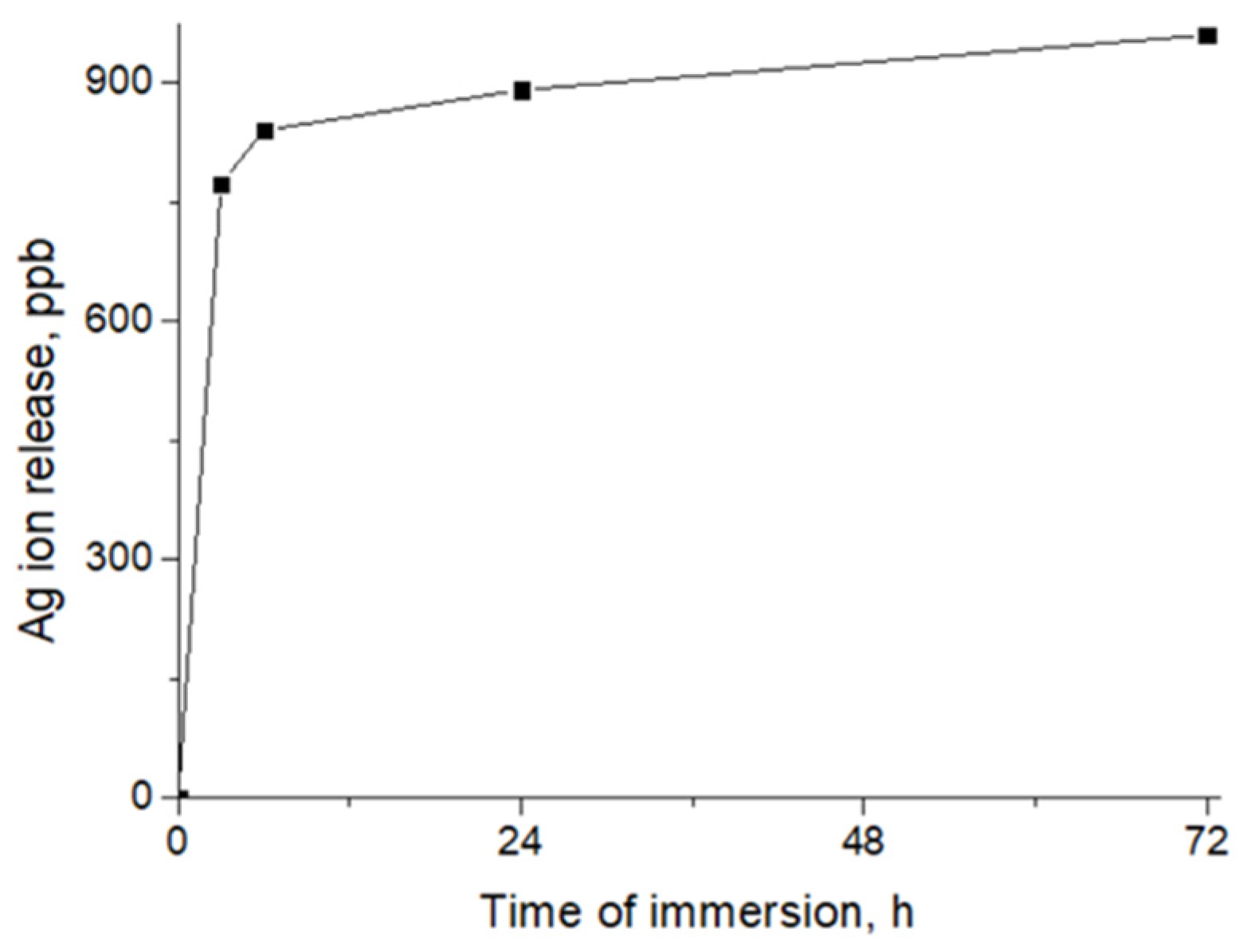
2.5. Ag Ion Release
2.6. Antipathogen Activity
2.7. Biofilm Formation
3. Results
3.1. PCL-Ag Nanofibers’ Structures and Ag+ Release
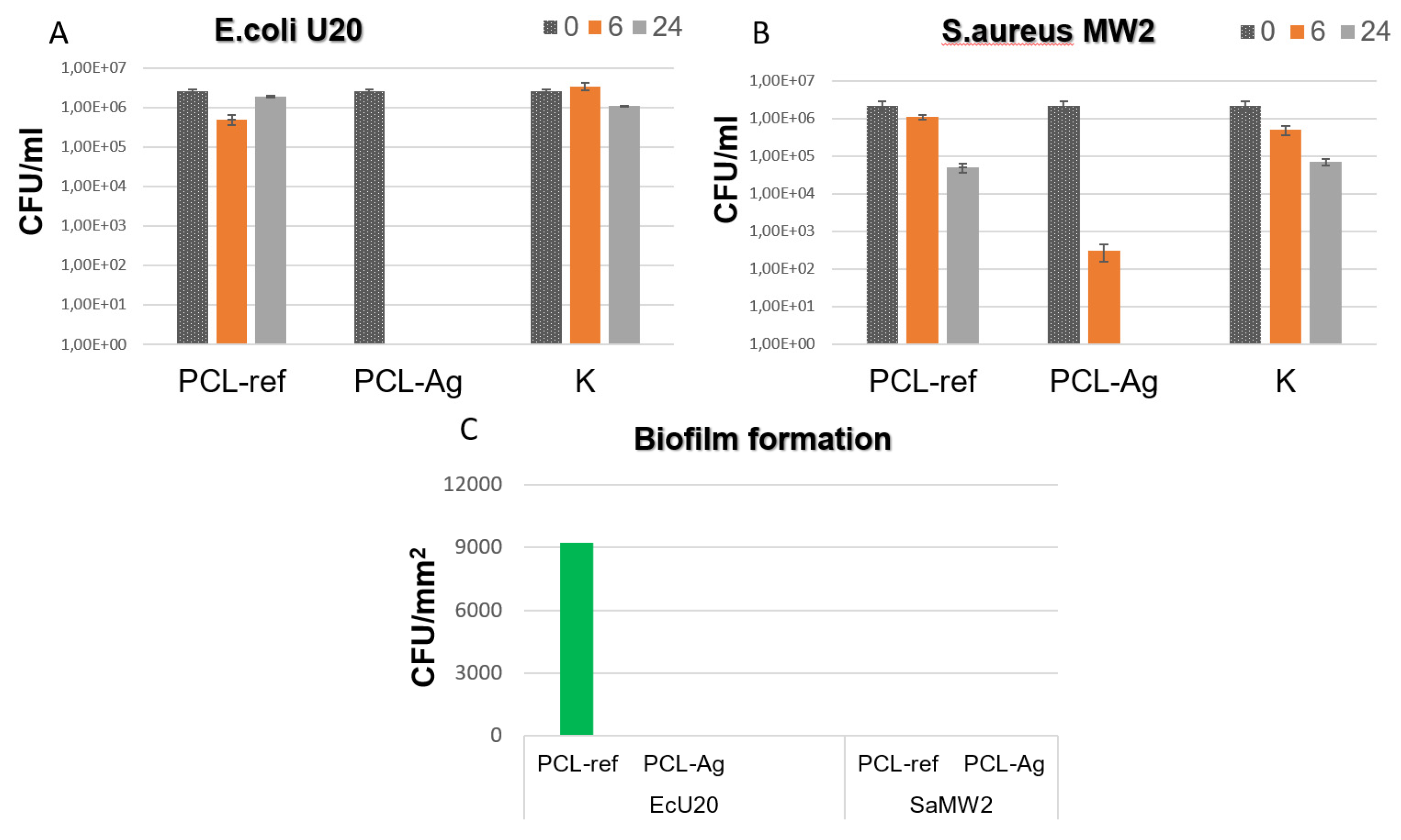
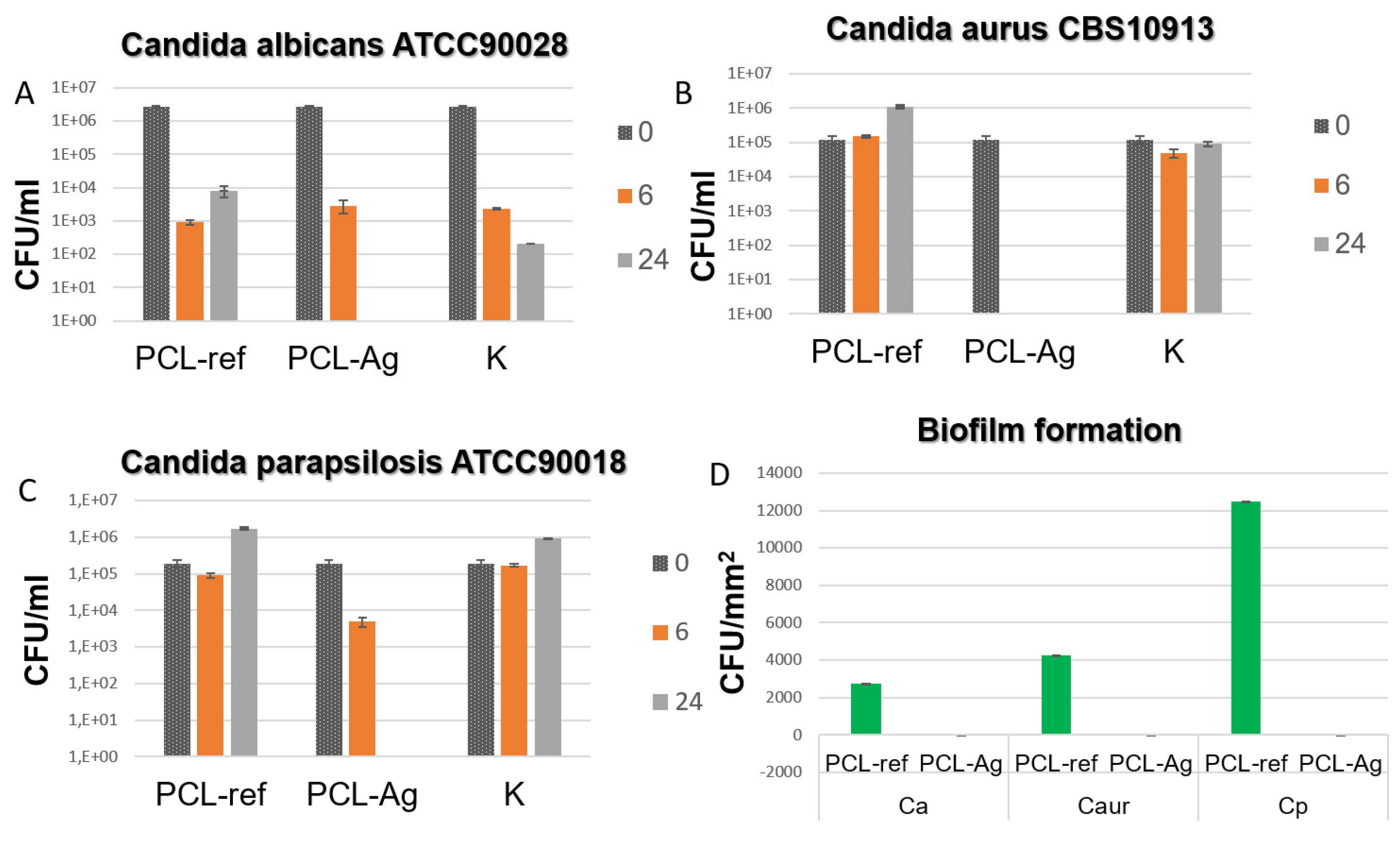
3.2. Antipathogen Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ju, J.T.J.; Boisvert, L.N.; Zuo, Y.Y. Face Masks against COVID-19: Standards, Efficacy, Testing and Decontamination Methods. Adv. Colloid Interface Sci. 2021, 292, 102435. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, M.; Gao, L.; Ayaz Ahmed, M.; Uy, J.P.; Cheng, C.; Zhou, Q.; Sun, C. Face Masks to Prevent Transmission of COVID-19: A Systematic Review and Meta-Analysis. Am. J. Infect. Control 2021, 49, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Oginni, O. COVID-19 Disposable Face Masks: A Precursor for Synthesis of Valuable Bioproducts. Environ. Sci. Pollut. Res. 2022, 29, 85574–85576. [Google Scholar] [CrossRef] [PubMed]
- Mészáros, E.; Bodor, A.; Szierer, Á.; Kovács, E.; Perei, K.; Tölgyesi, C.; Bátori, Z.; Feigl, G. Indirect Effects of COVID-19 on the Environment: How Plastic Contamination from Disposable Surgical Masks Affect Early Development of Plants. J. Hazard. Mater. 2022, 436, 129255. [Google Scholar] [CrossRef]
- Deng, W.; Sun, Y.; Yao, X.; Subramanian, K.; Ling, C.; Wang, H.; Chopra, S.S.; Xu, B.B.; Wang, J.X.; Chen, J.F.; et al. Masks for COVID-19. Adv. Sci. 2022, 9, 2102189. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.; Yadav, B.; Patel, P. State-of-the-Art and Projected Developments of Nanofiber Filter Material for Face Mask Against COVID-19. Recent Pat. Nanotechnol. 2021, 15, 262–270. [Google Scholar] [CrossRef]
- Manakhov, A.M.; Permyakova, E.S.; Sitnikova, N.A.; Tsygankova, A.R.; Alekseev, A.Y.; Solomatina, M.V.; Baidyshev, V.S.; Popov, Z.I.; Blahová, L.; Eliáš, M.; et al. Biodegradable Nanohybrid Materials as Candidates for Self-Sanitizing Filters Aimed at Protection from SARS-CoV-2 in Public Areas. Molecules 2022, 27, 1333. [Google Scholar] [CrossRef]
- Alavi, M.; Kamarasu, P.; McClements, D.J.; Moore, M.D. Metal and Metal Oxide-Based Antiviral Nanoparticles: Properties, Mechanisms of Action, and Applications. Adv. Colloid Interface Sci. 2022, 306, 102726. [Google Scholar] [CrossRef]
- Permyakova, E.S.; Manakhov, A.M.; Kiryukhantsev-korneev, P.V.; Shtansky, D.V. Electrospun Polycaprolactone/ZnO Nanocomposite Membranes with High Antipathogen Activity. Polymers 2022, 14, 5364. [Google Scholar] [CrossRef]
- Cheang, T.; Huang, W.; Li, W.; Ren, S.; Wen, H.; Zhou, T.; Zhang, Y.; Lin, W. Exposed Carboxyl Functionalized MIL-101 Derivatives for Rapid and Efficient Extraction of Heavy Metals from Aqueous Solution. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129517. [Google Scholar] [CrossRef]
- DuChanois, R.M.; Heiranian, M.; Yang, J.; Porter, C.J.; Li, Q.; Zhang, X.; Verduzco, R.; Elimelech, M. Designing Polymeric Membranes with Coordination Chemistry for High-Precision Ion Separations. Sci. Adv. 2022, 8, eabm9436. [Google Scholar] [CrossRef]
- Permyakova, E.S.; Polčak, J.; Slukin, P.V.; Ignatov, S.G.; Gloushankova, N.A.; Zajíčková, L.; Shtansky, D.V.; Manakhov, A. Antibacterial Biocompatible PCL Nanofibers Modified by COOH-Anhydride Plasma Polymers and Gentamicin Immobilization. Mater. Des. 2018, 153, 60–70. [Google Scholar] [CrossRef]
- Ponomarev, V.A.; Sukhorukova, I.V.; Sheveyko, A.N.; Permyakova, E.S.; Manakhov, A.M.; Ignatov, S.G.; Gloushankova, N.A.; Zhitnyak, I.Y.; Lebedev, O.I.; Polčak, J.; et al. Antibacterial Performance of TiCaPCON Films Incorporated with Ag, Pt, and Zn: Bactericidal Ions Versus Surface Microgalvanic Interactions. ACS Appl. Mater. Interfaces 2018, 10, 24406–24420. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, G.; Mia, R.; Wang, W.; Xie, L.; Lü, S.; Mahmud, S.; Liu, H. Bioreduction (Ag+ to Ag0) and Stabilization of Silver Nanocatalyst Using Hyaluronate Biopolymer for Azo-Contaminated Wastewater Treatment. J. Alloys Compd. 2022, 894, 162502. [Google Scholar] [CrossRef]
- Puccetti, M.; Donnadio, A.; Ricci, M.; Latterini, L.; Quaglia, G.; Pietrella, D.; Di Michele, A.; Ambrogi, V. Alginate Ag/AgCl Nanoparticles Composite Films for Wound Dressings with Antibiofilm and Antimicrobial Activities. J. Funct. Biomater. 2023, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Cortese-Krott, M.M.; Münchow, M.; Pirev, E.; Heßner, F.; Bozkurt, A.; Uciechowski, P.; Pallua, N.; Kröncke, K.D.; Suschek, C.V. Silver Ions Induce Oxidative Stress and Intracellular Zinc Release in Human Skin Fibroblasts. Free Radic. Biol. Med. 2009, 47, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, R.V.; Rosemary, M.J.; Nandkumar, M.A.; Krishnan, K.V.; Krishnan, L.K. Silver Nanoparticle Impregnated Poly (ɛ-Caprolactone) Scaffolds: Optimization of Antimicrobial and Noncytotoxic Concentrations. Tissue Eng. Part A 2011, 17, 439–449. [Google Scholar] [CrossRef]
- Gibała, A.; Żeliszewska, P.; Gosiewski, T.; Krawczyk, A.; Duraczyńska, D.; Szaleniec, J.; Szaleniec, M.; Oćwieja, M. Antibacterial and Antifungal Properties of Silver Nanoparticles—Effect of a Surface-Stabilizing Agent. Biomolecules 2021, 11, 1481. [Google Scholar] [CrossRef]
- Lansdown, A.B.G. Silver I: Its Antibacterial Properties and Mechanism of Action. J. Wound Care 2002, 11, 125–130. [Google Scholar] [CrossRef]
- Kędziora, A.; Wieczorek, R.; Speruda, M.; Matolínová, I.; Goszczyński, T.M.; Litwin, I.; Matolín, V.; Bugla-Płoskońska, G. Comparison of Antibacterial Mode of Action of Silver Ions and Silver Nanoformulations With Different Physico-Chemical Properties: Experimental and Computational Studies. Front. Microbiol. 2021, 12, 659614. [Google Scholar] [CrossRef]
- Applications, B.; Oprea, M. Nanocellulose Hybrids with Metal Oxides Nanoparticles for Biomedical Applications. Molecules 2020, 25, 4045. [Google Scholar]
- Hetta, H.F.; Ramadan, Y.N.; Al-Harbi, A.I.; Ahmed, E.A.; Battah, B.; Abd Ellah, N.H.; Zanetti, S.; Donadu, M.G. Nanotechnology as a Promising Approach to Combat Multidrug Resistant Bacteria: A Comprehensive Review and Future Perspectives. Biomedicines 2023, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Suleyman, G.; Alangaden, G.J. Nosocomial Fungal Infections: Epidemiology, Infection Control, and Prevention. Infect. Dis. Clin. N. Am. 2021, 35, 1027–1053. [Google Scholar] [CrossRef] [PubMed]
- Sati, H.; Alastruey-Izquierdo, A.; Beardsley, J.; Morrissey, O.; Jan-Will, P.G.-V. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; Beyer, P., Gigante, V., Getahun, H., Eds.; World Health Organization: Geneva, Switzerland, 2022; ISBN 9789240060241.
- Horton, M.V.; Nett, J.E. Candida auris Infection and Biofilm Formation: Going beyond the Surface. Curr. Clin. Microbiol. Rep. 2020, 7, 51–56. [Google Scholar] [CrossRef]
- Varma, A.; Warghane, A.; Dhiman, N.K.; Paserkar, N.; Upadhye, V.; Modi, A.; Saini, R. The Role of Nanocomposites against Biofilm Infections in Humans. Front. Cell. Infect. Microbiol. 2023, 13, 1104615. [Google Scholar] [CrossRef]
- Eix, E.F.; Nett, J.E. How Biofilm Growth Affects Candida-Host Interactions. Front. Microbiol. 2020, 11, 1437. [Google Scholar] [CrossRef]
- Kupka, V.; Dvořáková, E.; Manakhov, A.; Michlíček, M.; Petruš, J.; Vojtová, L.; Zajíčková, L. Well-Blended PCL/PEO Electrospun Nanofibers with Functional Properties Enhanced by Plasma Processing. Polymers 2020, 12, 1403. [Google Scholar] [CrossRef]
- Manakhov, A.; Kedroňová, E.; Medalová, J.; Černochová, P.; Obrusník, A.; Michlíček, M.; Shtansky, D.V.; Zajíčková, L. Carboxyl-Anhydride and Amine Plasma Coating of PCL Nanofibers to Improve Their Bioactivity. Mater. Des. 2017, 132, 257–265. [Google Scholar] [CrossRef]
- Ponomarev, V.A.; Sheveyko, A.N.; Sukhorukova, I.V.; Shvindina, N.V.; Manakhov, A.M.; Zhitnyak, I.Y.; Gloushankova, N.A.; Fursova, N.K.; Ignatov, S.G.; Permyakova, E.S.; et al. Microstructure, Chemical and Biological Performance of Boron-Modified TiCaPCON Films. Appl. Surf. Sci. 2019, 465, 486–497. [Google Scholar] [CrossRef]
- Konopatsky, A.S.; Firestein, K.L.; Leybo, D.V.; Popov, Z.I.; Larionov, K.V.; Steinman, A.E.; Kovalskii, A.M.; Matveev, A.T.; Manakhov, A.M.; Sorokin, P.B.; et al. BN Nanoparticle/Ag Hybrids with Enhanced Catalytic Activity: Theory and Experiments. Catal. Sci. Technol. 2018, 8, 1652–1662. [Google Scholar] [CrossRef]
- Manakhov, A.; Landová, M.; Medalová, J.; Michlíček, M.; Polčák, J.; Nečas, D.; Zajíčková, L. Cyclopropylamine Plasma Polymers for Increased Cell Adhesion and Growth. Plasma Process. Polym. 2017, 14, 1600123. [Google Scholar] [CrossRef]
- Manakhov, A.; Kiryukhantsev-Korneev, P.; Michlíček, M.; Permyakova, E.; Dvořáková, E.; Polčák, J.; Popov, Z.; Visotin, M.; Shtansky, D.V. Grafting of Carboxyl Groups Using CO2/C2H4/Ar Pulsed Plasma: Theoretical Modeling and XPS Derivatization. Appl. Surf. Sci. 2018, 435, 1220–1227. [Google Scholar] [CrossRef]
- Manakhov, A.; Permyakova, E.; Ershov, S.; Miroshnichenko, S.; Pykhtina, M.; Beklemishev, A.; Kovalskii, A.; Solovieva, A. XPS Modeling of Immobilized Recombinant Angiogenin and Apoliprotein A1 on Biodegradable Nanofibers. Nanomaterials 2020, 10, 879. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.; Bhushan, B.; Sachdev, A.; Matai, I.; Uday Kumar, S.; Gopinath, P. Silver-Nanoparticle-Incorporated Composite Nanofibers for Potential Wound-Dressing Applications. J. Appl. Polym. Sci. 2015, 132, 42473. [Google Scholar] [CrossRef]
- Annur, D.; Wang, Z.K.; Liao, J.D.; Kuo, C. Plasma-Synthesized Silver Nanoparticles on Electrospun Chitosan Nanofiber Surfaces for Antibacterial Applications. Biomacromolecules 2015, 16, 3248–3255. [Google Scholar] [CrossRef] [PubMed]
- Heydari Foroushani, P.; Rahmani, E.; Alemzadeh, I.; Vossoughi, M.; Pourmadadi, M.; Rahdar, A.; Díez-Pascual, A.M. Curcumin Sustained Release with a Hybrid Chitosan-Silk Fibroin Nanofiber Containing Silver Nanoparticles as a Novel Highly Efficient Antibacterial Wound Dressing. Nanomaterials 2022, 12, 3426. [Google Scholar] [CrossRef]
- Mohiti-Asli, M.; Pourdeyhimi, B.; Loboa, E.G. Novel, Silver-Ion-Releasing Nanofibrous Scaffolds Exhibit Excellent Antibacterial Efficacy without the Use of Silver Nanoparticles. Acta Biomater. 2014, 10, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.K.; Ullah, A.; Sarwar, M.N.; Yamaguchi, T.; Wang, Q.; Ullah, S.; Park, S.; Kim, I.S. Fabricating Antibacterial and Antioxidant Electrospun Hydrophilic Polyacrylonitrile Nanofibers Loaded with AgNPs by Lignin-Induced In-Situ Method. Polymers 2021, 13, 748. [Google Scholar] [CrossRef]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, D.G.; Liu, Y.; Liu, Y.N. Progress of Electrospun Nanofibrous Carriers for Modifications to Drug Release Profiles. J. Funct. Biomater. 2022, 13, 289. [Google Scholar] [CrossRef]
- Sitnikova, N.A.; Solovieva, A.O.; Permyakova, E.S.; Sheveyko, A.N.; Shtansky, D.V.; Manakhov, A.M. Silver Ions Incorporation into Nanofibers for Enhanced HMSC Viability. Chemistry 2022, 4, 931–939. [Google Scholar] [CrossRef]
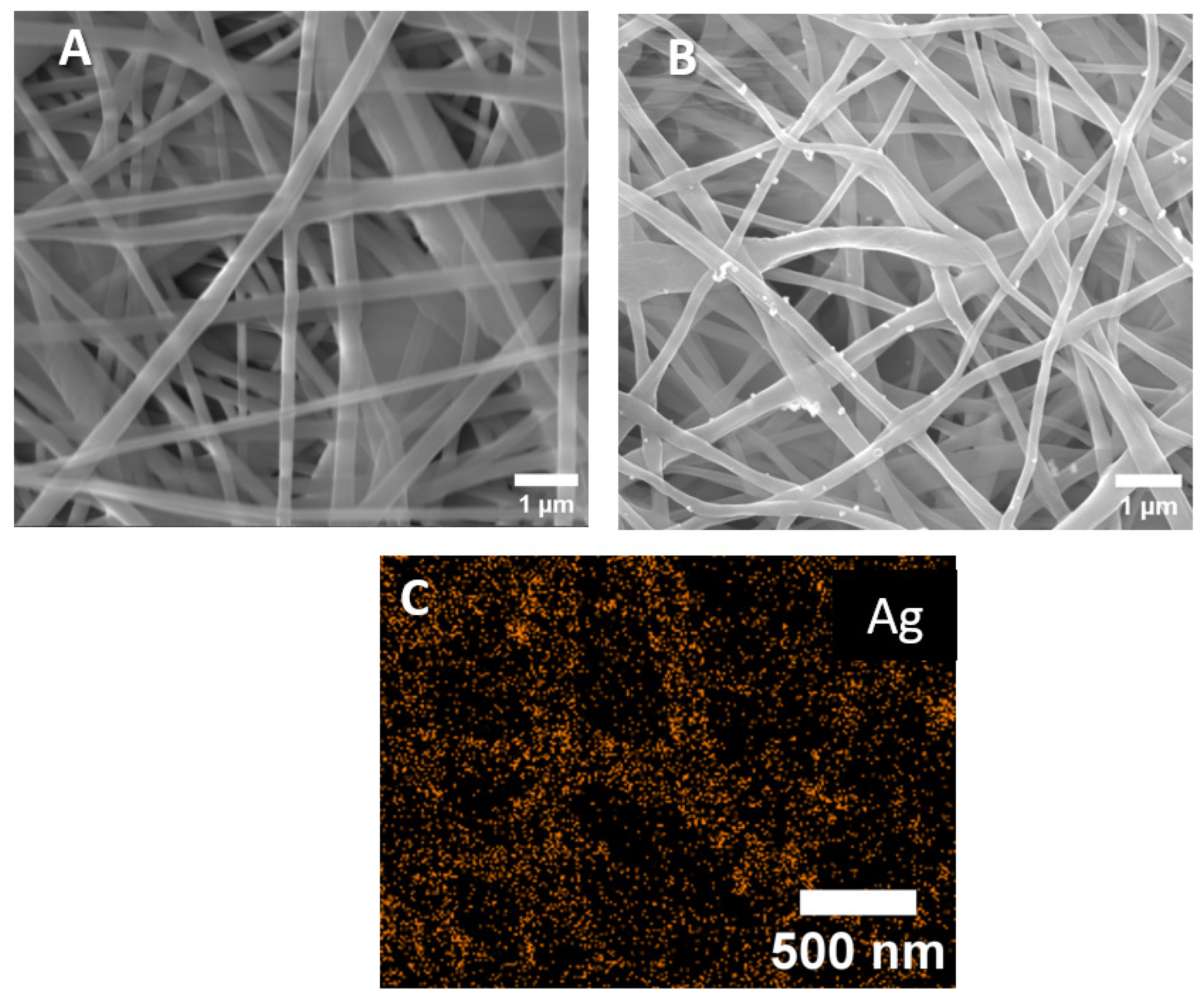
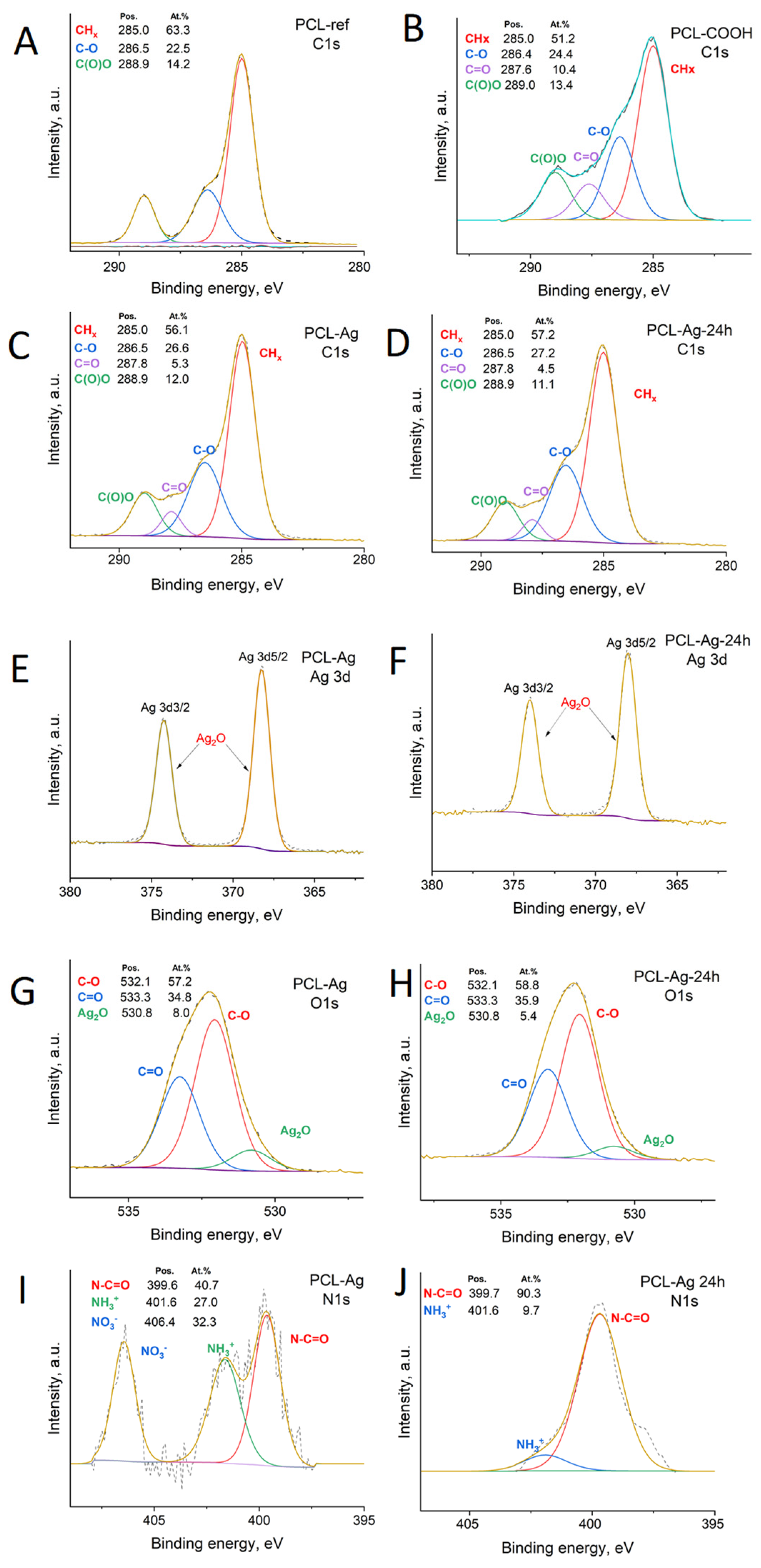
| Sample | [C], at.% | [O], at.% | [Ag], at.% | [Pt], at.% |
|---|---|---|---|---|
| PCL-ref | 73.9 | 26.0 | 0.1 | |
| PCL-Ag | 89.9 | 9.9 | 1.1 | 0.1 |
| PCL-Ag-24 h | 91.1 | 8.4 | 0.4 | 0.1 |
| Sample | C1s, at.% | O1s, at.% | Ag3d, at.% | N1s, at.% |
|---|---|---|---|---|
| PCL-ref | 74.0 | 26.0 | - | - |
| PCL-COOH | 72.5 | 27.5 | - | - |
| PCL-Ag | 67.7 | 26.7 | 3.5 | 2.1 |
| PCL-Ag-24 h | 71.9 | 23.9 | 2.3 | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Permyakova, E.S.; Manakhov, A.; Kiryukhantsev-Korneev, P.V.; Konopatsky, A.S.; Makarets, Y.A.; Kotyakova, K.Y.; Filippovich, S.Y.; Ignatov, S.G.; Solovieva, A.O.; Shtansky, D.V. Self-Sanitizing Polycaprolactone Electrospun Nanofiber Membrane with Ag Nanoparticles. J. Funct. Biomater. 2023, 14, 336. https://doi.org/10.3390/jfb14070336
Permyakova ES, Manakhov A, Kiryukhantsev-Korneev PV, Konopatsky AS, Makarets YA, Kotyakova KY, Filippovich SY, Ignatov SG, Solovieva AO, Shtansky DV. Self-Sanitizing Polycaprolactone Electrospun Nanofiber Membrane with Ag Nanoparticles. Journal of Functional Biomaterials. 2023; 14(7):336. https://doi.org/10.3390/jfb14070336
Chicago/Turabian StylePermyakova, Elizaveta S., Anton Manakhov, Philipp V. Kiryukhantsev-Korneev, Anton S. Konopatsky, Yulia A. Makarets, Kristina Yu. Kotyakova, Svetlana Yu. Filippovich, Sergey G. Ignatov, Anastasiya O. Solovieva, and Dmitry V. Shtansky. 2023. "Self-Sanitizing Polycaprolactone Electrospun Nanofiber Membrane with Ag Nanoparticles" Journal of Functional Biomaterials 14, no. 7: 336. https://doi.org/10.3390/jfb14070336
APA StylePermyakova, E. S., Manakhov, A., Kiryukhantsev-Korneev, P. V., Konopatsky, A. S., Makarets, Y. A., Kotyakova, K. Y., Filippovich, S. Y., Ignatov, S. G., Solovieva, A. O., & Shtansky, D. V. (2023). Self-Sanitizing Polycaprolactone Electrospun Nanofiber Membrane with Ag Nanoparticles. Journal of Functional Biomaterials, 14(7), 336. https://doi.org/10.3390/jfb14070336