Multi-Scale Characterization of Conventional and Immediate Dental Implant Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Dental Implants
2.3. Surgical Procedure
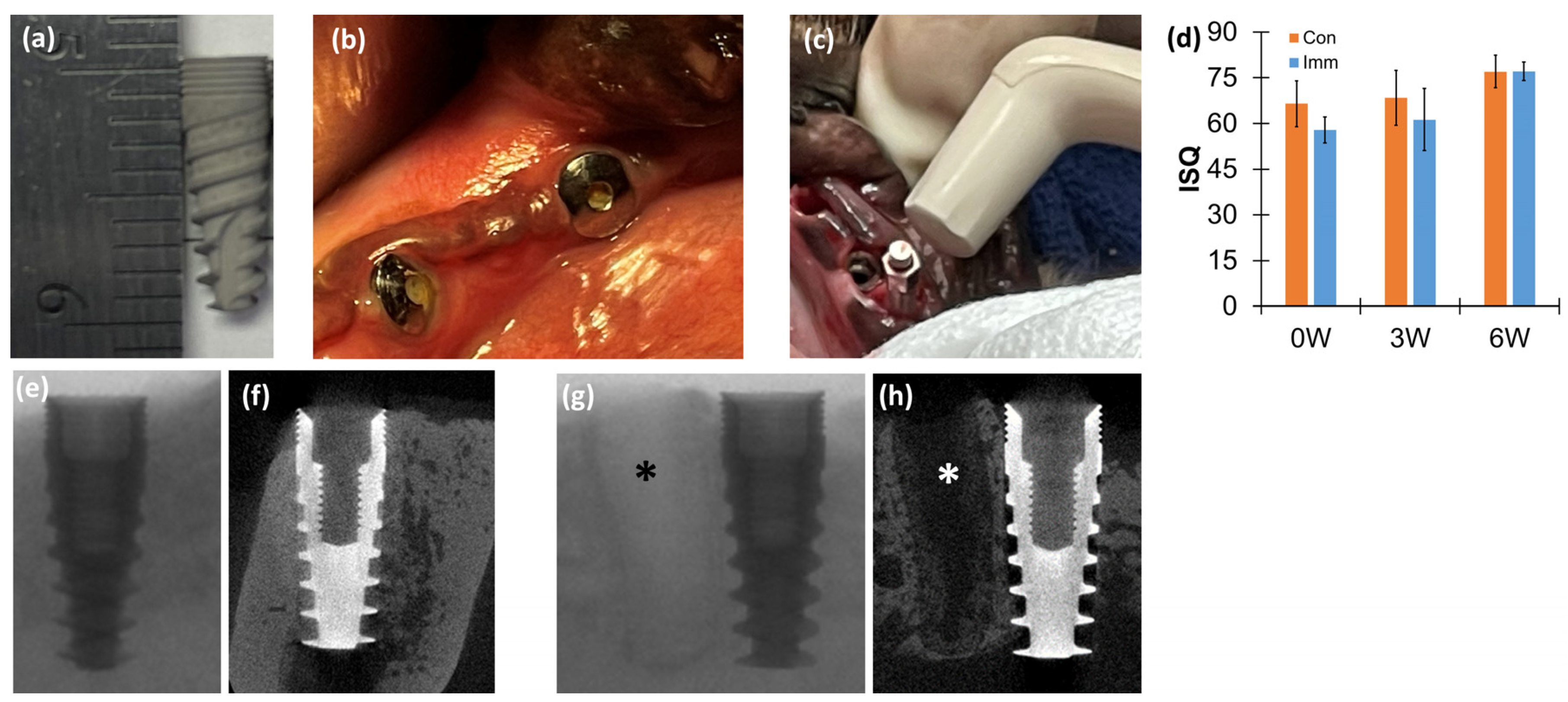
2.4. Resonance Frequency Analysis (RFA)
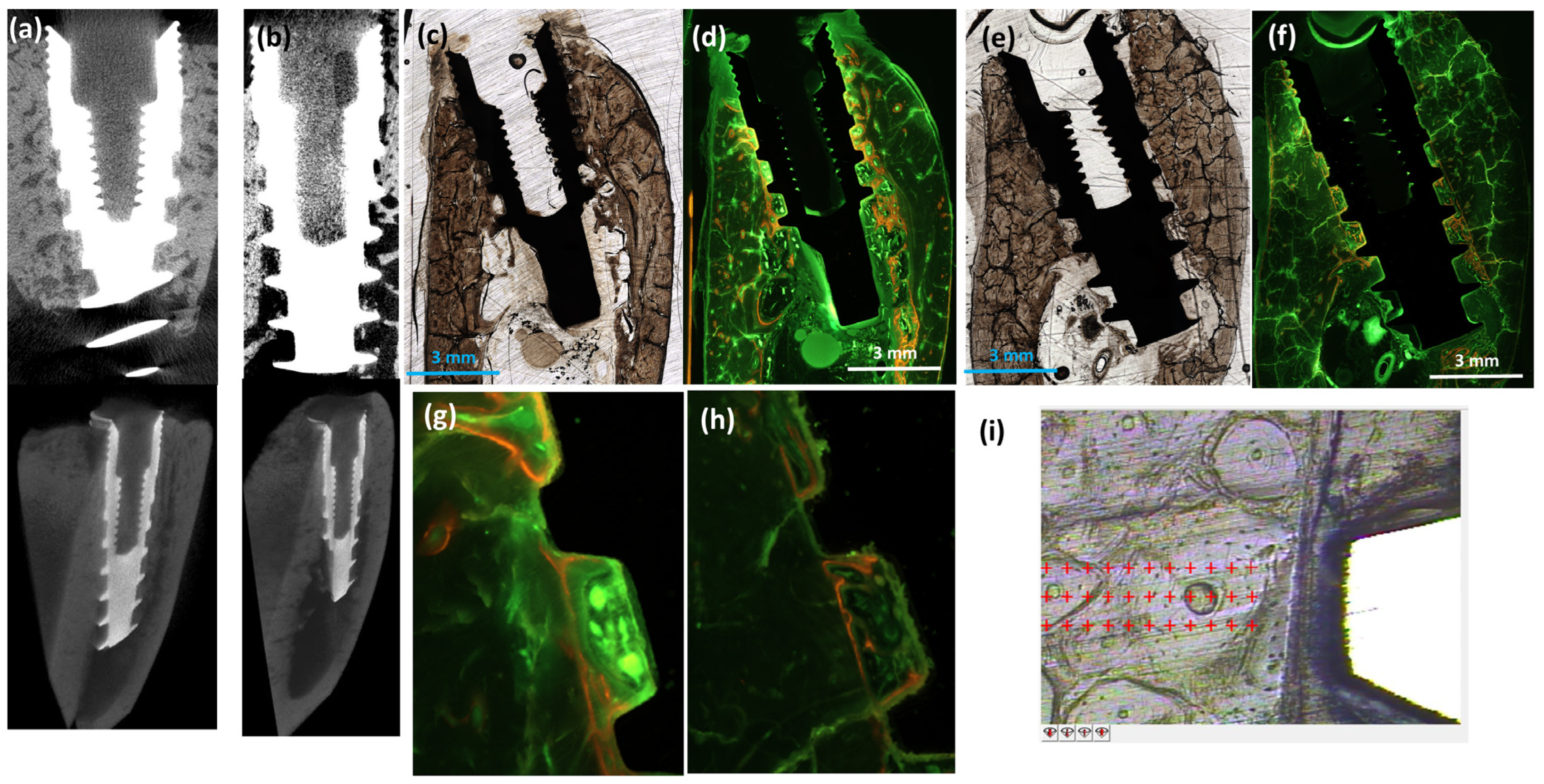
2.5. Computed Tomography (CT)
2.6. Nanoindentation
2.7. Histologic Examination
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quirynen, M.; Herrera, D.; Teughels, W.; Sanz, M. Implant therapy: 40 years of experience. Periodontology 2000 2014, 66, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Elani, H.W.; Starr, J.R.; Da Silva, J.D.; Gallucci, G.O. Trends in Dental Implant Use in the U.S., 1999–2016, and Projections to 2026. J. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Klinge, B.; Lundstrom, M.; Rosen, M.; Bertl, K.; Klinge, A.; Stavropoulos, A. Dental Implant Quality Register-A possible tool to further improve implant treatment and outcome. Clin. Oral. Implant. Res. 2018, 29 (Suppl. S18), 145–151. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontology 2000 2017, 73, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Brånemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindström, J.; Hallén, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. Suppl. 1977, 16, 1–132. [Google Scholar]
- Heimke, G. Osseo-Integrated Implants; CRC Press: Boca Raton, FL, USA, 1990; Volume 2. [Google Scholar]
- Udeabor, S.E.; Heselich, A.; Al-Maawi, S.; Alqahtani, A.F.; Sader, R.; Ghanaati, S. Current Knowledge on the Healing of the Extraction Socket: A Narrative Review. Bioengineering 2023, 10, 1145. [Google Scholar] [CrossRef]
- Yuan, X.; Pei, X.; Zhao, Y.; Li, Z.; Chen, C.H.; Tulu, U.S.; Liu, B.; Van Brunt, L.A.; Brunski, J.B.; Helms, J.A. Biomechanics of Immediate Postextraction Implant Osseointegration. J. Dent. Res. 2018, 97, 987–994. [Google Scholar] [CrossRef]
- Garetto, L.P.; Chen, J.; Parr, J.A.; Roberts, W.E. Remodeling dynamics of bone supporting rigidly fixed titanium implants: A histomorphometric comparison in four species including humans. Implant. Dent. 1995, 4, 235–243. [Google Scholar] [CrossRef]
- Kim, D.G.; Kim, K.H.; Jo, Y.; Lee, J.Y.; Park, Y.J.; Chung, C.P.; Seol, Y.J.; Han, J.S. Bone regeneration into side openings and hollow inner channel of a dental implant. J. Mech. Behav. Biomed. Mater. 2020, 101, 103416. [Google Scholar] [CrossRef]
- Kim, D.G.; Huja, S.S.; Tee, B.C.; Larsen, P.E.; Kennedy, K.S.; Chien, H.H.; Lee, J.W.; Wen, H.B. Bone Ingrowth and Initial Stability of Titanium and Porous Tantalum Dental Implants: A Pilot Canine Study. Implant. Dent. 2013, 22, 399–405. [Google Scholar] [CrossRef]
- Huang, Y.C.; Huang, Y.C.; Ding, S.J. Primary stability of implant placement and loading related to dental implant materials and designs: A literature review. J. Dent. Sci. 2023, 18, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Hoshaw, S.J.; Brunski, J.B.; Cochran, G.V. Mechanical Loading of Brånemark Implants Affects Interfacial Bone Modeling and Remodeling. Int. J. Oral. Maxillofac. Implant. 1994, 9, 345–360. [Google Scholar]
- Tian, Y.; Sadowsky, S.J.; Brunski, J.B.; Yuan, X.; Helms, J.A. Effects of masticatory loading on bone remodeling around teeth versus implants: Insights from a preclinical model. Clin. Oral. Implant. Res. 2022, 33, 342–352. [Google Scholar] [CrossRef]
- Boonrungsiman, S.; Gentleman, E.; Carzaniga, R.; Evans, N.D.; McComb, D.W.; Porter, A.E.; Stevens, M.M. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc. Natl. Acad. Sci. USA 2012, 109, 14170–14175. [Google Scholar] [CrossRef] [PubMed]
- Roschger, P.; Paschalis, E.P.; Fratzl, P.; Klaushofer, K. Bone mineralization density distribution in health and disease. Bone 2008, 42, 456–466. [Google Scholar] [CrossRef]
- Martin, R.B.; Burr, D.B.; Sharkey, N.A. Skeletal Tissue Mechanics; Springer: New York, NY, USA, 1998; p. 79. [Google Scholar]
- Brunski, J.B. In vivo bone response to biomechanical loading at the bone/dental-implant interface. Adv. Dent. Res. 1999, 13, 99–119. [Google Scholar] [CrossRef]
- Roberts, W.E.; Turley, P.K.; Brezniak, N.; Fielder, P.J. Implants: Bone physiology and metabolism. CDA J. Calif. Dent. Assoc. 1987, 15, 54–61. [Google Scholar]
- Baldassarri, M.; Bonfante, E.; Suzuki, M.; Marin, C.; Granato, R.; Tovar, N.; Coelho, P.G. Mechanical properties of human bone surrounding plateau root form implants retrieved after 0.3–24 years of function. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2012, 100, 2015–2021. [Google Scholar] [CrossRef]
- Piattelli, A.; Artese, L.; Penitente, E.; Iaculli, F.; Degidi, M.; Mangano, C.; Shibli, J.A.; Coelho, P.G.; Perrotti, V.; Iezzi, G. Osteocyte density in the peri-implant bone of implants retrieved after different time periods (4 weeks to 27 years). J. Biomed. Mater. Res. Part. B Appl. Biomater. 2014, 102, 239–243. [Google Scholar] [CrossRef]
- Stoilov, M.; Shafaghi, R.; Stark, H.; Marder, M.; Kraus, D.; Enkling, N. Influence of Implant Macro-Design, -Length, and -Diameter on Primary Implant Stability Depending on Different Bone Qualities Using Standard Drilling Protocols—An In Vitro Analysis. J. Funct. Biomater. 2023, 14, 469. [Google Scholar] [CrossRef]
- Folkman, M.; Becker, A.; Meinster, I.; Masri, M.; Ormianer, Z. Comparison of bone-to-implant contact and bone volume around implants placed with or without site preparation: A histomorphometric study in rabbits. Sci. Rep. 2020, 10, 12446. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, V.; Vayron, R.; Richard, G.; Lambert, G.; Naili, S.; Meningaud, J.P.; Haiat, G. Biomechanical determinants of the stability of dental implants: Influence of the bone-implant interface properties. J. Biomech. 2014, 47, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Hoshaw, S.J.; Watson, J.T.; Schaffler, M.B.D.P.F. Microdamage at bone-implant interfaces affects bone remodeling activity. In Proceedings of the Trans 41st Orthopaedic Research Society, Orlando, FL, USA, 13–16 February 1995; p. 188. [Google Scholar]
- Chang, P.C.; Seol, Y.J.; Goldstein, S.A.; Giannobile, W.V. Determination of the dynamics of healing at the tissue-implant interface by means of microcomputed tomography and functional apparent moduli. Int. J. Oral. Maxillofac. Implant. 2013, 28, 68–76. [Google Scholar] [CrossRef]
- Schulte, W.; Kleineikenscheidt, H.; Lindner, K.; Schareyka, R. The Tübingen immediate implant in clinical studies. Dtsch. Zahnarztl. Z. 1978, 33, 348–359. [Google Scholar] [PubMed]
- Ibrahim, A.; Chrcanovic, B.R. Dental Implants Inserted in Fresh Extraction Sockets versus Healed Sites: A Systematic Review and Meta-Analysis. Materials 2021, 14, 7903. [Google Scholar] [CrossRef]
- Hong, D.G.K.; Oh, J.H. Recent advances in dental implants. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 33. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.G.; Jeong, Y.H.; Kosel, E.; Agnew, A.M.; McComb, D.W.; Bodnyk, K.; Hart, R.T.; Kim, M.K.; Han, S.Y.; Johnston, W.M. Regional variation of bone tissue properties at the human mandibular condyle. Bone 2015, 77, 98–106. [Google Scholar] [CrossRef]
- Carosi, P.; Lorenzi, C.; Di Gianfilippo, R.; Papi, P.; Laureti, A.; Wang, H.-L.; Arcuri, C. Immediate vs. Delayed Placement of Immediately Provisionalized Self-Tapping Implants: A Non-Randomized Controlled Clinical Trial with 1 Year of Follow-Up. J. Clin. Med. 2023, 12, 489. [Google Scholar] [CrossRef]
- Ersanli, S.; Karabuda, C.; Beck, F.; Leblebicioglu, B. Resonance frequency analysis of one-stage dental implant stability during the osseointegration period. J. Periodontol. 2005, 76, 1066–1071. [Google Scholar] [CrossRef]
- Stavropoulos, A.; Nyengaard, J.R.; Lang, N.P.; Karring, T. Immediate loading of single SLA implants: Drilling vs. osteotomes for the preparation of the implant site. Clin. Oral. Implant. Res. 2008, 19, 55–65. [Google Scholar] [CrossRef]
- Choi, J.-Y.C.; Choi, C.A.; Yeo, I.-S.L. Spiral scanning imaging and quantitative calculation of the 3-dimensional screw-shaped bone-implant interface on micro-computed tomography. J. Periodontal Implant. Sci. 2018, 48, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Mulder, L.; Koolstra, J.H.; den Toonder, J.M.; van Eijden, T.M. Relationship between tissue stiffness and degree of mineralization of developing trabecular bone. J. Biomed. Mater. Res. Part. A 2008, 84, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Mulder, L.; Koolstra, J.H.; den Toonder, J.M.J.; van Eijden, T.M.G.J. Intratrabecular distribution of tissues stiffness and mineralization in developing trabecular bone. Bone 2007, 41, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.G.; Kwon, H.J.; Jeong, Y.H.; Kosel, E.; Lee, D.J.; Han, J.S.; Kim, H.L.; Kim, D.J. Mechanical properties of bone tissues surrounding dental implant systems with different treatments and healing periods. Clin. Oral. Investig. 2016, 20, 2211–2220. [Google Scholar] [CrossRef] [PubMed]
- Fraulob, M.; Pang, S.; Le Cann, S.; Vayron, R.; Laurent-Brocq, M.; Todatry, S.; Soares, J.; Jasiuk, I.; Haïat, G. Multimodal characterization of the bone-implant interface using Raman spectroscopy and nanoindentation. Med. Eng. Phys. 2020, 84, 60–67. [Google Scholar] [CrossRef]
- Rho, J.Y.; Pharr, G.M. Effects of drying on the mechanical properties of bovine femur measured by nanoindentation. J. Mater. Sci. Mater. Med. 1999, 10, 485–488. [Google Scholar] [CrossRef]
- Cha, J.Y.; Pereira, M.D.; Smith, A.A.; Houschyar, K.S.; Yin, X.; Mouraret, S.; Brunski, J.B.; Helms, J.A. Multiscale Analyses of the Bone-implant Interface. J. Dent. Res. 2015, 94, 482–490. [Google Scholar] [CrossRef]
- Kitamura, E.; Stegaroiu, R.; Nomura, S.; Miyakawa, O. Influence of marginal bone resorption on stress around an implant—A three-dimensional finite element analysis. J. Oral. Rehabil. 2005, 32, 279–286. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, C.; Wang, Z.; Zeng, T.; Wang, Y. Optimization of stress distribution of bone-implant interface (BII). Biomater. Adv. 2023, 147, 213342. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mok, S.; Naftulin, M.E.; Meirelles, L.; Kim, M.; Liu, J.; Lee, C.H.; Emam, H.A.; Jatana, C.A.; Chien, H.-H.; Ko, C.-C.; et al. Multi-Scale Characterization of Conventional and Immediate Dental Implant Systems. J. Funct. Biomater. 2024, 15, 317. https://doi.org/10.3390/jfb15110317
Mok S, Naftulin ME, Meirelles L, Kim M, Liu J, Lee CH, Emam HA, Jatana CA, Chien H-H, Ko C-C, et al. Multi-Scale Characterization of Conventional and Immediate Dental Implant Systems. Journal of Functional Biomaterials. 2024; 15(11):317. https://doi.org/10.3390/jfb15110317
Chicago/Turabian StyleMok, Seeun, Mori E. Naftulin, Luiz Meirelles, Minji Kim, Jie Liu, Christine H. Lee, Hany A. Emam, Courtney A. Jatana, Hua-Hong Chien, Ching-Chang Ko, and et al. 2024. "Multi-Scale Characterization of Conventional and Immediate Dental Implant Systems" Journal of Functional Biomaterials 15, no. 11: 317. https://doi.org/10.3390/jfb15110317
APA StyleMok, S., Naftulin, M. E., Meirelles, L., Kim, M., Liu, J., Lee, C. H., Emam, H. A., Jatana, C. A., Chien, H.-H., Ko, C.-C., & Kim, D.-G. (2024). Multi-Scale Characterization of Conventional and Immediate Dental Implant Systems. Journal of Functional Biomaterials, 15(11), 317. https://doi.org/10.3390/jfb15110317







