Fabrication and Evaluation of Porous dECM/PCL Scaffolds for Bone Tissue Engineering
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
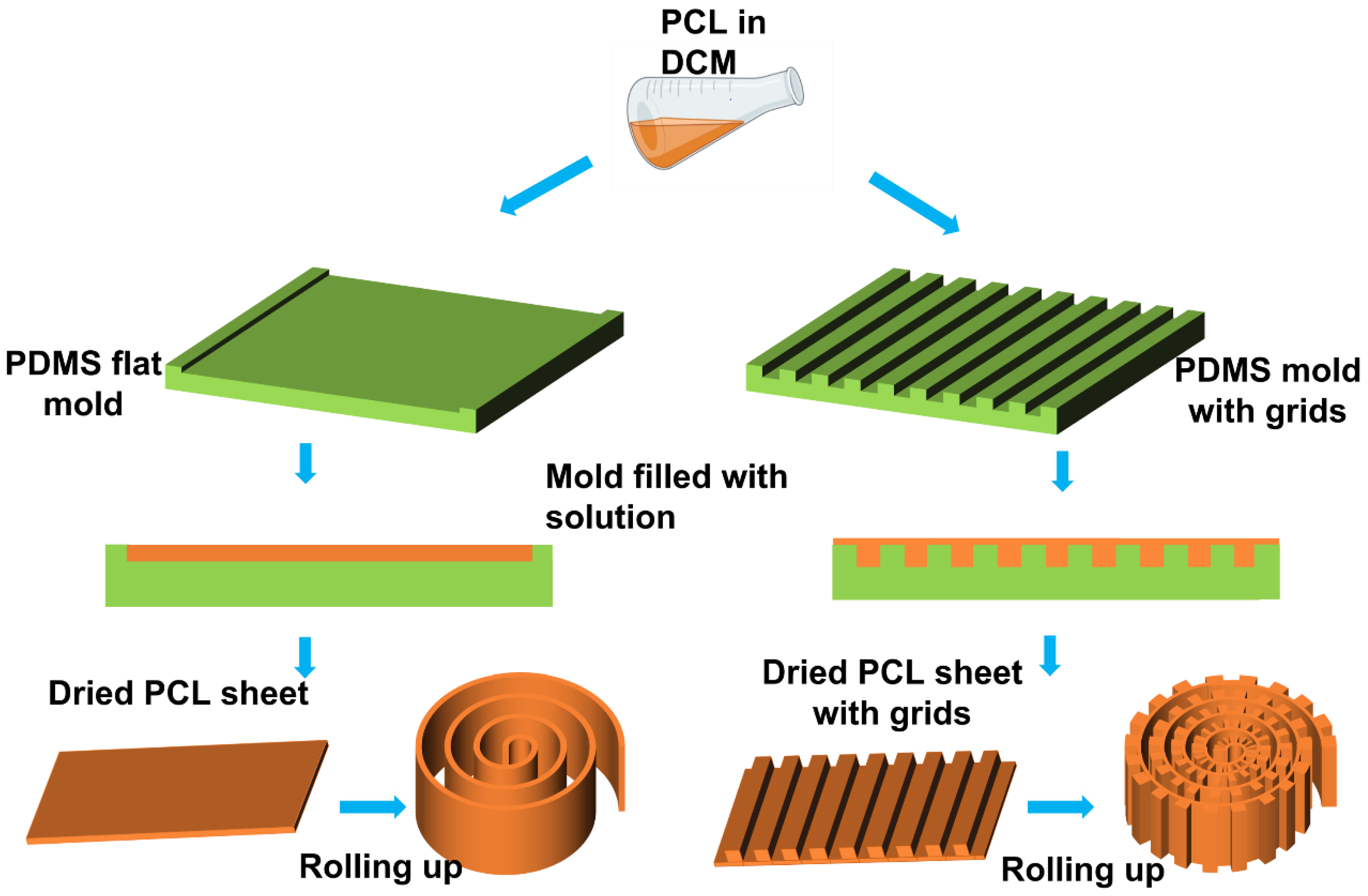
2.2. PCL Scaffold Fabrication
2.3. Test of the Mechanical Properties
2.4. Porosity Test
2.5. Cell Culture
2.6. Decellularization
2.7. Evaluation of Decellularization Based on Protein and DNA Content
2.8. Cell Differentiation on Scaffolds
2.9. ALP Assay
2.10. Biodeposition
2.11. Morphological Characterization
2.11.1. Scanning Electron Microscopy
2.11.2. Stereomicroscopy
2.11.3. Fluorescent Microscopy
2.12. Statistical Analysis
3. Results and Discussion
3.1. Fabrication of the PCL Scaffolds
3.2. Mechanical Tests
3.3. The Porosity of Different PCL Scaffolds
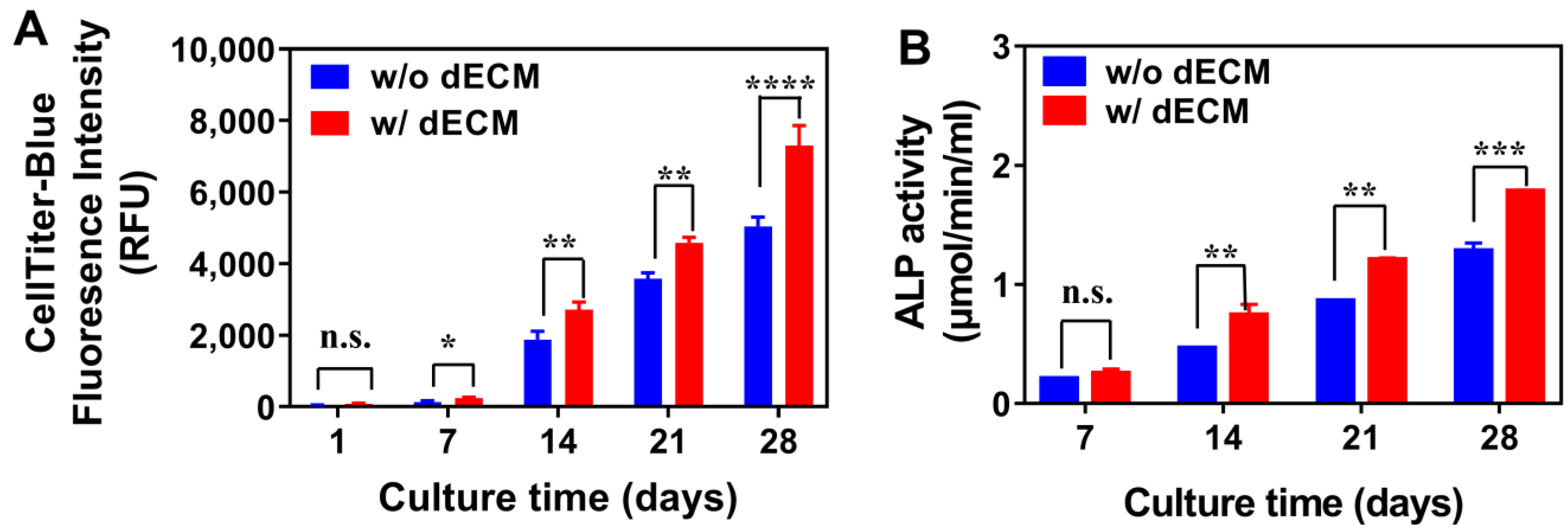
3.4. Cell Attachment and Proliferation
3.5. Decellularization
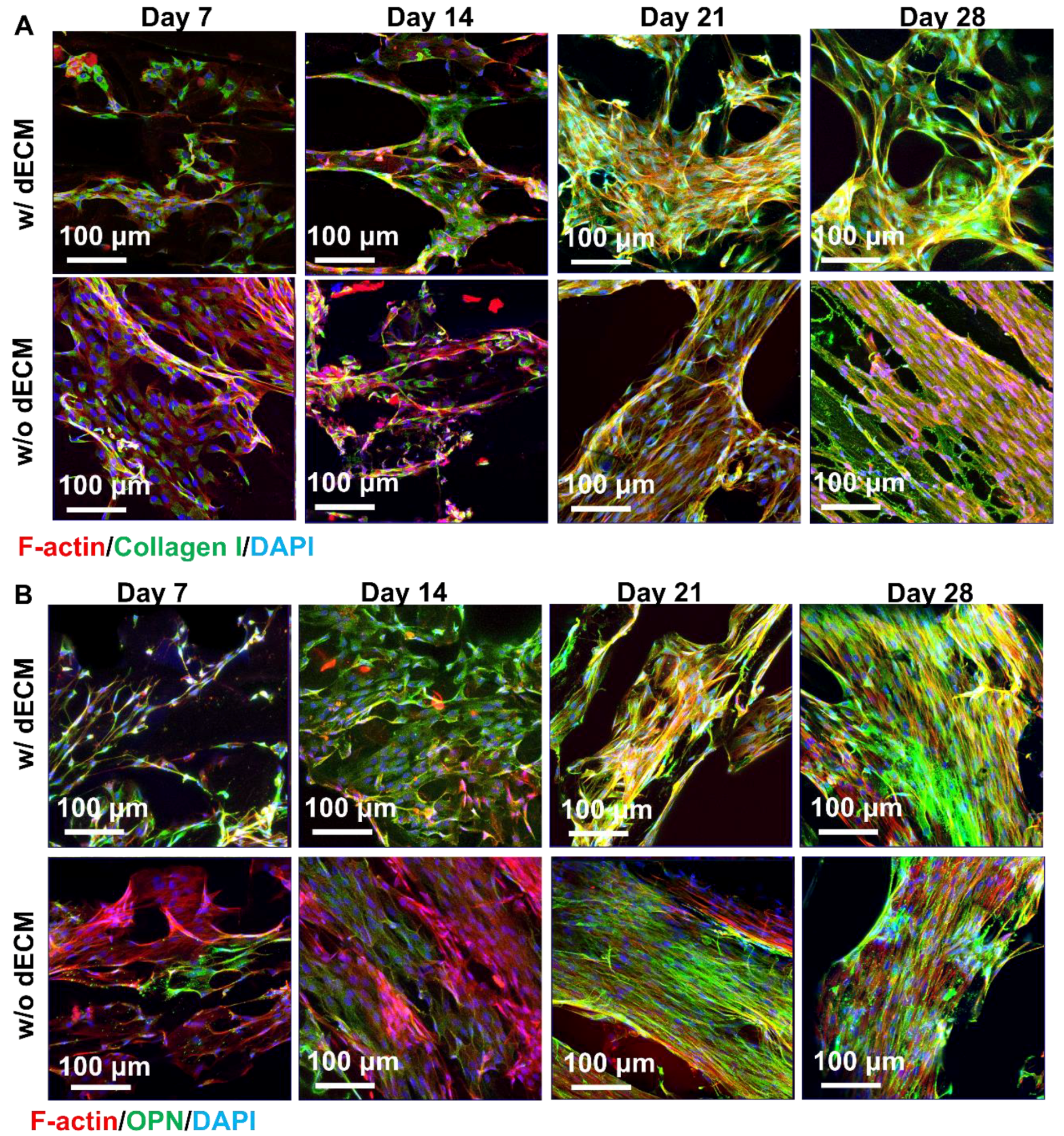
3.6. Reseeding Cells to dECM Scaffolds
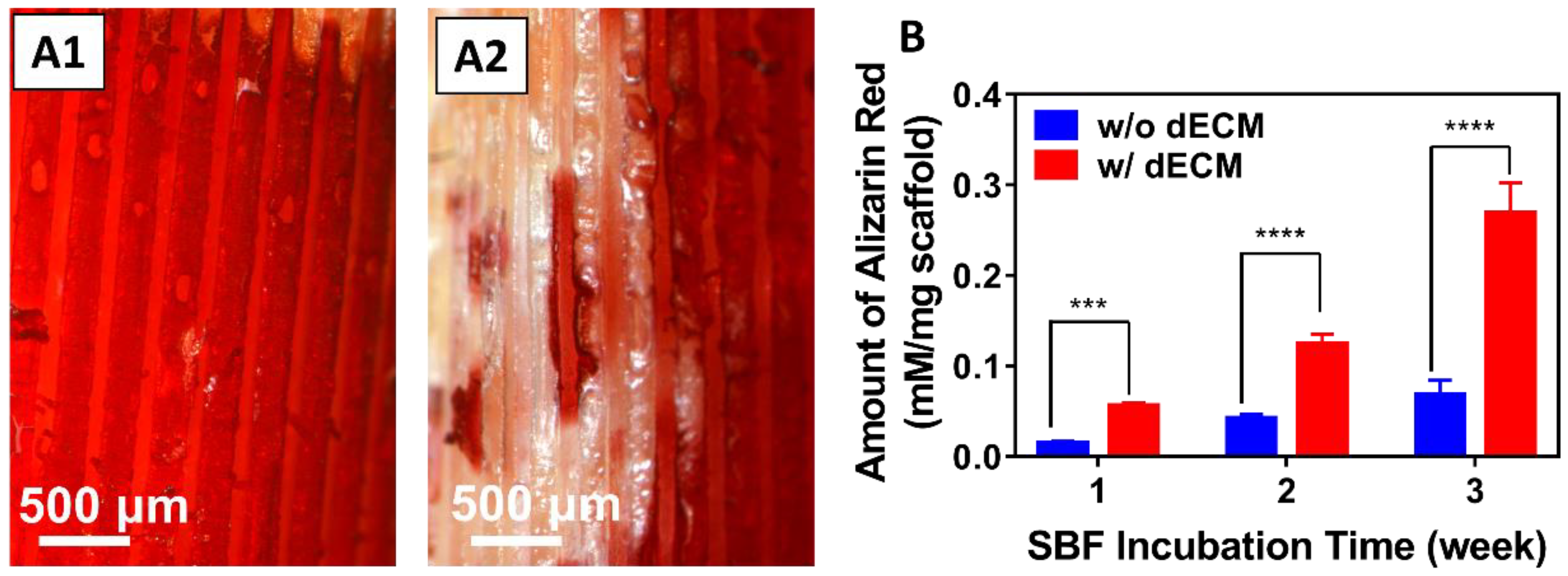
3.7. Biodeposition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Betz, R.R. Limitations of autograft and allograft: New synthetic solutions. Orthopedics 2002, 25 (Suppl. S5), s561–s570. [Google Scholar] [CrossRef]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma. 2019, 33, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, E.; Cadossi, M.; Tedesco, G.; Capra, P.; Calamelli, C.; Shehu, A.; Giannini, S. Autograft, allograft and bone substitutes in reconstructive orthopedic surgery. Aging Clin. Exp. Res. 2013, 25 (Suppl. S1), S101–S103. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G.; Moghaddam, A. Allograft bone matrix versus synthetic bone graft substitutes. Injury 2011, 42 (Suppl. S2), S16–S21. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Vidal, L.; Kampleitner, C.; Brennan, M.A.; Hoornaert, A.; Layrolle, P. Reconstruction of Large Skeletal Defects: Current Clinical Therapeutic Strategies and Future Directions Using 3D Printing. Front. Bioeng. Biotechnol. 2020, 8, 61. [Google Scholar] [CrossRef] [Green Version]
- Frohlich, M.; Grayson, W.L.; Wan, L.Q.; Marolt, D.; Drobnic, M.; Vunjak-Novakovic, G. Tissue engineered bone grafts: Biological requirements, tissue culture and clinical relevance. Curr. Stem Cell Res. Ther. 2008, 3, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Petite, H.; Viateau, V.; Bensaid, W.; Meunier, A.; de Pollak, C.; Bourguignon, M.; Oudina, K.; Sedel, L.; Guillemin, G. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963. [Google Scholar] [CrossRef]
- Zhu, T.; Cui, Y.; Zhang, M.; Zhao, D.; Liu, G.; Ding, J. Engineered three-dimensional scaffolds for enhanced bone regeneration in osteonecrosis. Bioact. Mater. 2020, 5, 584–601. [Google Scholar] [CrossRef]
- Yeo, A.; Wong, W.J.; Teoh, S.H. Surface modification of PCL-TCP scaffolds in rabbit calvaria defects: Evaluation of scaffold degradation profile, biomechanical properties and bone healing patterns. J. Biomed. Mater. Res. A 2010, 93, 1358–1367. [Google Scholar] [CrossRef]
- Fernandez, J.M.; Molinuevo, M.S.; Cortizo, M.S.; Cortizo, A.M. Development of an osteoconductive PCL-PDIPF-hydroxyapatite composite scaffold for bone tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, e126–e135. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, Q.; Wootton, D.; Chiou, R.; Li, D.; Lu, B.; Lelkes, P.; Zhou, J. Biocompatibility and biodegradation studies of PCL/beta-TCP bone tissue scaffold fabricated by structural porogen method. J. Mater. Sci. Mater. Med. 2012, 23, 2217–2226. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tankus, E.B.; Santarella, F.; Rohr, N.; Sharma, N.; Martin, S.; Michalscheck, M.; Maintz, M.; Cao, S.; Thieringer, F.M. Fabrication and Characterization of PCL/HA Filament as a 3D Printing Material Using Thermal Extrusion Technology for Bone Tissue Engineering. Polymers 2022, 14, 669. [Google Scholar] [CrossRef] [PubMed]
- Moghaddaszadeh, A.; Seddiqi, H.; Najmoddin, N.; Abbasi Ravasjani, S.; Klein-Nulend, J. Biomimetic 3D-printed PCL scaffold containing a high concentration carbonated-nanohydroxyapatite with immobilized-collagen for bone tissue engineering: Enhanced bioactivity and physicomechanical characteristics. Biomed. Mater. 2021, 16, 065029. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, Y.; Suo, H.; Yan, Y.; Liu, L.; Wang, Q.; Ge, Y.; Xu, Y. Fabricating a pearl/PLGA composite scaffold by the low-temperature deposition manufacturing technique for bone tissue engineering. Biofabrication 2010, 2, 025002. [Google Scholar] [CrossRef]
- Chen, S.H.; Lei, M.; Xie, X.H.; Zheng, L.Z.; Yao, D.; Wang, X.L.; Li, W.; Zhao, Z.; Kong, A.; Xiao, D.M.; et al. PLGA/TCP composite scaffold incorporating bioactive phytomolecule icaritin for enhancement of bone defect repair in rabbits. Acta Biomater. 2013, 9, 6711–6722. [Google Scholar] [CrossRef]
- Pouroutzidou, G.K.; Papadopoulou, L.; Lazaridou, M.; Tsachouridis, K.; Papoulia, C.; Patsiaoura, D.; Tsamesidis, I.; Chrissafis, K.; Vourlias, G.; Paraskevopoulos, K.M.; et al. Composite PLGA-Nanobioceramic Coating on Moxifloxacin-Loaded Akermanite 3D Porous Scaffolds for Bone Tissue Regeneration. Pharmaceutics 2023, 15, 819. [Google Scholar] [CrossRef]
- Chen, F.; Han, J.; Guo, Z.; Mu, C.; Yu, C.; Ji, Z.; Sun, L.; Wang, Y.; Wang, J. Antibacterial 3D-Printed Silver Nanoparticle/Poly Lactic-Co-Glycolic Acid (PLGA) Scaffolds for Bone Tissue Engineering. Materials 2023, 16, 3895. [Google Scholar] [CrossRef]
- Hirota, M.; Matsui, Y.; Mizuki, N.; Kishi, T.; Watanuki, K.; Ozawa, T.; Fukui, T.; Shoji, S.; Adachi, M.; Monden, Y.; et al. Combination with allogenic bone reduces early absorption of beta-tricalcium phosphate (beta-TCP) and enhances the role as a bone regeneration scaffold. Experimental animal study in rat mandibular bone defects. Dent. Mater. J. 2009, 28, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Kuboyama, N. A biodegradable porous composite scaffold of PGA/beta-TCP for bone tissue engineering. Bone 2010, 46, 386–395. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, J.; Zhang, L.; Xu, K. A multi-scaled hybrid orthopedic implant: Bone ECM-shaped Sr-HA nanofibers on the microporous walls of a macroporous titanium scaffold. Nanotechnology 2011, 22, 275603. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zuo, Y.; Zou, Q.; Yang, B.; Lin, L.; Li, J.; Li, Y. Hierarchical Structure and Mechanical Improvement of an n-HA/GCO-PU Composite Scaffold for Bone Regeneration. ACS Appl. Mater. Interfaces 2015, 7, 22618–22629. [Google Scholar] [CrossRef] [PubMed]
- Klimek, K.; Przekora, A.; Benko, A.; Niemiec, W.; Blazewicz, M.; Ginalska, G. The use of calcium ions instead of heat treatment for beta-1,3-glucan gelation improves biocompatibility of the beta-1,3-glucan/HA bone scaffold. Carbohydr. Polym. 2017, 164, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, H.; Lee, J.; Kim, G. Mechanically and biologically enhanced 3D-printed HA/PLLA/dECM biocomposites for bone tissue engineering. Int. J. Biol. Macromol. 2022, 218, 9–21. [Google Scholar] [CrossRef]
- Gang, F.; Ye, W.; Ma, C.; Wang, W.; Xiao, Y.; Liu, C.; Sun, X. 3D Printing of PLLA/Biomineral Composite Bone Tissue Engineering Scaffolds. Materials 2022, 15, 4280. [Google Scholar] [CrossRef]
- Williams, D.F. The biomaterials conundrum in tissue engineering. Tissue Eng. Part A 2014, 20, 1129–1131. [Google Scholar] [CrossRef]
- Dawson, J.I.; Oreffo, R.O. Bridging the regeneration gap: Stem cells, biomaterials and clinical translation in bone tissue engineering. Arch. Biochem. Biophys. 2008, 473, 124–131. [Google Scholar] [CrossRef]
- Griffith, L.G.; Naughton, G. Tissue engineering-current challenges and expanding opportunities. Science 2002, 295, 1009–1014. [Google Scholar] [CrossRef]
- Abdulmaged, A.I.; Soon, C.F.; Talip, B.A.; Zamhuri, S.A.A.; Mostafa, S.A.; Zhou, W. Characterization of Alginate-Gelatin-Cholesteryl Ester Liquid Crystals Bioinks for Extrusion Bioprinting of Tissue Engineering Scaffolds. Polymer 2022, 14, 1021. [Google Scholar] [CrossRef]
- Jablonska-Trypuc, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2016, 31 (Suppl. S1), 177–183. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; Del Rio Hernandez, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef] [Green Version]
- Khatiwala, C.B.; Peyton, S.R.; Metzke, M.; Putnam, A.J. The regulation of osteogenesis by ECM rigidity in MC3T3-E1 cells requires MAPK activation. J. Cell. Physiol. 2007, 211, 661–672. [Google Scholar] [CrossRef]
- Khatiwala, C.B.; Kim, P.D.; Peyton, S.R.; Putnam, A.J. ECM compliance regulates osteogenesis by influencing MAPK signaling downstream of RhoA and ROCK. J. Bone Min. Res. 2009, 24, 886–898. [Google Scholar] [CrossRef] [Green Version]
- Hoshiba, T.; Lu, H.; Kawazoe, N.; Chen, G. Decellularized matrices for tissue engineering. Expert. Opin. Biol. Ther. 2010, 10, 1717–1728. [Google Scholar] [CrossRef]
- Hoshiba, T.; Chen, G.; Endo, C.; Maruyama, H.; Wakui, M.; Nemoto, E.; Kawazoe, N.; Tanaka, M. Decellularized Extracellular Matrix as an In Vitro Model to Study the Comprehensive Roles of the ECM in Stem Cell Differentiation. Stem Cells Int. 2016, 2016, 6397820. [Google Scholar] [CrossRef] [Green Version]
- Badylak, S.F. The extracellular matrix as a biologic scaffold material. Biomaterials 2007, 28, 3587–3593. [Google Scholar] [CrossRef]
- Taylor, D.A.; Sampaio, L.C.; Ferdous, Z.; Gobin, A.S.; Taite, L.J. Decellularized matrices in regenerative medicine. Acta Biomater. 2018, 74, 74–89. [Google Scholar] [CrossRef]
- Diniz, M.G.; Soares, G.A.; Coelho, M.J.; Fernandes, M.H. Surface topography modulates the osteogenesis in human bone marrow cell cultures grown on titanium samples prepared by a combination of mechanical and acid treatments. J. Mater. Sci. Mater. Med. 2002, 13, 421–432. [Google Scholar] [CrossRef]
- Jiang, H.; Zuo, Y.; Zou, Q.; Wang, H.; Du, J.; Li, Y.; Yang, X. Biomimetic spiral-cylindrical scaffold based on hybrid chitosan/cellulose/nano-hydroxyapatite membrane for bone regeneration. ACS Appl. Mater. Interfaces 2013, 5, 12036–12044. [Google Scholar] [CrossRef]
- Zhou, S.; Zheng, T.; Chen, Y.; Zhang, J.; Li, L.; Lu, F.; Zhu, J.J. Toward therapeutic effects evaluation of chronic myeloid leukemia drug: Electrochemical platform for caspase-3 activity sensing. Biosens. Bioelectron. 2014, 61, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, X. Preparation, characterization and in vitro analysis of novel structured nanofibrous scaffolds for bone tissue engineering. Acta Biomater. 2010, 6, 3004–3012. [Google Scholar] [CrossRef]
- Junka, R.; Quevada, K.; Yu, X. Acellular polycaprolactone scaffolds laden with fibroblast/endothelial cell-derived extracellular matrix for bone regeneration. J. Biomed. Mater. Res. A 2020, 108, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Junka, R.; Yu, X. Novel Acellular Scaffold Made from Decellularized Schwann Cell Sheets for Peripheral Nerve Regeneration. Regen. Eng. Transl. Med. 2015, 1, 22–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokubo, T.; Yamaguchi, S. Simulated body fluid and the novel bioactive materials derived from it. J. Biomed. Mater. Res. A 2019, 107, 968–977. [Google Scholar] [CrossRef]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [Green Version]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef]
- Kim, I.G.; Hwang, M.P.; Du, P.; Ko, J.; Ha, C.W.; Do, S.H.; Park, K. Bioactive cell-derived matrices combined with polymer mesh scaffold for osteogenesis and bone healing. Biomaterials 2015, 50, 75–86. [Google Scholar] [CrossRef]
- Li, M.; Zhang, A.; Li, J.; Zhou, J.; Zheng, Y.; Zhang, C.; Xia, D.; Mao, H.; Zhao, J. Osteoblast/fibroblast coculture derived bioactive ECM with unique matrisome profile facilitates bone regeneration. Bioact. Mater. 2020, 5, 938–948. [Google Scholar] [CrossRef]
- Quarles, L.D.; Yohay, D.A.; Lever, L.W.; Caton, R.; Wenstrup, R.J. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: An in vitro model of osteoblast development. J. Bone Min. Res. 1992, 7, 683–692. [Google Scholar] [CrossRef]
- Beresford, J.N.; Graves, S.E.; Smoothy, C.A. Formation of mineralized nodules by bone derived cells in vitro: A model of bone formation? Am. J. Med. Genet. 1993, 45, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Junka, R.; Zhou, X.; Wang, W.; Yu, X. Albumin-Coated Polycaprolactone (PCL)-Decellularized Extracellular Matrix (dECM) Scaffold for Bone Regeneration. ACS Appl. Bio Mater. 2022, 12, 5634–5644. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Zhou, X.; Yin, Z.; Yu, X. Fabrication and Evaluation of Porous dECM/PCL Scaffolds for Bone Tissue Engineering. J. Funct. Biomater. 2023, 14, 343. https://doi.org/10.3390/jfb14070343
Wang W, Zhou X, Yin Z, Yu X. Fabrication and Evaluation of Porous dECM/PCL Scaffolds for Bone Tissue Engineering. Journal of Functional Biomaterials. 2023; 14(7):343. https://doi.org/10.3390/jfb14070343
Chicago/Turabian StyleWang, Weiwei, Xiaqing Zhou, Zhuozhuo Yin, and Xiaojun Yu. 2023. "Fabrication and Evaluation of Porous dECM/PCL Scaffolds for Bone Tissue Engineering" Journal of Functional Biomaterials 14, no. 7: 343. https://doi.org/10.3390/jfb14070343
APA StyleWang, W., Zhou, X., Yin, Z., & Yu, X. (2023). Fabrication and Evaluation of Porous dECM/PCL Scaffolds for Bone Tissue Engineering. Journal of Functional Biomaterials, 14(7), 343. https://doi.org/10.3390/jfb14070343






