Synthesis, Physicochemical Characteristics, and Biocompatibility of Multi-Component Collagen-Based Hydrogels Developed by E-Beam Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Formulation of Multi-Component Hydrogels
2.3. E-Beam Cross-Linking of Multi-Component Hydrogels
2.4. Characterization of Multi-Component Hydrogels
2.4.1. Sol-Gel Analysis
2.4.2. Swelling Degree
2.4.3. Moisture Retention Capability and Water Vapor Transmission Rate
2.4.4. Network Structural Parameters
2.4.5. Rheological Analysis
2.4.6. Fourier Transform Infrared (FTIR) Analysis
2.4.7. Scanning Electron Microscopy (SEM)
2.5. In Vitro Biological Characterization
2.5.1. Cell Culture Model and Extraction Medium Preparation
2.5.2. Indirect Contact Studies
2.5.3. Direct Contact Studies
2.6. Statistical Analysis
3. Results and Discussion
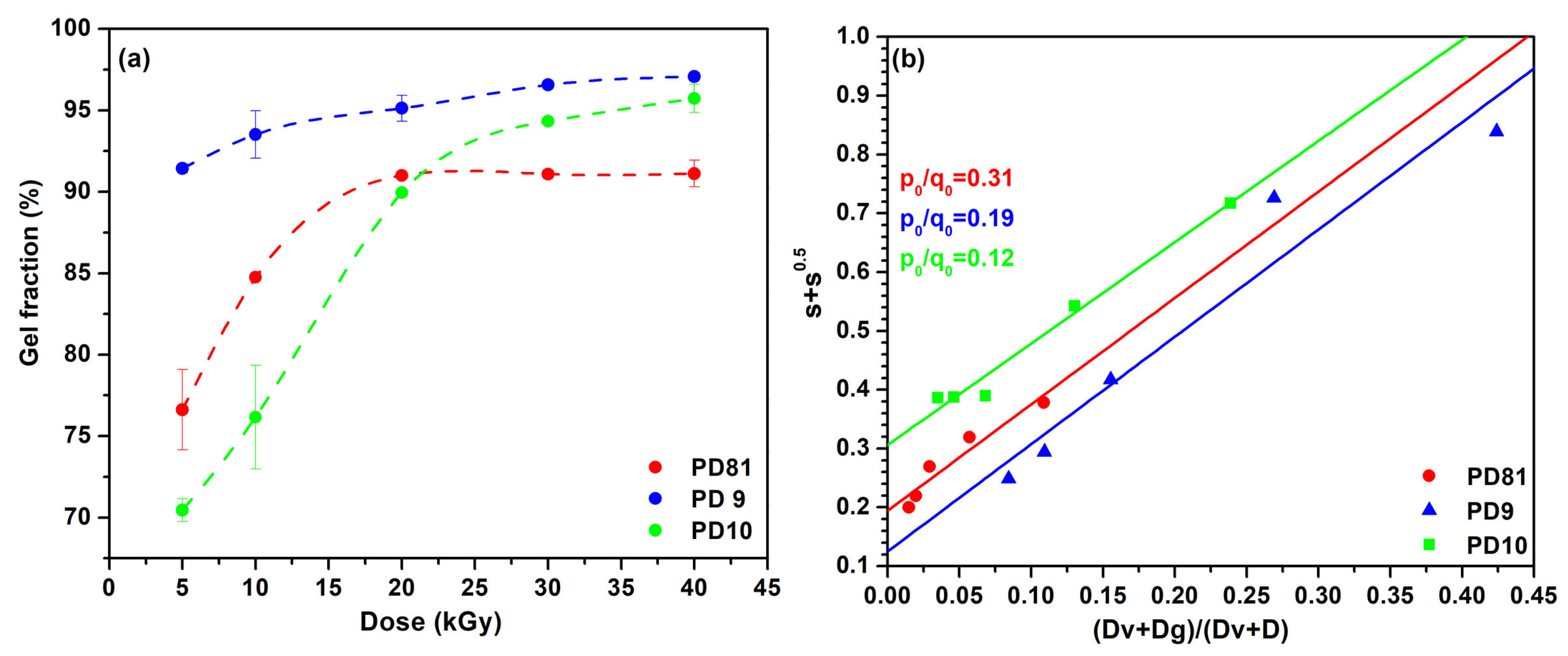
3.1. Sol-Gel Analysis
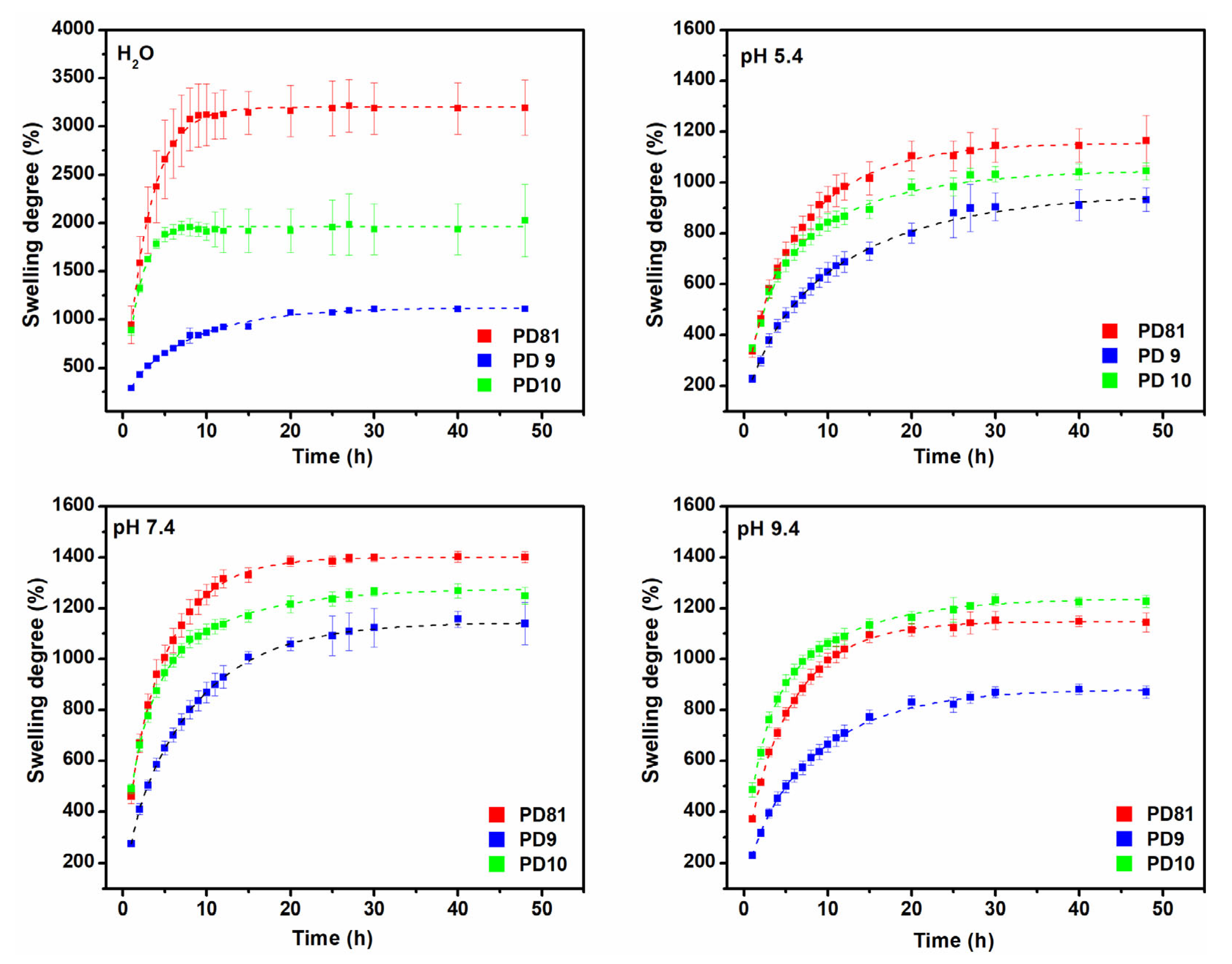
3.2. Swelling Degree
3.3. Network Parameters
3.4. Rheological Analysis
3.5. Retention Capability and Water Vapor Transmission Rate (WVTR)
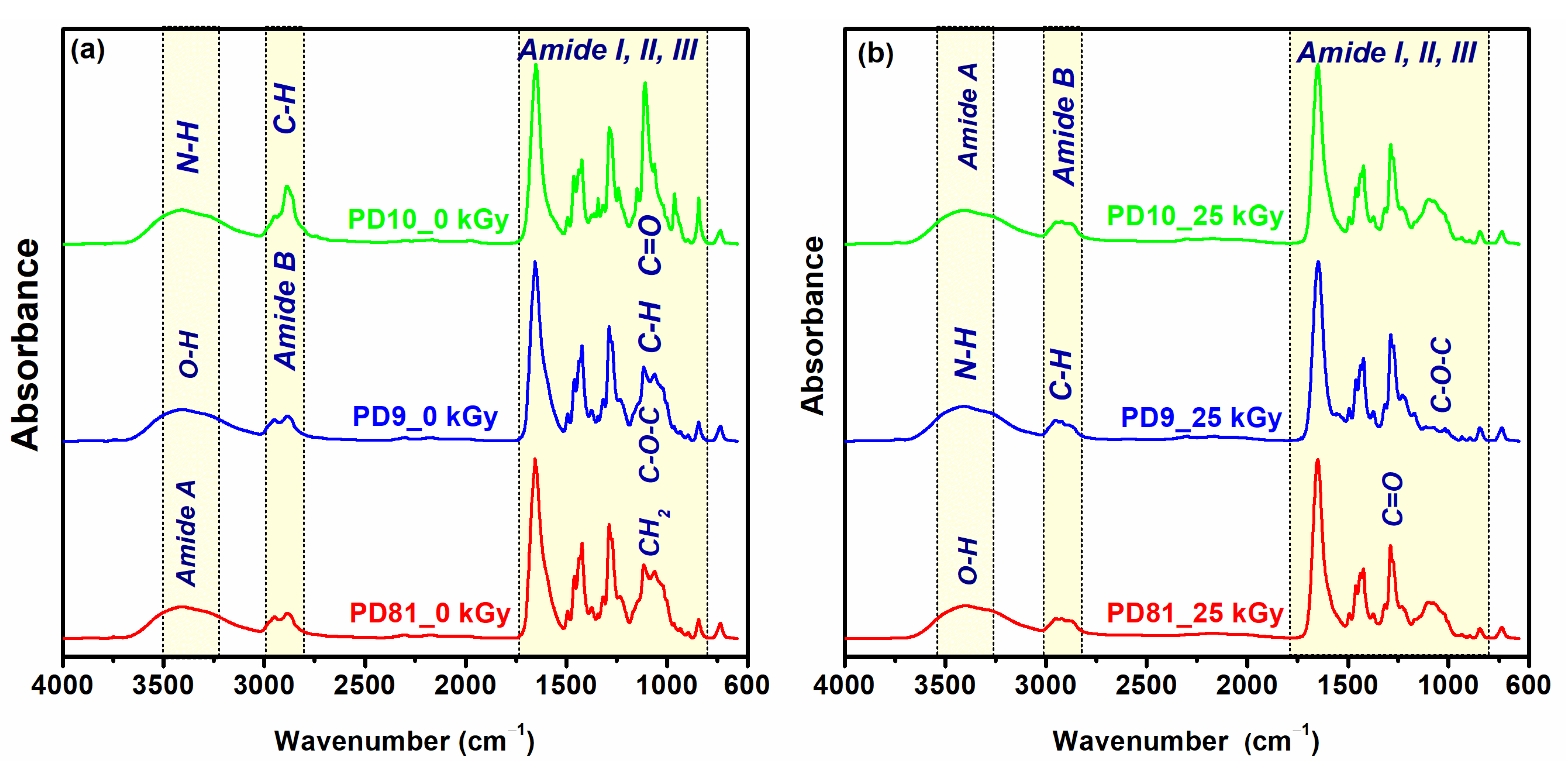
3.6. ATR-FTIR
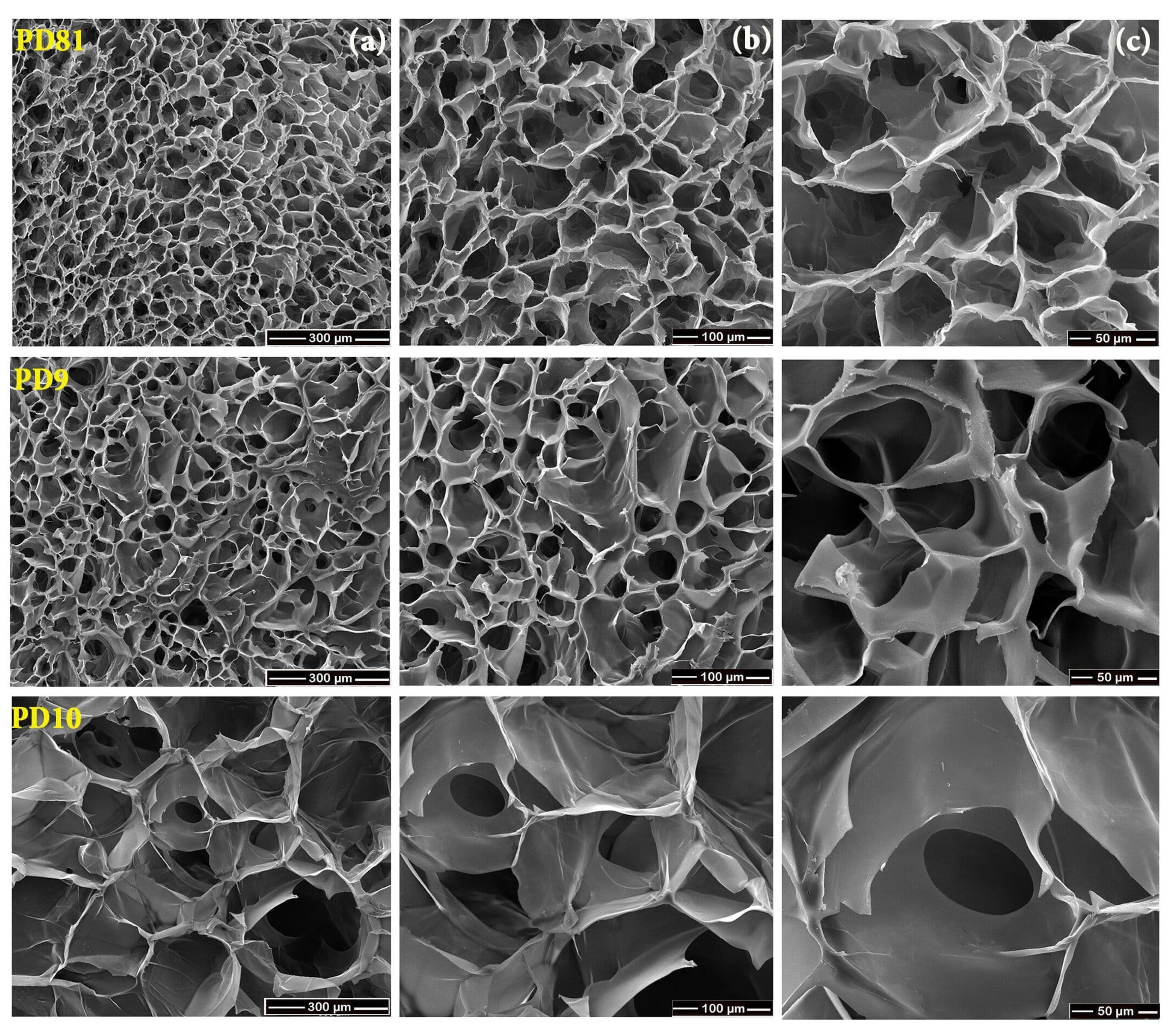
3.7. SEM
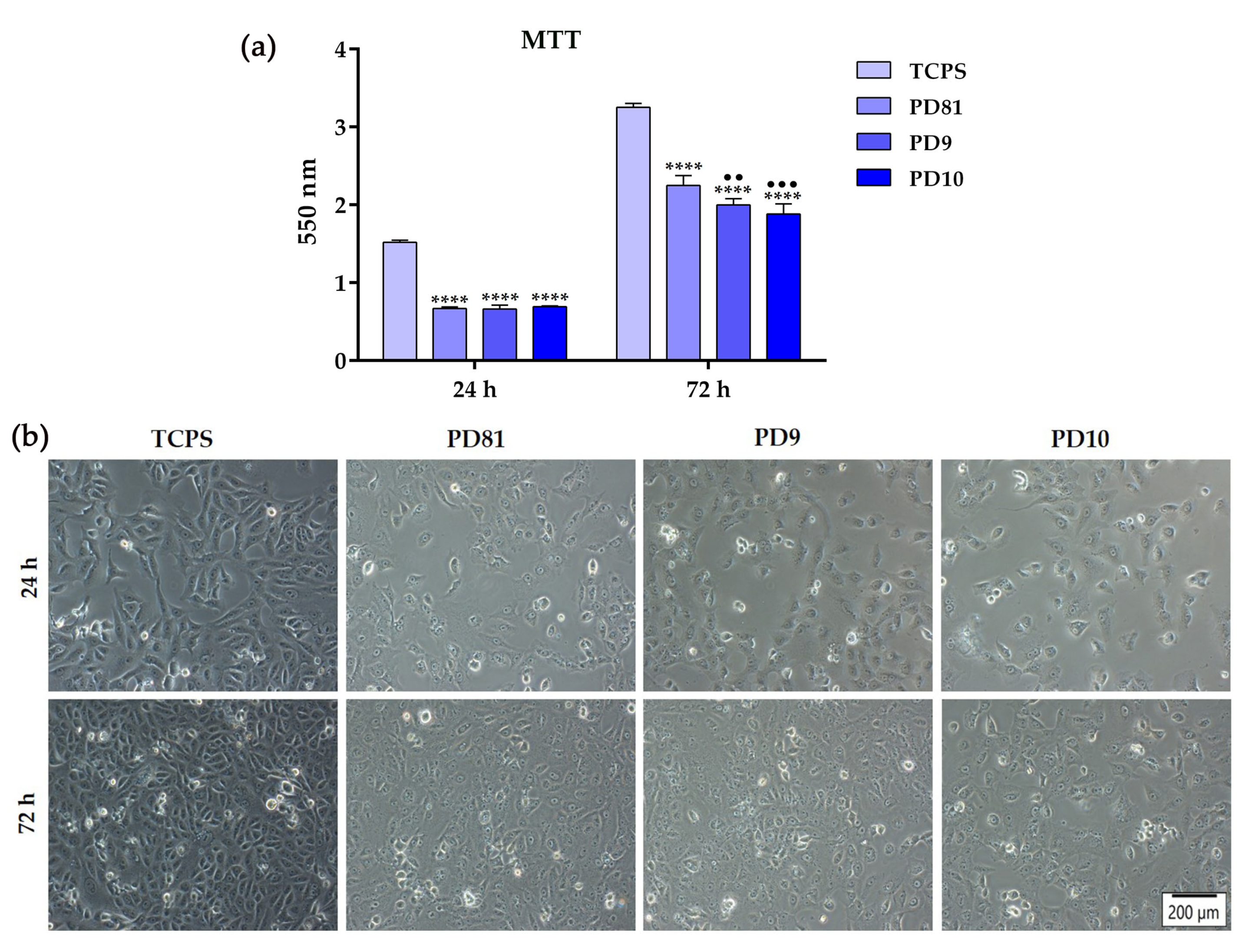
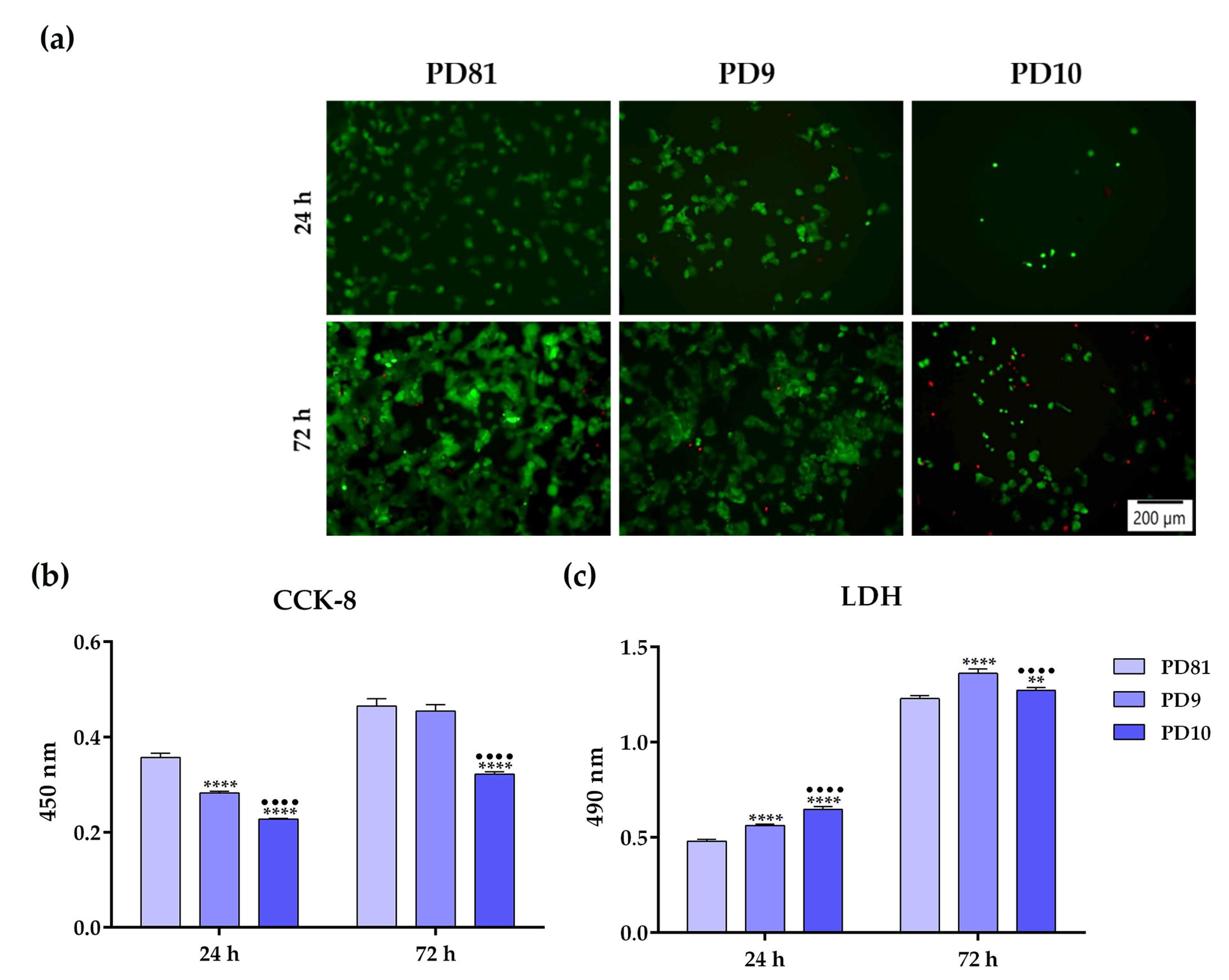
3.8. In Vitro Biological Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pal, S.; Mehta, D.; Dasgupta, U.; Bajaj, A. Advances in Engineering of Low Molecular Weight Hydrogels for Chemotherapeutic Applications. Biomed. Mater. 2021, 16, 024102. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, J.; Barati, A.; Ai, J.; Nooshabadi, V.T.; Mirzaei, Z. Employing Hydrogels in Tissue Engineering Approaches to Boost Conventional Cancer-Based Research and Therapies. RSC Adv. 2021, 11, 10646–10669. [Google Scholar] [CrossRef] [PubMed]
- Askari, E.; Seyfoori, A.; Amereh, M.; Gharaie, S.S.; Ghazali, H.S.; Ghazali, Z.S.; Khunjush, B.; Akbari, M. Stimuli-Responsive Hydrogels for Local Post-Surgical Drug Delivery. Gels 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Datta, A. Characterization of Polyethylene Glycol Hydrogels for Biomedical Applications; Louisiana State University and Agricultural and Mechanical College: Baton Rouge, LA, USA, 2007. [Google Scholar]
- Rosiak, J.M.; Olejniczak, J. Medical Applications of Radiation Formed Hydrogels. Radiat. Phys. Chem. 1993, 42, 903–906. [Google Scholar] [CrossRef]
- Yoshii, F.; Zhanshan, Y.; Isobe, K.; Shinozaki, K.; Makuuchi, K. Electron Beam Crosslinked PEO and PEO/PVA Hydrogels for Wound Dressing. Radiat. Phys. Chem. 1999, 55, 133–138. [Google Scholar] [CrossRef]
- Han, L.; Lin, J.; Du, C.; Zhang, C.; Wang, X.; Feng, Q. Effect of Mechanical Microenvironment on Collagen Self-Assembly In Vitro. J. Funct. Biomater. 2023, 14, 235. [Google Scholar] [CrossRef]
- Sklenářová, R.; Akla, N.; Latorre, M.J.; Ulrichová, J.; Franková, J. Collagen as a Biomaterial for Skin and Corneal Wound Healing. J. Funct. Biomater. 2022, 13, 249. [Google Scholar] [CrossRef] [PubMed]
- Neacsu, I.A.; Leau, S.-A.; Marin, S.; Holban, A.M.; Vasile, B.-S.; Nicoara, A.-I.; Ene, V.L.; Bleotu, C.; Albu Kaya, M.G.; Ficai, A. Collagen-Carboxymethylcellulose Biocomposite Wound-Dressings with Antimicrobial Activity. Materials 2021, 14, 1153. [Google Scholar] [CrossRef]
- Călina, I.; Demeter, M.; Scărișoreanu, A.; Sătulu, V.; Mitu, B. One Step E-Beam Radiation Cross-Linking of Quaternary Hydrogels Dressings Based on Chitosan-Poly(Vinyl-Pyrrolidone)-Poly(Ethylene Glycol)-Poly(Acrylic Acid). Int. J. Mol. Sci. 2020, 21, 9236. [Google Scholar] [CrossRef] [PubMed]
- Demeter, M.; Călina, I.; Scărișoreanu, A.; Micutz, M.; Kaya, M.A. Correlations on the Structure and Properties of Collagen Hydrogels Produced by E-Beam Crosslinking. Materials 2022, 15, 7663. [Google Scholar] [CrossRef]
- Demeter, M.; Meltzer, V.; Călina, I.; Scărișoreanu, A.; Micutz, M.; Albu Kaya, M.G. Highly Elastic Superabsorbent Collagen/PVP/PAA/PEO Hydrogels Crosslinked via e-Beam Radiation. Radiat. Phys. Chem. 2020, 174, 108898. [Google Scholar] [CrossRef]
- Tichý, E.; Murányi, A.; Pšenková, J. The Effects of Moist Heat Sterilization Process and the Presence of Electrolytes on Rheological and Textural Properties of Hydrophilic Dispersions of Polymers-Hydrogels. Adv. Polym. Technol. 2016, 35, 198–207. [Google Scholar] [CrossRef]
- Wang, M.; Xu, L.; Hu, H.; Zhai, M.; Peng, J.; Nho, Y.; Li, J.; Wei, G. Radiation Synthesis of PVP/CMC Hydrogels as Wound Dressing. Nucl. Instrum. Methods Phys. Res. B 2007, 265, 385–389. [Google Scholar] [CrossRef]
- Abd El-Mohdy, H.L.; Hegazy, E.-S.A. Preparation of Polyvinyl Pyrrolidone-Based Hydrogels by Radiation-Induced Crosslinking with Potential Application as Wound Dressing. J. Macromol. Sci. A 2008, 45, 995–1002. [Google Scholar] [CrossRef]
- Ajji, Z.; Othman, I.; Rosiak, J.M. Production of Hydrogel Wound Dressings Using Gamma Radiation. Nucl. Instrum. Methods Phys. Res. B 2005, 229, 375–380. [Google Scholar] [CrossRef]
- Soler, D.M.; Rodríguez, Y.; Correa, H.; Moreno, A.; Carrizales, L. Pilot Scale-up and Shelf Stability of Hydrogel Wound Dressings Obtained by Gamma Radiation. Radiat. Phys. Chem. 2012, 81, 1249–1253. [Google Scholar] [CrossRef]
- Salmawi, K.M. El Gamma Radiation-Induced Crosslinked PVA/Chitosan Blends for Wound Dressing. J. Macromol. Sci. A 2007, 44, 541–545. [Google Scholar] [CrossRef]
- Mozalewska, W.; Czechowska-Biskup, R.; Olejnik, A.K.; Wach, R.A.; Ulański, P.; Rosiak, J.M. Chitosan-Containing Hydrogel Wound Dressings Prepared by Radiation Technique. Radiat. Phys. Chem. 2017, 134, 1–7. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Y.; Wang, L.; Xu, L.; Zhai, M.; Wei, S. Radiation Synthesis and Characterization of Nanosilver/Gelatin/Carboxymethyl Chitosan Hydrogel. Radiat. Phys. Chem. 2012, 81, 553–560. [Google Scholar] [CrossRef]
- Yang, C.; Xu, L.; Zhou, Y.; Zhang, X.; Huang, X.; Wang, M.; Han, Y.; Zhai, M.; Wei, S.; Li, J. A Green Fabrication Approach of Gelatin/CM-Chitosan Hybrid Hydrogel for Wound Healing. Carbohydr. Polym. 2010, 82, 1297–1305. [Google Scholar] [CrossRef]
- Albu, M.G. Collagen Gels and Matrices for Biomedical Applications: The Obtaining and Characterization of Collagen-Based Biomaterials as Support for Local Release; Lambert Academic Publishing: Saarbrücken, Germany, 2011. [Google Scholar]
- Capanema, N.S.V.; Mansur, A.A.P.; Carvalho, S.M.; Carvalho, I.C.; Chagas, P.; de Oliveira, L.C.A.; Mansur, H.S. Bioengineered Carboxymethyl Cellulose-Doxorubicin Prodrug Hydrogels for Topical Chemotherapy of Melanoma Skin Cancer. Carbohydr. Polym. 2018, 195, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, N.; Yagi, T.; Kume, T.; Yoshii, F. Radiation Crosslinking of Carboxymethyl Starch. Carbohydr. Polym. 2004, 58, 109–113. [Google Scholar] [CrossRef]
- Olejniczak, J.; Rosiak, J.; Charlesby, A. Gel/Dose Curves for Polymers Undergoing Simultaneous Crosslinking and Scission. Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem. 1991, 37, 499–504. [Google Scholar] [CrossRef]
- Wach, R.A.; Mitomo, H.; Nagasawa, N.; Yoshii, F. Radiation Crosslinking of Methylcellulose and Hydroxyethylcellulose in Concentrated Aqueous Solutions. Nucl. Instrum. Methods Phys. Res. B 2003, 211, 533–544. [Google Scholar] [CrossRef]
- Charlesby, A. Past and Future Trends in Polymer Irradiation. Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem. 1991, 37, 5–10. [Google Scholar] [CrossRef]
- Chen, J.; Park, H.; Park, K. Synthesis of Superporous Hydrogels: Hydrogels with Fast Swelling and Superabsorbent Properties. J. Biomed. Mater. Res. 1999, 44, 53–62. [Google Scholar] [CrossRef]
- Boonkaew, B.; Suwanpreuksa, P.; Cuttle, L.; Barber, P.M.; Supaphol, P. Hydrogels Containing Silver Nanoparticles for Burn Wounds Show Antimicrobial Activity without Cytotoxicity. J. Appl. Polym. Sci. 2014, 131, 9. [Google Scholar] [CrossRef]
- Razzak, M.T.; Darwis, D.; Zainuddin, S. Irradiation of Polyvinyl Alcohol and Polyvinyl Pyrrolidone Blended Hydrogel for Wound Dressing. Radiat. Phys. Chem. 2001, 62, 107–113. [Google Scholar] [CrossRef]
- Mahmudi, N.; Sen, M.; Rendevski, S.; Güven, O. Radiation Synthesis of Low Swelling Acrylamide Based Hydrogels and Determination of Average Molecular Weight between Cross-Links. Nucl. Instrum. Methods Phys. Res. B 2007, 265, 375–378. [Google Scholar] [CrossRef]
- Canal, T.; Peppas, N.A. Correlation between Mesh Size and Equilibrium Degree of Swelling of Polymeric Networks. J. Biomed. Mater. Res. 1989, 23, 1183–1193. [Google Scholar] [CrossRef]
- Cao, A.; Tang, Y.; Liu, Y.; Yuan, H.; Liu, L. A Strategy for Antimicrobial Regulation Based on Fluorescent Conjugated Oligomer–DNA Hybrid Hydrogels. Chem. Comm. 2013, 49, 5574–5576. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.A.; Peppas, N.A. Molecular Structure of Physiologically-Responsive Hydrogels Controls Diffusive Behavior. Macromol. Biosci. 2009, 9, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Gu, H.; Lang, M. Molecular Simulation to Predict Miscibility and Phase Separation Behavior of Chitosan/Poly(ϵ-Caprolactone) Binary Blends: A Comparison with Experiments. Macromol. Theory Simul. 2013, 22, 377–384. [Google Scholar] [CrossRef]
- Robinson, G.; Ross-Murphy, S.B.; Morris, E.R. Viscosity-Molecular Weight Relationships, Intrinsic Chain Flexibility, and Dynamic Solution Properties of Guar Galactomannan. Carbohydr. Res. 1982, 107, 17–32. [Google Scholar] [CrossRef]
- Gudeman, L.F.; Peppas, N.A. PH-Sensitive Membranes from Poly(Vinyl Alcohol)/Poly(Acrylic Acid) Interpenetrating Networks. J. Memb. Sci. 1995, 107, 239–248. [Google Scholar] [CrossRef]
- Shipovskaya, A.; Malinkina, O.; Fomina, V.; Rudenko, D.; Shchyogolev, S. Optical Activity of Solutions and Films of Chitosan Acetate. Russ. Chem. Bull. 2015, 64, 1172–1177. [Google Scholar] [CrossRef]
- Parlato, M.; Reichert, S.; Barney, N.; Murphy, W.L. Poly(Ethylene Glycol) Hydrogels with Adaptable Mechanical and Degradation Properties for Use in Biomedical Applications. Macromol. Biosci. 2014, 14, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Wallin, R.F. Practical Guide to ISO 10993-12: Sample Preparation and Reference Materials; MD&DI: Los Angeles, CA, USA, 1998; Available online: https://www.mddionline.com/news/practical-guide-iso-10993-12-sample-preparation-and-reference-materials (accessed on 28 July 2023).
- Fekete, T.; Borsa, J.; Takács, E.; Wojnárovits, L. Synthesis of Carboxymethylcellulose/Acrylic Acid Hydrogels with Superabsorbent Properties by Radiation-Initiated Crosslinking. Radiat. Phys. Chem. 2016, 124, 135–139. [Google Scholar] [CrossRef]
- Kadłubowski, S.; Henke, A.; Ulański, P.; Rosiak, J.M. Hydrogels of Polyvinylpyrrolidone (PVP) and Poly(Acrylic Acid) (PAA) Synthesized by Radiation-Induced Crosslinking of Homopolymers. Radiat. Phys. Chem. 2010, 79, 261–266. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Janik, I.; Kadlubowski, S.; Kozicki, M.; Kujawa, P.; Stasica, P.; Ulanski, P. Radiation Formation of Hydrogels for Biomedical Application; International Atomic Energy Agency (IAEA): Vienna, Austria, 2002. [Google Scholar]
- Drobny, J.G. 2 Fundamentals of Radiation Chemistry and Physics. In Ionizing Radiation and Polymers; Drobny, J.G., Ed.; William Andrew Publishing: Norwich, NY, USA, 2013; pp. 11–26. ISBN 978-1-455,7-7881-2. [Google Scholar]
- Wach, R.A.; Adamus-Wlodarczyk, A.; Olejnik, A.K.; Matusiak, M.; Tranquilan-Aranilla, C.; Ulanski, P. Carboxymethylchitosan Hydrogel Manufactured by Radiation-Induced Crosslinking as Potential Nerve Regeneration Guide Scaffold. React. Funct. Polym. 2020, 152, 104588. [Google Scholar] [CrossRef]
- Ghaffarlou, M.; Sütekin, S.D.; Güven, O. Preparation of Nanogels by Radiation-Induced Cross-Linking of Interpolymer Complexes of Poly (Acrylic Acid) with Poly (Vinyl Pyrrolidone) in Aqueous Medium. Radiat. Phys. Chem. 2018, 142, 130–136. [Google Scholar] [CrossRef]
- Riedel, S.; Hietschold, P.; Krömmelbein, C.; Kunschmann, T.; Konieczny, R.; Knolle, W.; Mierke, C.T.; Zink, M.; Mayr, S.G. Design of Biomimetic Collagen Matrices by Reagent-Free Electron Beam Induced Crosslinking: Structure-Property Relationships and Cellular Response. Mater. Des. 2019, 168, 107606. [Google Scholar] [CrossRef]
- Treloar, L.R.G. The Physics of Rubber Elasticity, 3rd ed.; Clarendon: Oxford, UK, 1975. [Google Scholar]
- Makuuchi, K.; Cheng, S. Radiation Processing of Aqueous Polymer Systems. In Radiation Processing of Polymer Materials and Its Industrial Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012; p. 282. ISBN 9780470587690. [Google Scholar]
- Baby, D.K. Chapter 9 Rheology of Hydrogels. In Rheology of Polymer Blends and Nanocomposites; Thomas, S., Sarathchandran, C., Chandran, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 193–204. ISBN 978-0-12-816957-5. [Google Scholar]
- Zhang, M.; Xu, D.; Yan, X.; Chen, J.; Dong, S.; Zheng, B.; Huang, F. Self-Healing Supramolecular Gels Formed by Crown Ether Based Host–Guest Interactions. Angew. Chem. Int. Ed. Engl. 2012, 51, 7011–7015. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.; Hayrabolulu, H. Radiation Synthesis and Characterisation of the Network Structure of Natural/Synthetic Double-Network Superabsorbent Polymers. Radiat. Phys. Chem. 2012, 81, 1378–1382. [Google Scholar] [CrossRef]
- Yu, G.; Yan, X.; Han, C.; Huang, F. Characterization of Supramolecular Gels. Chem. Soc. Rev. 2013, 42, 6697–6722. [Google Scholar] [CrossRef]
- Brazdaru, L.; Micutz, M.; Staicu, T.; Albu, M.; Sulea, D.; Leca, M. Structural and Rheological Properties of Collagen Hydrogels Containing Tannic Acid and Chlorhexidine Digluconate Intended for Topical Applications. Comptes Rendus Chim. 2015, 18, 160–169. [Google Scholar] [CrossRef]
- Mezger, T. The Rheology Handbook; Vincentz Network GmbH & Co. KG: Hannover, Germany, 2020; ISBN 9783748603702. [Google Scholar]
- Bruin, P.; Jonkman, M.F.; Meijer, H.J.; Pennings, A.J. A New Porous Polyetherurethane Wound Covering. J. Biomed. Mater. Res. 1990, 24, 217–226. [Google Scholar] [CrossRef]
- Bahadoran, M.; Shamloo, A.; Dorri, Y. Development of a Polyvinyl Alcohol/Sodium Alginate Hydrogel-Based Scaffold Incorporating BFGF-Encapsulated Microspheres for Accelerated Wound Healing. Sci. Rep. 2020, 10, 7342. [Google Scholar] [CrossRef]
- Yang, Y.; Campbell Ritchie, A.; Everitt, N.M. Recombinant Human Collagen/Chitosan-Based Soft Hydrogels as Biomaterials for Soft Tissue Engineering. Mater. Sci. Eng. C 2021, 121, 111846. [Google Scholar] [CrossRef]
- Deng, A.; Yang, Y.; Du, S.; Yang, X.; Pang, S.; Wang, X.; Yang, S. Preparation of a Recombinant Collagen-Peptide (RHC)-Conjugated Chitosan Thermosensitive Hydrogel for Wound Healing. Mater. Sci. Eng. C 2021, 119, 111555. [Google Scholar] [CrossRef]
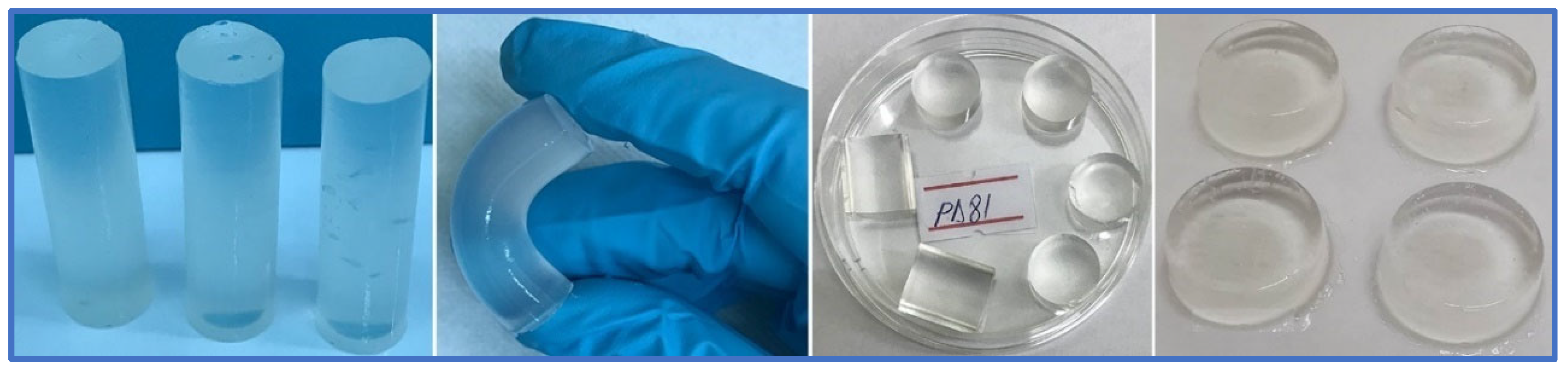


| Pre-Hydrogel Component | Conc. (%) | Hydrogel Code | ||
|---|---|---|---|---|
| PD81 | PD9 | PD10 | ||
| Collagen gel | 0.3−0.5 | 18.18 mL | 30.30 mL | 30.30 mL |
| Chitosan | 0.5 | − | − | 0.5 g |
| PVP 1300 | 3−3.1 | 15.5 mL | − | 3 g |
| PVP 360 | 7 | − | 7 g | − |
| PEO | 0.2 | 5.71 mL | − | − |
| CMCNa | 0.625 | 31.25 mL | − | − |
| PEG | 1 | − | − | 1 g |
| MBA | 0.25 | − | 0.25 g | 0.25 g |
| Acrylic acid 0.05 M | 3.6 | − | − | 343 μL |
| DI−water | 92.2−95.8 | 29.26 mL | 62.45 mL | 65.20 mL |
| Sol-Gel Analysis | p0/q0 | Dg (kGy) | Dv (kGy) | R2 |
|---|---|---|---|---|
| PD81 | 0.31 | 0.44 | 0.99 | 0.99 |
| PD9 | 0.19 | 0.08 | 0.52 | 0.99 |
| PD10 | 0.12 | 0.02 | 3.68 | 0.99 |
| Hydrogel Code | G(X) a/G(S) b (µmol/J) | ||||
|---|---|---|---|---|---|
| 5 kGy | 10 kGy | 20 kGy | 30 kGy | 40 kGy | |
| PD81 | 0.66 a/0.39 b | 0.27 a/0.16 b | 0.13 a/0.07 b | 0.13 a/0.07 b | 0.12 a/0.07 b |
| PD9 | 1.78 a/0.67 b | 1.28 a/0.48 b | 1.07 a/0.40 b | 1.00 a/0.38 b | 1.10 a/0.41 b |
| PD10 | 2.88 a/0.63 b | 2.12 a/0.46 b | 0.42 a/0.09 b | 0.36 a/0.07 b | 0.32 a/0.07 b |
| Hydrogel Code | ρ (kg/m3) | G′ (Pa) | (kg/mol) | ξ (nm) | |
|---|---|---|---|---|---|
| PD81 | 1007.8 ± 2.7 | 9697 ± 24 | 8.56 | 1.17 | 15.8 |
| PD9 | 1017.6 ± 2.3 | 14,980 ± 42 | 13.04 | 0.78 | 13.6 |
| PD10 | 1009.6 ± 5.0 | 5625 ± 3.1 | 27.16 | 0.37 | 26.7 |
| Time (h) | PD81 (%) | PD9 (%) | PD10 (%) |
|---|---|---|---|
| 2 | 96.8 ± 0.04 | 96.8 ± 0.08 | 97.6 ± 0.08 |
| 4 | 93.8 ± 0.06 | 93.7 ± 0.15 | 95.2 ± 0.15 |
| 6 | 91.3 ± 0.09 | 91.1 ± 0.18 | 93.2 ± 0.20 |
| 8 | 88.6 ± 0.12 | 88.2 ± 0.18 | 91.2 ± 0.18 |
| 10 | 86.7 ± 0.12 | 86.3 ± 0.16 | 90.0 ± 0.16 |
| 12 | 85.2 ± 0.13 | 84.8 ± 0.16 | 89.2 ± 0.13 |
| Hydrogel | WVTR (g/m2∙h) |
|---|---|
| PD81 | 53.37 ± 3.58 |
| PD9 | 69.10 ± 1.52 |
| PD10 | 55.04 ± 1.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demeter, M.; Negrescu, A.M.; Calina, I.; Scarisoreanu, A.; Albu Kaya, M.; Micutz, M.; Dumitru, M.; Cimpean, A. Synthesis, Physicochemical Characteristics, and Biocompatibility of Multi-Component Collagen-Based Hydrogels Developed by E-Beam Irradiation. J. Funct. Biomater. 2023, 14, 454. https://doi.org/10.3390/jfb14090454
Demeter M, Negrescu AM, Calina I, Scarisoreanu A, Albu Kaya M, Micutz M, Dumitru M, Cimpean A. Synthesis, Physicochemical Characteristics, and Biocompatibility of Multi-Component Collagen-Based Hydrogels Developed by E-Beam Irradiation. Journal of Functional Biomaterials. 2023; 14(9):454. https://doi.org/10.3390/jfb14090454
Chicago/Turabian StyleDemeter, Maria, Andreea Mariana Negrescu, Ion Calina, Anca Scarisoreanu, Mădălina Albu Kaya, Marin Micutz, Marius Dumitru, and Anisoara Cimpean. 2023. "Synthesis, Physicochemical Characteristics, and Biocompatibility of Multi-Component Collagen-Based Hydrogels Developed by E-Beam Irradiation" Journal of Functional Biomaterials 14, no. 9: 454. https://doi.org/10.3390/jfb14090454
APA StyleDemeter, M., Negrescu, A. M., Calina, I., Scarisoreanu, A., Albu Kaya, M., Micutz, M., Dumitru, M., & Cimpean, A. (2023). Synthesis, Physicochemical Characteristics, and Biocompatibility of Multi-Component Collagen-Based Hydrogels Developed by E-Beam Irradiation. Journal of Functional Biomaterials, 14(9), 454. https://doi.org/10.3390/jfb14090454












