Catecholamine Derivatives as Novel Crosslinkers for the Synthesis of Versatile Biopolymers
Abstract
:1. Introduction
2. Common Biochemical Pathway of Catecholamines
3. Catecholamines Destined to Become Biopolymers
3.1. Melanin Biopolymers
3.2. N-Acyldopamine Derivatives and Cuticular Sclerotization
3.3. Tunichromes and Related Marine Compounds
3.4. Peptidyl Dopa Derivatives
4. Chemical Reactivities of Catechols
4.1. Reactivity Exhibited through Quinone Formation
4.2. Reactivity Exhibited through Quinone Methide Formation
4.3. Reactivity Exhibited through Dehydrodopa Formation
4.4. Reactivity Exhibited through Free Radicals
4.5. Catechols and Their Derivatives as Metal Chelators
5. Catecholamine-Based Biomaterials
5.1. Peptidyl Dopa-Derived Biomaterial
5.2. Tunichrome-Based Biomaterials
5.3. N-Acyldopamine Derivatives and Cuticular Hardening
5.4. Synthetic Biomaterial Mimics
6. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Micillo, R.; Penzella, L.; Koike, K.; Monfrecola, G.; Napolitano, A.; d’Ischia, M. “Fifty Shades” of black and red or how carboxyl groups fine tune eumelanin and pheomelanin properties. Int. J. Mol. Sci. 2016, 17, 746. [Google Scholar] [CrossRef] [PubMed]
- Penzella, L.; Ebato, A.; Napolitano, A.; Koike, K. The late stages of melanogenesis: Exploring the chemical facets and the application opportunities. Int. J. Mol. Sci. 2018, 19, 1753. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Chemistry of mixed melanogenesis—Pivotal role of dopaquinone. Photochem. Photobiol. 2008, 84, 582–592. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K.; d’Ischia, M.; Napolitano, A.; Pezzella, A. Structure of melanins. In Melanins and Melanosomes: Biosynthesis, Physiological and Pathological Functions; Riley, P.A., Borovansky, J., Eds.; Wiley-Blackwell: Weinheim, Germany, 2011; pp. 167–185. [Google Scholar]
- Ito, S.; Sugumaran, M.; Wakamatsu, K. Chemical reactivities of ortho-quinone produced in living Organisms. Fate of quinonoid products formed by tyrosinase and phenoloxidase action on phenols and catechols. Int. J. Mol. Sci. 2020, 21, 6080. [Google Scholar] [CrossRef]
- Proto, G. Melanins and Melanogenesis; Academic Press: New York, NY, USA, 1992; pp. 1–260. [Google Scholar]
- Sugumaran, M.; Barek, H. Critical analysis of melanogenic pathway in insects and higher animals. Int. J Mol. Sci. 2016, 17, 1753. [Google Scholar] [CrossRef]
- Kobayashi, T.; Urabe, K.; Winder, A.; Jimenez-Cervantes, C.; Imokawa, G.; Brewington, T.; Solano, F.; Garcia-Borron, J.C.; Hearing, V.J. Tyrosinase-related protein-1 (Trp-1) functions as DHICA oxidase in melanin biosynthesis. EMBO J. 1994, 13, 5818–5825. [Google Scholar] [CrossRef]
- Barek, H.; Sugumaran, M.; Ito, S.; Wakamatsu, K. Insect cuticular melanins are distinctly different from those of mammalian epidermal melanins. Pig. Cell Melanoma Res. 2018, 31, 384–392. [Google Scholar] [CrossRef]
- Karlson, P.; Sekeris, C.E. N-Acetyldopamine as sclerotizing agent of the insect cuticle. Nature 1962, 195, 183–184. [Google Scholar] [CrossRef]
- Hopkins, T.L.; Morgan, T.D.; Aso, Y.; Kramer, K.J. N-β-Alanyldopamine: Major role in insect cuticle tanning. Science 1982, 217, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, T.L.; Kramer, K.J. Insect cuticle sclerotization. Ann. Rev. Entomol. 1992, 37, 273–302. [Google Scholar] [CrossRef]
- Andersen, S.O.; Peter, M.G.; Roepstorff, P. Cuticular sclerotization in insects. Comp. Biochem. Physiol. B 1996, 113, 689–705. [Google Scholar] [CrossRef]
- Andersen, S.O. Insect cuticular sclerotization—A review. Insect Biochem. Mol. Biol. 2010, 40, 166–178. [Google Scholar] [CrossRef]
- Brunet, P.C.J. The metabolism of amino acids concerned in the crosslinking of insect cuticle. Insect Biochem. 1980, 10, 467–500. [Google Scholar] [CrossRef]
- Sugumaran, M. Cuticular sclerotization in insect—A critical review. Adv. Insect Physiol. 2022, 62, 111–214. [Google Scholar]
- Aust, S.; Brusselbach, F.; Putz, S.; Hovermann, B.T. Alternative tasks of Drosophila tan in neurotransmitter recycling versus cuticle sclerotization disclosed by kinetic properties. J. Biol. Chem. 2010, 285, 20740–20747. [Google Scholar] [CrossRef]
- Barek, H.; Zhao, H.; Heath, K.; Veraksa, A.; Sugumaran, M. Drosophila yellow-h encodes dopaminechrome tautomerase: A new enzyme in eumelanin biosynthetic pathway. Pig. Cell Melanoma Res. 2021, 35, 26–37. [Google Scholar] [CrossRef]
- Kramer, K.J.; Hopkins, T.L.; Schaefer, J. Application of solids NMR to the analysis of insect sclerotized structures. Insect Biochem. Mol. Biol. 1995, 25, 1067–1080. [Google Scholar] [CrossRef]
- Rzepecki, L.M.; Nagafuchi, T.; Waite, H.J. α,β-Dehydro-3,4-dihydroxy phenylalanine derivatives: Potential sclerotization intermediates in natural composite materials. Arch. Biochem. Biophys. 1991, 285, 17–26. [Google Scholar] [CrossRef]
- Rzepecki, L.M.; Waite, H.J. α,β-Dehydro-3,4-dihydroxy phenylalanine derivatives: Rate and mechanism of formation. Arch. Biochem. Biophys. 1991, 285, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Kim, D.; Horenstein, B.; Nakanishi, K.; Kustin, K. Unraveling the chemistry of tunichromes. Acc. Chem. Res. 1991, 24, 117–124. [Google Scholar] [CrossRef]
- Kustin, K.; Robinson, W.E.; Smith, M.J. Tunichromes, vanadium, and vacuolated blood cells in tunicates. Invert Reprod. Develop. 1990, 17, 129–139. [Google Scholar] [CrossRef]
- Taylor, S.; Kammerer, B.; Bayer, E. New perspectives in the chemistry and biochemistry of the tunichromes and related compounds. Chem. Rev. 1997, 97, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, M.; Robinson, W.E. Bioactive dehydrotyrosyl and dehydrodopyl compounds of marine origin. Marine Drugs. 2010, 8, 2906–2935. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, M.; Robinson, W.E. Structure, biosynthesis, and possible function of tunichrome and related compounds. Comp. Biochem. Physiol. B 2012, 163, 1–25. [Google Scholar] [CrossRef]
- Tincu, J.A.; Taylor, S.W. Tunichrome Sp-1: New pentapeptide tunichrome from the hemocytes of Styela plicata. J. Nat. Prod. 2002, 65, 377–378. [Google Scholar] [CrossRef]
- Tincu, J.A.; Menzel, L.P.; Azimov, R.; Sands, J.; Hong, T.; Waring, A.J.; Taylor, S.W.; Lehrer, R.I. Plicatamide, antibacterial octapeptide from Styela pilicata hemocytes. J. Biol. Chem. 2003, 278, 13546–13553. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, R.I.; Tincu, J.A.; Taylor, S.W.; Menzel, L.P.; Waring, A.J. Natural peptide antibiotics from tunicates: Structures, functions and potential uses. Integr. Comp. Biol. 2003, 43, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Macara, I.G.; McLeod, G.C.; Kustin, K. Isolation, properties and structural studies on a compound from tunicate blood cells that may be involved in vanadium accumulation. Biochem. J. 1979, 181, 457–465. [Google Scholar] [CrossRef]
- Bruening, R.C.; Oltz, E.M.; Furukawa, J.; Nakanishi, K. Isolation and structure elucidation of tunichrome B-1, a reducing blood pigment from the tunicate Ascidia nigra L. J. Amer Chem. Soc. 1985, 107, 5298–5300. [Google Scholar] [CrossRef]
- Waite, J.H. Phylogeny and chemical diversity of quinone tanned glues and varnishes. Comp. Biochem. Physiol. 1990, 97, 19–29. [Google Scholar] [CrossRef]
- Waite, J.H. Adhesion a la Moule. Integr. Comp. Biol. 2002, 42, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.H. Mussel power. Nat. Mater. 2008, 7, 8–9. [Google Scholar] [CrossRef]
- Waite, J.H. Mussel adhesion—Essential footwork. J. Exp. Biol. 2017, 220, 517–530. [Google Scholar] [CrossRef]
- Rubin, D.J.; Miserez, A.; Waite, J.H. Diverse strategies of protein sclerotization in marine invertebrates: Structure-property relationships in natural biomaterials. Adv. Insect. physiol. 2010, 38, 75–133. [Google Scholar]
- Andersen, N.H.; Jewhurst, S.; Sun, C. Mussel adhesion: Finding the tricks worth mimicking, J. Adhes 2005, 81, 297–317. [Google Scholar]
- Lee, B.P.; Messersmith, P.B.; Israelachville, J.N.; Waite, J.H. Mussel inspired adhesives and coatings. Ann. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef] [PubMed]
- Holten-Andersen, N.; Waite, J.H. Mussel-designed protective coatings for compliant substrates. J. Dental Res. 2008, 87, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Wilker, J.J. Rare metal, precious adhesion. Science 2021, 374, 148–149. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Zhong, W. Mussel-inspired hydrogel tissue adhesives for wound closure. RSC Adv. 2017, 7, 47380–47396. [Google Scholar] [CrossRef]
- Yamazaki, I.; Mason, H.S.; Piette, L. Identification of electron para-magnetic resonance spectroscopy of free radicals generated from substrates by peroxidase. J. Biol. Chem. 1960, 235, 24444–24449. [Google Scholar] [CrossRef]
- Nakamura, T. On the process of enzymatic oxidation of hydroquinone. Biochem. Biophys. Res. Commun. 1960, 2, 111–113. [Google Scholar] [CrossRef]
- Wang, S.X.; Mure, M.; Medzihradszky, K.F.; Burlingame, A.I.; Brown, D.E.; Dooley, D.M.; Smith, A.J.; Kagan, M.; Klinman, J.P. A crosslinked cofactor in lysyl oxidase: Redox function for amino acid side chains. Science 2006, 273, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.O.; Jacobsen, J.P.; Roepstorff, P.; Peter, M.G. Catecholamine protein conjugates: Isolation of an adduct of N-acetyl histidine to the side chain of N-acetyldopamine from an insect-enzyme catalyzed reaction. Tetrahedron Lett. 1991, 32, 4287–4290. [Google Scholar] [CrossRef]
- Andersen, S.O.; Peter, M.G.; Roepstorff, P. Cuticle-catalyzed coupling between N-acetyl histidine and N-acetyldopamine. Insect Biochem. Mol. Biol. 1992, 22, 459–469. [Google Scholar] [CrossRef]
- Kerwin, J.L.; Turecek, F.; Xu, R.; Kramer, K.J.; Hopkins, T.L.; Gatlin, C.L.; Yates, J.R. Mass spectrometric analysis of catechol-histidine adducts from insect cuticle. Anal. Biochem. 1999, 268, 229–237. [Google Scholar] [CrossRef]
- Xu, R.; Huang, X.; Morgan, T.D.; Prakash, O.; Kramer, K.J.; Hawley, M.D. Characterization of products from the reactions of N-acetyldopamine quinone with N-acetyl histidine. Arch. Biochem. Biophys. 1996, 329, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Huang, X.; Hopkins, T.L.; Kramer, K.J. Catecholamine and histidyl protein cross-linked structures in sclerotized insect cuticle. Insect Biochem. Mol. Biol. 1997, 27, 101–108. [Google Scholar] [CrossRef]
- Jane, S.M.; Mu, D.; Wemmer, D.; Smith, A.I.; Kaur, S.; Malby, D.; Burlingame, A.I.; Klinman, J.P. A new redox cofactor in eukaryotic enzymes: 6-Hydroxydopa at the active site of bovine amine oxidase. Science 1990, 248, 981–987. [Google Scholar] [CrossRef]
- Ito, S.; Prota, G. A facile one step synthesis of cysteinyldopas using mushroom tyrosinase. Experientia 1977, 33, 1118–1119. [Google Scholar] [CrossRef]
- Land, E.J.; Ramsden, C.A.; Riley, P.A. Quinone chemistry and melanogenesis. Meth. Enzymol. 2004, 378, 88–102. [Google Scholar]
- Alfieri, M.L.; Cariola, A.; Panzella, L.; Napolitano, A.; d’Ischia, M.; Valgimigli, L.; Crescenzi, O. Disentangling the puzzling regiochemistry of thiol addition to o-quinones. J. Org Chem. 2021, 87, 4580–4589. [Google Scholar] [CrossRef] [PubMed]
- Vithayathil, P.J.; Murthy, G.S. New reaction of o-benzoquinone at the thioether group of methionine. Nature 1972, 236, 101–103. [Google Scholar] [CrossRef]
- Vithayathil, P.J.; Gupta, M.N. Reaction of methionine with some biologically important o-quinones. Indian J. Biochem. Biophys. 1981, 18, 82–83. [Google Scholar]
- Bolton, J.L.; Acay, N.M.; Vukomanovic, V. Evidence that 4-allyl-orthoquinones spontaneously rearrange to their more electrophilic quinone methides: Potential bioactivation mechanism for the hepatocarcinogen safrole. Chem. Res. Toxicol. 1994, 7, 443–450. [Google Scholar] [CrossRef]
- Bolton, J.L.; Wu, H.M.; Hu, L.Q. Mechanism of isomerization of 4-propyl-o-quinone to its tautomeric quinone methide. Chem Res. Toxicol. 1996, 9, 109–113. [Google Scholar] [CrossRef]
- Cheah, Y.S.; Santhanakrishnan, S.; Sullivan, M.B.; Neoh, K.G.; Chai, C.L. The chemical reactivities of dopa and dopamine derivatives and their regioselectivities upon oxidative nucleophilic trapping. Tetrahedron 2016, 72, 6543–6550. [Google Scholar] [CrossRef]
- Kuang, Q.F.; Abebe, A.; Evans, J.; Sugumaran, M. Oxidative transformation of tunichromes—Model studies with 1,2-dehydro-N-acetyldopamine and N-acetylcysteine. Bioorg. Chem. 2017, 73, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Abebe, A.; Zheng, D.; Evans, J.; Sugumaran, M. Novel post translational oligomerization of peptidyl dehydrodopa model compound, 1,2-dehydro-N-acetyldopa methyl ester. Bioorg. Chem. 2016, 66, 34–40. [Google Scholar] [CrossRef]
- Sugumaran, M.; Umit, K.; Evans, J.; Muriph, R.; Ito, S.; Wakamatsu, K. Oxidative oligomerization of DBL catechol, a potential cytotoxic compound for melanocytes, reveals the occurrence of novel ionic Diels-Alder type additions. Inter. J Mol. Sci. 2020, 21, 6774. [Google Scholar] [CrossRef]
- Burzio, L.A.; Waite, J.H. Reactivity of peptidyl-tyrosine to hydroxylation and cross-linking. Protein Sci. 2001, 10, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cohen Stuart, M.A.; Kamperman, M. Jack of all trades: Versatile catechol crosslinking mechanisms. Chem Soc. Rev. 2014, 43, 8271–8298. [Google Scholar] [CrossRef]
- Xu, Z. Mechanics of metal-catecholate complexes: The role of coordination state and metal types. Sci. Rep. 2013, 3, 2914. [Google Scholar] [CrossRef]
- Tamarin, A.; Keller, P.J. An ultrastructural study of the byssal thread forming system in Mytilus. J. Ultrastruc. Res. 1972, 40, 401–416. [Google Scholar] [CrossRef]
- Martinez Rodriguez, N.R.; Das, S.; Kaufman, Y.; Israelachvili, J.N.; Waite, J.H. Interfacial pH during mussel adhesive plaque formation. Biofouling 2015, 31, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.S.; Zeng, H.; Srivastava, A.; Krogstad, D.V.; Tirrell, M.; Israelachvili, J.N.; Waite, J.H. Viscosity and interfacial properties in a mussel-inspired adhesive coacervate. Soft Matter. 2010, 21, 3232–3236. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R.; Spahn, J.E.; Waite, J.H. The staying power of adhesion associated antioxidant activity in Mytilus californianus. J. Roy. Soc. Interface 2015, 12, 20150614. [Google Scholar] [CrossRef]
- Nicklisch, S.C.T.; Waite, J.H. Role of redox in dopa-mediated marine adhesion. Biofouling 2012, 28, 865–877. [Google Scholar] [CrossRef]
- Priemel, T.; Palia, G.; Forrste, F.; Jehle, F.; Sviben, S.; Mantouvalou, I.; Zaslansky, P.; Bertinetti, L.; Harrington, M.J. Microfluidic-like fabrication of metal ion-cured bioadhesives by mussels. Science 2021, 374, 206–211. [Google Scholar] [CrossRef]
- Zeng, H.; Hwang, D.S.; Israelachvili, J.N.; Waite, J.H. Strong reversible Fe3+ mediated bridging between Dopa-containing protein films in water. Proc. Nat. Acad. Sci. USA 2010, 107, 12850–12853. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.S.; Zeng, H.; Harrington, M.J.; Masic, A.; Fratzl, P.; Israelachvili, J.N.; Waite, J.H. Fe3+-dependent cohesion of a prominent protein of mussel adhesive plaques. J. Biol. Chem. 2010, 285, 25850–25858. [Google Scholar] [CrossRef]
- Holten Andersen, N.; Toprak, M.; Stucky, G.D.; Zok, F.W.; Waite, J.H. Metals and the integrity of a biological coating: The cuticle of mussel byssus. Langmuir 2009, 25, 3323–3326. [Google Scholar] [CrossRef] [PubMed]
- Broomell, C.C.; Zok, F.W.; Waite, J.H. Role of transition metals in sclerotization of biological tissue. Acta Biomater. 2008, 4, 2045–2051. [Google Scholar] [CrossRef]
- Lichtenegger, H.C.; Birkedal, H.; Waite, J.H. Heavy metals in the jaws of invertebrates. Met. Ions Life Sci. 2008, 4, 295–325. [Google Scholar]
- Nette, G.; Scippa, S.; de Vincentiis, M. Origin of the Heinze solution/precipitate from morula cells of the blood of the ascidian Phallusia mammillata. Naturwissenchaften 2000, 87, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Ciancio, A.; Scippa, S.; Nette, G.; de Vincentiis, M. Analysis of the Heinze precipitate from the blood cells of the ascidian Phallusia mammillata. Naturwissenchaften 2004, 91, 366–370. [Google Scholar] [CrossRef]
- De Leo, G.; Patricolo, E.; D’Arncona Lunetta, G. Studies on the fibrous components of the test of Ciona intestinalis Linnaeus. I. Cellulose-like polysaccharide. Acta Zool. 1977, 58, 135–141. [Google Scholar] [CrossRef]
- Andersen, S.O. Covalent crosslinks in a structural protein, resilin. Acta Physiol. Scand. Suppl. 1966, 66, 1–81. [Google Scholar]
- Li, J.; Hodgeman, B.A.; Christensen, B.M. Involvement of peroxidase in chorion hardening in Aedes aegypti. Insect Biochem. Mol. Biol. 1996, 26, 309–317. [Google Scholar] [CrossRef]
- Schofield, R.M.S.; Nesson, M.H.; Richardson, K.A. Tooth hardness increases with zinc-content in mandibles of young adult leaf cutter ants. Naturwissenschaften 2002, 89, 579–583. [Google Scholar] [CrossRef]
- Miserez, A.; Schneberk, T.; Sun, C.; Zok, F.W.; Waite, J.H. The transition from stiff to compliant materials in squid beaks. Science 2008, 319, 1816–1819. [Google Scholar] [CrossRef]
- Miserez, A.; Rubin, D.; Waite, J.H. Crosslinking chemistry of squid beak. J. Biol. Chem. 2010, 285, 38115–38124. [Google Scholar] [CrossRef] [PubMed]
- Broomell, C.C.; Chase, S.F.; Laue, T.; Waite, J.H. Cutting edge structural protein from the jaws of Nereis virens. Biomacromolecules 2008, 9, 1669–1677. [Google Scholar] [CrossRef]
- Sugumaran, M. Comparative biochemistry of eumelanogenesis and the protective roles of phenoloxidase and melanin in insects. FEBS Lett. 2002, 15, 2–9. [Google Scholar] [CrossRef]
- Theopold, U.; Sschmidt, O.; Soderhall, K.; Dushay, M.S. Coagulation in arthropods: Defense, wound closure, and healing. Trends Immunol. 2004, 25, 289–294. [Google Scholar] [CrossRef]
- Nappi, A.J.; Christensen, B.M. Melanogenesis and associated cytotoxic reactions: Applications to insect innate immunity. Insect Biochem. Mol. Biol. 2005, 35, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zou, Y.; Li, Y.; Cheng, Y. Metal-containing polydopamine nanomaterials: Catalysis, energy, and theranostics. Small 2020, 16, 1907042. [Google Scholar] [CrossRef]
- Oh, D.X.; Kim, S.; Lee, D.; Hwang, D.S. Tunicate mimic nanofibrous hydrogel adhesive with improved wet adhesion. Acta Biomater. 2015, 20, 104–112. [Google Scholar] [CrossRef]
- Montroni, D.; Palanca, M.; Morellato, K.; Fermani, S.; Crisofolini, L.; Falini, G. Hierarchical chitinous matrices byssus-inspired with mechanical properties tunable by Fe(II) and oxidation. Carbohydr. Polym. 2021, 251, 116984. [Google Scholar] [CrossRef]
- Kim, C.; Ejima, H.; Yoshie, N. Non-swellable self-healing polymer with long-term stability under seawater. RSC Adv. 2017, 7, 19288. [Google Scholar] [CrossRef]
- Fan, C.; Fu, J.; Zhu, W.; Wang, D. A mussel-inspired double-crosslinked tissue adhesive intended for internal medical use. Acta Mater. 2016, 33, 51–63. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J.S.; Lee, C.; Park, H.J.; Yang, K.; Jin, Y.; Ryu, J.H.; Hong, K.S.; Moon, S.H.; Chung, H.M.; et al. Tissue adhesive catechol-modified hyaluronic acid hydrogel for effective, minimally invasive cell therapy. Adv. Funct. Mater. 2015, 25, 3814. [Google Scholar] [CrossRef]
- Yan, G.; Chen, G.; Peng, Z.; Tang, X.; Sun, Y.; Zeng, X.; Lin, L. The cross-linking mechanism and applications of catechol-metal polymer materials. Adv. Mater. Interfaces. 2021, 8, 2100239. [Google Scholar] [CrossRef]
- Olofsson, K.; Granskog, V.; Cai, Y.; Hult, A.; Malkoch, M. Activated dopamine derivatives as primers for adhesive patch fixation of bone fracture. RSC Adv. 2016, 6, 26398–26405. [Google Scholar] [CrossRef]
- Wang, Y.; Jeon, E.J.; Lee, J.; Hwang, H.; Cho, H.S.W.; Lee, H. A phenol-amine superglue inspired by insect cuticle sclerotization process. Adv. Mater. 2020, 32, 2002118. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Han, L.; Lu, X.; Liu, K.; Wang, K.; Fang, L.; Weng, L.-T.; Zhang, H.; Tang, Y.; Ren, F.; Zhao, C.; et al. Mussel inspired adhesive and tough hydrogel based on nano clay confined dopamine polymerization. ACS Nano 2017, 11, 2561–2574. [Google Scholar] [CrossRef]
- Budisa, N. Engineering the Genetic Code; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Lepthien, S.; Merkel, L.; Budisa, N. In vivo double and triple labeling of proteins using synthetic amino acids. Angew. Chem. Inter. Ed. Engl. 2010, 49, 5446–5450. [Google Scholar] [CrossRef]
- Hoesl, M.G.; Oehm, S.; Durkin, P.; Darmon, E.; Peil, L.; Aerni, H.-R.; Rappsiber, J.; Rinehart, J.; Leach, D.; Scll, D.; et al. Chemical Evolution of a Bacterial Proteome. Angew. Commun. Internatl. Edtn. 2015, 54, 10030–10034. [Google Scholar]
- Hauf, M.; Richter, F.; Schneider, T.; Faidt, T.; Martins, B.M.; Baumann, T.; Durkin, P.; Dobbek, H.; Jacobs, K.; Moglich, A.; et al. Photoactivatable mussel based underwater adhesive protein by an expanded genetic code. ChemBioChem 2017, 18, 1819–1823. [Google Scholar] [CrossRef]
- Behboodi-Sadabad, F.; Li, S.; Lei, W.; Liu, Y.; Sommer, T.; Friederich, P.; Sobek, C.; Messersmith, P.B.; Levkin, P.A. High-throughput screening of multifunctional nanocoating based on combination of polyphenols and catecholamines. Mater. Today Bio 2021, 10, 100108. [Google Scholar] [CrossRef]





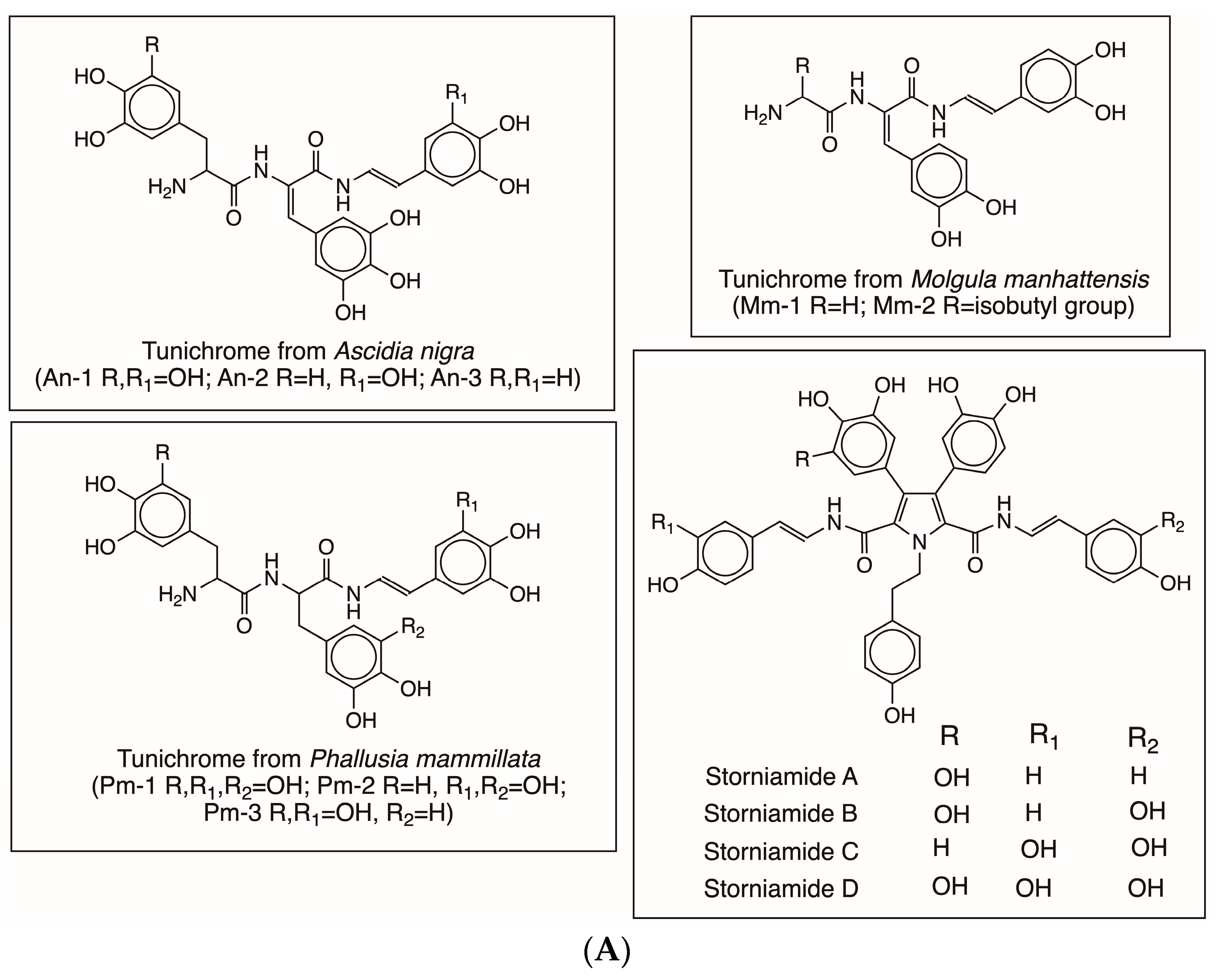




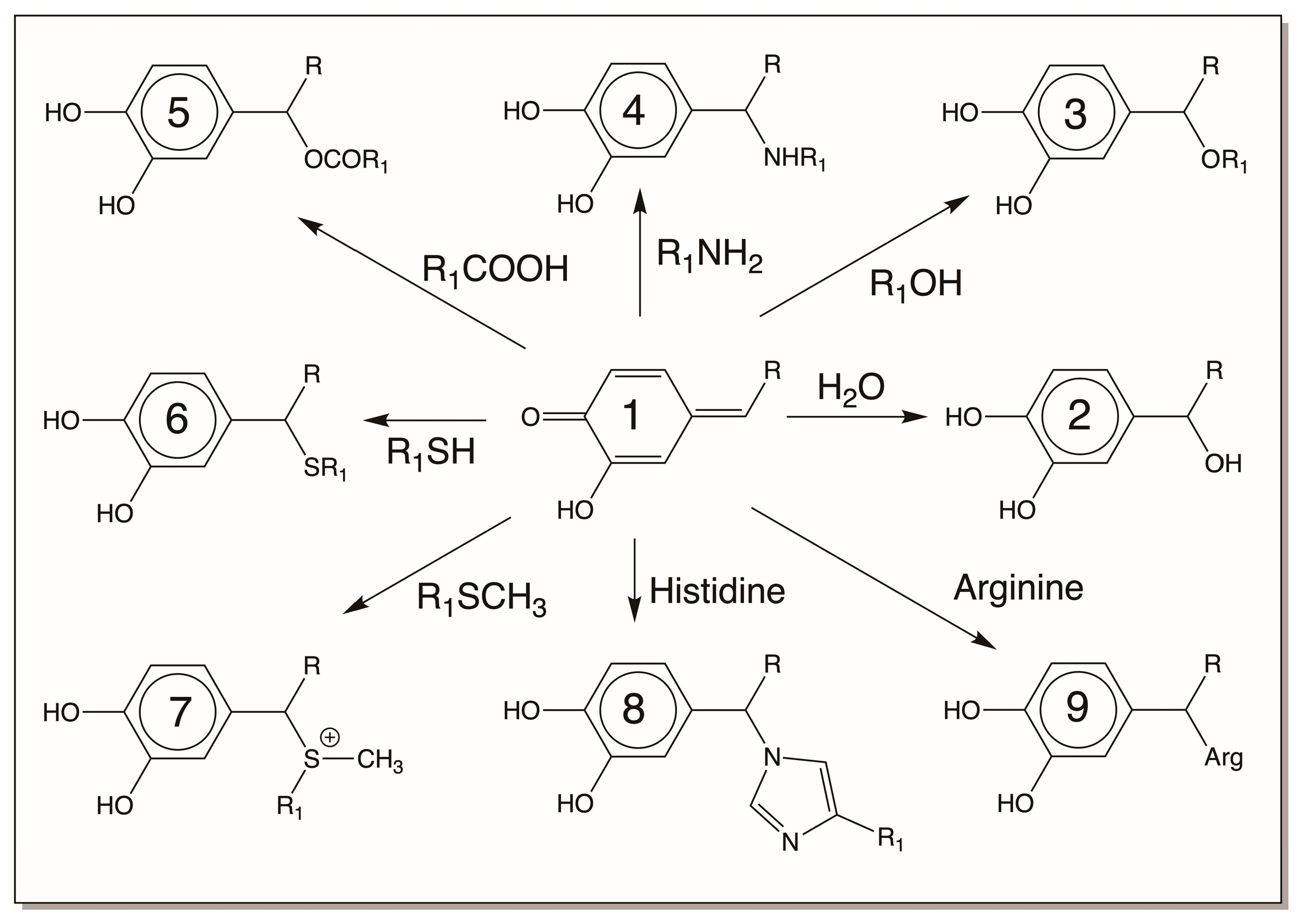












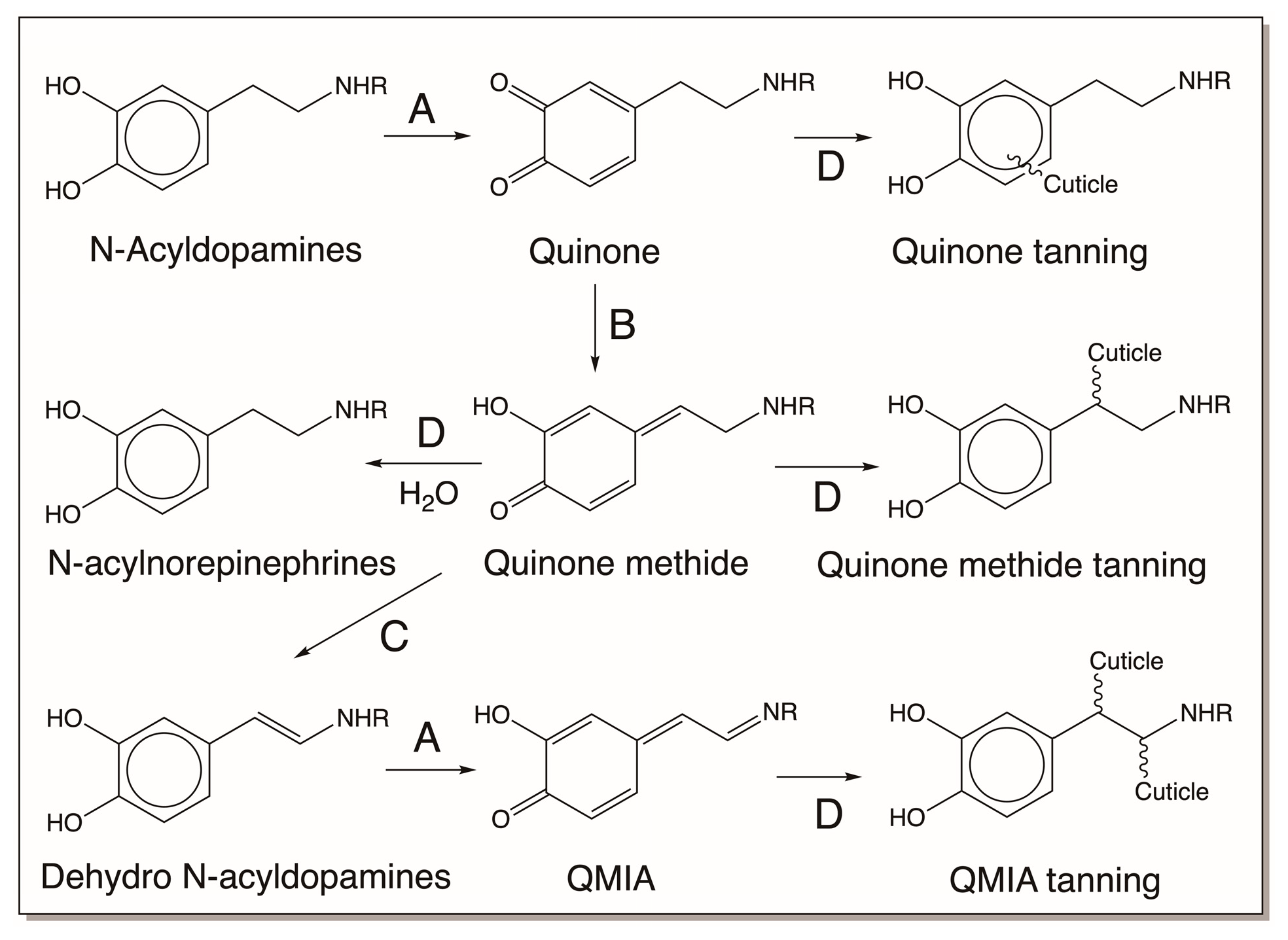

| Compound Name | Chemical Name |
|---|---|
| Tunichrome An-1 | Topa-Dehydrotopa-Dehydrotopamine |
| Tunichrome An-2 | Dopa-Dehydrotopa-Dehydrotopamine |
| Tunichrome An-3 | Dopa-Dehydrotopa-Dehydrodopamine |
| Tunichrome Pm-1 | Topa-Topa-Dehydrotopamine |
| Tunichrome Pm-2 | Dopa-Topa-Dehydrotopamine |
| Tunichrome Pm-3 | Topa-Topa-Dehydrodopamine |
| Tunichrome Mm-1 | Gly-Dehydrodopa-Dehydrodopamine |
| Tunichrome Mm-2 | Leu-Dehydrodopa-Dehydrodopamine |
| Tunichrome Sp-1 | Dopa-Dopa-Gly-Pro-Dehydrodopamine |
| Plicatamide | Phe-Phe-His-Leu-His-Phe-His-Dehydrodopamine |
| Clionamide 1 | 6-BrTrp-Dehydrotopamine |
| Celenamide A | Leu-Dehydrotopa-6-BrTrp-Dehydrodopamine |
| Celenamide B | Val-Dehydrotopa-6-BrTrp-Dehydrodopamine |
| Celenamide C | Leu-Dehydrotopa-6-BrTrp-Dehydrotyramine |
| Celenamide D | Leu-Dehydrotopa-Dehydrotopa-Dehydrodopamine |
| Celenamide E | Dehydrotopa-6-BrTrp-Dehydrodopamine |
| Morulin Pm | Polycyclic compound with 6-BrTrp & dehydrodopamine |
| Purpurone | Polycyclic compound with dehydrodopamine |
| Lamillarins | Polycyclic compounds with dehydrodopamine |
| Ningalins A-D | Polycyclic compounds with dehydrodopamine |
| Storniamides A-D | Polycyclic compounds with dehydrodopamine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugumaran, M.; Evans, J.J. Catecholamine Derivatives as Novel Crosslinkers for the Synthesis of Versatile Biopolymers. J. Funct. Biomater. 2023, 14, 449. https://doi.org/10.3390/jfb14090449
Sugumaran M, Evans JJ. Catecholamine Derivatives as Novel Crosslinkers for the Synthesis of Versatile Biopolymers. Journal of Functional Biomaterials. 2023; 14(9):449. https://doi.org/10.3390/jfb14090449
Chicago/Turabian StyleSugumaran, Manickam, and Jason J. Evans. 2023. "Catecholamine Derivatives as Novel Crosslinkers for the Synthesis of Versatile Biopolymers" Journal of Functional Biomaterials 14, no. 9: 449. https://doi.org/10.3390/jfb14090449
APA StyleSugumaran, M., & Evans, J. J. (2023). Catecholamine Derivatives as Novel Crosslinkers for the Synthesis of Versatile Biopolymers. Journal of Functional Biomaterials, 14(9), 449. https://doi.org/10.3390/jfb14090449









