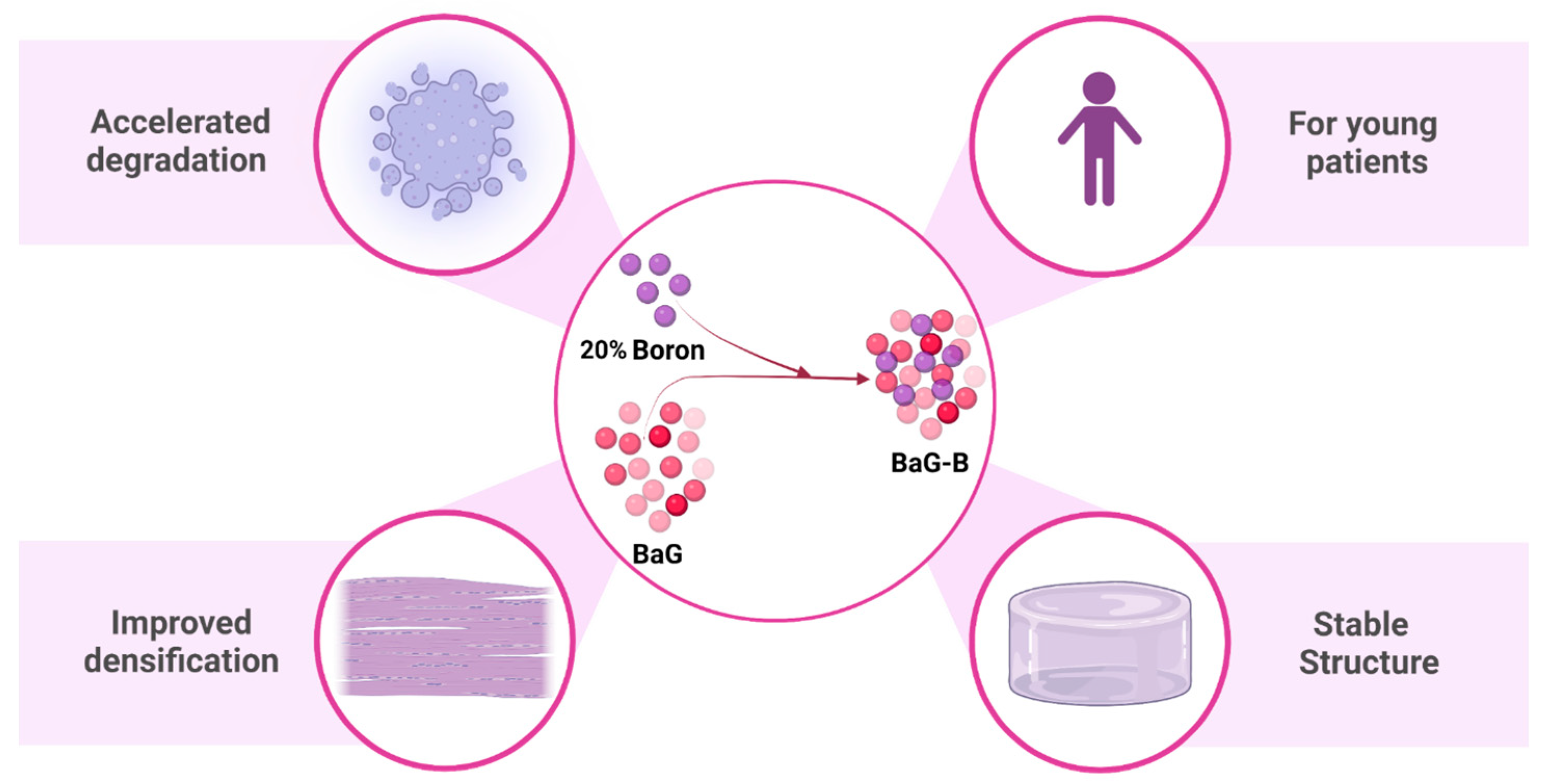
High Boron Content Enhances Bioactive Glass Biodegradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Glass Preparation
2.2. In Vitro Assays
2.3. Structural Characterization
2.3.1. X-ray Diffraction (XRD)
2.3.2. Infrared Spectroscopy (IR)
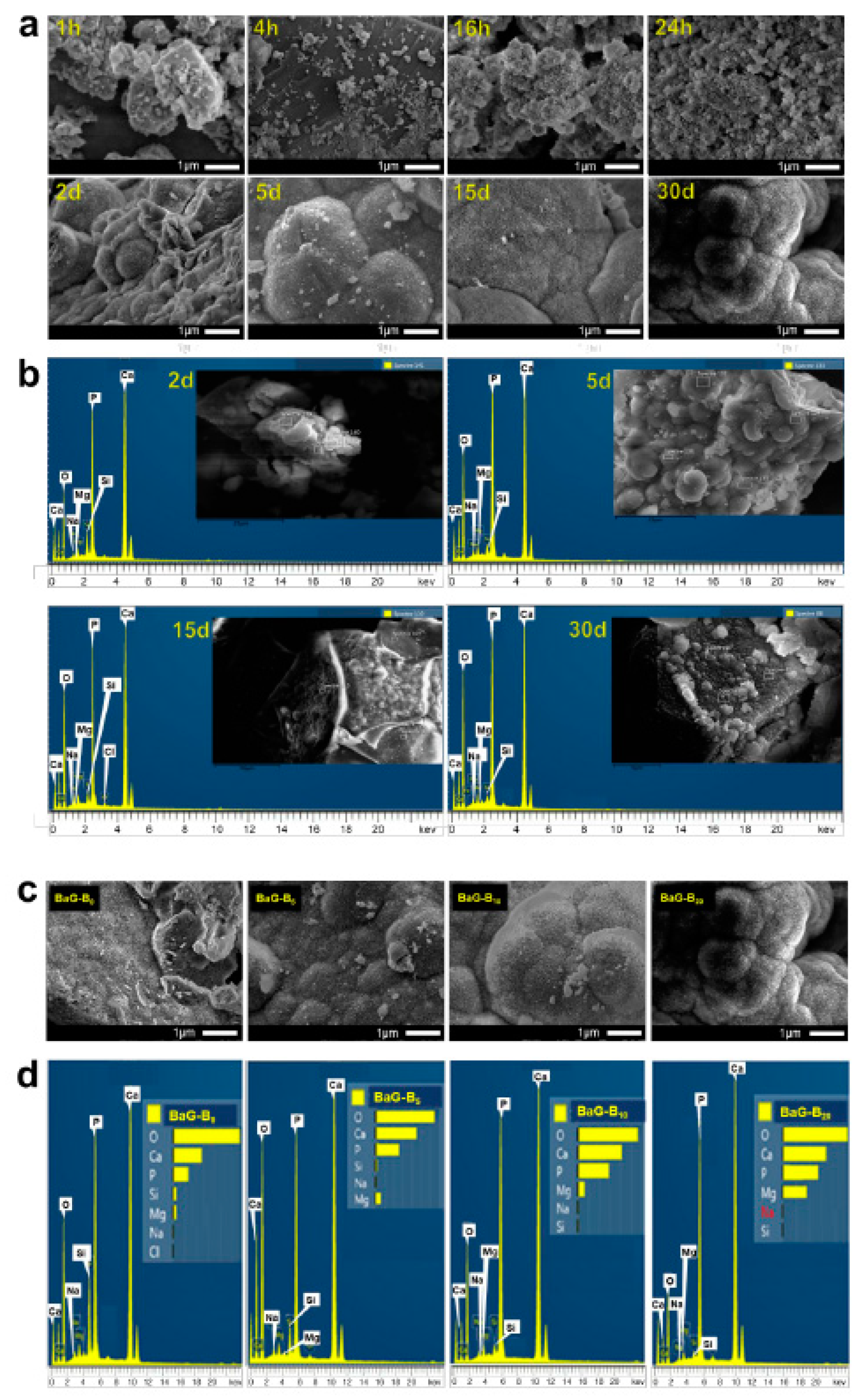
2.3.3. Scanning Electron Microscopy-Energy-Dispersive Spectrometry (SEM-EDS)
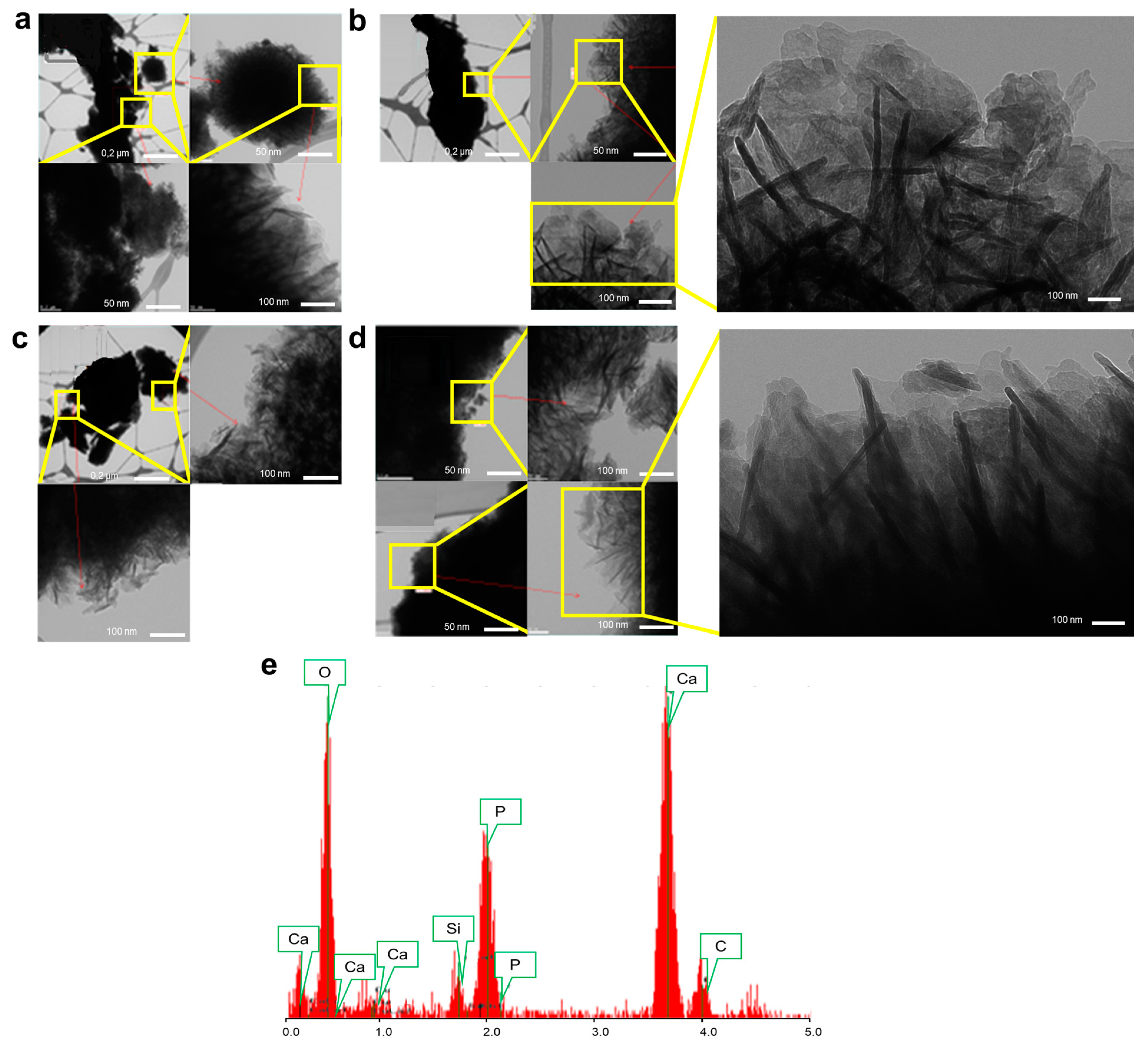
2.3.4. Transmission Electron Microscope (TEM)
2.4. Chemical Characterization
3. Results and Discussion
3.1. Physico-Chemical Characterizations after Immersion in SBF
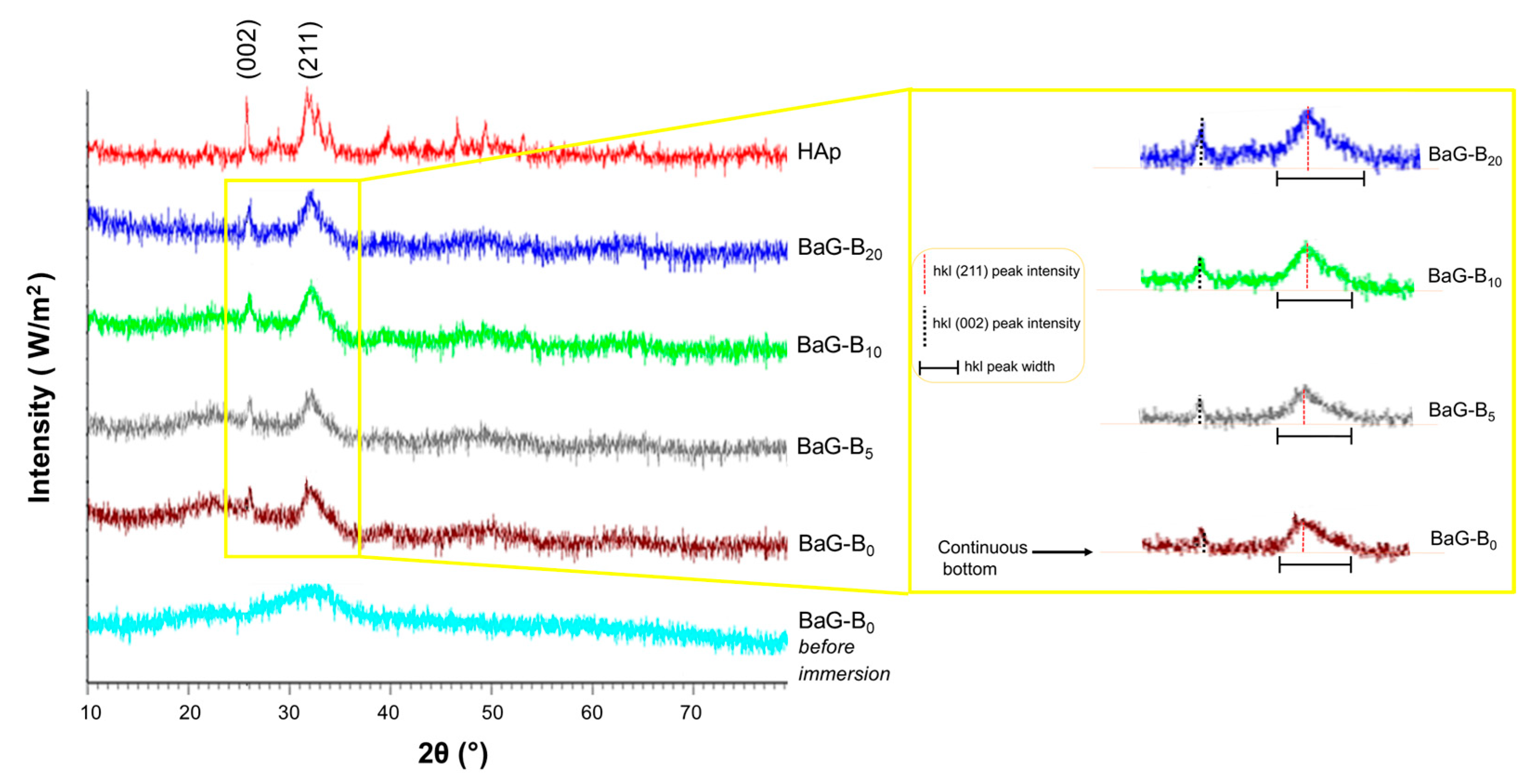
3.1.1. XRD
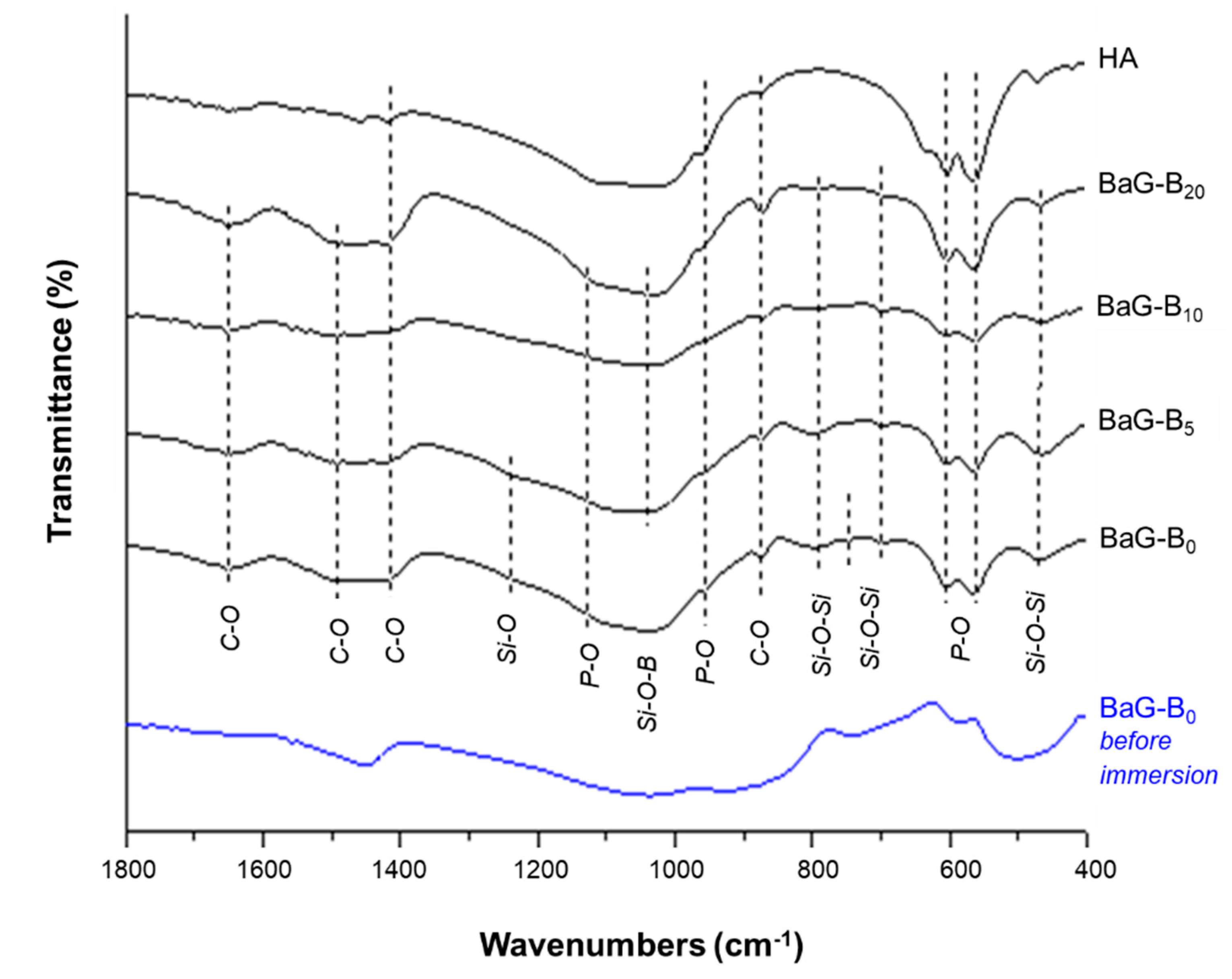
3.1.2. Infra-Red Spectroscopic Analysis
3.1.3. BaG-Bx Surface Modifications
3.1.4. Nano-Structural Changes in BaG-B
3.1.5. Ion Exchange between BaG-B and SBF
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arealis, G.; Nikolaou, V.S. Bone printing: New frontiers in the treatment of bone defect. Injury 2015, 46, S20–S22. [Google Scholar] [CrossRef] [PubMed]
- Bara, J.J.; Dresing, I.; Zeiter, S.; Anton, M.; Daculsi, G.; Eglin, D.; Nehrbass, D.; Stadelmann, V.A.; Betts, D.C.; Müller, R.; et al. A doxycycline inducible, adenoviral bone morphogenetic protein-2 gene delivery system to bone. J. Tissue Eng. Regen. Med. 2018, 12, e106–e118. [Google Scholar] [CrossRef] [PubMed]
- Ashammakhi, N.; Peltoniemi, H.; Waris, E.; Suuronen, R.; Serlo, W.; Kellomäki, M.; Törmälä, P.; Waris, T. Developments in craniomaxillofacial surgery: Use of self-reinforced bioabsorbable osteofixation devices. Plast. Reconstr. Surg. 2001, 108, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Ashammakhi, N.; Renier, D.; Arnaud, E.; Marchac, D.; Ninkovic, M.; Donaway, D.; Jones, B.; Serlo, W.; Laurikainen, K.; Törmälä, P.; et al. Successful use of biosorb osteofixation devices in 165 cranial and maxillofacial cases: A multicenter report. J. Craniofacial Surg. 2004, 15, 692–701. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Waris, T.; Serlo, W.; Pertii, T. Self-reinforced bioabsorbable devices for osteofixation of craniofacial bones. In Biomaterials in Orthopedics; CRC Press: Boca Raton, FL, USA, 2005; pp. 169–184. [Google Scholar]
- Ashammakhi, N.; Mäkelä, E.A.; Törmälä, P.; Waris, T.; Rokkanen, P. Effect of self-reinforced polyglycolide membrane on osteogenesis: An experimental study in rats. Eur. J. Plast. Surg. 2000, 23, 423–428. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Serlo, W. Reflections on complications to bioresorbable osteofixation devices. J. Craniofac. Surg. 2007, 18, 1242–1243. [Google Scholar] [CrossRef]
- Jukola, H.; Nikkola, L.; Gomes, M.E.; Chiellini, F.; Tukiainen, M.; Kellomäki, M.; Chiellini, E.; Reis, R.L.; Ashammakhi, N. Development of a bioactive glass fiber reinforced starch–polycaprolactone composite. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 87B, 197. [Google Scholar] [CrossRef]
- Hench, L.L.; Latorre, G.P.; Andersson, Ö.H. The kinetics of bioactive ceramics part III: Surface reactions for bioactive glasses compared with an inactive glass. In Bioceramics; Butterworth-Heinemann: Oxford, UK, 1991; pp. 155–162. [Google Scholar]
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Hench, L.L.; Andersson, Ö. Bioactive glasses. Wilson J. Ed. Introd. Bioceram. 1993, 1, 41–62. [Google Scholar]
- Priyadarshini, B.; Rama, M.; Chetan; Vijayalakshmi, U. Bioactive coating as a surface modification technique for biocompatible metallic implants: A review. J. Asian Ceram. Soc. 2019, 7, 397–406. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, P.; Dubey, A.K. Effect of incorporation of piezoelectric phases on antibacterial and cellular response of borate bioactive glass. Open Ceram. 2022, 9, 100234. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, W.; Gu, Y.; Xiao, W.; Liu, X.; Wang, D.; Zhang, C.; Huang, W.; Rahaman, M.N.; Day, D.E.; et al. Teicoplanin-loaded borate bioactive glass implants for treating chronic bone infection in a rabbit tibia osteomyelitis model. Biomaterials 2010, 31, 5865–5874. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Sonny Bal, B.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef]
- Margha, F.H.; Abdelghany, A.M. Bone bonding ability of some borate bio-glasses and their corresponding glass-ceramic derivatives. Process. Appl. Ceram. 2012, 6, 183–192. [Google Scholar] [CrossRef]
- Badry, K.M.; Moustafa, F.A.; Azooz, M.A.; El Batal, F.H. Corrosion behaviour of some selected bioglasses by different aqueous solutions. Glass Technol. 2002, 43, 162–170. [Google Scholar]
- El Batal, F.H.; ElKheshen, A. Preparation and characterization of some substituted bioglasses and their ceramic derivatives from the system SiO2–Na2O–CaO–P2O5 and effect of gamma irradiation. Mater. Chem. Phys. 2008, 110, 352–362. [Google Scholar] [CrossRef]
- Jung, S.B.; Day, D.E. Conversion kinetics of silicate, borosilicate, and borate bioactive glasses to hydroxyapatite. Phys. Chem. Glasses Eur. J. Glass Sci. Technol. Part B 2009, 50, 85–88. [Google Scholar]
- Richard, M.N. Bioactive Behavior of a Borate Glass. Master’s Thesis, University of Missouri, Rolla, MO, USA, 2000. [Google Scholar]
- Huang, W.; Day, D.E.; Kittiratanapiboon, K.; Rahaman, M.N. Kinetics and mechanisms of the conversion of silicate (45S5), borate, and borosilicate glasses to hydroxyapatite in dilute phosphate solutions. J. Mater. Sci. Mater. Med. 2006, 17, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Radu, T.; Chiriac, M.T.; Popescu, O.; Simon, V.; Simon, S. In vitro evaluation of the effects of yttria–alumina–silica microspheres on human keratinocyte cells. J. Biomed. Mater. Res. Part A 2013, 101A, 472–477. [Google Scholar] [CrossRef]
- Cavalu, S.; Banica, F.; Gruian, C.; Vanea, E.; Goller, G.; Simon, V. Microscopic and spectroscopic investigation of bioactive glasses for antibiotic controlled reléase. J. Mol. Struct. 2013, 1040, 47–52. [Google Scholar] [CrossRef]
- Singh, R.K.; Srinivasan, A.; Kothiyal, G.P. Evaluation of CaO–SiO2–P2O5–Na2O–Fe2O3 bioglass-ceramics for hyperthermia application. J. Mater. Sci. Mater. Med. 2008, 20, 147–151. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Büttner, T.; Miguez Pacheco, V.; Boccaccini, A.R. Boron-containing bioactive glasses in bone and soft tissue engineering. J. Eur. Ceram. Soc. 2018, 38, 855–869. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Grünewald, A.; Detsch, R.; Hupa, L.; Jokic, B.; Tallia, F.; Solanki, A.K.; Jones, J.R.; Boccaccini, A.R. Ion release, hydroxyapatite conversion, and cytotoxicity of boron-containing bioactive glass scaffolds. Int. J. Appl. Glass Sci. 2016, 7, 206–215. [Google Scholar] [CrossRef]
- Schuhladen, K.; Pantulap, U.; Engel, K.; Jeleń, P.; Olejniczak, Z.; Hupa, L.; Sitarz, M.; Boccaccini, A.R. Influence of the replacement of silica by boron trioxide on the properties of bioactive glass scaffolds. Int. J. Appl. Glass Sci. 2021, 12, 293–312. [Google Scholar] [CrossRef]
- Mohini, G.J.; Krishnamacharyulu, N.; Sahaya Baskaran, G.; Rao, P.V.; Veeraiah, N. Studies on influence of aluminium ions on the bioactivity of B2O3–SiO2–P2O5–Na2O–CaO glass system by means of spectroscopic studies. Appl. Surf. Sci. 2013, 287, 46–53. [Google Scholar] [CrossRef]
- Gharbi, A.; Kallel, A.Y.; Kanoun, O.; Cheikhrouhou-Koubaa, W.; Contag, C.H.; Antoniac, I.; Derbel, N.; Ashammakhi, N. A Biodegradable Bioactive Glass-Based Hydration Sensor for Biomedical Applications. Micromachines 2023, 14, 226. [Google Scholar] [CrossRef]
- Gharbi, A.; Oudadesse, H.; Ashammakhi, N.; Cheikhrouhou-Koubaa, W.; Blaeser, A.; Rau, J.V.; Antoniac, I.; Derbel, N.; El Feki, H. Thermodynamic behavior of bioactive glass in relationship with high fluorine content. Ceram. Int. 2023, 49, 18238–18247. [Google Scholar] [CrossRef]
- Huang, H.; Sakurai, F.; Higuchi, Y.; Kawakami, S.; Hashida, M.; Kawabata, K.; Mizuguchi, H. Suppressive effects of sugar-modified cationic liposome/NF-κB decoy complexes on adenovirus vector-induced innate immune responses. J. Control. Release 2009, 133, 139–145. [Google Scholar] [CrossRef]
- Miguel, B.S.; Kriauciunas, R.; Tosatti, S.; Ehrbar, M.; Ghayor, C.; Textor, M.; Weber, F.E. Enhanced osteoblastic activity and bone regeneration using surface-modified porous bioactive glass scaffolds. J. Biomed. Mater. Res. Part A 2010, 94A, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, W.; Fu, H.; Yao, A.; Wang, D.; Pan, H.; Lu, W.W.; Jiang, X.; Zhang, X. Bioactive borosilicate glass scaffolds: In vitro degradation and bioactivity behaviors. J. Mater. Sci. Mater. Med. 2009, 20, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Saranti, A.; Koutselas, I.; Karakassides, M.A. Bioactive glasses in the system CaO–B2O3–P2O5: Preparation, structural study and in vitro evaluation. J. Non Cryst. Solids 2006, 352, 390–398. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, C.K.; Chang, B.S.; Ryu, H.S.; Seo, J.H.; Hong, K.S.; Kim, H. In vivo study of novel biodegradable and osteoconductive CaO-SiO2-B2O3 glass-ceramics. J. Biomed. Mater. Res. Part A 2006, 77A, 362–369. [Google Scholar] [CrossRef]
- Ryu, S.H.; Lee, K.J.; Seo, H.J.; Kim, H.; Hong, K.S.; Kim, D.J.; Lee, J.H.; Lee, H.D.; Chang, S.B.; Lee, K.C.; et al. Novel bioactive and biodegradable glass ceramics with high mechanical strength in the CaO-SiO2-B2O3 system. J. Biomed. Mater. Res. Part A 2004, 68A, 79–89. [Google Scholar] [CrossRef]
- Dzondo-Gadet, M.; Mayap-Nzietchueng, R.; Hess, K.; Nabet, P.; Belleville, F.; Dousset, B. Action of boron at the molecular level: Effects on transcription and translation in an acellular system. Biol. Trace Elem. Res. 2002, 85, 23–33. [Google Scholar] [CrossRef]
- Hakki, S.S.; Bozkurt, B.S.; Hakki, E.E. Boron regulates mineralized tissue-associated proteins in osteoblasts (MC3T3-E1). J. Trace Elem. Med. Biol. 2010, 24, 243–250. [Google Scholar] [CrossRef]
- Lee, J.H.; Nam, H.; Ryu, H.S.; Seo, J.H.; Chang, B.S.; Lee, C.K. Bioactive ceramic coating of cancellous screws improves the osseointegration in the cancellous bone. J. Orthop. Sci. 2011, 16, 291–297. [Google Scholar] [CrossRef]
- Lee, J.H.; Ryu, S.H.; Seo, H.J.; Chang, S.B.; Lee, K.C. A 90-day intravenous administration toxicity study of CaO-SiO2-P2O5-B2O3 glass-ceramics (BGS-7) in rat. Drug Chem. Toxicol. 2010, 33, 38–47. [Google Scholar] [CrossRef]
- Sitarz, M.; Bulat, K.; Olejniczak, Z. Structure and microstructure of glasses from a NaCaPO4–SiO2–BPO4 system. Vib. Spectrosc. 2012, 61, 72–77. [Google Scholar] [CrossRef]
- Gharbi, A.; Ayadi, S.; Jouini, N.; Schoenstein, F.; Oudadess, H.; Feki, H.E.; Cheikhrouhou-Koubaa, W. Original implementation of low-temperature SPS for bioactive glass used as a bone biomaterial. J. Mech. Behav. Biomed. Mater. 2022, 126, 104988. [Google Scholar] [CrossRef]
- Siqueira, R.L.; Peitl, O.; Zanotto, E.D. Gel-derived SiO2–CaO–Na2O–P2O5 bioactive powders: Synthesis and in vitro bioactivity. Mater. Sci. Eng. C 2011, 31, 983–991. [Google Scholar] [CrossRef]
- Gharbi, A.; El Feki, H.; Oudadesse, H. Novel alkali borosilicate glasses: Preparation, structural investigation and thermal study. Korean J. Chem. Eng. 2016, 33, 1456–1461. [Google Scholar] [CrossRef]
- Doyle, C.; Tanner, E.T.; Bonfield, W. In vitro and in vivo evaluation of polyhydroxybutyrate and of polyhydroxybutyrate reinforced with hydroxyapatite. Biomaterials 1991, 12, 841–847. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Múzquiz-Ramos, E.M.; Cortés-Hernández, D.A.; Escobedo-Bocardo, J. Biomimetic apatite coating on magnetite particles. Mater. Lett. 2010, 64, 1117–1119. [Google Scholar] [CrossRef]
- Reyes-Gasga, J.; Martínez-Piñeiro, E.L.; Rodríguez-Álvarez, G.; Tiznado-Orozco, G.E.; García-García, R.; Brès, E.F. XRD and FTIR crystallinity indices in sound human tooth enamel and synthetic hydroxyapatite. Mater. Sci. Eng. C 2013, 33, 4568–4574. [Google Scholar] [CrossRef]
- Palard, M. Synthèse, Frittage et Evaluation Biologique D’hydroxyapatites Silicatées. Ph.D. Thesis, University of Limoges, Limoges, France, 2007. [Google Scholar]
- Lafon, P.J. Synthesis, thermal stability and sintering of carbonated hydroxyapatites. Ph.D. Thesis, University of Limoges, Limoges, France, 2004. [Google Scholar]
- Raynaud, S. Synthèse, Frittage et Propriétés Mécaniques de Phosphates de Calcium Dans le Système Hydroxyapatite—Phosphate Tricalcique. Ph.D. Thesis, University of Limoges, Limoges, France, 1999. [Google Scholar]
- Brown, R.F.; Rahaman, M.N.; Dwilewicz, A.B.; Huang, W.; Day, D.E.; Li, Y.; Bal, B.S. Effect of borate glass composition on its conversion to hydroxyapatite and on the proliferation of MC3T3-E1 cells. J. Biomed. Mater. Res. Part A 2009, 88A, 392–400. [Google Scholar] [CrossRef]
- Heughebaert, J.C. Contribution à L’étude de L’évolution des Orthophosphates de Calcium Précipités Amorphes en Orthophosphates Apatitiques. Ph.D. Thesis, These d’Etat. Institut National Polytechnique de Toulouse, Toulouse, France, 1977. [Google Scholar]
- Marchat, D.; Zymelka, M.; Coelho, C.; Gremillard, L.; Joly-Pottuz, L.; Babonneau, F.; Esnouf, C.; Chevalier, J.; Bernache-Assollant, D. Accurate characterization of pure silicon-substituted hydroxyapatite powders synthesized by a new precipitation route. Acta Biomater. 2013, 9, 6992–7004. [Google Scholar] [CrossRef]
- Johan, Z.; Gilbert, M. La Kanemite, nouveau silicate de sodium hydraté de néoformation. Bull. De Minéralogie 1972, 95, 371–382. [Google Scholar] [CrossRef]
- Fowler, B.O. Infrared studies of apatites. I. Vibrational assignments for calcium, strontium, and barium hydroxyapatites utilizing isotopic substitution. Inorg. Chem. 1974, 13, 194–207. [Google Scholar] [CrossRef]
- Rey, C.; Renugopalakrishnan, V.; Shimizu, M.; Collins, B.; Glimcher, M.J. A resolution-enhanced Fourier transform infrared spectroscopic study of the environment of the CO3 2− ion in the mineral phase of enamel during its formation and maturation. Calcif. Tissue Int. 1991, 49, 259–268. [Google Scholar] [CrossRef]
- Fleet, M.E. Infrared spectra of carbonate apatites: ν2-Region bands. Biomaterials 2009, 30, 1473–1481. [Google Scholar] [CrossRef]
- Rey, C.; Collins, B.; Goehl, T.; Dickson, I.R.; Glimcher, M.J. The carbonate environment in bone mineral: A resolution-enhanced Fourier transform infrared spectroscopy study. Calcif. Tissue Int. 1989, 45, 157–164. [Google Scholar] [CrossRef]
- Aktas, B.; Acikgoz, A.; Yilmaz, D.; Yalcin, S.; Dogru, K.; Yorulmaz, N. The role of TeO2 insertion on the radiation shielding, structural and physical properties of borosilicate glasses. J. Nucl. Mater. 2022, 563, 153619. [Google Scholar] [CrossRef]
- Vignoles, M.; Bonel, G.; Holcomb, D.W.; Young, R.A. Influence of preparation conditions on the composition of type B carbonated hydroxyapatite and on the localization of the carbonate ions. Calcif. Tissue Int. 1988, 43, 33–40. [Google Scholar] [CrossRef]
- El Feki, H.; Savariault, J.M.; Ben Salah, A. Structure refinements by the Rietveld method of partially substituted hydroxyapatite: Ca9Na0.5 (PO4)4.5 (CO3) 1.5(OH)2. J. Alloys Compd. 1999, 287, 114–120. [Google Scholar] [CrossRef]
- El Feki, H.; Michel Savariault, J.; Ben Salah, A.; Jemal, M. Sodium and carbonate distribution in substituted calcium hydroxyapatite. Solid State Sci. 2000, 2, 577–586. [Google Scholar] [CrossRef]
- Maqbool, M. Substituted Hydroxyapatites for Antibacterial Applications. Ph.D. Thesis, Universitaet Erlangen, Nuernberg, Germany, 2021. [Google Scholar]
- Radin, S.R.; Ducheyne, P. The effect of calcium phosphate ceramic composition and structure on in vitro behavior. II. Precipitation. J. Biomed. Mater. Res. 1993, 27, 35–45. [Google Scholar] [CrossRef]
- Henao, J.; Poblano-Salas, C.; Monsalve, M.; Corona-Castuera, J.; Barceinas-Sanchez, O. Bio-active glass coatings manufactured by thermal spray: A status report. J. Mater. Res. Technol. 2019, 8, 4965–4984. [Google Scholar] [CrossRef]
- Tilocca, A.; Cormack, A.N. Exploring the surface of bioactive glasses: Water adsorption and reactivity. J. Phys. Chem. 2008, 112, 11936–11945. [Google Scholar] [CrossRef]
- Mezahi, Z.F.; Girot, A.L.; Oudadesse, H.; Harabi, A. Reactivity kinetics of 52S4 glass in the quaternary system SiO2–CaO–Na2O–P2O5: Influence of the synthesis process: Melting versus sol–gel. J. Non Cryst. Solids 2013, 361, 111–118. [Google Scholar] [CrossRef]
- Pan, H.B.; Zhao, X.L.; Zhang, X.; Zhang, K.B.; Li, L.C.; Li, Z.Y.; Lam, W.M.; Lu, W.W.; Wang, D.P.; Huang, W.H.; et al. Strontium borate glass: Potential biomaterial for bone regeneration. J. R. Soc. Interface 2010, 7, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.K.; Swamiappan, S. Review on calcium-and magnesium-based silicates for bone tissue engineering applications. J. Biomed. Mater. Res. Part A 2020, 108, 1546–1562. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, A. Role of magnesium in genomic stability. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2001, 475, 113–121. [Google Scholar] [CrossRef]
- Zreiqat, H.; Howlett, C.R.; Zannettino, A.; Evans, P.; Schulze-Tanzil, G.; Knabe, C.; Shakibaei, M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J. Biomed. Mater. Res. 2002, 62, 175–184. [Google Scholar] [CrossRef]
- Agathopoulos, S.; Tulyaganov, D.U.; Ventura, J.M.G.; Kannan, S.; Karakassides, M.A.; Ferreira, J.M.F. Formation of hydroxyapatite onto glasses of the CaO–MgO–SiO2 system with B2O3, Na2O, CaF2 and P2O5 additives. Biomaterials 2006, 27, 1832–1840. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Yoshida, Y.; Okazaki, M.; Shimazu, A.; Kubo, T.; Akagawa, Y.; Uchida, T. Action of FGMgCO3Ap-collagen composite in promoting bone formation. Biomaterials 2003, 24, 4913–4920. [Google Scholar] [CrossRef]
- Abo-Naf, S.M.; Khalil, S.M.E.; El-Sayed, E.-S.M.; Zayed, H.A.; Youness, R.A. In vitro bioactivity evaluation, mechanical properties and microstructural characterization of Na2O–CaO–B2O3–P2O5 glasses. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 144, 88–98. [Google Scholar] [CrossRef]
- Tabia, Z.; El Mabrouk, K.; Bricha, M.; Nouneh, K. Mesoporous bioactive glass nanoparticles doped with magnesium: Drug delivery and acellular in vitro bioactivity. RSC Adv. 2019, 9, 12232–12246. [Google Scholar] [CrossRef]
- Tian, K.V.; Chass, G.A.; Tommaso, D.D. Simulations reveal the role of composition into the atomic-level flexibility of bioactive glass cements. Phys. Chem. Chem. Phys. 2016, 18, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Abodunrin, O.D.; El Mabrouk, K.; Bricha, M. A review on borate bioactive glasses (BBG): Effect of doping elements, degradation, and applications. J. Mater. Chem. B 2023, 11, 955. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A. Bioactive Glass Derived Scaffolds with Therapeutic Ion Releasing Capability for Bone Tissue Engineering. Ph.D. Thesis, Technische Fakultät, Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 2014. [Google Scholar]
- Rahaman, M.N. Bioactive Ceramics and Glasses for Tissue Engineering. In Tissue Engineering Using Ceramics and Polymers; Woodhead Publishing: Sawston, UK, 2014; pp. 67–114. [Google Scholar]
- Schumacher, M.; Habibovic, P.; van Rijt, S. Mesoporous bioactive glass composition effects on degradation and bioactivity. Bioact. Mater. 2021, 6, 1921–1931. [Google Scholar] [CrossRef] [PubMed]
- Antoniac, I.V. Handbook of Bioceramics and Biocomposites; Springer: Berlin/Heidelberg, Germany, 2016; Volume 1. [Google Scholar]
- Rau, J.V.; Antoniac, I.; Fosca, M.; De Bonis, A.; Blajan, A.I.; Cotrut, C.; Graziani, V.; Curcio, M.; Cricenti, A.; Niculescu, M.; et al. Glass-ceramic coated Mg-Ca alloys for biomedical implant applications. Mater. Sci. Eng. C 2016, 64, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.V.; Antoniac, I.; Cama, G.; Komlev, V.S.; Ravaglioli, A. Bioactive materials for bone tissue engineering. Biomed Res. Int. 2016, 2016, 3741428. [Google Scholar] [CrossRef]
- Piatti, E.; Verné, E.; Miola, M. Synthesis and characterization of sol-gel bioactive glass nanoparticles doped with boron and copper. Ceram. Int. 2022, 48, 13706–13718. [Google Scholar] [CrossRef]
- Stone-Weiss, N.; Pierce, E.M.; Youngman, R.E.; Gulbiten, O.; Smith, N.J.; Du, J.; Goel, A. Understanding the structural drivers governing glass–water interactions in borosilicate based model bioactive glasses. Acta Biomater. 2018, 65, 436–449. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]

| BaG | SiO2 | CaO | Na2O | P2O5 | B2O3 |
|---|---|---|---|---|---|
| BaG-B0 | 46 | 24 | 24 | 6 | 0 |
| BaG-B5 | 41 | 24 | 24 | 6 | 5 |
| BaG-B10 | 36 | 24 | 24 | 6 | 10 |
| BaG-B20 | 26 | 24 | 24 | 6 | 20 |
| Wave Numbers (cm−1) | Assignment | Mode |
|---|---|---|
| 1121 | P-O, antisymmetric elongation of ions PO43− | υ3 |
| 960 | P-O, symmetric elongation of ions PO43− | υ1 |
| 603–562 | P-O, antisymmetric deformation of ions PO43− | υ4 |
| Wave Numbers (cm−1) | Assignment | Mode |
|---|---|---|
| 1235 | Si-O | |
| 1045 | Si-O-B | |
| 699–794 | SiO4 on HAp | |
| 458 | Si-O-Si | υ1 |
| 475 | SiO4 on HAp | υ2 |
| Wave Numbers (cm−1) | Assignment | Mode |
|---|---|---|
| 1651 | CO3 on site A et B | |
| 1492 | CO3 on site B | υ3 |
| 1410 | CO3 on site B | υ3 |
| 873 | CO3 on site B | υ2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gharbi, A.; Oudadesse, H.; el Feki, H.; Cheikhrouhou-Koubaa, W.; Chatzistavrou, X.; V. Rau, J.; Heinämäki, J.; Antoniac, I.; Ashammakhi, N.; Derbel, N. High Boron Content Enhances Bioactive Glass Biodegradation. J. Funct. Biomater. 2023, 14, 364. https://doi.org/10.3390/jfb14070364
Gharbi A, Oudadesse H, el Feki H, Cheikhrouhou-Koubaa W, Chatzistavrou X, V. Rau J, Heinämäki J, Antoniac I, Ashammakhi N, Derbel N. High Boron Content Enhances Bioactive Glass Biodegradation. Journal of Functional Biomaterials. 2023; 14(7):364. https://doi.org/10.3390/jfb14070364
Chicago/Turabian StyleGharbi, Amina, Hassane Oudadesse, Hafedh el Feki, Wissem Cheikhrouhou-Koubaa, Xanthippi Chatzistavrou, Julietta V. Rau, Jyrki Heinämäki, Iulian Antoniac, Nureddin Ashammakhi, and Nabil Derbel. 2023. "High Boron Content Enhances Bioactive Glass Biodegradation" Journal of Functional Biomaterials 14, no. 7: 364. https://doi.org/10.3390/jfb14070364
APA StyleGharbi, A., Oudadesse, H., el Feki, H., Cheikhrouhou-Koubaa, W., Chatzistavrou, X., V. Rau, J., Heinämäki, J., Antoniac, I., Ashammakhi, N., & Derbel, N. (2023). High Boron Content Enhances Bioactive Glass Biodegradation. Journal of Functional Biomaterials, 14(7), 364. https://doi.org/10.3390/jfb14070364











