Evaluation of How Methacrylate Gelatin Hydrogel Loaded with Ximenia americana L. Extract (Steam Bark) Effects Bone Repair Activity Using Rats as Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ximenia americana L. Extract
2.2. Incorporation of Ximenia americana L. in GelMA Hydrogel
2.3. Hydrogel Characterization
2.4. Controlled Release Test of Epicatechin—Main Compound of Ximenia americana L.
2.5. In Vivo Study
2.5.1. Ethical and Legal Aspects, and Experimental Animals
- Control Group (CG): induced fracture and no treatment;
- GelMA Group (GG): induced fracture and GelMA as treatment;
- GelMA + LED Group (GLEDG): induced fracture and GelMA + LED as treatment;
- GelMA/Ximenia americana L. Group (GXG): induced fractures and GelMA/Ximenia americana L. as treatment;
- GelMA/Ximenia americana L. + LED Group (GXLEDG): induced fractures and GelMA/Ximenia americana L. + LED as treatment.
2.5.2. Surgical Procedure
2.5.3. LED Therapy
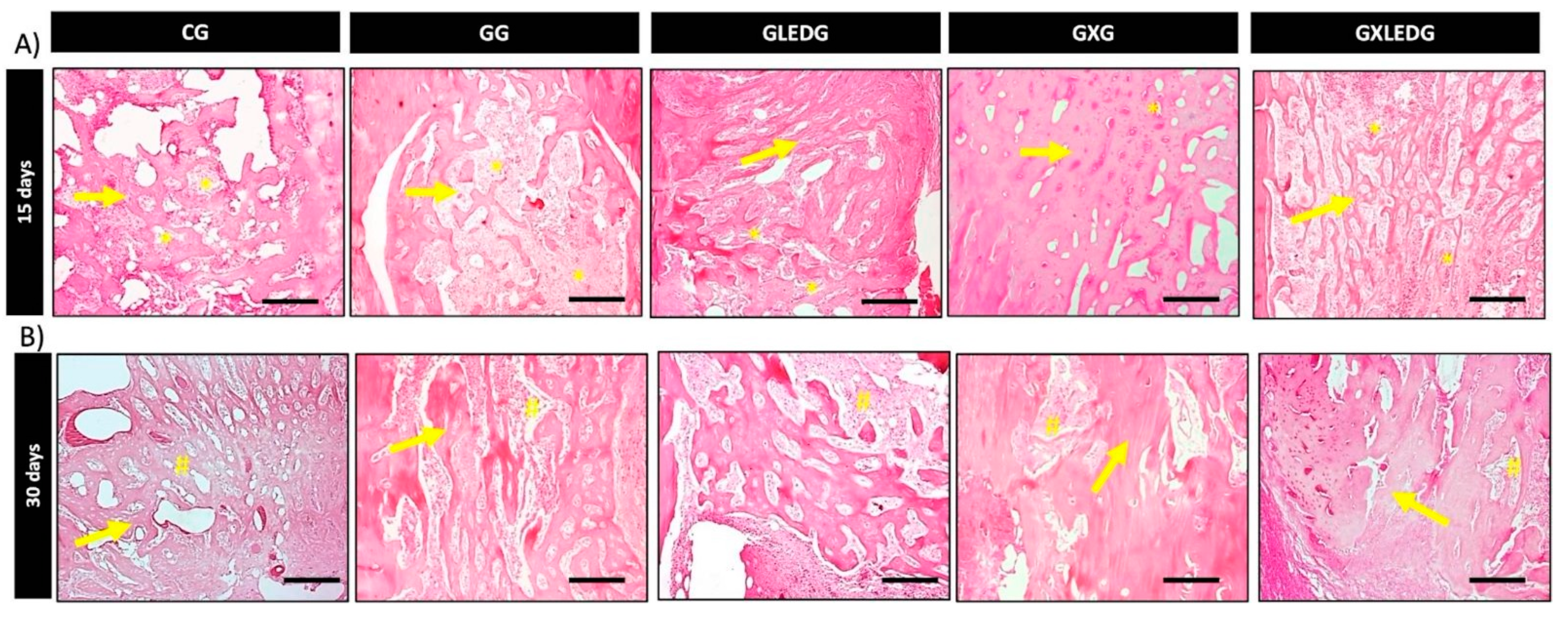
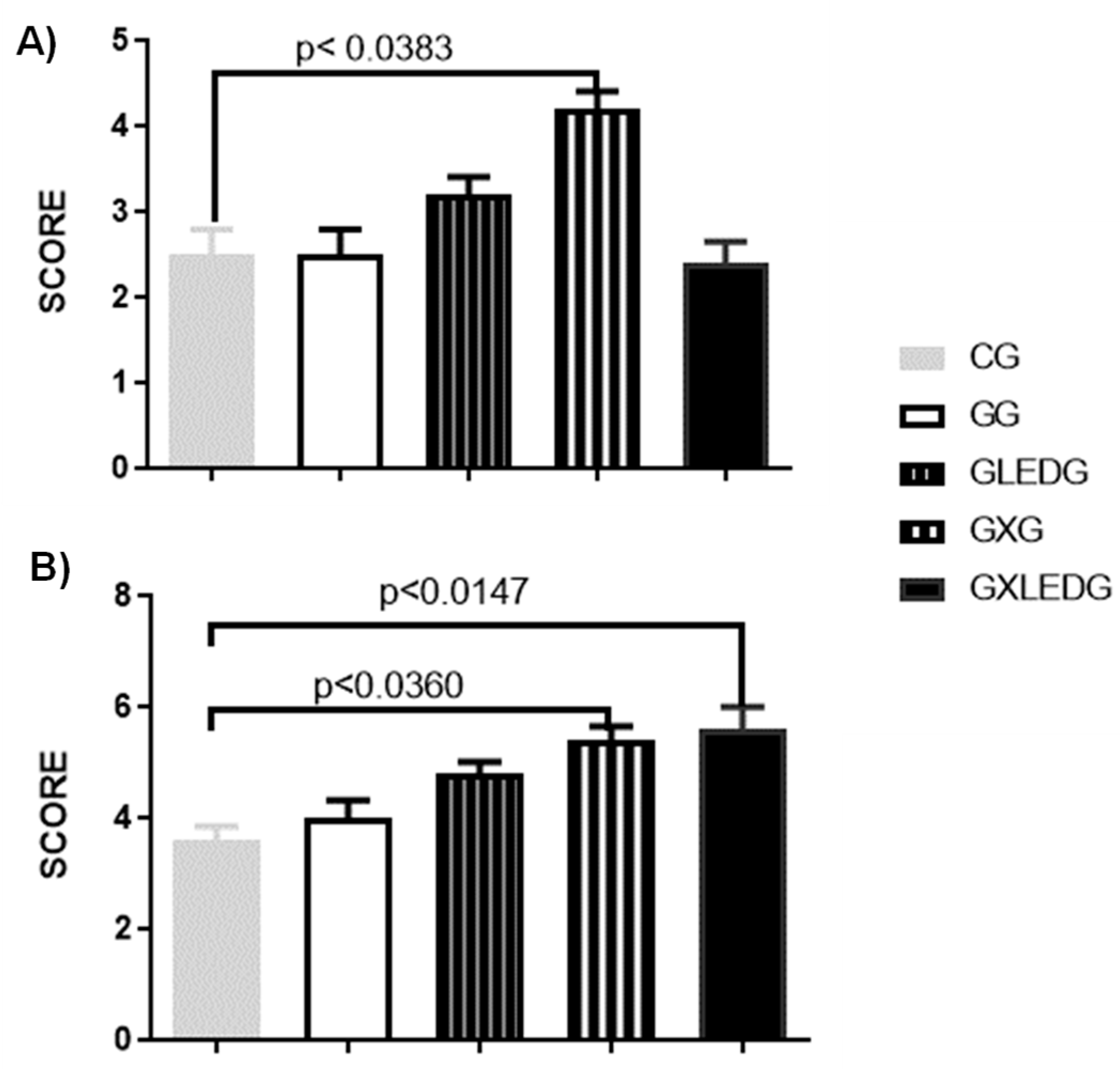
2.5.4. Histological Analysis
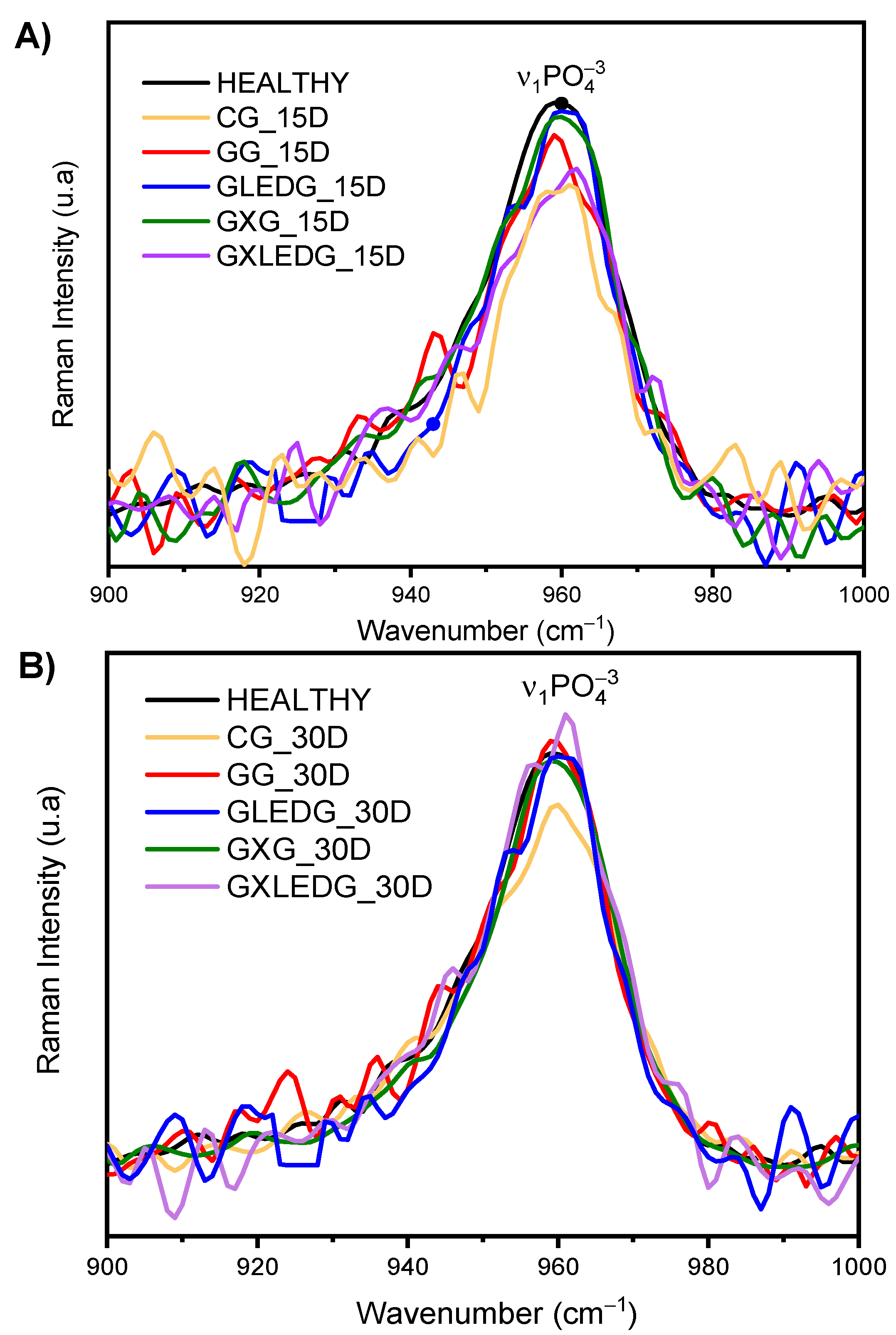
2.5.5. Dispersive Raman Spectroscopy
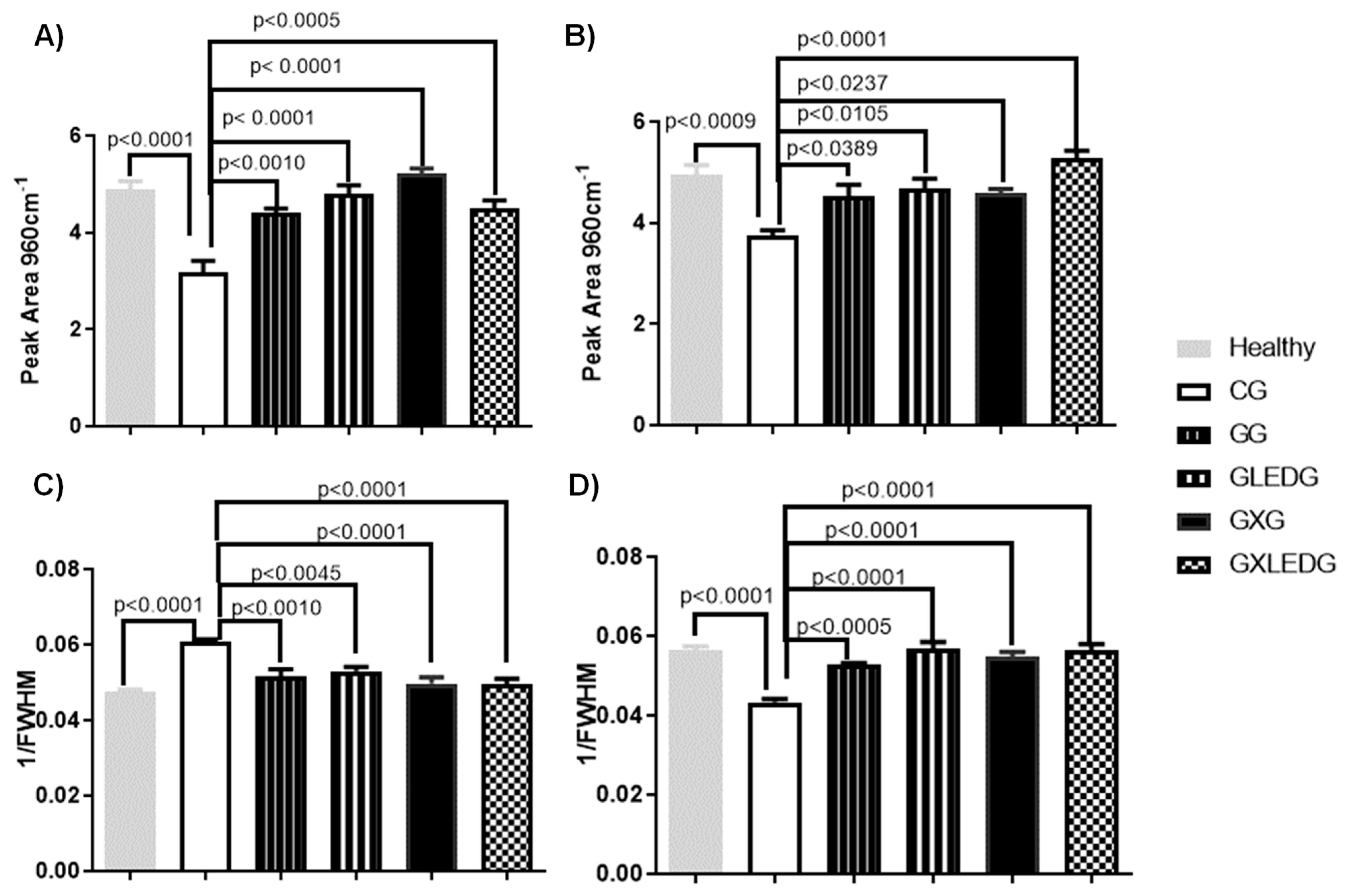
2.5.6. Statical Analysis
3. Results and Discussion
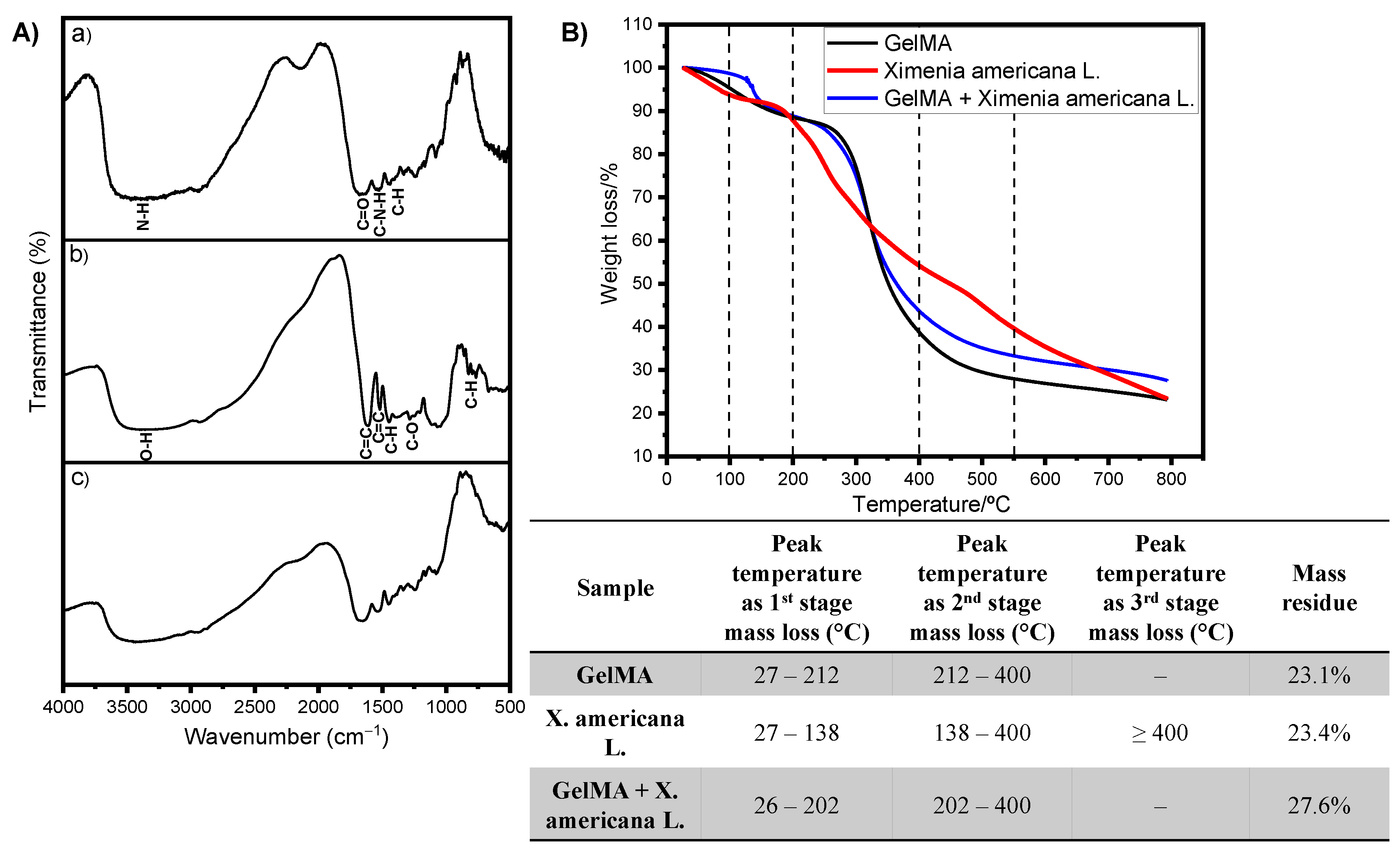
3.1. Production and Characterization of Raw Materials and GelMA and GelMA + Ximenia americana L. Hydrogels
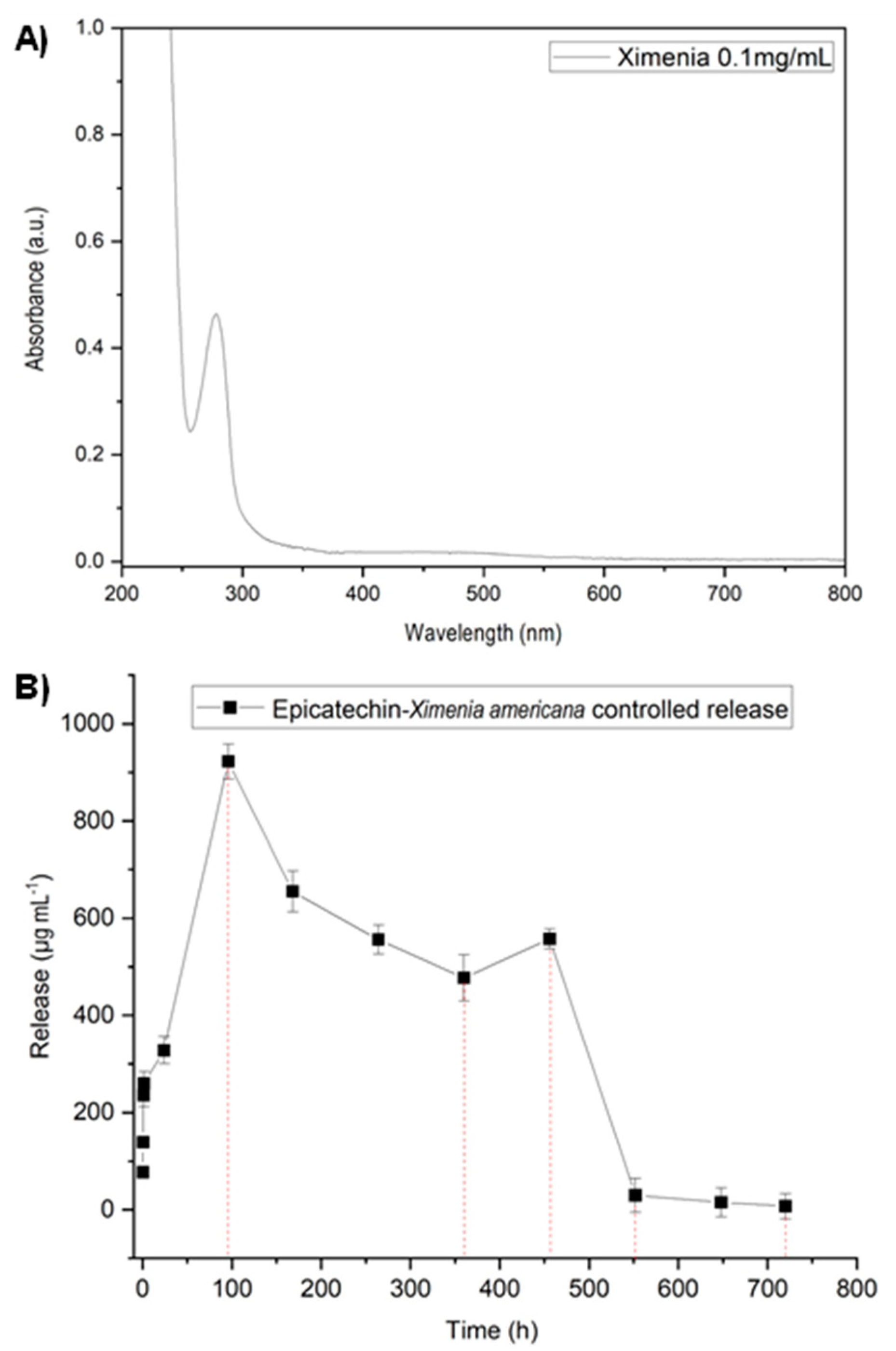
3.2. Controlled Release Test of Epicatechin—Main Compound of Ximenia americana L.
3.3. In Vivo Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nicholson, J.A.; Makaram, N.; Simpson, A.H.R.W.; Keating, J.F. Fracture Nonunion in Long Bones: A Literature Review of Risk Factors and Surgical Management. Injury 2021, 52, S3–S11. [Google Scholar] [CrossRef]
- Qiao, K.; Xu, L.; Tang, J.; Wang, Q.; Lim, K.S.; Hooper, G.; Woodfield, T.B.F.; Liu, G.; Tian, K.; Zhang, W.; et al. The Advances in Nanomedicine for Bone and Cartilage Repair. J. Nanobiotechnol. 2022, 20, 141. [Google Scholar] [CrossRef]
- Qi, J.; Yu, T.; Hu, B.; Wu, H.; Ouyang, H. Current Biomaterial-Based Bone Tissue Engineering and Translational Medicine. Int. J. Mol. Sci. 2021, 22, 10233. [Google Scholar] [CrossRef]
- Feng, Y.; Zhu, S.; Mei, D.; Li, J.; Zhang, J.; Yang, S.; Guan, S. Application of 3D Printing Technology in Bone Tissue Engineering: A Review. Curr. Drug Deliv. 2020, 18, 847–861. [Google Scholar] [CrossRef]
- Cui, Z.K.; Kim, S.; Baljon, J.J.; Wu, B.M.; Aghaloo, T.; Lee, M. Microporous Methacrylated Glycol Chitosan-Montmorillonite Nanocomposite Hydrogel for Bone Tissue Engineering. Nat. Commun. 2019, 10, 3523. [Google Scholar] [CrossRef]
- Lin, H.; Sohn, J.; Shen, H.; Langhans, M.T.; Tuan, R.S. Bone Marrow Mesenchymal Stem Cells: Aging and Tissue Engineering Applications to Enhance Bone Healing. Biomaterials 2019, 203, 96–110. [Google Scholar] [CrossRef]
- Dumic-Cule, I.; Peric, M.; Kucko, L.; Grgurevic, L.; Pecina, M.; Vukicevic, S. Bone Morphogenetic Proteins in Fracture Repair. Int. Orthop. 2018, 42, 2619–2626. [Google Scholar] [CrossRef]
- Gao, J.; Liu, X.; Cheng, J.; Deng, J.; Han, Z.; Li, M.; Wang, X.; Liu, J.; Zhang, L. Application of Photocrosslinkable Hydrogels Based on Photolithography 3D Bioprinting Technology in Bone Tissue Engineering. Regen. Biomater. 2023, 10, rbad037. [Google Scholar] [CrossRef]
- Oh, W.T.; Yang, Y.S.; Xie, J.; Ma, H.; Kim, J.M.; Park, K.H.; Oh, D.S.; Park-Min, K.H.; Greenblatt, M.B.; Gao, G.; et al. WNT-Modulating Gene Silencers as a Gene Therapy for Osteoporosis, Bone Fracture, and Critical-Sized Bone Defects. Mol. Ther. 2023, 31, 435–453. [Google Scholar] [CrossRef]
- Ullah, S.; Hussain, Z.; Ullah, I.; Wang, L.; Mehmood, S.; Liu, Y.; Mansoorianfar, M.; Liu, X.; Ma, F.; Pei, R. Mussel Bioinspired, Silver-Coated and Insulin-Loaded Mesoporous Polydopamine Nanoparticles Reinforced Hyaluronate-Based Fibrous Hydrogel for Potential Diabetic Wound Healing. Int. J. Biol. Macromol. 2023, 247, 125738. [Google Scholar] [CrossRef]
- Lantigua, D.; Wu, X.; Suvarnapathaki, S.; Nguyen, M.A.; Camci-Unal, G. Composite Scaffolds from Gelatin and Bone Meal Powder for Tissue Engineering. Bioengineering 2021, 8, 169. [Google Scholar] [CrossRef]
- Goto, R.; Nishida, E.; Kobayashi, S.; Aino, M.; Ohno, T.; Iwamura, Y.; Kikuchi, T.; Hayashi, J.I.; Yamamoto, G.; Asakura, M.; et al. Gelatin Methacryloyl–Riboflavin (Gelma–Rf) Hydrogels for Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 1635. [Google Scholar] [CrossRef]
- Jiang, Q.; Bai, G.; Liu, X.; Chen, Y.; Xu, G.; Yang, C.; Zhang, Z. 3D Gelma Icc Scaffolds Combined with Sw033291 for Bone Regeneration by Modulating Macrophage Polarization. Pharmaceutics 2021, 13, 1934. [Google Scholar] [CrossRef]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.W.P.; Malda, J.; Melchels, P.W. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef]
- Miranda, L.L.; Guimarães-Lopes, V.D.P.; Altoé, L.S.; Sarandy, M.M.; Melo, F.C.S.A.; Novaes, R.D.; Gonçalves, R.V. Plant Extracts in the Bone Repair Process: A Systematic Review. Mediat. Inflamm. 2019, 2019, 1296153. [Google Scholar] [CrossRef]
- Park, K.R.; Park, J.E.; Kim, B.; Kwon, I.K.; Hong, J.T.; Yun, H.M. Calycosin-7-o-β-Glucoside Isolated from Astragalus Membranaceus Promotes Osteogenesis and Mineralization in Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 11362. [Google Scholar] [CrossRef]
- Hanafiah, O.A.; Hanafiah, D.S.; Dohude, G.A.; Satria, D.; Livita, L.; Moudy, N.S.; Rahma, R. Effects of 3% Binahong (Anredera Cordifolia) Leaf Extract Gel on Alveolar Bone Healing in Post-Extraction Tooth Socket Wound in Wistar Rats (Rattus Norvegicus). F1000Research 2022, 10, 923. [Google Scholar] [CrossRef]
- Singh, P.; Gupta, A.; Qayoom, I.; Singh, S.; Kumar, A. Orthobiologics with Phytobioactive Cues: A Paradigm in Bone Regeneration. Biomed. Pharmacother. 2020, 130, 110754. [Google Scholar] [CrossRef]
- Santana, C.P.; Medeiros, F.D.; Correia, L.P.; Diniz, P.H.G.D.; Véras, G.; Medeiros, A.C.D. Dissolution and Uniformity of Content of Tablets Developed with Extract of Ximenia americana L. PLoS ONE 2018, 13, e0197323. [Google Scholar] [CrossRef]
- Leal, S.S.; Uchôa, V.T.; Figuerêdo-Silva, J.; Soares, R.B.; Mota, D.M.; De Alencar, R.C.; Filho, A.L.M.M.; Sant’Ana, A.E.G.; Beltrame Junior, M. Eficácia Da Fonoforese Com Ximenia americana L. Na Inflamação de Tendão de Ratos. Rev. Bras. Med. Esporte 2016, 22, 355–360. [Google Scholar] [CrossRef]
- da Silva, K.M.A.; Chaves, T.P.; Santos, R.L.; Brandão, D.O.; Fernandes, F.H.A.; de Ramos Júnior, F.J.L.; dos Santos, V.L.; de Felismino, D.C.; Medeiros, A.C.D. Modulation of the Erythromycin Resistance in Staphylococcus Aureus by Ethanolic Extracts of Ximenia americana L. and Schinopsis Brasiliensis Engl. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2015, 14, 92–98. [Google Scholar]
- Silva, M.S.P.; Brando, D.O.; Chaves, T.P.; Formiga Filho, A.L.N.; Costa, E.M.M.D.B.; Santos, V.L.; Medeiros, A.C.D. Study Bioprospecting of Medicinal Plant Extracts of the Semiarid Northeast: Contribution to the Control of Oral Microorganisms. Evidence-based Complement. Altern. Med. 2012, 2012, 681207. [Google Scholar] [CrossRef]
- Da Palma, A.F.M.; Marques, L.K.M.; Carneiro, R.D.S.; Carvalho, G.F.S.; Ferreira, D.C.L.; Sant’Ana, A.E.G.; Maia Filho, A.L.M.; Marques, R.B.; Alves, W.D.S.; Uchôa, V.T. Evaluation of Hydroalcoholic Extracts of Stem and Leaves of Ximenia americana L. In the Healing of Excisional Acute Wounds in Mice. Rev. Virtual Quim. 2020, 12, 37–50. [Google Scholar] [CrossRef]
- Almeida, L.; Júnior, J.A.O.; Silva, M.; Nóbrega, F.; Andrade, J.; Santos, W.; Ribeiro, A.; Conceição, M.; Veras, G.; Medeiros, A.C. Tablet of Ximenia americana L. Developed from Mucoadhesive Polymers for Future Use in Oral Treatment of Fungal Infections. Polymers 2019, 11, 379. [Google Scholar] [CrossRef]
- Dias, T.L.M.F.; Melo, G.M.A.; Da Silva, Y.K.C.; Queiroz, A.C.; Goulart, H.F.; Alexandre-Moreira, M.S.; Santana, A.E.G.; Uchôa, V.T. Antinociceptive and Anti-Inflammatory Activities of the Ethanolic Extract, of Fractions and of Epicatechin Isolated from the Stem Bark of Ximenia americana L. (Oleacaceae). Rev. Virtual Quim. 2018, 10, 86–101. [Google Scholar] [CrossRef]
- Jin, Y.; Koh, R.H.; Kim, S.H.; Kim, K.M.; Park, G.K.; Hwang, N.S. Injectable Anti-Inflammatory Hyaluronic Acid Hydrogel for Osteoarthritic Cartilage Repair. Mater. Sci. Eng. C 2020, 115, 111096. [Google Scholar] [CrossRef]
- Gao, Z.R.; Feng, Y.Z.; Zhao, Y.Q.; Zhao, J.; Zhou, Y.H.; Ye, Q.; Chen, Y.; Tan, L.; Zhang, S.H.; Feng, Y.; et al. Traditional Chinese Medicine Promotes Bone Regeneration in Bone Tissue Engineering. Chin. Med. 2022, 17, 86. [Google Scholar] [CrossRef]
- Ortiz, A.d.C.; Fideles, S.O.M.; Reis, C.H.B.; Bellini, M.Z.; Pereira, E.d.S.B.M.; Pilon, J.P.G.; de Marchi, M.Â.; Detregiachi, C.R.P.; Flato, U.A.P.; Trazzi, B.F.d.M.; et al. Therapeutic Effects of Citrus Flavonoids Neohesperidin, Hesperidin and Its Aglycone, Hesperetin on Bone Health. Biomolecules 2022, 12, 626. [Google Scholar] [CrossRef]
- Sun, W.; Li, M.; Zhang, Y.; Huang, Y.; Zhan, Q.; Ren, Y.; Dong, H.; Chen, J.; Li, Z.; Fan, C.; et al. Total Flavonoids of Rhizoma Drynariae Ameliorates Bone Formation and Mineralization in BMP-Smad Signaling Pathway Induced Large Tibial Defect Rats. Biomed. Pharmacother. 2021, 138, 111480. [Google Scholar] [CrossRef]
- Carlos Rodríguez-Merchán, E. The Molecular Mechanisms of Bone Healing. Int. J. Mol. Sci 2021, 22, 767. [Google Scholar] [CrossRef]
- Zuo, Y.; Li, Q.; Xiong, Q.; Li, J.; Tang, C.; Zhang, Y.; Wang, D. Naringin Release from a Nano-Hydroxyapatite / Collagen Tissue Reconstruction. Polymers 2022, 14, 3260. [Google Scholar] [CrossRef] [PubMed]
- Uchôa, V.T.; Sousa, C.M.M.; Carvalho, A.A.; Sant’Ana, A.E.G.; Chaves, M.H. Free Radical Scavenging Ability of Ximenia americana L. Stem Bark and Leaf Extracts. J. Appl. Pharm. Sci. 2016, 6, 091–096. [Google Scholar] [CrossRef]
- Carvalho, G.F.S.; Marques, L.K.; Sousa, H.G.; Silva, L.R.; Leão Ferreira, D.C.; Pires de Moura do Amaral, F.; Martins Maia Filho, A.L.; Figueredo-Silva, J.; Alves, W.d.S.; Oliveira, M.d.D.A.d.; et al. Phytochemical Study, Molecular Docking, Genotoxicity and Therapeutic Efficacy of the Aqueous Extract of the Stem Bark of Ximenia americana L. in the Treatment of Experimental COPD in Rats. J. Ethnopharmacol. 2020, 247, 112259. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, R.d.S.; Canuto, M.R.; Ribeiro, L.K.; Ferreira, D.C.L.; Assunção, A.F.C.; Costa, C.A.C.B.; de Freitas, J.D.; Rai, M.; Cavalcante, L.S.; Alves, W. dos S.; et al. Novel Antibacterial Efficacy of ZnO Nanocrystals/Ag Nanoparticles Loaded with Extract of Ximenia americana L. (Stem Bark) for Wound Healing. S. Afr. J. Bot. 2022, 151, 18–32. [Google Scholar] [CrossRef]
- Deniz, E.; Arslan, A.; Diker, N.; Olgac, V.; Kilic, E. Evaluation of Light-Emitting Diode Photobiomodulation on Bone Healing of Rat Calvarial Defects. Biotechnol. Biotechnol. Equip. 2015, 29, 758–765. [Google Scholar] [CrossRef]
- dos Santos, S.A.; dos Santos Vieira, M.A.; Simões, M.C.B.; Serra, A.J.; Leal-Junior, E.C.; de Carvalho, P.d.T.C. Photobiomodulation Therapy Associated with Treadmill Training in the Oxidative Stress in a Collagen-Induced Arthritis Model. Lasers Med. Sci. 2017, 32, 1071–1079. [Google Scholar] [CrossRef]
- Mostafavinia, A.; Razavi, S.; Abdollahifar, M.; Amini, A.; Ghorishi, S.K.; Rezaei, F.; Pouriran, R.; Bayat, M. Evaluation of the Effects of Photobiomodulation on Bone Healing in Healthy and Streptozotocin-Induced Diabetes in Rats. Photomed. Laser Surg. 2017, 35, 537–545. [Google Scholar] [CrossRef]
- Pinheiro, A.L.B.; Soares, L.G.P.; da Silva, A.C.P.; Santos, N.R.S.; da Silva, A.P.L.T.; Neves, B.L.R.C.; Soares, A.P.; Gerbi, M.E.M.M.; dos Santos, J.N. The Use of Photobiomodulation Therapy or LED and Mineral Trioxide Aggregate Improves the Repair of Complete Tibial Fractures Treated with Wire Osteosynthesis in Rodents. Lasers Med. Sci. 2021, 36, 735–742. [Google Scholar] [CrossRef]
- Chen, A.C.H.; Huang, Y.Y.; Sharma, S.K.; Hamblin, M.R. Effects of 810-Nm Laser on Murine Bone-Marrow-Derived Dendritic Cells. Photomed. Laser Surg. 2011, 29, 383–389. [Google Scholar] [CrossRef]
- Barbosa, D.; Villaverde, A.G.J.B.; LoschiavoArisawa, E.Â.; de Souza, R.A. Laser Therapy in Bone Repair in Rats: Analysis of Bone Optical Density. Acta Ortop. Bras. 2014, 22, 71–74. [Google Scholar] [CrossRef]
- Sella, V.R.G.; do Bomfim, F.R.C.; Machado, P.C.D.; da Silva Morsoleto, M.J.M.; Chohfi, M.; Plapler, H. Effect of Low-Level Laser Therapy on Bone Repair: A Randomized Controlled Experimental Study. Lasers Med. Sci. 2015, 30, 1061–1068. [Google Scholar] [CrossRef]
- Mayer, L.; Freddo, A.L.; Blaya, D.S. Effects of Low-Level Laser Therapy on Distraction Osteogenesis: A Histological Analysis. RFO UPF 2012, 17, 326–331. [Google Scholar]
- Ekizer, A.; Uysal, T.; Güray, E.; Akkuş, D. Effect of LED-Mediated-Photobiomodulation Therapy on Orthodontic Tooth Movement and Root Resorption in Rats. Lasers Med. Sci. 2015, 30, 779–785. [Google Scholar] [CrossRef]
- Kloukos, D. The Effect of Light-Emitting Diode Phototherapy on Rate of Orthodontic Tooth Movement: A Split Mouth, Controlled Clinical Trial. J. Orthod. 2015, 42, 271. [Google Scholar] [CrossRef]
- Lv, L.; Cheng, W.; Wang, S.; Lin, S.; Dang, J.; Ran, Z.; Zhu, H.; Xu, W.; Huang, Z.; Xu, P.; et al. Poly(β-Amino Ester) Dual-Drug-Loaded Hydrogels with Antibacterial and Osteogenic Properties for Bone Repair. ACS Biomater. Sci. Eng. 2023, 9, 1976–1990. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.; Bae, H.; Hwang, C.M.; Khademhosseini, A. 2010 Biomaterials Ali. Cell-Laden Microengineered Gelatin Methacrylate Hydrogels. Biomaterials 2011, 31, 5536–5544. [Google Scholar] [CrossRef]
- Lee, J.Y.; Son, S.J.; Son, J.S.; Kang, S.S.; Choi, S.H. Bone-Healing Capacity of PCL/PLGA/Duck Beak Scaffold in Critical Bone Defects in a Rabbit Model. BioMed Res. Int. 2016, 2016, 2136215. [Google Scholar] [CrossRef]
- Schlickewei, C.; Klatte, T.O.; Wildermuth, Y.; Laaff, G.; Rueger, J.M.; Ruesing, J.; Chernousova, S.; Lehmann, W.; Epple, M. A Bioactive Nano-Calcium Phosphate Paste for In-Situ Transfection of BMP-7 and VEGF-A in a Rabbit Critical-Size Bone Defect: Results of an In Vivo Study. J. Mater. Sci. 2019, 30, 15. [Google Scholar] [CrossRef]
- Kido, H.W.; Brassolatti, P.; Tim, C.R.; Gabbai-Armelin, P.R.; Magri, A.M.P.; Fernandes, K.R.; Bossini, P.S.; Parizotto, N.A.; Crovace, M.C.; Malavazi, I.; et al. Porous Poly (D,L-Lactide-Co-Glycolide) Acid/Biosilicate® Composite Scaffolds for Bone Tissue Engineering. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2017, 105, 63–71. [Google Scholar] [CrossRef]
- Alves, A.M.M.; de Miranda Fortaleza, L.M.; Filho, A.L.M.M.; Ferreira, D.C.L.; da Costa, C.L.S.; Viana, V.G.F.; Santos, J.Z.L.V.; de Oliveira, R.A.; de Meira Gusmão, G.O.; Soares, L.E.S. Evaluation of Bone Repair after Application of a Norbixin Membrane Scaffold with and without Laser Photobiomodulation (λ 780 Nm). Lasers Med. Sci. 2018, 33, 1493–1504. [Google Scholar] [CrossRef]
- Nascimento, L.D.E.S.; Nicolau, R.A.; Filho, A.L.M.M.; Santos, J.Z.L.V.; Fonseca, K.M.; Ferreira, D.C.L.; de Sousa, R.C.; Viana, V.G.F.; Carvalho, L.F.M.; Figueredo-Silva, J. Effect of Norbixin-Based Poly(Hydroxybutyrate) Membranes on the Tendon Repair Process after Tenotomy in Rats. Acta Cir. Bras. 2019, 34, e201901101. [Google Scholar] [CrossRef]
- Cardoso, Á.B. Estudo Histomorfométrico Comparativo Da Reparação Óssea Em Ratos Após o Uso de Biomateriais de Origem Bovina e Sintética; Universidade Federal do Pernambuco: Recife, Brazil, 2008. [Google Scholar]
- Kido, H.W.; Bossini, P.S.; Tim, C.R.; Parizotto, N.A.; da Cunha, A.F.; Malavazi, I.; Renno, A.C.M. Evaluation of the Bone Healing Process in an Experimental Tibial Bone Defect Model in Ovariectomized Rats. Aging Clin. Exp. Res. 2014, 26, 473–481. [Google Scholar] [CrossRef]
- Radev, L.; Fernandes, M.H.V.; Salvado, I.M.; Kovacheva, D. Organic/Inorganic Bioactive Materials Part III: In Vitro Bioactivity of Gelatin/Silicocarnotite Hybrids. Cent. Eur. J. Chem. 2009, 7, 721–730. [Google Scholar] [CrossRef]
- Aldana, A.A.; Malatto, L.; Ur Rehman, M.A.; Boccaccini, A.R.; Abraham, G.A. Fabrication of Gelatin Methacrylate (GelMA) Scaffolds with Nano-and Micro-Topographical and Morphological Features. Nanomaterials 2019, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- El-Maghawry, E.; Tadros, M.I.; Elkheshen, S.A.; Abd-Elbary, A. Eudragit®-S100 Coated Plga Nanoparticles for Colon Targeting of Etoricoxib: Optimization and Pharmacokinetic Assessments in Healthy Human Volunteers. Int. J. Nanomed. 2020, 15, 3965–3980. [Google Scholar] [CrossRef] [PubMed]
- De Salvi, D.T.B.; da Barud, H.S.; Treu-Filho, O.; Pawlicka, A.; Mattos, R.I.; Raphael, E.; Ribeiro, S.J.L. Preparation, Thermal Characterization, and DFT Study of the Bacterial Cellulose: Triethanolamine System. J. Therm. Anal. Calorim. 2014, 118, 205–215. [Google Scholar] [CrossRef]
- Aragão, Ticiana Parente. ATIVIDADE GASTROPROTETORA DA CASCA DO CAULE DE Ximenia americana L. (Ameixa de Espinho) (OLACACEAE) EM RATOS, 2019. Available online: https://repositorio.ufpe.br/handle/123456789/33692 (accessed on 5 July 2023).
- Aciole, J.; Barbosa, P. Avaliação Da Fotobiomodulação Laser/LED Em Defeito Ósseo No Fêmur de Ratas Osteoporóticas: Estudo Histológico, Histomorfométrico e Por Espectroscopia Raman Em Modelo Animal, 2016. Available online: https://repositorio.ufba.br/handle/ri/20782 (accessed on 5 July 2023).
- Akkus, O.; Adar, F.; Schaffler, M.B. Age-Related Changes in Physicochemical Properties of Mineral Crystals Are Related to Impaired Mechanical Function of Cortical Bone. Bone 2004, 34, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Awonusi, A.; Morris, M.D.; Tecklenburg, M.M.J. Carbonate Assignment and Calibration in the Raman Spectrum of Apatite. Calcif. Tissue Int. 2007, 81, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.D.; Mandair, G.S. Raman Assessment of Bone Quality. Clin. Orthop. Relat. Res. 2011, 469, 2160–2169. [Google Scholar] [CrossRef] [PubMed]
- Wan Osman, W.N.F.; Che Ahmad Tantowi, N.A.; Lau, S.F.; Mohamed, S. Epicatechin and Scopoletin Rich Morinda Citrifolia (Noni) Leaf Extract Supplementation, Mitigated Osteoarthritis via Anti-Inflammatory, Anti-Oxidative, and Anti-Protease Pathways. J. Food Biochem. 2019, 43, e12755. [Google Scholar] [CrossRef]
- Comunian, C.R.; Custódio, A.L.N.; de Oliveira, L.J.; Dutra, C.E.A.; D’almeida Ferreira Neto, M.; Rezende, C.M.F. Photobiomodulation with LED and Laser in Repair of Mandibular Socket Rabbit: Clinical Evaluation, Histological, and Histomorphometric. Oral Maxillofac. Surg. 2017, 21, 201–206. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Patrocínio-Silva, T.L.; de Souza, A.M.F.; Goulart, R.L.; Pegorari, C.F.; Oliveira, J.R.; Fernandes, K.R.; Magri, A.M.P.; Pereira, R.M.R.; Ribeiro, D.A.; Nagaoka, M.R.; et al. Low-Level Laser Therapy Associated to a Resistance Training Protocol on Bone Tissue in Diabetic Rats. Arch. Endocrinol. Metab. 2016, 60, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Chaves, T.P.; Santana, C.P.; Véras, G.; Brandão, D.O.; Felismino, D.C.; Cláudia, A.; Medeiros, D.; De, D.M.; Trovão, B.M. Seasonal Variation in the Production of Secondary Metabolites and Antimicrobial Activity of Two Plant Species Used in Brazilian Traditional Medicine. Afr. J. Biotechnol. 2013, 12, 847–853. [Google Scholar] [CrossRef]
- Nogueira Albino Calland, F.; de Castro Brito, G.; Fernandes de Sousa, G.; de Carvalho Oliveira, F.; Roberta Marciano, F.; Oliveira Lobo, A. Evaluation of Wound Healing Activity of GelMA/PCLMA Fibrous Composites in Diabetic Model Rats. Mater. Lett. 2023, 336, 133893. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Jiang, G.; Li, S.; Yu, K.; He, B.; Hong, J.; Xu, T.; Meng, J.; Ye, C.; Chen, Y.; Shi, Z.; et al. A 3D-Printed PRP-GelMA Hydrogel Promotes Osteochondral Regeneration through M2 Macrophage Polarization in a Rabbit Model. Acta Biomater. 2021, 128, 150–162. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leal, S.S.; Gusmão, G.O.d.M.; Uchôa, V.T.; Figueiredo-Silva, J.; Pinto, L.S.S.; Tim, C.R.; Assis, L.; Maia-Filho, A.L.M.; Oliveira, R.A.d.; Lobo, A.O.; et al. Evaluation of How Methacrylate Gelatin Hydrogel Loaded with Ximenia americana L. Extract (Steam Bark) Effects Bone Repair Activity Using Rats as Models. J. Funct. Biomater. 2023, 14, 438. https://doi.org/10.3390/jfb14090438
Leal SS, Gusmão GOdM, Uchôa VT, Figueiredo-Silva J, Pinto LSS, Tim CR, Assis L, Maia-Filho ALM, Oliveira RAd, Lobo AO, et al. Evaluation of How Methacrylate Gelatin Hydrogel Loaded with Ximenia americana L. Extract (Steam Bark) Effects Bone Repair Activity Using Rats as Models. Journal of Functional Biomaterials. 2023; 14(9):438. https://doi.org/10.3390/jfb14090438
Chicago/Turabian StyleLeal, Seânia Santos, Gustavo Oliveira de Meira Gusmão, Valdiléia Teixeira Uchôa, José Figueiredo-Silva, Lucielma Salmito Soares Pinto, Carla R. Tim, Lívia Assis, Antonio Luiz Martins Maia-Filho, Rauirys Alencar de Oliveira, Anderson Oliveira Lobo, and et al. 2023. "Evaluation of How Methacrylate Gelatin Hydrogel Loaded with Ximenia americana L. Extract (Steam Bark) Effects Bone Repair Activity Using Rats as Models" Journal of Functional Biomaterials 14, no. 9: 438. https://doi.org/10.3390/jfb14090438
APA StyleLeal, S. S., Gusmão, G. O. d. M., Uchôa, V. T., Figueiredo-Silva, J., Pinto, L. S. S., Tim, C. R., Assis, L., Maia-Filho, A. L. M., Oliveira, R. A. d., Lobo, A. O., & Pavinatto, A. (2023). Evaluation of How Methacrylate Gelatin Hydrogel Loaded with Ximenia americana L. Extract (Steam Bark) Effects Bone Repair Activity Using Rats as Models. Journal of Functional Biomaterials, 14(9), 438. https://doi.org/10.3390/jfb14090438






