Injectable Pectin–Alginate Hydrogels for Improving Vascularization and Adipogenesis of Human Fat Graft
Abstract
1. Introduction
2. Materials and Methods
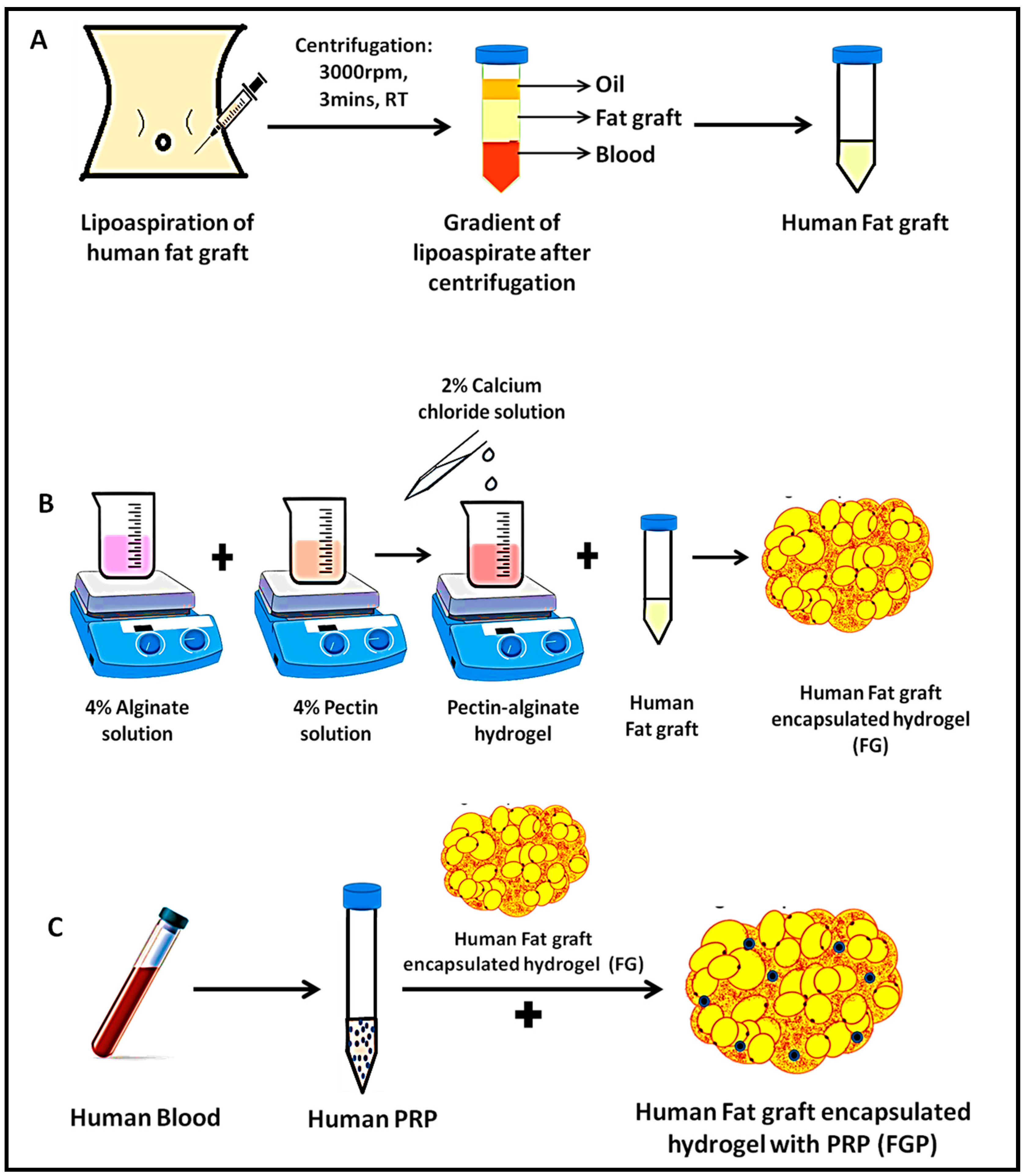
2.1. Extraction of Human Fat Graft (F)
2.2. Preparation of Fat-Graft-Encapsulated Pectin–Alginate Hydrogel (FG)
2.3. Preparation of Fat-Graft-Encapsulated Pectin–Alginate Hydrogel with PRP (FGP)
2.4. Physiochemical Characterization of the Developed Hydrogel
2.4.1. Inversion and Injectability
2.4.2. Fourier-Transform Infrared Spectrometry (FTIR) Analysis
2.4.3. Rheological Studies
2.5. In Vitro Cytocompatibility Studies
2.6. In Vivo Study
2.7. Histological Analysis
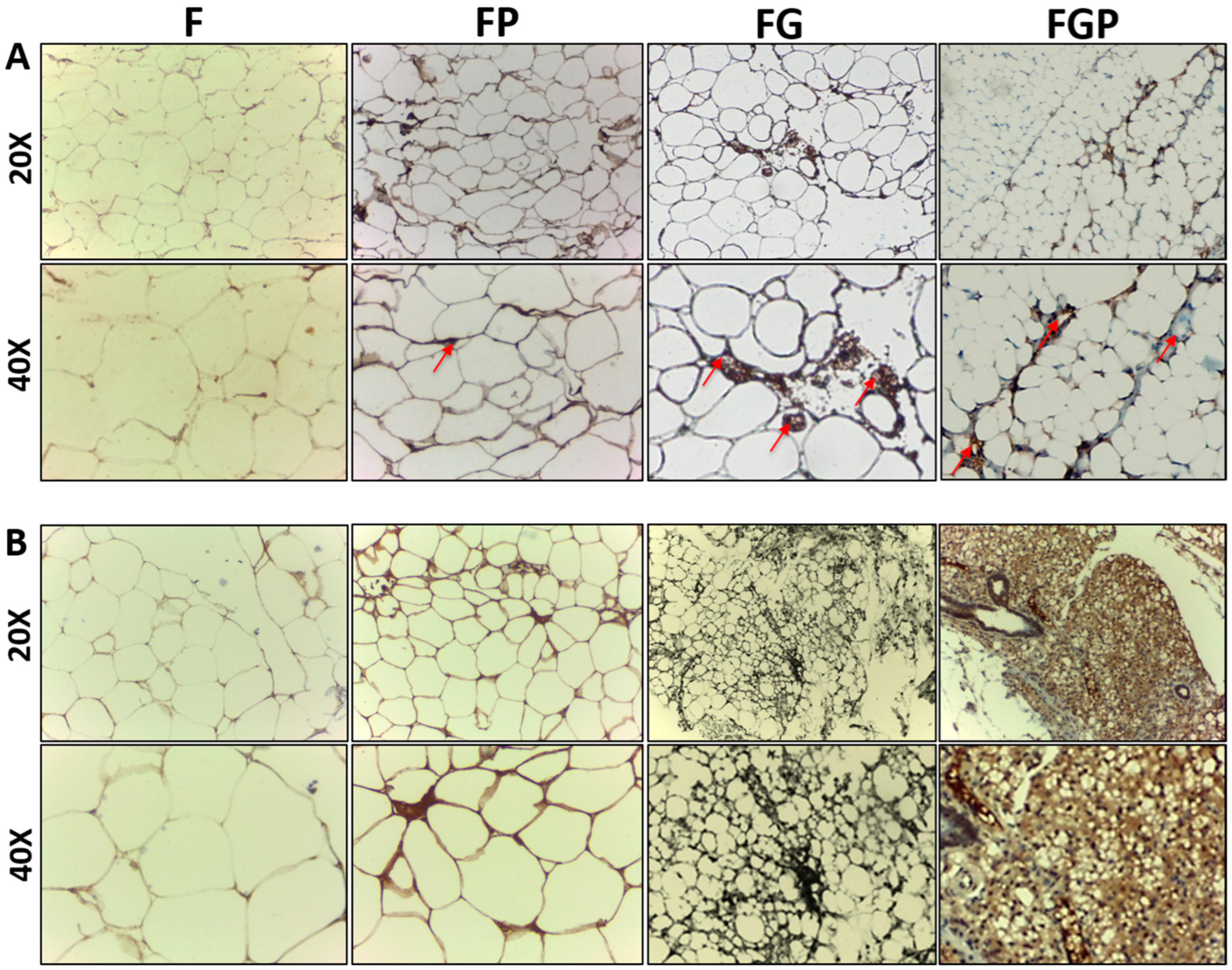
2.8. Immunohistochemistry
3. Results
3.1. Preparation of Fat-Graft-Encapsulated Hydrogel
3.2. Physiochemical Characterization of the Developed Hydrogel
3.3. Rheological Properties of the Developed Hydrogel
3.4. In Vitro Cytocompatibility
3.5. In Vivo Studies
3.6. Histological Analysis
3.7. Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFG | Autologous fat grafting |
| G | Pectin–alginate hydrogel |
| F | Human fat graft |
| PRP | Platelet-rich plasma |
| FG | Human-fat-graft-encapsulated pectin–alginate hydrogel |
| FGP | Human-fat-graft-encapsulated pectin–alginate hydrogel |
| IHC | Immunohistochemistry studies |
| ATE | Adipose tissue engineering |
| VEGF | Vascular endothelial growth factor |
| IGF-1 | Insulin-like growth factor-1 |
| FGF | Fibroblast growth factor |
| EGF | Epidermal growth factor |
| FTIR | Fourier-transform infrared spectrometry |
| HUVECs | Human umbilical vein endothelial cells |
| ADSCs | Adipose-derived stem cells |
References
- Fiedler, L.S.; Saleh, D.B.; Mukrowsky, A. Autologous fat grafting in the face and neck: Multinational trends and knowledge of the safety, applications, and indications considering oncologic risk potential. Laryngoscope Investig. Otolaryngol. 2021, 6, 1024–1030. [Google Scholar]
- Meier, J.D.; Glasgold, R.A.; Glasgold, M.J. Autologous fat grafting: Long-term evidence of its efficacy in midfacial rejuvenation. Arch. Facial. Plast. Surg. 2009, 11, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.A.; Schwarzman, G.; Eivazi, M.; Zachary, L. Autologous staged fat tissue transfer in post-traumatic lower extremity reconstruction. Int. J. Surg. Case Rep. 2015, 11, rjv141. [Google Scholar] [CrossRef] [PubMed]
- Arcuri, F.; Brucoli, M.; Baragiotta, N.; Stellin, L.; Giarda, M.; Benech, A. The role of fat grafting in the treatment of posttraumatic maxillofacial deformities. Craniomaxillofac. Trauma Reconstr. 2013, 6, 121–126. [Google Scholar] [CrossRef]
- Ducic, Y. Fat grafting in trauma and reconstructive surgery. Facial Plast. Surg. Clin. N. Am. 2008, 16, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Krumboeck, A.; Giovanoli, P.; Plock, J.A. Fat grafting and stem cell enhanced fat grafting to the breast under oncological aspects–recommendations for patient selection. Breast J. 2013, 22, 579–584. [Google Scholar] [CrossRef]
- Klinger, M.; Marazzi, M.; Vigo, D.; Torre, M. Fat injection for cases of severe burn outcomes: A new perspective of scar remodeling and reduction. Aesthetic Plast. Surg. 2008, 32, 465–469. [Google Scholar]
- Fredman, R.; Edkins, R.E.; Hultman, C.S. Fat grafting for neuropathic pain after severe burns. Ann. Plast. Surg. 2016, 76, S298–S303. [Google Scholar] [CrossRef]
- Shukla, L.; Morrison, W.A.; Shayan, R. Adipose-derived stem cells in radiotherapy injury: A new frontier. Front. Surg. 2015, 2, 1–12. [Google Scholar]
- Krastev, T.; van Turnhout, A.; Vriens, E.; Smits, L.; van der Hulst, R. Long-term follow-up of autologous fat transfer vs conventional breast reconstruction and association with cancer relapse in patients with breast cancer. JAMA Surg. 2019, 154, 56–63. [Google Scholar] [CrossRef]
- Tanna, N.; Wan, D.C.; Kawamoto, H.K.; Bradley, J.P. Craniofacial microsomia soft tissue reconstruction comparison: Inframammary extended circumflex scapular flap versus serial fat grafting. Plast. Reconstr. Surg. 2011, 127, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Winters, R.; Moulthrop, T. Is autologous fat grafting superior to other fillers for facial rejuvenation? Laryngoscope 2013, 123, 1068–1069. [Google Scholar] [CrossRef] [PubMed]
- Shuck, J.; Iorio, M.L.; Hung, R.; Davison, S.P. Autologous fat grafting and injectable dermal fillers for human immunodeficiency virus–associated facial lipodystrophy: A comparison of safety, efficacy, and long-term treatment outcomes. Plast. Reconstr. Surg. 2013, 131, 499–506. [Google Scholar] [PubMed]
- Pfaff, M.; Wu, W.; Zellner, E.; Steinbacher, D.M. Processing technique for lipofilling influences adipose-derived stem cell concentration and cell viability in lipoaspirate. Aesthetic Plast. Surg. 2014, 38, 224–229. [Google Scholar] [CrossRef]
- Lui, Y.F.; Lp, W.Y. Application of gel in reconstruction Surgery: Gel/fat graft complex filler for volume reconstruction in critical sized muscle defects. Biomed. Res. Int. 2016, 2016, 3459431. [Google Scholar] [CrossRef]
- Tan, S.S.; Ng, Z.Y.; Zhan, W.; Rozen, W. Role of adipose-derived stem cells in fat grafting and reconstructive surgery. J. Cutan. Aesthet. Surg. 2016, 9, 152–156. [Google Scholar] [CrossRef]
- Topcu, A.; Aydin, O.E.; Ünlü, M.; Barutcu, A.; Atabey, A. Increasing the viability of fat grafts by vascular endothelial growth factor. Arch. Facial Plast. Surg. 2012, 14, 270–276. [Google Scholar] [CrossRef]
- Coleman, S.R. Structural fat grafting: More than permanent filler. Plast. Reconstr. Surg. 2006, 118, 108S–120S. [Google Scholar] [CrossRef]
- Pu, L.L.; Coleman, S.R.; Cui, X.; Ferguson, R.E., Jr.; Vasconez, H.C. Autologous fat grafts harvested and refined by the coleman technique: A comparative study. Plast. Reconstr. Surg. 2008, 122, 932–937. [Google Scholar]
- Simonacci, F.; Bertozzi, N.; Grieco, M.P.; Grignaffini, E.; Raposio, E. Procedure, applications, and outcomes of autologous fat grafting. Ann. Med. Surg. 2017, 20, 49–60. [Google Scholar] [CrossRef]
- Zielins, E.R.; Brett, E.A.; Longaker, M.T.; Wan, D.C. Autologous fat grafting: The science behind the surgery. Aesthet. Surg. J. 2016, 36, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, A.; Champaneria, M.C.; Maxwell, G.P. Fat grafting and breast reconstruction: Tips for ensuring predictability. Gland Surg. 2015, 4, 232–243. [Google Scholar]
- Shahzad, F.; Mehrara, B.J. The future of fat grafting. Aesthet. Surg. J. 2017, 37, S69–S74. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zou, Y.; Arno, M.C.; Chen, S.; Wang, T.; Gao, J.; Dove, A.P.; Du, J. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem. Soc. Rev. 2017, 46, 6255–6275. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Heo, C.Y. Current applications of adipose-derived stem cells and their future perspectives. World J. Stem Cells 2014, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Butler, P.E.; Seifalian, A.M. Adipose-derived stem cells for clinical applications: A review. Cell Prolif. 2011, 44, 86–98. [Google Scholar]
- Mousaei Ghasroldasht, M.; Seok, J.; Park, H.S.; Liakath Ali, F.B.; Al-Hendy, A. Stem cell therapy: From idea to clinical practice. Int. J. Mol. Sci. 2022, 23, 2850. [Google Scholar] [CrossRef]
- Sivashanmugam, A.; Kumar, R.A.; Priya, M.V.; Nair, S.V.; Jayakumar, R. An overview of injectable polymeric gels for tissue engineering. Eur. Polym. J. 2015, 72, 543–565. [Google Scholar] [CrossRef]
- Karaçal, N.; Çobanoğlu, Ü.; Ambarcioğlu, Ö.; Kutlu, N. The effect of fibrin glue on fat graft survival. J. Plast. Reconstr. Aesthet. Surg. 2007, 60, 300–303. [Google Scholar] [CrossRef]
- Hillel, A.T.; Unterman, S.; Nahas, Z.; Reid, B.; Coburn, J.M.; Axelman, J.; Chae, J.J.; Guo, Q.; Trow, R.; Thomas, A.; et al. Photoactivated composite biomaterial for soft tissue restoration in rodents and in humans. Sci. Transl. Med. 2011, 3, 93ra67. [Google Scholar] [CrossRef]
- Piasecki, J.H.; Gutowski, K.A.; Moreno, K.M.; Lahvis, G.L. Purified viable fat suspended in matrigel improves volume longevity. Aesthet. Surg. J. 2008, 28, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Alghoul, M.; Mendiola, A.; Seth, R.; Rubin, B.P.; Zins, J.E.; Calabro, A.; Siemionow, M.; Kusuma, S. The effect of hyaluronan gel on fat graft survival. Aesthet. Surg. J. 2012, 32, 622–633. [Google Scholar] [CrossRef][Green Version]
- Bidarra, S.J.; Barrias, C.C.; Granja, P.L. Injectable alginate gels for cell delivery in tissue engineering. Acta Biomater. 2014, 10, 1646–1662. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.; Kim, S.; Shin, B.H.; Lee, M.H.; Choy, Y.B.; Lee, K.; Heo, C.Y.; Koh, W.G. Preparation of alginate hydrogel with human-derived adipose tissue to improve fat graft survival and adipogenesis. J. Ind. Eng. Chem. 2021, 95, 148–155. [Google Scholar] [CrossRef]
- Munarin, F.; Guerreiro, S.G.; Grellier, M.A.; Tanzi, M.C.; Barbosa, M.A.; Petrini, P.; Granja, P.L. Pectin-based injectable biomaterials for bone tissue engineering. Biomacromolecules 2011, 12, 568–577. [Google Scholar] [CrossRef]
- Liao, H.T.; James, I.B.; Marra, K.G.; Rubin, J.P. The effects of platelet-rich plasma on cell proliferation and adipogenic potential of adipose-derived stem cells. Tissue Eng. Part A 2015, 21, 2714–2722. [Google Scholar] [CrossRef]
- Aksoy, M.A.; Acikalin, M.F.; Gurbuz, M.K.; Ozudogru, E.N.; Canaz, F.; Kaya, E.; Pinarbasli, M.O.; Incesulu, A.; Cakli, H.; Cingi, C. Efficacy of platelet-rich plasma on fat grafts in the repair of tympanic membrane perforations: An experimental study. J. Int. Adv. Otol. 2018, 14, 58–63. [Google Scholar]
- Cervelli, V.; Scioli, M.G.; Gentile, P.; Doldo, E.; Bonanno, E.; Spagnoli, L.G.; Orlandi, A. Platelet-rich plasma greatly potentiates insulin-induced adipogenic differentiation of human adipose-derived stem cells through a serine/threonine kinase akt-dependent mechanism and promotes clinical fat graft maintenance. Stem Cells Transl. Med. 2012, 1, 206–220. [Google Scholar] [CrossRef]
- Cervelli, V.; Bocchini, I.; Di Pasquali, C.; De Angelis, B.; Cervelli, G.; Curcio, C.B.; Orlandi, A.; Scioli, M.G.; Tati, E.; Delogu, P.; et al. PRL platelet rich lipotransfert: Our experience and current state of art in the combined use of fat and PRP. BioMed Res. Int. 2013, 2013, 434191. [Google Scholar] [CrossRef]
- Wu, M.; Karvar, M.; Liu, Q.; Orgill, D.P.; Panayi, A.C. Comparison of conventional and platelet-rich plasma-assisted fat grafting: A systematic review and meta-analysis. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 2821–2830. [Google Scholar]
- Oh, D.S.; Cheon, Y.W.; Jeon, Y.R.; Lew, D.H. Activated platelet-rich plasma improves fat graft survival in nude mice: A pilot study. Dermatol. Surg. 2011, 37, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Audebrand, M.; Garnier, C.; Kolb, M.; Axelos, M.A. Gelation of pectin-alginate mixture: Ultrastructure and rheological properties. In Proceedings of the 3rd International Symposium on Food Rheology and Structure, Zurich, Switzerland, 9–13 February 2003; pp. 9–13. [Google Scholar]
- Siew, C.K.; Williams, P.A.; Young, N.W. New insights into the mechanism of gelation of alginate and pectin: Charge annihilation and reversal mechanism. Biomacromolecules 2005, 6, 963–969. [Google Scholar]
- Audebrand, M.; Kolb, M.; Axelos, M.A. Combined rheological and ultrasonic study of alginate and pectin gels near the sol−gel transition. Biomacromolecules 2006, 7, 2811–2817. [Google Scholar] [CrossRef]
- Braccini, I.; Pérez, S. Molecular basis of Ca2+-induced gelation in alginates and pectins: The egg-box model revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [PubMed]
- Cavallo, C.; Roffi, A.; Grigolo, B.; Mariani, E.; Pratelli, L.; Merli, G.; Kon, E.; Marcacci, M.; Filardo, G. Platelet-rich plasma: The choice of activation method affects the release of bioactive molecules. Biomed Res. Int. 2016, 2016, 6591717. [Google Scholar] [PubMed]
- Stillaert, F.; Findlay, M.; Palmer, J.; Idrizi, R.; Cheang, S.; Messina, A.; Abberton, K.; Morrison, W.; Thompson, E.W. Host rather than graft origin of matrigel-induced adipose tissue in the murine tissue-engineering chamber. Tissue Eng. 2007, 13, 2291–2300. [Google Scholar] [CrossRef]
- Haug, V.; Torio-Padron, N.; Stark, G.B.; Finkenzeller, G.; Strassburg, S. Comparison between endothelial progenitor cells and human umbilical vein endothelial cells on neovascularization in an adipogenesis mouse model. Microvasc. Res. 2015, 97, 159–166. [Google Scholar] [CrossRef]
- Hausman, G.J.; Richardson, R.L. Adipose tissue angiogenesis. J. Anim. Sci. 2004, 82, 925–934. [Google Scholar] [CrossRef]
- Fukumura, D.; Ushiyama, A.; Duda, D.G.; Xu, L.; Tam, J.; Krishna, V.; Chatterjee, K.; Garkavtsev, I.; Jain, R.K. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ. Res. 2003, 93, e88–e97. [Google Scholar] [CrossRef]
- Ting, A.C.; Craft, R.O.; Palmer, J.A.; Gerrand, Y.W.; Penington, A.J.; Morrison, W.A.; Mitchell, G.M. The adipogenic potential of various extracellular matrices under the influence of an angiogenic growth factor combination in a mouse tissue engineering chamber. Acta Biomater. 2014, 10, 1907–1918. [Google Scholar] [CrossRef]
- Condé-Green, A.; Wu, I.; Graham, I.; Chae, J.J.; Drachenberg, C.B.; Singh, D.P.; Holton, L.; Slezak, S.; Elisseeff, J. Comparison of 3 techniques of fat grafting and cell-supplemented lipotransfer in athymic rats: A pilot study. Aesthet. Surg. J. 2013, 33, 713–721. [Google Scholar] [PubMed]
- Mashiko, T.; Yoshimura, K. How does fat survive and remodel after grafting? Clin. Plast. Surg. 2015, 42, 181–190. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janarthanan, R.; Jayakumar, R.; Iyer, S. Injectable Pectin–Alginate Hydrogels for Improving Vascularization and Adipogenesis of Human Fat Graft. J. Funct. Biomater. 2023, 14, 409. https://doi.org/10.3390/jfb14080409
Janarthanan R, Jayakumar R, Iyer S. Injectable Pectin–Alginate Hydrogels for Improving Vascularization and Adipogenesis of Human Fat Graft. Journal of Functional Biomaterials. 2023; 14(8):409. https://doi.org/10.3390/jfb14080409
Chicago/Turabian StyleJanarthanan, Ramu, Rangasamy Jayakumar, and Subramania Iyer. 2023. "Injectable Pectin–Alginate Hydrogels for Improving Vascularization and Adipogenesis of Human Fat Graft" Journal of Functional Biomaterials 14, no. 8: 409. https://doi.org/10.3390/jfb14080409
APA StyleJanarthanan, R., Jayakumar, R., & Iyer, S. (2023). Injectable Pectin–Alginate Hydrogels for Improving Vascularization and Adipogenesis of Human Fat Graft. Journal of Functional Biomaterials, 14(8), 409. https://doi.org/10.3390/jfb14080409






