Targeted EGFR Nanotherapy in Non-Small Cell Lung Cancer
Abstract
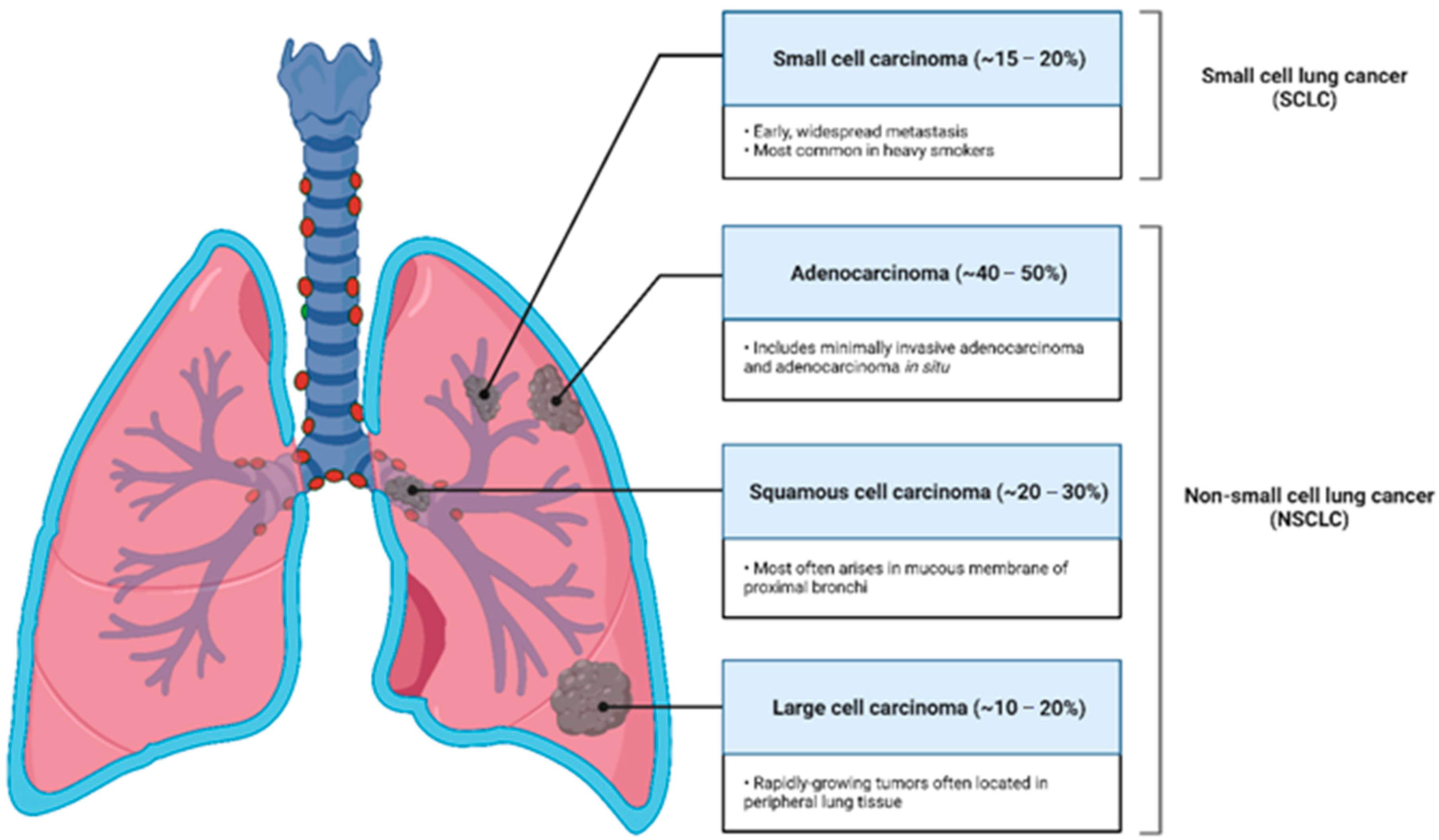
:1. Lung Cancer Awareness
2. Epidermal Growth Factor Receptor in Pulmonary Pathology

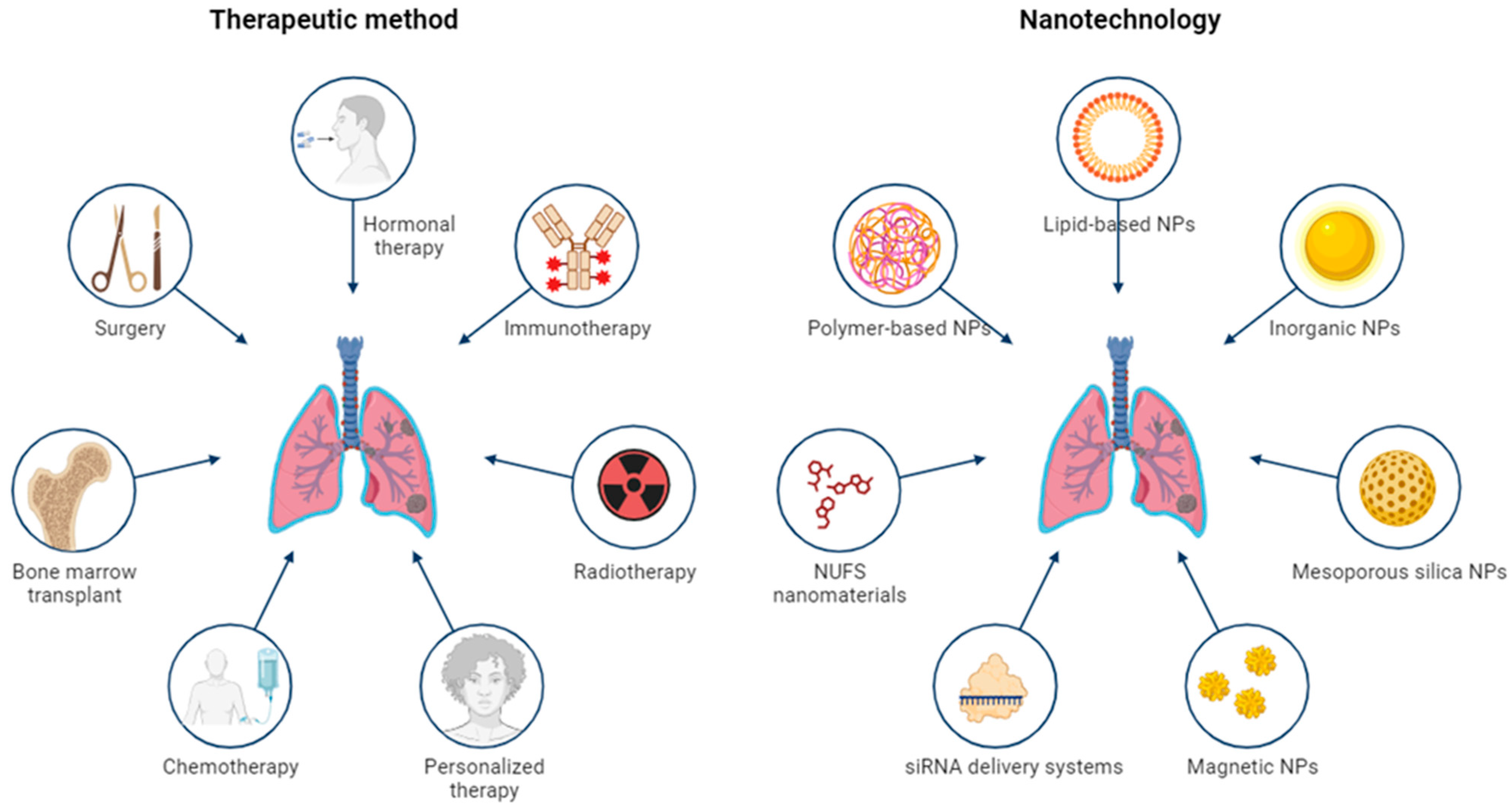
3. Therapeutic Management of NSCLC
3.1. Management of Early-Stage NSCLC
3.2. Management of Locally Advanced (Stage III) NSCLC
3.3. Management of Late-Stage IV(A) and IV(B) NSCLC
3.4. Management of Recurrent NSCLC and Palliative Care

4. Mutations-Related EGFR Treatment and Targeted Nanotherapy in NSLC
| Mechanisms of primary resistance | References |
|---|---|
| Exon 20 insertions | [107] |
| T790M mutation | [46,108] |
| HGF overexpression | [108] |
| BCL2L11 deletion | [46,109] |
| Mechanisms of acquired resistance | References |
| T790M gatekeeper mutation in the ATP binding pocket of EGFR | [109,110] |
| D761Y, L747S and T854A mutations | [48] |
| MET gene amplification | [108,110] |
| PI3KCA mutation | [110] |
| Histological transformation | [111,112] |
| HGF overexpression | [113,114] |
| IGF-1R hyperphosphorylation | [114] |
| C797S mutation | [49] |
| G796R/S/, l792H, L718Q, and G724S substitutions | [110,115] |
5. Nano-Immunotherapies for EGFR Mutated NSCLC
6. Nanoparticles Suitable for NSCLC
6.1. Organic Nanomaterials
6.1.1. Polymer-Based Particles
6.1.2. Lipid-Based Particles
6.2. Magnetic Nanomaterials
6.3. Inorganic Nanomaterials
6.4. siRNA Delivery Systems
6.5. Mesoporous Silica Nanomaterials
6.6. NUFS Nanomaterials
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of Lung Cancer. Contemp. Oncol. Poznan Pol. 2021, 25, 45–52. [Google Scholar] [CrossRef]
- Furrukh, M. Tobacco Smoking and Lung Cancer: Perception-Changing Facts. Sultan Qaboos Univ. Med. J. 2013, 13, 345–358. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer Lung Cancer Today; Globocan 2020; World Health Organisation: Lyon, France, 2020; pp. 1–2.
- NHS UK Lung Cancer. Available online: https://www.nhs.uk/conditions/lung-cancer/ (accessed on 1 May 2023).
- Cai, L.; Chen, Y.; Tong, X. The Genomic Landscape of Young and Old Lung Cancer Patients Highlights Age-Dependent Mutation Frequencies and Clinical Actionability in Young Patients. Int. J. Cancer 2021, 10, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Fidler-Benaoudia, M.M.; Torre, L.A.; Bray, F.; Ferlay, J.; Jemal, A. Lung Cancer Incidence in Young Women vs. Young Men: A Systematic Analysis in 40 Countries. Int. J. Cancer 2020, 147, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Tolwin, Y.; Gillis, R.; Peled, N. Gender and Lung Cancer-SEER-Based Analysis. Ann. Epidemiol. 2020, 46, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Cancer of the Lung and Bronchus—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 1 May 2023).
- Ning, J.; Ge, T.; Jiang, M. Early Diagnosis of Lung Cancer: Which Is the Optimal Choice? Aging 2021, 13, 6214–6227. [Google Scholar] [CrossRef]
- Cassim, S.; Chepulis, L.; Keenan, R.; Kidd, J.; Firth, M.; Lawrenson, R. Patient and Carer Perceived Barriers to Early Presentation and Diagnosis of Lung Cancer: A Systematic Review. BMC Cancer 2019, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Blandin Knight, S.; Crosbie, P.A.; Balata, H.; Chudziak, J.; Hussell, T.; Dive, C. Progress and Prospects of Early Detection in Lung Cancer. Open Biol. 2017, 7, 170070. [Google Scholar] [CrossRef]
- Casagrande, G.M.S.; Silva, M.O.; Reis, R.M.; Leal, L.F. Liquid Biopsy for Lung Cancer: Up-to-Date and Perspectives for Screening Programs. Int. J. Mol. Sci. 2023, 24, 2505. [Google Scholar] [CrossRef]
- Dama, E.; Colangelo, T.; Fina, E. Biomarkers and Lung Cancer Early Detection: State of the Art. Cancers 2021, 13, 3919. [Google Scholar] [CrossRef]
- Veronesi, G.; Baldwin, D.R.; Henschke, C.I.; Ghislandi, S.; Iavicoli, S.; Oudkerk, M.; Koning, H.J.; Shemesh, J.; Field, J.K.; Zulueta, J.J.; et al. Recommendations for Implementing Lung Cancer Screening with Low-Dose Computed Tomography in Europe. Cancers 2020, 12, 1672. [Google Scholar] [CrossRef] [PubMed]
- Saadat, M.; Manshadi, M.K.D.; Mohammadi, M. Magnetic Particle Targeting for Diagnosis and Therapy of Lung Cancers. J. Control Release 2020, 328, 776–791. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Yadav, K.; Mishra, A.; Singh, M.; Chaudhary, S.; Manohar, R.; Parmar, A. Early Diagnosis of Lung Cancer Using Magnetic Nanoparticles-Integrated Systems. Nanotechnol. Rev. 2022, 11, 544–574. [Google Scholar] [CrossRef]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-Cell Lung Cancer. Nat. Rev. Primer 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.-O.; Vo, T.H.; Lam, L.H.T.; Le, N.Q.K. ALDH2 as a Potential Stem Cell-Related Biomarker in Lung Adenocarcinoma: Comprehensive Multi-Omics Analysis. Comput. Struct. Biotechnol. J. 2023, 21, 1921–1929. [Google Scholar] [CrossRef]
- Hutchinson, B.D.; Shroff, G.S.; Truong, M.T.; Ko, J.P. Spectrum of Lung Adenocarcinoma. Semin. Ultrasound CT MR 2019, 40, 255–264. [Google Scholar] [CrossRef]
- Gandara, D.R.; Hammerman, P.S.; Sos, M.L.; Lara, P.N.; Hirsch, F.R. Squamous Cell Lung Cancer: From Tumor Genomics to Cancer Therapeutics. Clin. Cancer Res. 2015, 21, 2236–2243. [Google Scholar] [CrossRef]
- Suarez, E.; Knollmann-Ritschel, B.E.C. Squamous Cell Carcinoma of the Lung. Acad. Pathol. 2017, 4, 2374289517705950. [Google Scholar] [CrossRef]
- Kini, S.R. Large Cell Undifferentiated Carcinoma. In Color Atlas of Pulmonary Cytopathology; Kini, S.R., Ed.; Springer: New York, NY, USA, 2002; pp. 117–122. ISBN 978-0-387-21641-6. [Google Scholar]
- Tai, Q.; Zhang, L.; Hu, X. Clinical Characteristics, and Treatments of Large Cell Lung Carcinoma: A Retrospective Study Using SEER Data. Transl. Cancer Res. 2020, 9, 1455–1464. [Google Scholar] [CrossRef]
- Alvarado-Luna, G.; Morales-Espinosa, D. Treatment for Small Cell Lung Cancer, Where Are We Now?—A Review. Transl. Lung Cancer Res. 2016, 5, 26–38. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Z.; Wang, Q. Emerging Therapies for Small Cell Lung Cancer. J. Hematol. Oncol. 2019, 12, 47. [Google Scholar] [CrossRef]
- Larsen, J.E.; Minna, J.D. Molecular Biology of Lung Cancer: Clinical Implications. Clin. Chest Med. 2011, 32, 703–740. [Google Scholar] [CrossRef] [PubMed]
- Andrini, E.; Marchese, P.V.; Biase, D. Large Cell Neuroendocrine Carcinoma of the Lung: Current Understanding and Challenges. J. Clin. Med. 2022, 11, 1461. [Google Scholar] [CrossRef] [PubMed]
- Simbolo, M.; Centonze, G.; Giudice, L.; Grillo, F.; Maisonneuve, P.; Gkountakos, A.; Ciaparrone, C.; Cattaneo, L.; Sabella, G.; Giugno, R.; et al. Combined Large Cell Neuroendocrine Carcinomas of the Lung: Integrative Molecular Analysis Identifies Subtypes with Potential Therapeutic Implications. Cancers 2022, 14, 4653. [Google Scholar] [CrossRef] [PubMed]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Runkle, K.B.; Kharbanda, A.; Stypulkowski, E.; Cao, X.-J.; Wang, W.; Garcia, B.A.; Witze, E.S. Inhibition of DHHC20-Mediated EGFR Palmitoylation Creates a Dependence on EGFR Signaling. Mol. Cell 2016, 62, 385–396. [Google Scholar] [CrossRef]
- Johnson, G.R.; Kannan, B.; Shoyab, M.; Stromberg, K. Amphiregulin Induces Tyrosine Phosphorylation of the Epidermal Growth Factor. Receptor and P185erbB2. Evidence That Amphiregulin Acts Exclusively through the Epidermal Growth Factor Receptor at the Surface of Human Epithelial Cells. J. Biol. Chem. 1993, 268, 2924–2931. [Google Scholar] [CrossRef]
- Chen, W.S.; Lazar, C.S.; Lund, K.A.; Welsh, J.B.; Chang, C.P.; Walton, G.M.; Der, C.J.; Wiley, H.S.; Gill, G.N.; Rosenfeld, M.G. Functional Independence of the Epidermal Growth Factor Receptor from a Domain Required for Ligand-Induced Internalization and Calcium Regulation. Cell 1989, 59, 33–43. [Google Scholar] [CrossRef]
- Arcaro, A.; Zvelebil, M.J.; Wallasch, C.; Ullrich, A.; Waterfield, M.D.; Domin, J. Class II Phosphoinositide 3-Kinases Are Downstream Targets of Activated Polypeptide Growth Factor Receptors. Mol. Cell. Biol. 2000, 20, 3817–3830. [Google Scholar] [CrossRef]
- Jurišić, V.; Obradovic, J.; Pavlović, S.; Djordjevic, N. Epidermal Growth Factor Receptor Gene in Non-Small-Cell Lung Cancer: The Importance of Promoter Polymorphism Investigation. Anal. Cell. Pathol. 2018, 6192187. [Google Scholar] [CrossRef]
- Karlsen, E.-A.; Kahler, S.; Tefay, J.; Joseph, S.R.; Simpson, F. Epidermal Growth Factor Receptor Expression and Resistance Patterns to Targeted Therapy in Non-Small Cell Lung Cancer: A Review. Cells 2021, 10, 1206. [Google Scholar] [CrossRef] [PubMed]
- UniProt Consortium. UniProt: The Universal Protein Knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.C.H.; Antignani, A.; Sarnovsky, R.; FitzGerald, D. Characterization of Monoclonal Antibodies Generated to the 287–302 Amino Acid Loop of the Human Epidermal Growth Factor Receptor. Antib. Ther. 2019, 2, 88–98. [Google Scholar] [CrossRef]
- Maruyama, I.N. Mechanisms of Activation of Receptor Tyrosine Kinases: Monomers or Dimers. Cells 2014, 3, 304–330. [Google Scholar] [CrossRef] [PubMed]
- Oda, K.; Matsuoka, Y.; Funahashi, A.; Kitano, H. A Comprehensive Pathway Map of Epidermal Growth Factor Receptor Signaling. Mol. Syst. Biol. 2005, 1, 2005.0010. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, L.; Lippman, S.M.; El-Naggar, A.K. Targeting the MAPK-RAS-RAF Signaling Pathway in Cancer Therapy. Expert Opin. Ther. Targets 2012, 16, 103–119. [Google Scholar] [CrossRef]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef]
- Seshacharyulu, P.; Ponnusamy, M.P.; Haridas, D.; Jain, M.; Ganti, A.K.; Batra, S.K. Targeting the EGFR Signaling Pathway in Cancer Therapy. Expert Opin. Ther. Targets 2012, 16, 15–31. [Google Scholar] [CrossRef]
- Sooro, M.A.; Zhang, N.; Zhang, P. Targeting EGFR-Mediated Autophagy as a Potential Strategy for Cancer Therapy. Int. J. Cancer 2018, 143, 2116–2125. [Google Scholar] [CrossRef]
- Shostak, K.; Chariot, A. EGFR and NF-ΚB: Partners in Cancer. Trends Mol. Med. 2015, 21, 385–393. [Google Scholar] [CrossRef]
- Campbell, P.; Morton, P.E.; Takeichi, T.; Salam, A.; Roberts, N.; Proudfoot, L.E.; Mellerio, J.E.; Aminu, K.; Wellington, C.; Patil, S.N.; et al. Epithelial Inflammation Resulting from an Inherited Loss-of-Function Mutation in EGFR. J. Investig. Dermatol. 2014, 134, 2570–2578. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.K.; Cvrljevic, A.N.; Johns, T.G. The Epidermal Growth Factor Receptor Variant III (EGFRvIII): Where Wild Things Are Altered. FEBS J. 2013, 280, 5350–5370. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Aksoy, O.; Zheng, T.; Fan, Q.W.; Weiss, W.A. Epidermal Growth Factor Receptor and EGFRvIII in Glioblastoma: Signaling Pathways and Targeted Therapies. Oncogene 2018, 37, 1561–1575. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Chekhonin, I.V.; Chekhonin, V.P. The EGFR Variant III Mutant as a Target for Immunotherapy of Glioblastoma Multiforme. Eur. J. Pharmacol. 2017, 810, 70–82. [Google Scholar] [CrossRef]
- Humphrey, P.A.; Wong, A.J.; Vogelstein, B.; Friedman, H.S.; Werner, M.H.; Bigner, D.D.; Bigner, S.H. Amplification and Expression of the Epidermal Growth Factor Receptor Gene in Human Glioma Xenografts. Cancer Res. 1988, 48, 2231–2238. [Google Scholar]
- Yamazaki, H.; Ohba, Y.; Tamaoki, N.; Shibuya, M. A Deletion Mutation within the Ligand Binding Domain Is Responsible for Activation of Epidermal Growth Factor Receptor Gene in Human Brain Tumors. Jpn. J. Cancer Res. Gann 1990, 81, 773–779. [Google Scholar] [CrossRef]
- Ramnarain, D.B.; Park, S.; Lee, D.Y.; Hatanpaa, K.J.; Scoggin, S.O.; Otu, H.; Libermann, T.A.; Raisanen, J.M.; Ashfaq, R.; Wong, E.T.; et al. Differential Gene Expression Analysis Reveals Generation of an Autocrine Loop by a Mutant Epidermal Growth Factor Receptor in Glioma Cells. Cancer Res. 2006, 66, 867–874. [Google Scholar] [CrossRef]
- Rutkowska, A.; Stoczyńska-Fidelus, E.; Janik, K.; Włodarczyk, A.; Rieske, P. EGFRvIII: An Oncogene with Ambiguous Role. J. Oncol. 2019, 1092587. [Google Scholar] [CrossRef]
- Pillay, V.; Allaf, L.; Wilding, A.L.; Donoghue, J.F.; Court, N.W.; Greenall, S.A.; Scott, A.M.; Johns, T.G. The Plasticity of Oncogene Addiction: Implications for Targeted Therapies Directed to Receptor Tyrosine Kinases. Neoplasia 2009, 11, 448–458. [Google Scholar] [CrossRef]
- Guo, D.; Hildebrandt, I.J.; Prins, R.M.; Soto, H.; Mazzotta, M.M.; Dang, J.; Czernin, J.; Shyy, J.Y.-J.; Watson, A.D.; Phelps, M.; et al. The AMPK Agonist AICAR Inhibits the Growth of EGFRvIII-Expressing Glioblastomas by Inhibiting Lipogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 12932–12937. [Google Scholar] [CrossRef]
- Swinnen, J.V.; Brusselmans, K.; Verhoeven, G. Increased Lipogenesis in Cancer Cells: New Players, Novel Targets. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Rahban, M.; Bostanian, F.; Esmaeilzadeh, F.; Bagherabadi, A.; Zolghadri, S.; Stanek, A. Targeting Protein Kinases and Epigenetic Control as Combinatorial Therapy Options for Advanced Prostate Cancer Treatment. Pharmaceutics 2022, 14, 515. [Google Scholar] [CrossRef] [PubMed]
- El Khayari, A.; Bouchmaa, N.; Taib, B.; Wei, Z.; Zeng, A.; El Fatimy, R. Metabolic Rewiring in Glioblastoma Cancer: EGFR, IDH and Beyond. Front. Oncol. 2022, 12, 901951. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Zhao, X.; Yuza, Y.; Shimamura, T.; Li, D.; Protopopov, A.; Jung, B.L.; McNamara, K.; Xia, H.; Glatt, K.A.; et al. Epidermal Growth Factor Receptor Variant III Mutations in Lung Tumorigenesis and Sensitivity to Tyrosine Kinase Inhibitors. Proc. Natl. Acad. Sci. USA 2006, 103, 7817–7822. [Google Scholar] [CrossRef]
- Yoshimoto, K.; Dang, J.; Zhu, S.; Nathanson, D.; Huang, T.; Dumont, R.; Seligson, D.B.; Yong, W.H.; Xiong, Z.; Rao, N.; et al. Development of a Real-Time RT-PCR Assay for Detecting EGFRvIII in Glioblastoma Samples. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Amelia, T.; Kartasasmita, R.E.; Ohwada, T.; Tjahjono, D.H. Structural Insight and Development of EGFR Tyrosine Kinase Inhibitors. Molecules 2022, 27, 819. [Google Scholar] [CrossRef]
- Arteaga, C.L. Overview of Epidermal Growth Factor Receptor Biology and Its Role as a Therapeutic Target in Human Neoplasia. Semin. Oncol. 2002, 29, 3–9. [Google Scholar] [CrossRef]
- AACR Project GENIE Consortium AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017, 7, 818–831. [CrossRef]
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagavatula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Supko, J.G.; Haluska, F.G.; et al. Activating Mutations in the Epidermal Growth Factor Receptor Underlying Responsiveness of Non-Small-Cell Lung Cancer to Gefitinib. N. Engl. J. Med. 2004, 350, 2129–2139. [Google Scholar] [CrossRef]
- Scodes, S.; Cappuzzo, F. Determining the Appropriate Treatment for Different EGFR Mutations in Non-Small Cell Lung Cancer Patients. Expert Rev. Respir. Med. 2020, 14, 565–576. [Google Scholar] [CrossRef]
- Yoneda, K.; Imanishi, N.; Ichiki, Y.; Tanaka, F. Treatment of Non-Small Cell Lung Cancer with EGFR-Mutations. J. UOEH 2019, 41, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yang, M.; Liang, N.; Li, S. Determining EGFR-TKI Sensitivity of G719X and Other Uncommon EGFR Mutations in Non-Small Cell Lung Cancer. Perplexity Solut. Rev. Oncol. Rep. 2017, 37, 1347–1358. [Google Scholar] [CrossRef]
- Yu, Q.; Huang, F.; Zhang, M.; Ji, H.; Wu, S.; Zhao, Y.; Zhang, C.; Wu, J.; Wang, B.; Pan, B.; et al. Multiplex Picoliter-Droplet Digital PCR for Quantitative Assessment of EGFR Mutations in Circulating Cell-Free DNA Derived from Advanced Non-Small Cell Lung Cancer Patients. Mol. Med. Rep. 2017, 16, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Ulivi, P.; Petracci, E.; Canale, M.; Priano, I.; Capelli, L.; Calistri, D.; Chiadini, E.; Cravero, P.; Rossi, A.; Delmonte, A.; et al. Liquid Biopsy for EGFR Mutation Analysis in Advanced Non-Small-Cell Lung Cancer Patients: Thoughts Drawn from a Real-Life Experience. Biomedicines 2021, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Gong, J.; Zhang, X.; Feng, X.; Huang, H.; Gao, M.; Chu, L. Plasma-Based Early Screening and Monitoring of EGFR Mutations in NSCLC Patients by a 3-Color Digital PCR Assay. Br. J. Cancer 2020, 123, 1437–1444. [Google Scholar] [CrossRef]
- Santaniello, A.; Napolitano, F.; Servetto, A.; De Placido, P.; Silvestris, N.; Bianco, C.; Formisano, L.; Bianco, R. Tumour Microenvironment and Immune Evasion in EGFR Addicted NSCLC: Hurdles and Possibilities. Cancers 2019, 11, 1419. [Google Scholar] [CrossRef]
- Lung Cancer Statistics|How Common Is Lung Cancer? Available online: https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html (accessed on 1 May 2023).
- Lung Cancer Survival Rates|5-Year Survival Rates for Lung Cancer. Available online: https://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 1 May 2023).
- Lung Cancer—Non-Small Cell—Types of Treatment. Available online: https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/types-treatment (accessed on 1 May 2023).
- Lung Cancer—Non-Small Cell—Stages. Available online: https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/stages (accessed on 1 May 2023).
- Indini, A.; Rijavec, E.; Bareggi, C.; Grossi, F. Novel Treatment Strategies for Early-Stage Lung Cancer: The Oncologist’s Perspective. J. Thorac. Dis. 2020, 12, 3390–3398. [Google Scholar] [CrossRef]
- Ottlakan, A.; Martucci, N.; Rocco, G. Is Surgery Still. the Best. Management Option for Early Stage NSCLC? Transl. Lung Cancer Res. 2014, 3, 159–163. [Google Scholar] [CrossRef]
- Das, M.; Abdelmaksoud, M.H.K.; Loo, B.W.; Kothary, N. Alternatives to Surgery for Early Stage Non-Small Cell Lung Cancer-Ready for Prime Time? Curr. Treat. Options Oncol. 2010, 11, 24–35. [Google Scholar] [CrossRef]
- Ginsberg, R.J.; Rubinstein, L.V. Randomized Trial of Lobectomy versus Limited Resection for T1N0 Non-Small Cell Lung Cancer. Lung Cancer Study Group. Ann. Thorac. Surg. 1995, 60, 615–622. [Google Scholar]
- Pignon, J.-P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.-Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung Adjuvant Cisplatin Evaluation: A Pooled Analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008, 26, 3552–3559. [Google Scholar] [CrossRef]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S.; ESMO Guidelines Committee. Early and Locally Advanced Non-Small-Cell Lung Cancer (NSCLC): ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef]
- Non-Small Cell Lung Cancer Treatment by Stage. Available online: https://www.cancer.org/cancer/lung-cancer/treating-non-small-cell/by-stage.html (accessed on 1 May 2023).
- Daly, M.E.; Singh, N.; Ismaila, N.; Antonoff, M.B.; Arenberg, D.A.; Bradley, J.; David, E.; Detterbeck, F.; Früh, M.; Gubens, M.A.; et al. Management of Stage III Non-Small-Cell Lung Cancer: ASCO Guideline. J. Clin. Oncol. 2022, 40, 1356–1384. [Google Scholar] [CrossRef]
- Aupérin, A.; Le Péchoux, C.; Pignon, J.P. Concomitant Radio-Chemotherapy Based on Platin Compounds in Patients with Locally Advanced Non-Small Cell Lung Cancer (NSCLC): A Meta-Analysis of Individual Data from 1764 Patients. Ann. Oncol. 2006, 17, 473–483. [Google Scholar] [CrossRef]
- Butts, C.; Socinski, M.A.; Mitchell, P.L. Tecemotide (L-BLP25) versus Placebo after Chemoradiotherapy for Stage III Non-Small-Cell Lung Cancer (START): A Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2014, 15, 59–68. [Google Scholar] [CrossRef]
- Bradley, J.D.; Paulus, R.; Komaki, R. Standard-Dose versus High-Dose Conformal Radiotherapy with Concurrent and Consolidation Carboplatin plus Paclitaxel with or without Cetuximab for Patients with Stage IIIA or IIIB Non-Small-Cell Lung Cancer (RTOG 0617): A Randomised, Two-by-Two Factorial Phase 3 Study. Lancet Oncol. 2015, 16, 187–199. [Google Scholar] [PubMed]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic Non-Small Cell Lung Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- Bodor, J.N.; Kasireddy, V.; Borghaei, H. First-Line Therapies for Metastatic Lung Adenocarcinoma without a Driver Mutation. J. Oncol. Pract. 2018, 14, 529–535. [Google Scholar] [CrossRef] [PubMed]
- König, D.; Savic Prince, S.; Rothschild, S.I. Targeted Therapy in Advanced and Metastatic Non-Small Cell Lung Cancer. An Update on Treatment of the Most Important Actionable Oncogenic Driver Alterations. Cancers 2021, 13, 804. [Google Scholar] [CrossRef]
- Zimmermann, F.B.; Molls, M.; Jeremic, B. Treatment of Recurrent Disease in Lung Cancer. Semin. Surg. Oncol. 2003, 21, 122–127. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R. Pembrolizumab for the Treatment of Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 2018–2020. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Bass, P. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Lester, J.F.; Macbeth, F.R.; Toy, E.; Coles, B. Palliative Radiotherapy Regimens for Non-Small Cell Lung Cancer. Cochrane Database Syst. Rev. 2006, 4, CD002143. [Google Scholar] [CrossRef]
- Jumeau, R.; Vilotte, F.; Durham, A.D.; Ozsahin, E.M. Current Landscape of Palliative Radiotherapy for Non-Small-Cell Lung Cancer. Transl. Lung Cancer Res. 2019, 8, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Passaro, A.; Jänne, P.A.; Mok, T.; Peters, S. Overcoming Therapy Resistance in EGFR-Mutant Lung Cancer. Nat. Cancer 2021, 2, 377–391. [Google Scholar] [CrossRef]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging Functions of the EGFR in Cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Bethune, G.; Bethune, D.; Ridgway, N.; Xu, Z. Epidermal Growth Factor Receptor (EGFR) in Lung Cancer: An Overview and Update. J. Thorac. Dis. 2010, 2, 48–51. [Google Scholar]
- Chhouri, H.; Alexandre, D.; Grumolato, L. Mechanisms of Acquired Resistance and Tolerance to EGFR Targeted Therapy in Non-Small Cell Lung Cancer. Cancers 2023, 15, 504. [Google Scholar] [CrossRef]
- Metro, G.; Crinò, L. Advances on EGFR Mutation for Lung Cancer. Transl. Lung Cancer Res. 2012, 1, 5–13. [Google Scholar] [CrossRef]
- Huang, L.; Fu, L. Mechanisms of Resistance to EGFR Tyrosine Kinase Inhibitors. Acta Pharm. Sin. B 2015, 5, 390–401. [Google Scholar] [CrossRef]
- Niederst, M.J.; Hu, H.; Mulvey, H.E.; Lockerman, E.L.; Garcia, A.R.; Piotrowska, Z.; Sequist, L.V.; Engelman, J.A. The Allelic Context of the C797S Mutation Acquired upon Treatment with Third-Generation EGFR Inhibitors Impacts Sensitivity to Subsequent Treatment Strategies. Clin. Cancer Res. 2015, 21, 3924–3933. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, A.; Sharma, S.; Minari, R.; Perego, P.; Giovannetti, E.; Tiseo, M. Resistance Mechanisms to Osimertinib in EGFR-Mutated Non-Small Cell Lung Cancer. Br. J. Cancer 2019, 121, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; He, T.; Wang, K.; Wei, Y. Comparison of Gefitinib plus Chemotherapy versus Gefitinib Alone for Advanced Non-small-cell Lung Cancer: A Meta Analysis. Clin. Sao Paulo Braz. 2023, 78, 100152. [Google Scholar] [CrossRef] [PubMed]
- Rocco, D.; Della Gravara, L.; Palazzolo, G.; Gridelli, C. The Role of Antiangiogenic Monoclonal Antibodies Combined to EGFR-TKIs in the Treatment of Advanced Non-Small Cell Lung Cancer with Activating EGFR Mutations: Acquired Resistance Mechanisms and Strategies to Overcome Them. Cancer Drug Resist. 2022, 5, 1016–1024. [Google Scholar] [CrossRef]
- Wang, Q.; Zeng, A.; Zhu, M.; Song, L. Dual Inhibition of EGFR-VEGF: An Effective Approach to the Treatment of Advanced Non-small Cell Lung Cancer with EGFR Mutation (Review). Int. J. Oncol. 2023, 62, 26. [Google Scholar] [CrossRef]
- Deluce, J.; Maj, D.; Verma, S.; Breadner, D.; Boldt, G.; Raphael, J. Efficacy and Toxicity of Combined Inhibition of EGFR and VEGF in Patients with Advanced Non-Small Cell Lung Cancer Harboring Activating EGFR Mutations: A Systematic Review and Meta-Analysis. Am. J. Clin. Oncol. 2023, 46, 87–93. [Google Scholar] [CrossRef]
- Dai, J.; Liu, X.; Li, J.; Qu, T.; Cui, Y.; Jin, S.; Zhang, E.; Guo, R. Efficacy and Safety of Antiangiogenic Agents or Chemotherapy plus EGFR-TKIs in Advanced Non-Small Cell Lung Cancer: A Systematic Review and Network Meta-Analysis. Thorac. Cancer 2023, 14, 535–543. [Google Scholar] [CrossRef]
- Vasconcelos, P.E.N.S.; Gergis, C.; Viray, H.; Varkaris, A.; Fujii, M.; Rangachari, D.; VanderLaan, P.A.; Kobayashi, I.S.; Kobayashi, S.S.; Costa, D.B. EGFR-A763_Y764insFQEA Is a Unique Exon 20 Insertion Mutation That Displays Sensitivity to Approved and In-Development Lung Cancer EGFR Tyrosine Kinase Inhibitors. JTO Clin. Res. Rep. 2020, 1, 100051. [Google Scholar] [CrossRef]
- Rotow, J.; Bivona, T.G. Understanding and Targeting Resistance Mechanisms in NSCLC. Nat. Rev. Cancer 2017, 17, 637–658. [Google Scholar] [CrossRef]
- Yu, H.A.; Arcila, M.E.; Rekhtman, N.; Sima, C.S.; Zakowski, M.F.; Pao, W.; Kris, M.G.; Miller, V.A.; Ladanyi, M.; Riely, G.J. Analysis of Tumor Specimens at the Time of Acquired Resistance to EGFR TKI Therapy in 155 Patients with EGFR Mutant Lung Cancers. Clin. Cancer Res. 2013, 19, 2240–2247. [Google Scholar] [CrossRef]
- Johnson, M.; Garassino, M.C.; Mok, T.; Mitsudomi, T. Treatment Strategies and Outcomes for Patients with EGFR-Mutant Non-Small Cell Lung Cancer Resistant to EGFR Tyrosine Kinase Inhibitors: Focus on Novel Therapies. Lung Cancer 2022, 170, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Oxnard, G.R.; Hu, Y.; Mileham, K.F.; Husain, H.; Costa, D.B.; Tracy, P.; Feeney, N.; Sholl, L.M.; Dahlberg, S.E.; Redig, A.J.; et al. Assessment of Resistance Mechanisms and Clinical Implications in Patients with EGFR T790M–Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncol. 2018, 4, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.-H.; Chen, L.-Y.; Lin, Y.-C.; Shih, J.-Y.; Lin, Y.-C.; Tseng, R.-Y.; Chiu, A.-C.; Yeh, Y.-H.; Liu, C.; Lin, Y.-T.; et al. Epithelial-Mesenchymal Transition (EMT) beyond EGFR Mutations per Se Is a Common Mechanism for Acquired Resistance to EGFR TKI. Oncogene 2019, 38, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Wang, W.; Li, Q.; Matsumoto, K.; Sakurama, H.; Nakamura, T.; Ogino, H.; Kakiuchi, S.; Hanibuchi, M.; Nishioka, Y.; et al. Hepatocyte Growth Factor Induces Gefitinib Resistance of Lung Adenocarcinoma with Epidermal Growth Factor Receptor–Activating Mutations. Cancer Res. 2008, 68, 9479–9487. [Google Scholar] [CrossRef]
- Morgillo, F.; Corte, C.M.D.; Fasano, M.; Ciardiello, F. Mechanisms of Resistance to EGFR-Targeted Drugs: Lung Cancer. ESMO Open 2016, 1, E000060. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, N.; Ou, Q.; Xiang, Y.; Jiang, T.; Wu, X.; Bao, H.; Tong, X.; Wang, X.; Shao, Y.W.; et al. Investigating Novel Resistance Mechanisms to Third-Generation EGFR Tyrosine Kinase Inhibitor Osimertinib in Non–Small Cell Lung Cancer Patients. Clin. Cancer Res. 2018, 24, 3097–3107. [Google Scholar] [CrossRef]
- Crintea, A.; Dutu, A.G.; Samasca, G.; Florian, I.A.; Lupan, I.; Craciun, A.M. The Nanosystems Involved in Treating Lung Cancer. Life 2021, 11, 682. [Google Scholar] [CrossRef]
- Salata, O.V. Applications of Nanoparticles in Biology and Medicine. J. Nanobiotechnol. 2004, 2, 3. [Google Scholar] [CrossRef]
- Yin, B.; Wong, W.-K.; Ng, Y.-M.; Yang, M.; Leung, F.K.-C.; Wong, D.S.-H. Smart Design of Nanostructures for Boosting Tumor Immunogenicity in Cancer Immunotherapy. Pharmaceutics 2023, 15, 1427. [Google Scholar] [CrossRef]
- Crintea, A.; Motofelea, A.C.; Șovrea, A.S.; Constantin, A.-M.; Crivii, C.-B.; Carpa, R.; Duțu, A.G. Dendrimers: Advancements and Potential Applications in Cancer Diagnosis and Treatment-An Overview. Pharmaceutics 2023, 15, 1406. [Google Scholar] [CrossRef]
- Nichols, J.W.; Bae, Y.H. EPR: Evidence and Fallacy. J. Control. Release 2014, 190, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.V.; Allard-Vannier, E.; Chourpa, I.; Hervé-Aubert, K. Nanomedicines Functionalized with Anti-EGFR Ligands for Active Targeting in Cancer Therapy: Biological Strategy, Design and Quality Control. Int. J. Pharm. 2021, 605, 120795. [Google Scholar] [CrossRef] [PubMed]
- Markman, J.L.; Rekechenetskiy, A.; Holler, E.; Ljubimova, J.Y. Nanomedicine Therapeutic Approaches to Overcome Cancer Drug Resistance. Adv. Drug Deliv. Rev. 2013, 65, 1866–1879. [Google Scholar] [CrossRef]
- Peng, X.-H.; Wang, Y.; Huang, D.; Wang, Y.; Shin, H.J.; Chen, Z.; Spewak, M.B.; Mao, H.; Wang, X.; Wang, Y.; et al. Targeted Delivery of Cisplatin to Lung Cancer Using ScFvEGFR-Heparin-Cisplatin Nanoparticles. ACS Nano 2011, 5, 9480–9493. [Google Scholar] [CrossRef]
- Hayashi, H.; Kurata, T.; Nakagawa, K. Gemcitabine: Efficacy in the Treatment of Advanced Stage Nonsquamous Non-Small Cell Lung Cancer. Clin. Med. Insights Oncol. 2011, 5, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-B.; Zhou, H.-Y. Molecularly Targeted Gemcitabine-Loaded Nanoparticulate System towards the Treatment of EGFR Overexpressing Lung Cancer. BioMedicine 2015, 70, 123–128. [Google Scholar] [CrossRef]
- Nascimento, A.V.; Singh, A.; Bousbaa, H.; Ferreira, D.; Sarmento, B.; Amiji, M.M. Combinatorial-Designed Epidermal Growth Factor Receptor-Targeted Chitosan Nanoparticles for Encapsulation and Delivery of Lipid-Modified Platinum Derivatives in Wild-Type and Resistant Non-Small-Cell Lung Cancer Cells. Mol. Pharm. 2015, 12, 4466–4477. [Google Scholar] [CrossRef]
- Zhang, W.; Li, C.; Shen, C.; Liu, Y.; Zhao, X.; Liu, Y.; Zou, D.; Gao, Z.; Yue, C. Prodrug-Based Nano-Drug Delivery System for Co-Encapsulate Paclitaxel and Carboplatin for Lung Cancer Treatment. Drug Deliv. 2016, 23, 2575–2580. [Google Scholar] [CrossRef]
- Tian, J.; Min, Y.; Rodgers, Z.; Au, K.M.; Hagan, C.T.; Zhang, M.; Roche, K.; Yang, F.; Wagner, K.; Wang, A.Z. Co-Delivery of Paclitaxel and Cisplatin with Biocompatible PLGA-PEG Nanoparticles Enhances Chemoradiotherapy in Non-Small Cell Lung Cancer Models. J. Mater. Chem. B 2017, 5, 6049–6057. [Google Scholar] [CrossRef]
- K, S.K.; Choppala, A.D. Development and Optimization of Osimertinib-Loaded Biodegradable Polymeric Nanoparticles Enhance In-Vitro Cytotoxicity in Mutant EGFR NSCLC Cell Models and In-Vivo Tumor Reduction in H1975 Xenograft Mice Models. AAPS PharmSciTech 2022, 23, 159. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, F.; Wang, J.; He, H.; Duan, S.; Zhu, R.; Chen, C.; Yin, L.; Chen, Y. Biodegradable Nanoparticles Mediated Co-Delivery of Erlotinib (ELTN) and Fedratinib (FDTN) Toward the Treatment of ELTN-Resistant Non-Small Cell Lung Cancer (NSCLC) via Suppression of the JAK2/STAT3 Signaling Pathway. Front. Pharmacol. 2018, 9, 1214. [Google Scholar] [CrossRef]
- He, F.; Wang, Y.; Cai, W.; Li, M.; Dong, L. Reversal of EGFR Inhibitors’ Resistance by Co-Delivering EGFR and Integrin Avβ3 Inhibitors with Nanoparticles in Non-Small Cell Lung Cancer. Biosci. Rep. 2019, 39, BSR20181259. [Google Scholar] [CrossRef] [PubMed]
- Rethi, L.; Mutalik, C.; Rethi, L.; Chiang, W.-H.; Lee, H.-L.; Pan, W.-Y.; Yang, T.-S.; Chiou, J.-F.; Chen, Y.-J.; Chuang, E.-Y.; et al. Molecularly Targeted Photothermal Ablation of Epidermal Growth Factor Receptor-Expressing Cancer Cells with a Polypyrrole-Iron Oxide-Afatinib Nanocomposite. Cancers 2022, 14, 5043. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dai, X.; Jiang, S.; Qahar, M.; Feng, C.; Guo, D.; Wang, L.; Ma, S.; Huang, L. Targeted Co-Delivery of Gefitinib and Rapamycin by Aptamer-Modified Nanoparticles Overcomes EGFR-TKI Resistance in NSCLC via Promoting Autophagy. Int. J. Mol. Sci. 2022, 23, 8025. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiary, Z.; Barar, J.; Aghanejad, A.; Saei, A.A.; Nemati, E.; Ezzati Nazhad Dolatabadi, J.; Omidi, Y. Microparticles Containing Erlotinib-Loaded Solid Lipid Nanoparticles for Treatment of Non-Small Cell Lung Cancer. Drug Dev. Ind. Pharm. 2017, 43, 1244–1253. [Google Scholar] [CrossRef]
- Ganthala, P.D.; Alavala, S.; Chella, N.; Andugulapati, S.B.; Bathini, N.B.; Sistla, R. Co-Encapsulated Nanoparticles of Erlotinib and Quercetin for Targeting Lung Cancer through Nuclear EGFR and PI3K/AKT Inhibition. Colloids Surf. B Biointerfaces 2022, 211, 112305. [Google Scholar] [CrossRef]
- Song, Z.; Shi, Y.; Han, Q.; Dai, G. Endothelial Growth Factor Receptor-Targeted and Reactive Oxygen Species-Responsive Lung Cancer Therapy by Docetaxel and Resveratrol Encapsulated Lipid-Polymer Hybrid Nanoparticles. Biomed. Pharmacother. 2018, 105, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, Z.; Li, J.; Mo, Z.; Huang, Y.; Ma, C.; Wang, W.; Pan, X.; Wu, C. PLGA Porous Microspheres Dry Powders for Codelivery of Afatinib-Loaded Solid Lipid Nanoparticles and Paclitaxel: Novel Therapy for EGFR Tyrosine Kinase Inhibitors Resistant Nonsmall Cell Lung Cancer. Adv. Healthc. Mater. 2019, 8, e1900965. [Google Scholar] [CrossRef]
- Yokoyama, T.; Tam, J.; Kuroda, S.; Scott, A.W.; Aaron, J.; Larson, T.; Shanker, M.; Correa, A.M.; Kondo, S.; Roth, J.A.; et al. EGFR-Targeted Hybrid Plasmonic Magnetic Nanoparticles Synergistically Induce Autophagy and Apoptosis in Non-Small Cell Lung Cancer Cells. PLoS ONE 2011, 6, e25507. [Google Scholar] [CrossRef]
- Kuroda, S.; Tam, J.; Roth, J.A.; Sokolov, K.; Ramesh, R. EGFR-Targeted Plasmonic Magnetic Nanoparticles Suppress Lung Tumor Growth by Abrogating G2/M Cell-Cycle Arrest and Inducing DNA Damage. Int. J. Nanomed. 2014, 9, 3825–3839. [Google Scholar] [CrossRef]
- Sun, M.; Wang, T.; Li, L.; Li, X.; Zhai, Y.; Zhang, J.; Li, W. The Application of Inorganic Nanoparticles in Molecular Targeted Cancer Therapy: EGFR Targeting. Front. Pharmacol. 2021, 12, 702445. [Google Scholar] [CrossRef]
- Qian, Y.; Qiu, M.; Wu, Q.; Tian, Y.; Zhang, Y.; Gu, N.; Li, S.; Xu, L.; Yin, R. Enhanced Cytotoxic Activity of Cetuximab in EGFR-Positive Lung Cancer by Conjugating with Gold Nanoparticles. Sci. Rep. 2014, 4, 7490. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, W.; Tian, Y.; Yang, Z.; Wang, X.; Zhang, Y.; Tang, Y.; Zhao, S.; Wang, C.; Liu, Y.; et al. Anti-EGFR Peptide-Conjugated Triangular Gold Nanoplates for Computed Tomography/Photoacoustic Imaging-Guided Photothermal Therapy of Non-Small Cell Lung Cancer. ACS Appl. Mater. Interfaces 2018, 10, 16992–17003. [Google Scholar] [CrossRef] [PubMed]
- Duman, F.D.; Akkoc, Y.; Demirci, G.; Bavili, N.; Kiraz, A.; Gozuacik, D.; Acar, H.Y. Bypassing Pro-Survival and Resistance Mechanisms of Autophagy in EGFR-Positive Lung Cancer Cells by Targeted Delivery of 5FU Using Theranostic Ag2S Quantum Dots. J. Mater. Chem. B 2019, 7, 7363–7376. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.V.; Singh, A.; Bousbaa, H.; Ferreira, D.; Sarmento, B.; Amiji, M.M. Mad2 Checkpoint Gene Silencing Using Epidermal Growth Factor Receptor-Targeted Chitosan Nanoparticles in Non-Small Cell Lung Cancer Model. Mol. Pharm. 2014, 11, 3515–3527. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.V.; Gattacceca, F.; Singh, A.; Bousbaa, H.; Ferreira, D.; Sarmento, B.; Amiji, M.M. Biodistribution and Pharmacokinetics of Mad2 SiRNA-Loaded EGFR-Targeted Chitosan Nanoparticles in Cisplatin Sensitive and Resistant Lung Cancer Models. Nanomedicine 2016, 11, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.V.; Singh, A.; Bousbaa, H.; Ferreira, D.; Sarmento, B.; Amiji, M.M. Overcoming Cisplatin Resistance in Non-Small Cell Lung Cancer with Mad2 Silencing SiRNA Delivered Systemically Using EGFR-Targeted Chitosan Nanoparticles. Acta Biomater. 2017, 47, 71–80. [Google Scholar] [CrossRef]
- Kamrani Moghaddam, L.; Ramezani Paschepari, S.; Zaimy, M.A.; Abdalaian, A.; Jebali, A. The Inhibition of Epidermal Growth Factor Receptor Signaling by Hexagonal Selenium Nanoparticles Modified by SiRNA. Cancer Gene Ther. 2016, 23, 321–325. [Google Scholar] [CrossRef]
- Lv, T.; Li, Z.; Xu, L.; Zhang, Y.; Chen, H.; Gao, Y. Chloroquine in Combination with Aptamer-Modified Nanocomplexes for Tumor Vessel Normalization and Efficient Erlotinib/Survivin ShRNA Co-Delivery to Overcome Drug Resistance in EGFR-Mutated Non-Small Cell Lung Cancer. Acta Biomater. 2018, 76, 257–274. [Google Scholar] [CrossRef]
- Reda, M.; Ngamcherdtrakul, W.; Gu, S.; Bejan, D.S.; Siriwon, N.; Gray, J.W.; Yantasee, W. PLK1 and EGFR Targeted Nanoparticle as a Radiation Sensitizer for Non-Small Cell Lung Cancer. Cancer Lett. 2019, 467, 9–18. [Google Scholar] [CrossRef]
- Majumder, J.; Minko, T. Multifunctional Lipid-Based Nanoparticles for Codelivery of Anticancer Drugs and SiRNA for Treatment of Non-Small Cell Lung Cancer with Different Level of Resistance and EGFR Mutations. Pharmaceutics 2021, 13, 1063. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhou, D.; Dai, Y.; Li, Y.; Wu, J.; Liu, Q.; Deng, X. Construction of PEI-EGFR-PD-L1-SiRNA Dual Functional Nano-Vaccine and Therapeutic Efficacy Evaluation for Lung Cancer. Thorac. Cancer 2022, 13, 2941–2950. [Google Scholar] [CrossRef]
- Huang, H.; Yi, X.; Wei, Q.; Li, M.; Cai, X.; Lv, Y.; Weng, L.; Mao, Y.; Fan, W.; Zhao, M.; et al. Edible and Cation-Free Kiwi Fruit Derived Vesicles Mediated EGFR-Targeted SiRNA Delivery to Inhibit Multidrug Resistant Lung Cancer. J. Nanobiotechnol. 2023, 21, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, H.-Y.; Yang, L.; Zhang, Z.; Ji, H. Cetuximab-Modified Mesoporous Silica Nano-Medicine Specifically Targets EGFR-Mutant Lung Cancer and Overcomes Drug Resistance. Sci. Rep. 2016, 6, 25468. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Choi, Y.J.; Sung, K.J.; Yoo, S.-A.; Sung, Y.H.; Kim, J.K.; Choi, C.-M.; Yun, M.; Lee, E.Y.; Jin, Y.S.; et al. Efficacy of Nano-Particulated, Water-Soluble Erlotinib against Intracranial Metastases of EGFR-Mutant Lung Cancer. Mol. Oncol. 2018, 12, 2182–2190. [Google Scholar] [CrossRef]
- Wang, F.; Porter, M.; Konstantopoulos, A.; Zhang, P.; Cui, H. Preclinical Development of Drug Delivery Systems for Paclitaxel-Based Cancer Chemotherapy. J. Control. Release 2017, 267, 100–118. [Google Scholar] [CrossRef]
- Shih, Y.-H.; Luo, T.-Y.; Chiang, P.-F.; Yao, C.; Lin, W.-J.; Peng, C.-L.; Shieh, M.-J. EGFR-Targeted Micelles Containing near-Infrared Dye for Enhanced Photothermal Therapy in Colorectal Cancer. J. Control. Release 2017, 258, 196–207. [Google Scholar] [CrossRef]
- Gorachinov, F.; Mraiche, F.; Moustafa, D.A.; Hishari, O.; Ismail, Y.; Joseph, J.; Crcarevska, M.S.; Dodov, M.G.; Geskovski, N.; Goracinova, K. Nanotechnology—A Robust Tool for Fighting the Challenges of Drug Resistance in Non-Small Cell Lung Cancer. Beilstein J. Nanotechnol. 2023, 14, 240–261. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, W.; Xin, H.; Liu, R.; Wang, Q.; Cai, W.; Peng, X.; Yang, F.; Xin, H. Nanoparticles Advanced from Preclinical Studies to Clinical Trials for Lung Cancer Therapy. Cancer Nanotechnol. 2023, 14, 28. [Google Scholar] [CrossRef]
- da Silva Santos, E.; Nogueira, K.A.B.; Fernandes, L.C.C.; Martins, J.R.P.; Reis, A.V.F.; de Brito Vieira Neto, J.; da Silva Júnior, I.J.; Pessoa, C.; Petrilli, R.; Eloy, J.O. EGFR Targeting for Cancer Therapy: Pharmacology and Immunoconjugates with Drugs and Nanoparticles. Int. J. Pharm. 2021, 592, 120082. [Google Scholar] [CrossRef]
- Mamot, C.; Ritschard, R.; Wicki, A.; Stehle, G.; Dieterle, T.; Bubendorf, L.; Hilker, C.; Deuster, S.; Herrmann, R.; Rochlitz, C. Tolerability, Safety, Pharmacokinetics, and Efficacy of Doxorubicin-Loaded Anti-EGFR Immunoliposomes in Advanced Solid Tumours: A Phase 1 Dose-Escalation Study. Lancet Oncol. 2012, 13, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Kasenda, B.; König, D.; Manni, M.; Ritschard, R.; Duthaler, U.; Bartoszek, E.; Bärenwaldt, A.; Deuster, S.; Hutter, G.; Cordier, D.; et al. Targeting Immunoliposomes to EGFR-Positive Glioblastoma. ESMO Open 2022, 7, 100365. [Google Scholar] [CrossRef] [PubMed]
- Mamot, C.; Wicki, A.; Hasler-Strub, U.; Riniker, S.; Li, Q.; Holer, L.; Bärtschi, D.; Zaman, K.; von Moos, R.; Dedes, K.J.; et al. A Multicenter Phase II Trial of Anti-EGFR-Immunoliposomes Loaded with Doxorubicin in Patients with Advanced Triple Negative Breast Cancer. Sci. Rep. 2023, 13, 3705. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef]



| Ref. | Study Type | Composition | Drug Delivered | Size | Surface Mods | Mechanism | Weaknesses | Strengths |
|---|---|---|---|---|---|---|---|---|
| [123] | In Vivo Study | Polymer NPs | Heparin-Cisplatin | 20 nm | Negative charge, hydrophilic | EGFR targeting | Particle stability, organ conc., side effects | Enhanced antitumor effect, reduced toxicity |
| [125] | In Vitro and In Vivo | PLA NPs | Gemcitabine − Cetuximab | 120 nm | EDC activation | EGFR signal block | Cytotoxic to normal cells | Enhanced cell killing, passive targeting |
| [126] | In Vitro Study | Chitosan NPs | Lipid-Modified Cisplatin | 220–365 nm | Positively charged | Receptor-mediated endocytosis | Side effects | Improved cytotoxicity |
| [127] | In Vitro and In Vivo | PLGA-PEG NPs | Paclitaxel + Carboplatin | 125 nm | Negative charge | Drug release | Systemic side effects | Sustained drug release |
| [128] | In Vitro and In Vivo | PLGA-PEG NPs | PTX + Fatty Acid CPP | 80–85.5 nm | Negative charge | NP phagocytosis | Biodistribution, liver and kidney | Enhanced apoptosis |
| [129] | In Vitro and In Vivo | Chitosan-coated NPs | Osimertinib | 101.3–119.7 nm | Biodegradable NPs | Drug release | Side effects | Reduced tumor size |
| [130] | In Vitro and In Vivo | PEG-PLA NPs | Erlotinib + Fedratinib | 120 nm | Hydrophobic, dual-drug | Acidic microenvironment | Side effects | Enhanced therapeutic efficacy |
| [131] | In Vitro and In Vivo | Chitosan NPs | Osimertinib | 101.3–119.7 nm | Biodegradable NPs | Drug release | Side effects | Reduced tumor size |
| [134] | In Vitro | Solid Lipid NPs | Erlotinib microparticles | 1–5 μm | Dry powder inhaler | PI3K/AKT signaling | Inhalatory admin. | Suitable flowability |
| [135] | In Vitro and In Vivo | Polymer NPs | Erlotinib + Quercetin | 87.3 ± 0.78 nm | Chitosan-MA-TPGS | Nuclear EGFR | Low side effects | Minimal injury to healthy tissue |
| [136] | In Vitro and In Vivo | Core-Shell Lipid-Polymer NPs | Docetaxel + Resveratrol | 189.6 ± 5.6 nm | Dual-drug loaded NPS | Mitochondrial targeting | Mouse weight loss | Higher tumor inhibition |
| [137] | In Vitro and In Vivo | Solid Lipid NPs | Afatinib + Paclitaxel | 500 nm | Dual-drug loaded NPS | PI3K/Akt/mTOR pathway | Hepatic edema | Increased cell migration inhibition |
| [138] | In Vitro | Magnetic NPs | C225 + Hybrid Plasmonic NPs | 54 ± 11 nm | Gold-coated iron oxide NPs | Apoptosis, autophagy | Multivalency effect | Higher efficiency |
| [139] | In Vitro and In Vivo | Magnetic NPs | C225 + Hybrid Plasmonic NPs | 73 ± 35 nm | Gold-coated iron oxide NPs | Autophagy, apoptosis | Active on EGFR-positive cells | Greater tumor suppression |
| [141] | In Vitro and In Vivo | Gold NPs | Cetuximab | 25 nm | BSA-treated | EGFR endocytosis | Time/dose-dependent effect | Increased cytotoxicity |
| [142] | In Vitro and In Vivo | Gold Nanoplates | Anti-EGFR PTT agent | 77.9 ± 7.0 nm | Neutrally | Photothermal therapy | Requires light exposure to activate the photothermal effect | Selectively kill cancer cells, minimal side effects, can be used for imaging |
| [143] | In Vitro and In Vivo | Ag2S QDs | Cetuximab functionalization | <50 nm | PEGylated cationic NPs | Endocytosis | Fluorescence imaging | Enhanced apoptosis |
| [144] | In Vitro | PEG-CS NPs | Mad2 siRNA | 100–250 nm | Peptide-modified PEG-CS NPs | EGFR internalization | Efficiency dependent on MW | Increased selectivity |
| [145] | In Vitro and In Vivo | NTG and TG CS NPs | Mad 2 siRNA | 113.1–230.1 nm | Peptide-modified PEG-CS NPs | Apoptosis | Organ accumulation | Higher targeting efficiency |
| [146] | In Vitro and In Vivo | NTG and TG CS NPs | Mad 2 siRNA + Cisplatin | 126.7–202.7 nm | PEGylated CS derivatives | Apoptosis, mitotic failure | Decreased plasma exposure | Minimized side effects |
| [147] | In Vitro | Hexagonal Selenium NPs | siRNA | 20 nm | Oligonucleotide modification | Down-regulation of EGFR genes | Increased apoptosis, suppression | Effective tumor immunotherapy |
| [150] | In Vitro and In Vivo | Nanostructured Lipid Carriers | Gefitinib + Paclitaxel + siRNA | 100–300 nm | LHRH-coated NLCs | Suppression of EGF pathway | Instability of siRNA | Enhanced internalization |
| [151] | In Vitro and In Vivo | PEI Lipid NPs + siRNA | EGFR + PD-L1 siRNA | 30 nm | Peptide-modified PEI | Immune stimulation | T cells-related adverse effects | High biocompatibility, tumor immunotherapy |
| [152] | In Vitro and In Vivo | Kiwi-Derived Extracellular Vesicles | siSTAT3 | 186 nm | Aptamer surface mod. | STAT3-induced apoptosis | Side effects | High specificity, cytotoxicity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crintea, A.; Constantin, A.-M.; Motofelea, A.C.; Crivii, C.-B.; Velescu, M.A.; Coșeriu, R.L.; Ilyés, T.; Crăciun, A.M.; Silaghi, C.N. Targeted EGFR Nanotherapy in Non-Small Cell Lung Cancer. J. Funct. Biomater. 2023, 14, 466. https://doi.org/10.3390/jfb14090466
Crintea A, Constantin A-M, Motofelea AC, Crivii C-B, Velescu MA, Coșeriu RL, Ilyés T, Crăciun AM, Silaghi CN. Targeted EGFR Nanotherapy in Non-Small Cell Lung Cancer. Journal of Functional Biomaterials. 2023; 14(9):466. https://doi.org/10.3390/jfb14090466
Chicago/Turabian StyleCrintea, Andreea, Anne-Marie Constantin, Alexandru C. Motofelea, Carmen-Bianca Crivii, Maria A. Velescu, Răzvan L. Coșeriu, Tamás Ilyés, Alexandra M. Crăciun, and Ciprian N. Silaghi. 2023. "Targeted EGFR Nanotherapy in Non-Small Cell Lung Cancer" Journal of Functional Biomaterials 14, no. 9: 466. https://doi.org/10.3390/jfb14090466
APA StyleCrintea, A., Constantin, A.-M., Motofelea, A. C., Crivii, C.-B., Velescu, M. A., Coșeriu, R. L., Ilyés, T., Crăciun, A. M., & Silaghi, C. N. (2023). Targeted EGFR Nanotherapy in Non-Small Cell Lung Cancer. Journal of Functional Biomaterials, 14(9), 466. https://doi.org/10.3390/jfb14090466







