Osseointegration of Tantalum Trabecular Metal in Titanium Dental Implants: Histological and Micro-CT Study
Abstract
:1. Introduction
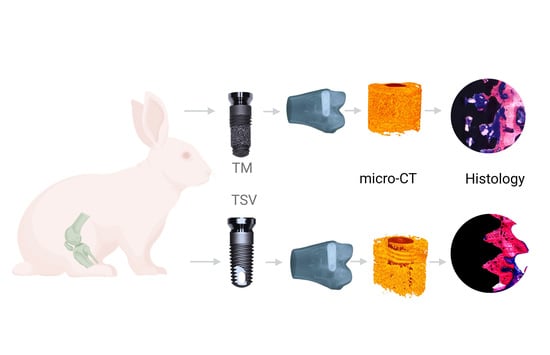
2. Materials and Methods
2.1. Implants
2.2. Micro-Computer Tomography
2.3. Histomorphometric Evaluation
2.4. Statistical Analysis
3. Results
3.1. General Observation
3.2. Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koller, B.; Att, W.; Strub, J.R. Survival rates of teeth, implants, and double crown-retained removable dental prostheses: A systematic literature review. Int. J. Prosthodont. 2011, 24, 109–117. [Google Scholar] [PubMed]
- Chung, W.E.; Rubenstein, J.E.; Phillips, K.M.; Raigrodski, A.J. Outcomes assessment of patients treated with osseointegrated dental implants at the university of washington graduate prosthodontic program, 1988 to 2000. Int. J. Oral Maxillofac. Implant. 2009, 24, 927–935. [Google Scholar]
- Holm-Pedersen, P.; Lang, N.P.; Muller, F. What are the longevities of teeth and oral implants? Clin. Oral Implant. Res. 2007, 18 (Suppl. 3), 15–19. [Google Scholar] [CrossRef]
- Romanos, G.E.; Delgado-Ruiz, R.; Sculean, A. Concepts for prevention of complications in implant therapy. Periodontology 2000 2019, 81, 7–17. [Google Scholar] [CrossRef]
- Kim, D.G.; Huja, S.S.; Tee, B.C.; Larsen, P.E.; Kennedy, K.S.; Chien, H.H.; Lee, J.W.; Wen, H.B. Bone ingrowth and initial stability of titanium and porous tantalum dental implants: A pilot canine study. Implant. Dent. 2013, 22, 399–405. [Google Scholar] [CrossRef]
- Trisi, P.; Marcato, C.; Todisco, M. Bone-to-implant apposition with machined and mtx microtextured implant surfaces in human sinus grafts. Int. J. Periodontics Restor. Dent. 2003, 23, 427–437. [Google Scholar]
- Gaikwad, A.M.; Joshi, A.A.; Nadgere, J.B. Biomechanical and histomorphometric analysis of endosteal implants placed by using the osseodensification technique in animal models: A systematic review and meta-analysis. J. Prosthet. Dent. 2022, 127, 61–70. [Google Scholar] [CrossRef]
- Okazaki, Y.; Katsuda, S.I. Biological safety evaluation and surface modification of biocompatible ti-15zr-4nb alloy. Materials 2021, 14, 731. [Google Scholar] [CrossRef] [PubMed]
- Bencharit, S.; Morelli, T.; Barros, S.; Seagroves, J.T.; Kim, S.; Yu, N.; Byrd, K.; Brenes, C.; Offenbacher, S. Comparing initial wound healing and osteogenesis of porous tantalum trabecular metal and titanium alloy materials. J. Oral Implantol. 2019, 45, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhao, D.; Wang, B.; Wang, W.; Kang, K.; Xie, H.; Liu, B.; Zhang, X.; Zhang, J.; Yang, Z. Tantalum coating of porous carbon scaffold supplemented with autologous bone marrow stromal stem cells for bone regeneration in vitro and in vivo. Exp. Biol. Med. 2016, 241, 592–602. [Google Scholar] [CrossRef] [Green Version]
- Bencharit, S.; Byrd, W.C.; Altarawneh, S.; Hosseini, B.; Leong, A.; Reside, G.; Morelli, T.; Offenbacher, S. Development and applications of porous tantalum trabecular metal-enhanced titanium dental implants. Clin. Implant. Dent. Relat. Res. 2014, 16, 817–826. [Google Scholar] [CrossRef] [Green Version]
- Farahat, A.; Nawar, N. Comparative study of bone height changes around immediately loaded porous tantalum parallel sided trabecular & screw shaped implants retaining mandibular implant- overdentures using cbct (rct). Egypt. Dent. J. 2019, 65, 3571–3580. [Google Scholar]
- Hefni, E.K.; Bencharit, S.; Kim, S.J.; Byrd, K.M.; Moreli, T.; Nociti, F.H.; Offenbacher, S.; Barros, S.P. Transcriptomic profiling of tantalum metal implant osseointegration in osteopenic patients. BDJ Open 2018, 4, 17042. [Google Scholar] [CrossRef] [Green Version]
- Carraro, F.; Bagno, A. Tantalum as trabecular metal for endosseous implantable applications. Biomimetics 2023, 8, 49. [Google Scholar] [CrossRef]
- Liu, Y.; Bao, C.; Wismeijer, D.; Wu, G. The physicochemical/biological properties of porous tantalum and the potential surface modification techniques to improve its clinical application in dental implantology. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ning, B.; Pei, X. Tantalum and its derivatives in orthopedic and dental implants: Osteogenesis and antibacterial properties. Colloids Surf. B Biointerfaces 2021, 208, 112055. [Google Scholar] [CrossRef] [PubMed]
- Bencharit, S.; Byrd, W.C.; Hosseini, B. Immediate placement of a porous-tantalum, trabecular metal-enhanced titanium dental implant with demineralized bone matrix into a socket with deficient buccal bone: A clinical report. J. Prosthet. Dent. 2015, 113, 262–269. [Google Scholar] [CrossRef] [Green Version]
- Schlee, M.; Pradies, G.; Mehmke, W.U.; Beneytout, A.; Stamm, M.; Meda, R.G.; Kamm, T.; Poiroux, F.; Weinlich, F.; del Canto Pingarron, M.; et al. Prospective, multicenter evaluation of trabecular metal-enhanced titanium dental implants placed in routine dental practices: 1-year interim report from the development period (2010 to 2011). Clin. Implant. Dent. Relat. Res. 2015, 17, 1141–1153. [Google Scholar] [CrossRef]
- van der Schoor, P.; Schlee, M.; Wen, H.-B. Prospective pilot study of immediately provisionalized restorations of trabecular metal-enhanced titanium dental implants: A 5-year follow-up report. Appl. Sci. 2022, 12, 942. [Google Scholar] [CrossRef]
- Lee, J.W.; Wen, H.B.; Gubbi, P.; Romanos, G.E. New bone formation and trabecular bone microarchitecture of highly porous tantalum compared to titanium implant threads: A pilot canine study. Clin. Oral Implant. Res. 2018, 29, 164–174. [Google Scholar] [CrossRef]
- Deglurkar, M.; Davy, D.T.; Stewart, M.; Goldberg, V.M.; Welter, J.F. Evaluation of machining methods for trabecular metal implants in a rabbit intramedullary osseointegration model. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, W.; Baneux, P.; Boot, R.; Decelle, T.; Deeny, A.A.; Fumanelli, M.; Illgen-Wilcke, B.; Felasa. Recommendations for the health monitoring of rodent and rabbit colonies in breeding and experimental units. Lab. Anim. 2002, 36, 20–42. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the arrive guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef] [PubMed]
- AlFarraj Aldosari, A.; Anil, S.; Alasqah, M.; Al Wazzan, K.A.; Al Jetaily, S.A.; Jansen, J.A. The influence of implant geometry and surface composition on bone response. Clin. Oral Implant. Res. 2014, 25, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Henkin, J. Micro-computed tomography assessment of human alveolar bone: Bone density and three-dimensional micro-architecture. Clin. Implant. Dent. Relat. Res. 2015, 17, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Pothuaud, L.; Van Rietbergen, B.; Mosekilde, L.; Beuf, O.; Levitz, P.; Benhamou, C.L.; Majumdar, S. Combination of topological parameters and bone volume fraction better predicts the mechanical properties of trabecular bone. J. Biomech. 2002, 35, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Kreve, S.; Ferreira, I.; da Costa Valente, M.L.; Dos Reis, A.C. Relationship between dental implant macro-design and osseointegration: A systematic review. Oral. Maxillofac. Surg. 2022, 1–14. [Google Scholar] [CrossRef]
- Hultin, M.; Fischer, J.; Gustafsson, A.; Kallus, T.; Klinge, B. Factors affecting late fixture loss and marginal bone loss around teeth and dental implants. Clin. Implant. Dent. Relat. Res. 2000, 2, 203–208. [Google Scholar] [CrossRef]
- Sfondrini, M.F.; Gandini, P.; Alcozer, R.; Vallittu, P.K.; Scribante, A. Failure load and stress analysis of orthodontic miniscrews with different transmucosal collar diameter. J. Mech. Behav. Biomed. Mater. 2018, 87, 132–137. [Google Scholar] [CrossRef]
- Vandeweghe, S.; Cosyn, J.; Thevissen, E.; Teerlinck, J.; De Bruyn, H. The influence of implant design on bone remodeling around surface-modified southern implants(r). Clin. Implant. Dent. Relat. Res. 2012, 14, 655–662. [Google Scholar] [CrossRef]
- Albrektsson, T.; Chrcanovic, B.R.; Jacobsson, M.; Wennerberg, A. Osseointegration of implants: A biological and clinical overview. JSM Dent. Surg. 2017, 2, 1022. [Google Scholar]
- Kittur, N.; Oak, R.; Dekate, D.; Jadhav, S.; Dhatrak, P. Dental implant stability and its measurements to improve osseointegration at the bone-implant interface: A review. Mater. Today Proc. 2021, 43, 1064–1070. [Google Scholar] [CrossRef]
- Levine, B.R.; Sporer, S.; Poggie, R.A.; Della Valle, C.J.; Jacobs, J.J. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials 2006, 27, 4671–4681. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 690–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.Y.; Huang, H.L.; Chen, Y.C.; Hsu, J.T.; Shieh, T.M.; Tsai, M.T. Biological characteristics of the mg-63 human osteosarcoma cells on composite tantalum carbide/amorphous carbon films. PLoS ONE 2014, 9, e95590. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.M.; Li, Y.; Gu, Y.X.; Zhang, C.N.; Lai, H.C.; Shi, J.Y. Ta-coated titanium surface with superior bacteriostasis and osseointegration. Int. J. Nanomed. 2019, 14, 8693–8706. [Google Scholar] [CrossRef] [Green Version]
- Fathi, M.H.; Azam, F. Novel hydroxyapatite/tantalum surface coating for metallic dental implant. Mater. Lett. 2007, 61, 1238–1241. [Google Scholar] [CrossRef]
- Spinato, S.; Zaffe, D.; Felice, P.; Checchi, L.; Wang, H.L. A trabecular metal implant 4 months after placement: Clinical-histologic case report. Implant. Dent. 2014, 23, 3–7. [Google Scholar] [CrossRef]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced bone apposition to a chemically modified sla titanium surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef]
- Cafiero, C.; Aglietta, M.; Iorio-Siciliano, V.; Salvi, G.E.; Blasi, A.; Matarasso, S. Implant surface roughness alterations induced by different prophylactic procedures: An in vitro study. Clin. Oral Implant. Res. 2017, 28, e16–e20. [Google Scholar] [CrossRef]
- Zareidoost, A.; Yousefpour, M.; Ghaseme, B.; Amanzadeh, A. The relationship of surface roughness and cell response of chemical surface modification of titanium. J. Mater. Sci. Mater. Med. 2012, 23, 1479–1488. [Google Scholar] [CrossRef] [Green Version]
- Khang, W.; Feldman, S.; Hawley, C.E.; Gunsolley, J. A multi-center study comparing dual acid-etched and machined-surfaced implants in various bone qualities. J. Periodontol. 2001, 72, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Fabbro, M.D.; Taschieri, S.; Canciani, E.; Addis, A.; Musto, F.; Weinstein, R.; Dellavia, C. Osseointegration of titanium implants with different rough surfaces: A histologic and histomorphometric study in an adult minipig model. Implant. Dent. 2017, 26, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K.; Bigerelle, M. Statistical demonstration of the relative effect of surface chemistry and roughness on human osteoblast short-term adhesion. J. Mater. Sci. Mater. Med. 2006, 17, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Lee, C.; Kim, D.G.; Choi, K.; Lee, K.H.; Kim, Y.D. Effect of surface structure on biomechanical properties and osseointegration. Mater. Sci. Eng. C 2008, 28, 1448–1461. [Google Scholar] [CrossRef]
- Kligman, S.; Ren, Z.; Chung, C.H.; Perillo, M.A.; Chang, Y.C.; Koo, H.; Zheng, Z.; Li, C. The impact of dental implant surface modifications on osseointegration and biofilm formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef]
- Schulze, C.; Weinmann, M.; Schweigel, C.; Kessler, O.; Bader, R. Mechanical properties of a newly additive manufactured implant material based on ti-42nb. Materials 2018, 11, 124. [Google Scholar] [CrossRef] [Green Version]
- Cooper, L.F. A role for surface topography in creating and maintaining bone at titanium endosseous implants. J. Prosthet. Dent. 2000, 84, 522–534. [Google Scholar] [CrossRef]
- Anil, S.; Cuijpers, V.M.; Preethanath, R.S.; Aldosari, A.A.; Jansen, J.A. Osseointegration of oral implants after delayed placement in rabbits: A microcomputed tomography and histomorphometric study. Int. J. Oral Maxillofac. Implant. 2013, 28, 1506–1511. [Google Scholar] [CrossRef]
- Duyck, J.; Slaets, E.; Sasaguri, K.; Vandamme, K.; Naert, I. Effect of intermittent loading and surface roughness on peri-implant bone formation in a bone chamber model. J. Clin. Periodontol. 2007, 34, 998–1006. [Google Scholar] [CrossRef]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wancket, L.M. Animal models for evaluation of bone implants and devices: Comparative bone structure and common model uses. Vet. Pathol. 2015, 52, 842–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Patil, D.J.; Soni, V.P.; Sarkate, L.B.; Khandekar, G.S.; Bellare, J.R. Bone healing performance of electrophoretically deposited apatite-wollastonite/chitosan coating on titanium implants in rabbit tibiae. J. Tissue Eng. Regen. Med. 2009, 3, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Hixon, K.R.; Miller, A.N. Animal models of impaired long bone healing and tissue engineering- and cell-based in vivo interventions. J. Orthop. Res. 2022, 40, 767–778. [Google Scholar] [CrossRef]







| Rabbit Serial No | Right Femur | Left Femur |
|---|---|---|
| SA-01 | TM | TSV |
| SA-02 | TSV | TM |
| SA-03 | TM | TSV |
| SA-04 | TSV | TM |
| SA-05 | TM | TSV |
| SA-06 | TSV | TM |
| SA-07 | TM | TSV |
| SA-08 | TSV | TM |
| SA-09 | TM | TSV |
| SA-10 | TSV | TM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Deeb, M.; Aldosari, A.A.; Anil, S. Osseointegration of Tantalum Trabecular Metal in Titanium Dental Implants: Histological and Micro-CT Study. J. Funct. Biomater. 2023, 14, 355. https://doi.org/10.3390/jfb14070355
Al Deeb M, Aldosari AA, Anil S. Osseointegration of Tantalum Trabecular Metal in Titanium Dental Implants: Histological and Micro-CT Study. Journal of Functional Biomaterials. 2023; 14(7):355. https://doi.org/10.3390/jfb14070355
Chicago/Turabian StyleAl Deeb, Modhi, Abdullah AlFarraj Aldosari, and Sukumaran Anil. 2023. "Osseointegration of Tantalum Trabecular Metal in Titanium Dental Implants: Histological and Micro-CT Study" Journal of Functional Biomaterials 14, no. 7: 355. https://doi.org/10.3390/jfb14070355
APA StyleAl Deeb, M., Aldosari, A. A., & Anil, S. (2023). Osseointegration of Tantalum Trabecular Metal in Titanium Dental Implants: Histological and Micro-CT Study. Journal of Functional Biomaterials, 14(7), 355. https://doi.org/10.3390/jfb14070355