Sericin Protein: Structure, Properties, and Applications
Abstract
1. Introduction
2. Meta-Analysis
2.1. Sericin-Based Studies
2.2. Fibroin-Based Studies
3. Silk Sericin Structure and Properties
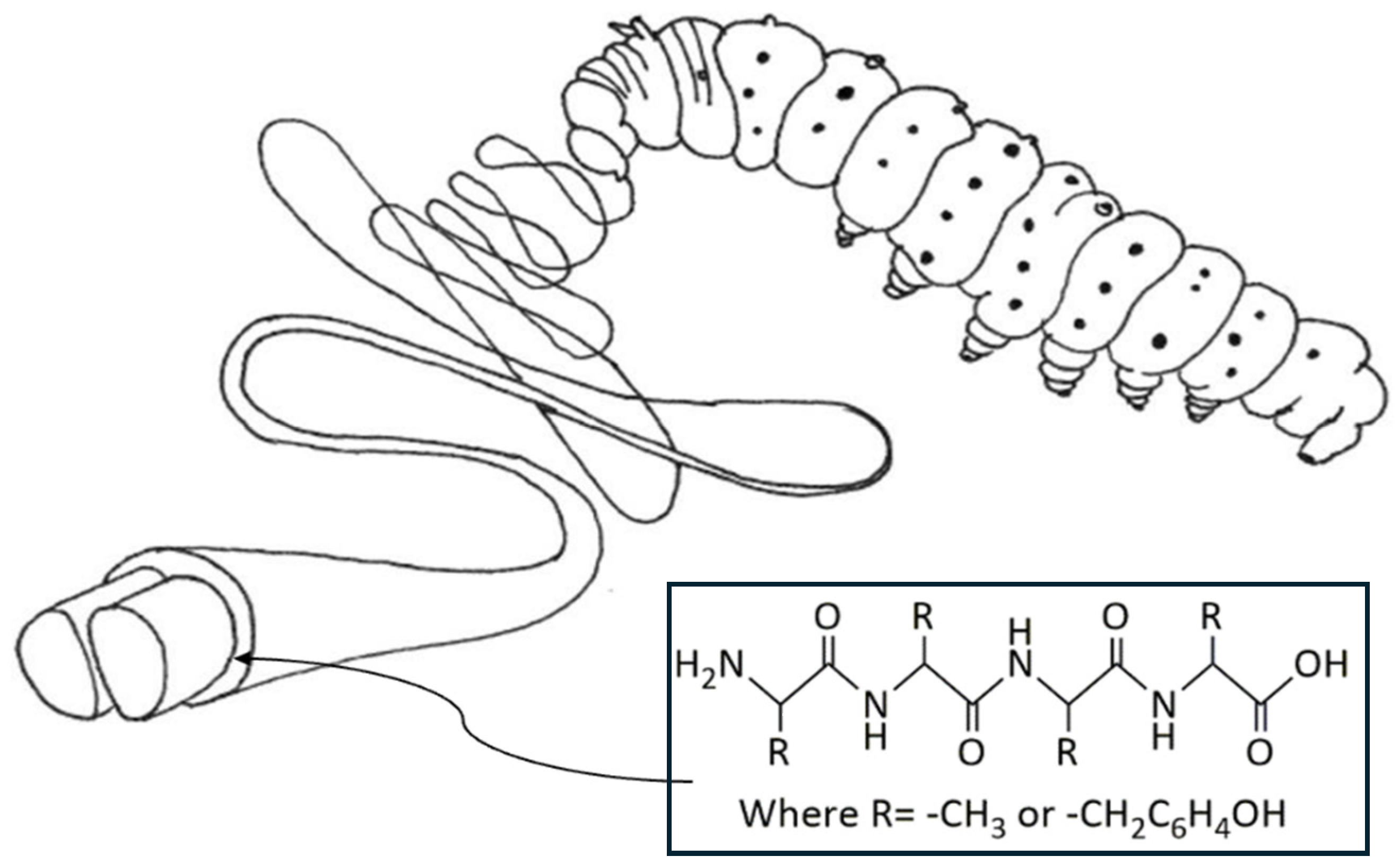
3.1. Chemical Composition and Structure
| Amino-Acid | 2000 [6] | 2006 [7] | 2009 [8] | 2014 [9] | 2015 [10] | 2018 [11] | Average |
|---|---|---|---|---|---|---|---|
| Ala | 4.60 | 4.30 | 3.86 | 4.30 | ND * | 3.28 | 4.07 |
| Arg | 2.80 | 4.90 | 6.16 | 3.60 | 11.95 | 4.71 | 5.69 |
| Asp | 19.10 | 18.80 | 17.64 | 14.80 | 14.00 | 11.52 | 15.98 |
| Cyst | <0.05 | 0.30 | ND * | 0.10 | ND * | 0.03 | 0.14 |
| Glu | 4.10 | 7.20 | 7.31 | 3.40 | 3.30 | 2.91 | 4.70 |
| Gly | 12.20 | 10.70 | 9.89 | 14.70 | 23.20 | 12.60 | 13.88 |
| His | 0.90 | 1.70 | 1.81 | 1.20 | 1.13 | 2.05 | 1.47 |
| Ile | 1.40 | 1.30 | 1.04 | 0.70 | 0.91 | 0.34 | 0.95 |
| Leu | 0.60 | 1.70 | 1.44 | 1.40 | 2.08 | 1.05 | 1.38 |
| Lys | 10.20 | 2.10 | 3.05 | 2.40 | 3.18 | 2.33 | 3.88 |
| Met | <0.05 | 0.50 | 0.11 | ND * | 0.77 | 0.13 | 0.38 |
| Phe | 0.40 | 1.60 | 1.08 | 0.30 | 1.29 | 0.53 | 0.87 |
| Pro | 0.80 | 1.20 | 0.59 | 0.70 | ND | 0.59 | 0.78 |
| Ser | 30.40 | 27.30 | 32.74 | 37.3 | 21.56 | 40.51 | 31.64 |
| Thr | 6.00 | 7.50 | 5.51 | 8.70 | 7.04 | 8.45 | 7.20 |
| Trp | ND * | 0.40 | ND * | ND * | ND * | ND * | 0.40 |
| Tyr | 3.80 | 4.60 | 4.63 | 2.60 | 6.23 | 5.42 | 4.55 |
| Val | 2.60 | 3.80 | 3.14 | 3.60 | 3.36 | 3.56 | 3.34 |
3.2. Physical Properties and Characteristics
3.2.1. Solubility
3.2.2. Molecular Weight
| Method of Extraction/Production | Molecular Weight (kDa) | References |
|---|---|---|
| High temperature | 100–200 | [15] |
| High temperature–high pressure | 25–150 | [14] |
| Urea-based | 10–225 | [14] |
| Acid-based | 50–150 | [14] |
| Alkali-based | 15–75 | [14] |
| Enzymatic | 5–25 | [19] |
| Recombinant protein | ≈25 | [5] |
3.3. Biophysical Characteristics
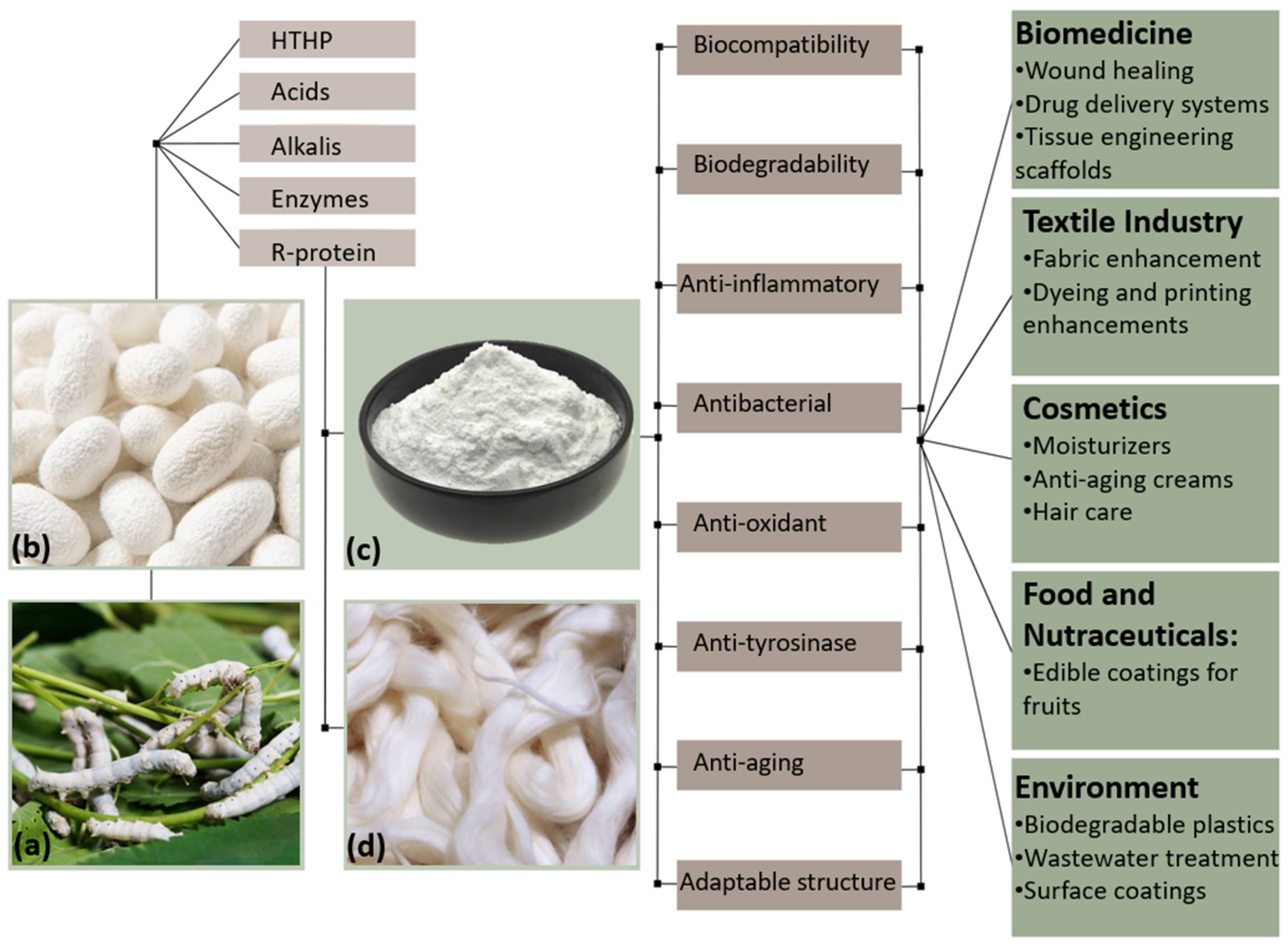
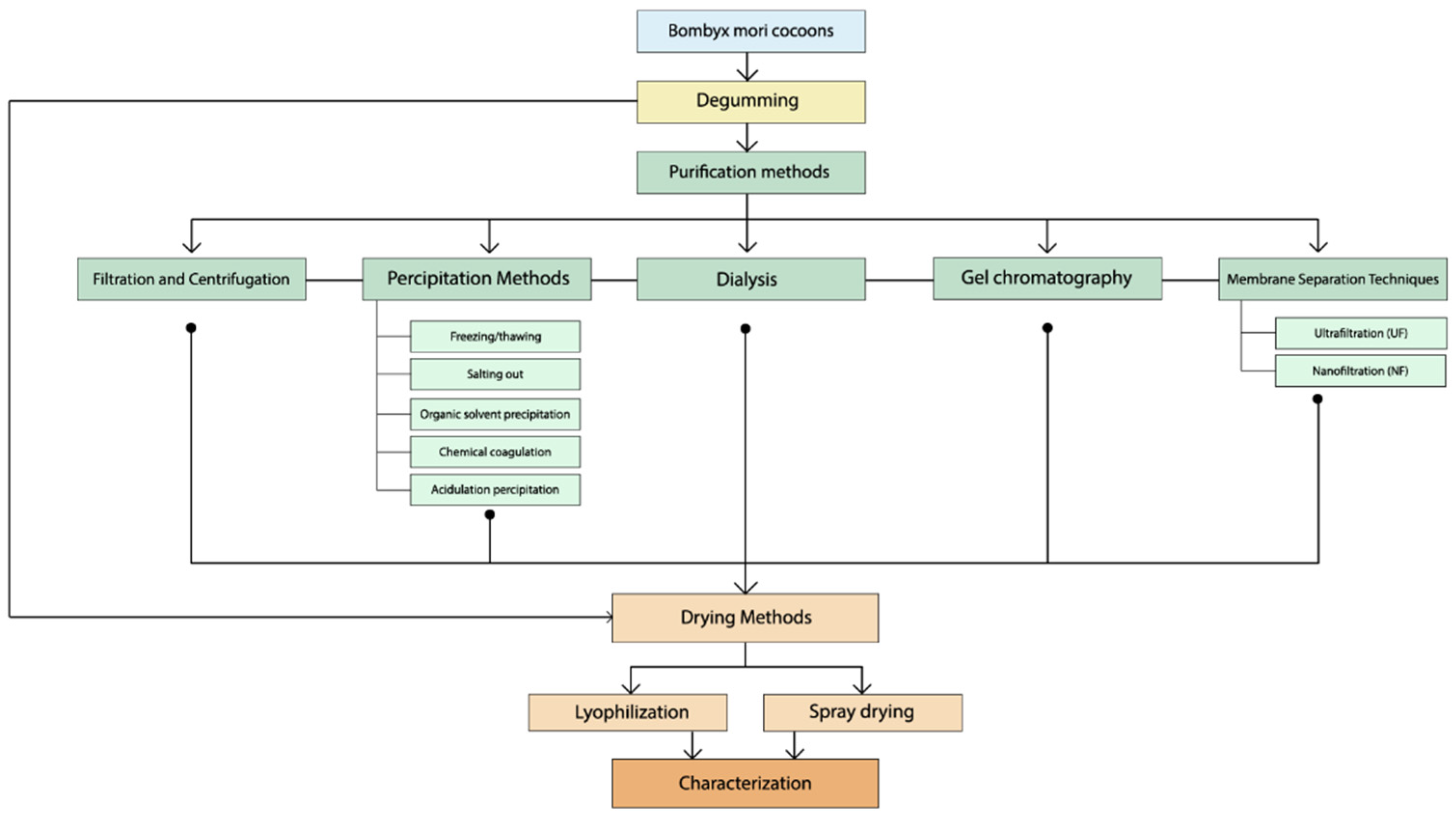
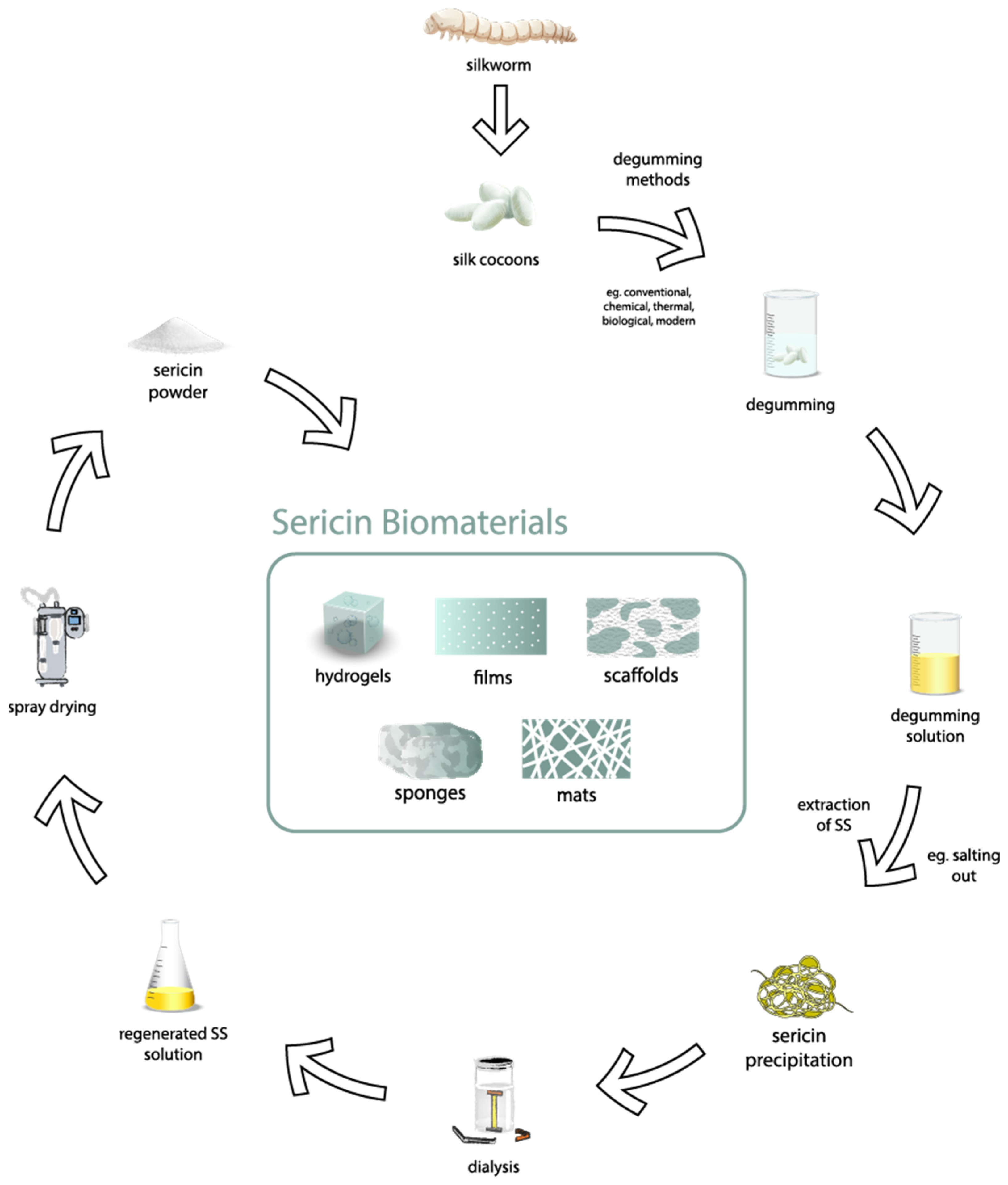
4. Extraction Methods
4.1. Degumming Process Overview
4.2. Conventional Extraction Methods
4.3. Chemical Extraction Methods
4.4. Biological Extraction Methods
4.5. Thermal Extraction Methods
4.6. Modern Extraction Methods
4.7. Sericin Extraction: Impact on Environment, Economy, and Functionality
5. Purification
6. Biological and Medical Applications
6.1. Drug Delivery Systems
| Materials | Medical Condition | Cell/Drug Delivered | Refs. |
|---|---|---|---|
| SS/PAC a | Ulcerative Colitis | Proanthocyanidins | [77] |
| SS@FeS b | Breast Cancer | Nano agent | [78] |
| SS-PLA c | Cancer Therapy | Doxorubicin (DOX) | [86] |
| SSC-NPs d | Cancer Phototherapy | Chlorin e6 (Ce6) | [87] |
| MR-SNC e | Breast Cancer | Resveratrol and Melatonin | [88] |
| Sericin Microparticles- MON | Metastatic Lung Cancer | Doxorubicin (DOX) | |
| Genipin/sericin hydrogels | Ischemic Stroke | neurotrophic cytokines | |
| Zein/sericin nanoblends | Antitumor | 5-Fluorouracil | [89,90] |
| SS-NPs f | Cancer Immunotherapy | Doxorubicin (DOX) and Indocyanine green (ICG) | [91] |
| Cispt-SNC g | Breast Cancer | Cisplatin | [92] |
| Fucoidan and Sericin | Chronic Inflammatory Diseases | Diclofenac sodium (DS) | [93] |
6.2. Tissue Engineering
| Materials | Form | Refs. |
|---|---|---|
| Fibroin, Sericin, Silver Nanoparticles and Gentamicin | Films | [110] |
| Silkworms, Sericin | Scaffold | [111] |
| Polyvinyl alcohol, Sericin, Azithromycin, Genipin | Hydrogel | [66] |
| Sericin, Chitosan, Silver Nanoparticles | Films | [112] |
| Sericin, Human Placenta-derived Extracellular Matrix | Scaffold | [113] |
| Gellan gum, Sericin, Halloysite nanotubes encapsulated with Polydopamine | Hydrogel | [114] |
| Carboxymethyl Chitosan, Sericin–Silver nanoparticles, Halloysite | Sponge | [115] |
| Sericin, Polyvinyl alcohol, Moringa oleifera leaves extract | Hydrogel | [116] |
| Sericin, Jasminum grandiflorum L. leaves extract | Cream | [103] |
| Sericin, heparin, basic fibroblast growth factor (bFGF) | Hydrogel | [117] |
6.3. Other Applications
6.4. Challenges and Limitations
7. Industrial and Commercial Applications
7.1. Textile Industry Impact
7.2. Food Packaging and Nutraceuticals
| Biomaterial | Experimental Method | Added Benefit | References |
|---|---|---|---|
| Glucose | Crosslinking | Overcome limitations in water resistance. Improve mechanical properties. | [185] |
| Glucomannan | Casting | Improve solubility and flexibility. | [189] |
| Glucomannan and glycerol | Casting | Improve solubility and flexibility. Increase water vapor permeability. | [189] |
| Chitosan and aloe vera | Casting | Edible food packaging films. | [181] |
| Bacterial cellulose | Solution impregnation | Improve water intake. | [190] |
| Nano-cellulose | Casting | Improve the mechanical properties. | [183] |
| Glycerol | Casting | Enhancement of elongation properties and increase in moisture content. | [191] |
| ZnONPs and AgNPs on sericin-agarose films a | Casting | Improve water absorption. Enhance mechanical properties. | [192] |
7.3. Cosmetics and Skincare Products
| Company | Product Type Example | References |
|---|---|---|
| Cicago (Englewood Cliffs, NJ, USA) | Facial moisturizer | EWG [198] |
| Drunk Elephant (Houston, TX, USA) | Moisturizing shampoo | EWG [198] |
| Imersa (Denver, CO, USA) | Moisturizing cream | Imersa [199] |
| Benefit (San Francisco, CA, USA) | Mascara | EWG [198] |
| Fondonatura (San Donato di Lecce, Italy) | Hair smotherer | Fondonatura [200] |
| J. And. C. (Como, Italy) | Facial cream | J&C [201] |
8. Discussions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-Based Biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Kunz, R.I.; Brancalhão, R.M.C.; Ribeiro, L.D.F.C.; Natali, M.R.M. Silkworm Sericin: Properties and Biomedical Applications. BioMed Res. Int. 2016, 2016, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.; Yeo, J.; Lee, K.; Park, Y.H.; Nahm, J.-H.; Cho, C. Effects of Poloxamer on the Gelation of Silk Sericin. Macromol. Rapid Commun. 2000, 21, 1302–1305. [Google Scholar] [CrossRef]
- Takasu, Y.; Yamada, H.; Tsubouchi, K. Isolation of Three Main Sericin Components from the Cocoon of the Silkworm, Bombyx mori. Biosci. Biotechnol. Biochem. 2002, 66, 2715–2718. [Google Scholar] [CrossRef]
- Das, G.; Shin, H.-S.; Campos, E.V.R.; Fraceto, L.F.; Del Pilar Rodriguez-Torres, M.; Mariano, K.C.F.; De Araujo, D.R.; Fernández-Luqueño, F.; Grillo, R.; Patra, J.K. Sericin Based Nanoformulations: A Comprehensive Review on Molecular Mechanisms of Interaction with Organisms to Biological Applications. J. Nanobiotechnology 2021, 19, 30. [Google Scholar] [CrossRef]
- Sasaki, M.; Yamada, H.; Kato, N. Consumption of Silk Protein, Sericin Elevates Intestinal Absorption of Zinc, Iron, Magnesium and Calcium in Rats. Nutr. Res. 2000, 20, 1505–1511. [Google Scholar] [CrossRef]
- Wu, J.-H.; Wang, Z.; Xu, S.-Y. Preparation and Characterization of Sericin Powder Extracted from Silk Industry Wastewater. Food Chem. 2007, 103, 1255–1262. [Google Scholar] [CrossRef]
- Sothornvit, R.; Chollakup, R.; Suwanruji, P. Extracted Sericin from Silk Waste for Film Formation. Songklanakarin J. Sci. Technol. 2010, 32, 17–22. [Google Scholar]
- Osorio, A.R. Generalidades de la seda y su proceso de teñido. Prospectiva 2014, 12, 7. [Google Scholar] [CrossRef][Green Version]
- Züge, L.C.B.; Silva, V.R.; Hamerski, F.; Ribani, M.; Gimenes, M.L.; Scheer, A.P. Emulsifying Properties of Sericin Obtained from Hot Water Degumming Process. J. Food Process Eng. 2017, 40, e12267. [Google Scholar] [CrossRef]
- Jena, K.; Pandey, J.P.; Kumari, R.; Sinha, A.K.; Gupta, V.P.; Singh, G.P. Tasar Silk Fiber Waste Sericin: New Source for Anti-Elastase, Anti-Tyrosinase and Anti-Oxidant Compounds. Int. J. Biol. Macromol. 2018, 114, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.T.B.; Smith, S.G. Amino-Acids of Silk Sericin. Nature 1951, 168, 745. [Google Scholar] [CrossRef]
- Aramwit, P.; Siritientong, T.; Srichana, T. Potential Applications of Silk Sericin, a Natural Protein from Textile Industry by-Products. Waste Manag. Res. J. Sustain. Circ. Econ. 2012, 30, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Sangcakul, A. The Effects of Sericin Cream on Wound Healing in Rats. Biosci. Biotechnol. Biochem. 2007, 71, 2473–2477. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.R.; Ribani, M.; Gimenes, M.L.; Scheer, A.P. High Molecular Weight Sericin Obtained by High Temperature and Ultrafiltration Process. Procedia Eng. 2012, 42, 833–841. [Google Scholar] [CrossRef]
- Bascou, R.; Hardouin, J.; Ben Mlouka, M.A.; Guénin, E.; Nesterenko, A. Detailed Investigation on New Chemical-Free Methods for Silk Sericin Extraction. Mater. Today Commun. 2022, 33, 104491. [Google Scholar] [CrossRef]
- Thomas, D.S.; Manoharan, C.; Rasalkar, S.; Mishra, R.K.; Gopalapillai, R. Recombinant Expression of Sericin-Cecropin Fusion Protein and Its Functional Activity. Biotechnol. Lett. 2020, 42, 1673–1682. [Google Scholar] [CrossRef]
- Zhang, Y.-Q. Applications of Natural Silk Protein Sericin in Biomaterials. Biotechnol. Adv. 2002, 20, 91–100. [Google Scholar] [CrossRef]
- Freddi, G.; Mossotti, R.; Innocenti, R. Degumming of Silk Fabric with Several Proteases. J. Biotechnol. 2003, 106, 101–112. [Google Scholar] [CrossRef]
- Hu, D.; Li, T.; Xu, Z.; Liu, D.; Yang, M.; Zhu, L. Self-Stabilized Silk Sericin-Based Nanoparticles: In Vivo Biocompatibility and Reduced Doxorubicin-Induced Toxicity. Acta Biomater. 2018, 74, 385–396. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Shi, X.; Qin, S.; Liu, J.; Lv, Q.; Liu, J.; Li, Q.; Wang, Z.; Wang, L. Development and Application of an Advanced Biomedical Material-Silk Sericin. Adv. Mater. 2024, 36, 2311593. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.S.; Costa, E.C.; Reis, S.; Spencer, C.; Calhelha, R.C.; Miguel, S.P.; Ribeiro, M.P.; Barros, L.; Vaz, J.A.; Coutinho, P. Silk Sericin: A Promising Sustainable Biomaterial for Biomedical and Pharmaceutical Applications. Polymers 2022, 14, 4931. [Google Scholar] [CrossRef] [PubMed]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthc. Mater. 2019, 8, 1800465. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign Body Reaction to Biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Caldeira, E.S. Biofuncionalização Do Algodão Com LCisteína: Estudo Do Efeito Antibacteriano e Dos Mecanismos de Acção Contra S. Aureus e K. Pneumoniae. Dissertação para Obtenção do Grau de Mestre em Bioquímica (2o Ciclo de Estudos). Master’s Thesis, Universidade Da Beira Interior, Covilhã, Portugal, 2012. [Google Scholar]
- Miguel, G.A.; Álvarez-López, C. Extraction and Antioxidant Activity of Sericin, a Protein from Silk. Braz. J. Food Technol. 2020, 23, e2019058. [Google Scholar] [CrossRef]
- Kitisin, T.; Maneekan, P.; Luplertlop, N. In-Vitro Characterization of Silk Sericin as an Anti-Aging Agent. J. Agric. Sci. 2013, 5, p54. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Arami, M.; Mazaheri, F.; Rahimi, S. Degradation of Sericin (Degumming) of Persian Silk by Ultrasound and Enzymes as a Cleaner and Environmentally Friendly Process. J. Clean. Prod. 2010, 18, 146–151. [Google Scholar] [CrossRef]
- Babu, K.M. The Dyeing of Silk. In Silk; Elsevier: Amsterdam, The Netherlands, 2019; pp. 109–128. ISBN 978-0-08-102540-6. [Google Scholar]
- Yun, H.; Oh, H.; Kim, M.K.; Kwak, H.W.; Lee, J.Y.; Um, I.C.; Vootla, S.K.; Lee, K.H. Extraction Conditions of Antheraea Mylitta Sericin with High Yields and Minimum Molecular Weight Degradation. Int. J. Biol. Macromol. 2013, 52, 59–65. [Google Scholar] [CrossRef]
- Lamboni, L.; Gauthier, M.; Yang, G.; Wang, Q. Silk Sericin: A Versatile Material for Tissue Engineering and Drug Delivery. Biotechnol. Adv. 2015, 33, 1855–1867. [Google Scholar] [CrossRef]
- Gulrajani, M.L. Degumming of Silk. Rev. Prog. Color. Relat. Top. 1992, 22, 79–89. [Google Scholar] [CrossRef]
- Gulrajani, M.L.; Purwar, R.; Prasad, R.K.; Joshi, M. Studies on Structural and Functional Properties of Sericin Recovered from Silk Degumming Liquor by Membrane Technology. J. Appl. Polym. Sci. 2009, 113, 2796–2804. [Google Scholar] [CrossRef]
- More, S.V.; Chavan, S.; Prabhune, A.A. Silk Degumming and Utilization of Silk Sericin by Hydrolysis Using Alkaline Protease from Beauveria sp. (MTCC 5184): A Green Approach. J. Nat. Fibers 2018, 15, 373–383. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, Y.; Shi, Z.; Jiang, W.; Liu, X.; Ni, Q.-Q. Degumming of Raw Silk via Steam Treatment. J. Clean. Prod. 2018, 203, 492–497. [Google Scholar] [CrossRef]
- Walters, R.H.; Hougen, O.A. Silk Degumming: I. Degradation of Silk Sericin by Alkalies. Text. Res. 1934, 5, 92–104. [Google Scholar] [CrossRef]
- Çapar, G.; Aygün, S.S. Characterization of Sericin Protein Recovered from Silk Wastewaters. Turk. Bull. Hyg. Exp. Biol. 2015, 72, 219–234. [Google Scholar] [CrossRef]
- Rangi, A.; Jajpura, L. The Biopolymer Sericin: Extraction and Applications. J. Text. Sci. Eng. 2015, 5, 1–5. [Google Scholar] [CrossRef]
- Aramwit, P.; Kanokpanont, S.; Nakpheng, T.; Srichana, T. The Effect of Sericin from Various Extraction Methods on Cell Viability and Collagen Production. Int. J. Mol. Sci. 2010, 11, 2200–2211. [Google Scholar] [CrossRef]
- Aramwit, P.; Damrongsakkul, S.; Kanokpanont, S.; Srichana, T. Properties and Antityrosinase Activity of Sericin from Various Extraction Methods. Biotechnol. Appl. Biochem. 2010, 55, 91–98. [Google Scholar] [CrossRef]
- Yang, M.; Shuai, Y.; Zhou, G.; Mandal, N.; Zhu, L.; Mao, C. Tuning Molecular Weights of Bombyx mori (B. mori) Silk Sericin to Modify Its Assembly Structures and Materials Formation. ACS Appl. Mater. Interfaces 2014, 6, 13782–13789. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Y.; Gong, K.; Zhou, Q.; Zhang, T.; Li, Q. A Comparative Study of Ultrasonic Degumming of Silk Sericin Using Citric Acid, Sodium Carbonate and Papain. Color. Technol. 2019, 135, 195–201. [Google Scholar] [CrossRef]
- Suwannaphan, S.; Fufeungsombut, E.; Promboon, A.; Chim-anage, P. A Serine Protease from Newly Isolated Bacillus sp. for Efficient Silk Degumming, Sericin Degrading and Colour Bleaching Activities. Int. Biodeterior. Biodegrad. 2017, 117, 141–149. [Google Scholar] [CrossRef]
- Devi, R. Biotechnological Application of Proteolytic Enzymes in Post Cocoon Technology. Int. J. Sci. Nat. 2012, 3, 237–240. [Google Scholar]
- Nakpathom, M.; Somboon, B.; Narumol, N. Papain Enzymatic Degumming of Thai Bombyx mori Silk Fibers. J. Microsc. Soc. Thail. 2009, 23, 142–146. [Google Scholar]
- Yakul, K.; Takenaka, S.; Nakamura, K.; Techapun, C.; Leksawasdi, N.; Seesuriyachan, P.; Watanabe, M.; Chaiyaso, T. Characterization of Thermostable Alkaline Protease from Bacillus halodurans SE5 and Its Application in Degumming Coupled with Sericin Hydrolysate Production from Yellow Cocoon. Process Biochem. 2019, 78, 63–70. [Google Scholar] [CrossRef]
- Gupta, D.; Agrawal, A.; Chaudhary, H.; Gulrajani, M.; Gupta, C. Cleaner Process for Extraction of Sericin Using Infrared. J. Clean. Prod. 2013, 52, 488–494. [Google Scholar] [CrossRef]
- Krishnamurthy, K.; Khurana, H.K.; Soojin, J.; Irudayaraj, J.; Demirci, A. Infrared Heating in Food Processing: An Overview. Compr. Rev. Food Sci. Food Saf. 2008, 7, 2–13. [Google Scholar] [CrossRef]
- Chirila, T.V.; Suzuki, S.; McKirdy, N.C. Further Development of Silk Sericin as a Biomaterial: Comparative Investigation of the Procedures for Its Isolation from Bombyx mori Silk Cocoons. Prog. Biomater. 2016, 5, 135–145. [Google Scholar] [CrossRef]
- Lo, C.-H.; Chao, Y. Degumming of Silk Fibers by CO2 Supercritical Fluid. J. Mater. Sci. Chem. Eng. 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Kumar, J.P.; Mandal, B.B. Antioxidant Potential of Mulberry and Non-Mulberry Silk Sericin and Its Implications in Biomedicine. Free Radic. Biol. Med. 2017, 108, 803–818. [Google Scholar] [CrossRef]
- Kurioka, A.; Kurioka, F.; Yamazaki, M. Characterization of Sericin Powder Prepared from Citric Acid-Degraded Sericin Polypeptides of the Silkworm, Bombyx mori. Biosci. Biotechnol. Biochem. 2004, 68, 774–780. [Google Scholar] [CrossRef]
- Yang, Y.; Lee, S.M.; Lee, H.S.; Lee, K.H. Recovery of Silk Sericin from Soap-Alkaline Degumming Solution. Int. J. Ind. Entomol. 2013, 27, 203–208. [Google Scholar] [CrossRef]
- Da Silva, T.L.; Junior, A.D.S.; Ribani, M.; Vieira, M.G.A.; Gimenes, M.L.; Da Silva, M.G. Evaluation of Molecular Weight Distribution of Sericin in Solutions Concentrated via Precipitation by Ethanol and Precipitation by Freezing/Thawing. Chem. Eng. Trans. 2014, 38, 103–108. [Google Scholar] [CrossRef]
- Sone, A.P.; Gimenes, M.L.; Hoscheid, J.; Tominc, G.C.; Dalmagro, M.; Zardeto, G.; Donadel, G.; Morejon, C.F.M. Purification and Drying Methods of Sericin from Bombyx mori Cocoons. Nat. Prod. Res. 2023, 37, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Takasu, Y.; Hiromi, R.; Kozo, T. Extraction and Chromatographic Analysis of Cocoon Sericin of the Silkworm, Bombyx mori. J. Insect Biotechnol. Sericology 2002, 71, 151–156. [Google Scholar] [CrossRef]
- Vaithanomsat, P.; Kitpreechavanich, V. Sericin Separation from Silk Degumming Wastewater. Sep. Purif. Technol. 2008, 59, 129–133. [Google Scholar] [CrossRef]
- Genc, G.; Narin, G.; Bayraktar, O. Spray drying as a method of producing silk sericin powders. J. Achiev. Mater. Manuf. Eng. 2009, 37, 78–86. [Google Scholar]
- Rocha, L.K.H.; Favaro, L.I.L.; Rios, A.C.; Silva, E.C.; Silva, W.F.; Stigliani, T.P.; Guilger, M.; Lima, R.; Oliveira, J.M.; Aranha, N.; et al. Sericin from Bombyx mori Cocoons. Part I: Extraction and Physicochemical-Biological Characterization for Biopharmaceutical Applications. Process Biochem. 2017, 61, 163–177. [Google Scholar] [CrossRef]
- Hong, S.M.; Choi, S.C.; Park, H.M.; Seok, Y.S. Preparation and Characterization of Sericin Powder Extracted with Deep Sea Water. 3 Biotech 2019, 9, 30. [Google Scholar] [CrossRef]
- Baptista-Silva, S.; Borges, S.; Costa-Pinto, A.R.; Costa, R.; Amorim, M.; Dias, J.R.; Ramos, Ó.; Alves, P.; Granja, P.L.; Soares, R.; et al. In Situ Forming Silk Sericin-Based Hydrogel: A Novel Wound Healing Biomaterial. ACS Biomater. Sci. Eng. 2021, 7, 1573–1586. [Google Scholar] [CrossRef]
- Chen, C.-S.; Zeng, F.; Xiao, X.; Wang, Z.; Li, X.-L.; Tan, R.-W.; Liu, W.-Q.; Zhang, Y.-S.; She, Z.-D.; Li, S.-J. Three-Dimensionally Printed Silk-Sericin-Based Hydrogel Scaffold: A Promising Visualized Dressing Material for Real-Time Monitoring of Wounds. ACS Appl. Mater. Interfaces 2018, 10, 33879–33890. [Google Scholar] [CrossRef]
- Han, C.M.; Kim, T.W.; Choi, H.W. Assessment of Laser Joining Quality by Visual Inspection, Computer Simulation, and Deep Learning. Appl. Sci. 2021, 11, 642. [Google Scholar] [CrossRef]
- Sapru, S.; Das, S.; Mandal, M.; Ghosh, A.K.; Kundu, S.C. Sericin-Chitosan-Glycosaminoglycans Hydrogels Incorporated with Growth Factors for in Vitro and in Vivo Skin Repair. Carbohydr. Polym. 2021, 258, 117717. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Cai, R.; Wang, Y.; Zuo, H.; He, H. Fabrication of Antibacterial Sericin Based Hydrogel as an Injectable and Mouldable Wound Dressing. Mater. Sci. Eng. C 2021, 119, 111597. [Google Scholar] [CrossRef] [PubMed]
- Bakadia, B.M.; Lamboni, L.; Qaed Ahmed, A.A.; Zheng, R.; Ode Boni, B.O.; Shi, Z.; Song, S.; Souho, T.; Mukole, B.M.; Qi, F.; et al. Antibacterial Silk Sericin/Poly (Vinyl Alcohol) Hydrogel with Antifungal Property for Potential Infected Large Burn Wound Healing: Systemic Evaluation. Smart Mater. Med. 2023, 4, 37–58. [Google Scholar] [CrossRef]
- Tyeb, S.; Kumar, N.; Kumar, A.; Verma, V. Flexible Agar-Sericin Hydrogel Film Dressing for Chronic Wounds. Carbohydr. Polym. 2018, 200, 572–582. [Google Scholar] [CrossRef]
- Gök, Z.G.; Yiğitoğlu, M.; Vargel, İ.; Şahin, Y.; Alçığır, M.E. Synthesis, Characterization and Wound Healing Ability of PET Based Nanofiber Dressing Material Coated with Silk Sericin Capped-Silver Nanoparticles. Mater. Chem. Phys. 2021, 259, 124043. [Google Scholar] [CrossRef]
- Akturk, O.; Tezcaner, A.; Bilgili, H.; Deveci, M.S.; Gecit, M.R.; Keskin, D. Evaluation of Sericin/Collagen Membranes as Prospective Wound Dressing Biomaterial. J. Biosci. Bioeng. 2011, 112, 279–288. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, R.; Tao, G.; Wang, P.; Zuo, H.; Zhao, P.; Umar, A.; He, H. A Novel AgNPs/Sericin/Agar Film with Enhanced Mechanical Property and Antibacterial Capability. Molecules 2018, 23, 1821. [Google Scholar] [CrossRef]
- Nayak, S.; Talukdar, S.; Kundu, S.C. Potential of 2D Crosslinked Sericin Membranes with Improved Biostability for Skin Tissue Engineering. Cell Tissue Res. 2012, 347, 783–794. [Google Scholar] [CrossRef]
- Anand, S.; Pandey, P.; Begum, M.Y.; Chidambaram, K.; Arya, D.K.; Gupta, R.K.; Sankhwar, R.; Jaiswal, S.; Thakur, S.; Rajinikanth, P.S. Electrospun Biomimetic Multifunctional Nanofibers Loaded with Ferulic Acid for Enhanced Antimicrobial and Wound-Healing Activities in STZ-Induced Diabetic Rats. Pharmaceuticals 2022, 15, 302. [Google Scholar] [CrossRef]
- Siritientong, T.; Angspatt, A.; Ratanavaraporn, J.; Aramwit, P. Clinical Potential of a Silk Sericin-Releasing Bioactive Wound Dressing for the Treatment of Split-Thickness Skin Graft Donor Sites. Pharm. Res. 2014, 31, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Peng, W.; Xiao, H.; Wu, X.; Chen, Y. Numerical Simulation and Microscopic Stress Mechanism for the Microscopic Pore Deformation during Soil Compression. Adv. Civ. Eng. 2019, 2019, 1542797. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, A.; Chauhan, N.S.; Yadav, G.; Goswami, M.; Packirisamy, G. Design and Fabrication of a Dual Protein-Based Trilayered Nanofibrous Scaffold for Efficient Wound Healing. ACS Appl. Bio Mater. 2022, 5, 2726–2740. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; He, H.; Wang, P.; Cai, R.; Tao, G.; Yang, M.; Liu, L.; Zuo, H.; Zhao, P.; Wang, Y. Rational Design and Fabrication of ZnONPs Functionalized Sericin/PVA Antimicrobial Sponge. Int. J. Mol. Sci. 2019, 20, 4796. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Han, X.; Liu, S.; Gao, X.; Guo, C.; Wu, X. Silk Sericin Stabilized Proanthocyanidins for Synergetic Alleviation of Ulcerative Colitis. Int. J. Biol. Macromol. 2022, 220, 1021–1030. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Wang, Y.; Zhang, X.; Zhang, X. Silk Sericin-Decorated Supramolecular Photothermal Nanocatalyst-Based Ferric Sulfide for Boosting High Therapeutic Performance of Tumor Cells. J. Drug Deliv. Sci. Technol. 2022, 69, 103104. [Google Scholar] [CrossRef]
- Yalcin, E.; Kara, G.; Celik, E.; Pinarli, F.A.; Saylam, G.; Sucularli, C.; Ozturk, S.; Yilmaz, E.; Bayir, O.; Korkmaz, M.H.; et al. Preparation and Characterization of Novel Albumin-Sericin Nanoparticles as siRNA Delivery Vehicle for Laryngeal Cancer Treatment. Prep. Biochem. Biotechnol. 2019, 49, 659–670. [Google Scholar] [CrossRef]
- Suktham, K.; Koobkokkruad, T.; Wutikhun, T.; Surassmo, S. Efficiency of Resveratrol-Loaded Sericin Nanoparticles: Promising Bionanocarriers for Drug Delivery. Int. J. Pharm. 2018, 537, 48–56. [Google Scholar] [CrossRef]
- Hardy, J.G.; Römer, L.M.; Scheibel, T.R. Polymeric Materials Based on Silk Proteins. Polymer 2008, 49, 4309–4327. [Google Scholar] [CrossRef]
- Huang, W.; Ling, S.; Li, C.; Omenetto, F.G.; Kaplan, D.L. Silkworm Silk-Based Materials and Devices Generated Using Bio-Nanotechnology. Chem. Soc. Rev. 2018, 47, 6486–6504. [Google Scholar] [CrossRef]
- Nishida, A.; Yamada, M.; Kanazawa, T.; Takashima, Y.; Ouchi, K.; Okada, H. Sustained-Release of Protein from Biodegradable Sericin Film, Gel and Sponge. Int. J. Pharm. 2011, 407, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.C.; Dash, B.C.; Dash, R.; Kaplan, D.L. Natural Protective Glue Protein, Sericin Bioengineered by Silkworms: Potential for Biomedical and Biotechnological Applications. Prog. Polym. Sci. 2008, 33, 998–1012. [Google Scholar] [CrossRef]
- Oh, H.; Lee, J.Y.; Kim, A.; Ki, C.S.; Kim, J.W.; Park, Y.H.; Lee, K.H. Preparation of Silk Sericin Beads Using LiCl/DMSO Solvent and Their Potential as a Drug Carrier for Oral Administration. Fibers Polym. 2007, 8, 470–476. [Google Scholar] [CrossRef]
- Boonpavanitchakul, K.; Bast, L.K.; Bruns, N.; Magaraphan, R. Silk Sericin-Polylactide Protein–Polymer Conjugates as Biodegradable Amphiphilic Materials and Their Application in Drug Release Systems. Bioconjug. Chem. 2020, 31, 2312–2324. [Google Scholar] [CrossRef]
- Gao, Y.-E.; Hou, S.; Cheng, J.; Li, X.; Wu, Y.; Tang, Y.; Li, Y.; Xue, P.; Kang, Y.; Xu, Z.; et al. Silk Sericin-Based Nanoparticle as the Photosensitizer Chlorin E6 Carrier for Enhanced Cancer Photodynamic Therapy. ACS Sustain. Chem. Eng. 2021, 9, 3213–3222. [Google Scholar] [CrossRef]
- Aghaz, F.; Asadi, Z.; Sajadimajd, S.; Kashfi, K.; Arkan, E.; Rahimi, Z. Codelivery of Resveratrol Melatonin Utilizing pH Responsive Sericin Based Nanocarriers Inhibits the Proliferation of Breast Cancer Cell Line at the Different pH. Sci. Rep. 2023, 13, 11090. [Google Scholar] [CrossRef]
- Gagliardi, A.; Ambrosio, N.; Voci, S.; Salvatici, M.C.; Fresta, M.; Cosco, D. Easy Preparation, Characterization and Cytotoxic Investigation of 5-Fluorouracil-Loaded Zein/Sericin Nanoblends. J. Mol. Liq. 2022, 366, 120344. [Google Scholar] [CrossRef]
- Zhao, H.; He, L. Fabrication of Neuroprotective Silk-Sericin Hydrogel: Potential Neuronal Carrier for the Treatment and Care of Ischemic Stroke. J. Exp. Nanosci. 2022, 17, 362–376. [Google Scholar] [CrossRef]
- Li, X.; Ye, M.; Huang, R.; Hou, S.; Xu, J.; Qiu, W.; Liang, M.; Gao, Y.; Zhang, H.; Xue, P.; et al. An Acid-Engineered Sericin Nanoplatform Enhances Photothermal Conversion and Chemotherapy Outcome for Inducing Immunogenic Cell Death. Chem. Eng. J. 2023, 477, 146938. [Google Scholar] [CrossRef]
- Bahremand, K.; Aghaz, F.; Bahrami, K. Enhancing Cisplatin Efficacy with Low Toxicity in Solid Breast Cancer Cells Using pH-Charge-Reversal Sericin-Based Nanocarriers: Development, Characterization, and In Vitro Biological Assessment. ACS Omega 2024, 9, 14017–14032. [Google Scholar] [CrossRef]
- Gagliardi, A.; Chiarella, E.; Voci, S.; Ambrosio, N.; Celano, M.; Salvatici, M.C.; Cosco, D. DIFUCOSIN: DIclofenac Sodium Salt Loaded FUCOidan-SericIN Nanoparticles for the Management of Chronic Inflammatory Diseases. Int. J. Pharm. 2024, 655, 124034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Huang, L.; Wang, Z.; Wang, L. Design and Performance of a Sericin-Alginate Interpenetrating Network Hydrogel for Cell and Drug Delivery. Sci. Rep. 2015, 5, 12374. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Dey, T.; Kundu, S.C. Fabrication of Sericin Nanoparticles for Controlled Gene Delivery. RSC Adv. 2014, 4, 2137–2142. [Google Scholar] [CrossRef]
- Deng, L.; Guo, W.; Li, G.; Hu, Y.; Zhang, L.-M. Hydrophobic IR780 Loaded Sericin Nanomicelles for Phototherapy with Enhanced Antitumor Efficiency. Int. J. Pharm. 2019, 566, 549–556. [Google Scholar] [CrossRef]
- Huang, L.; Tao, K.; Liu, J.; Qi, C.; Xu, L.; Chang, P.; Gao, J.; Shuai, X.; Wang, G.; Wang, Z.; et al. Design and Fabrication of Multifunctional Sericin Nanoparticles for Tumor Targeting and pH-Responsive Subcellular Delivery of Cancer Chemotherapy Drugs. ACS Appl. Mater. Interfaces 2016, 8, 6577–6585. [Google Scholar] [CrossRef]
- Kumar, S.S.D.; Abrahamse, H. Sericin-Based Nanomaterials and Their Applications in Drug Delivery. In Bio-Based Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 211–229. ISBN 978-0-323-85148-0. [Google Scholar]
- Wang, Z.; Zhang, Y.; Zhang, J.; Huang, L.; Liu, J.; Li, Y.; Zhang, G.; Kundu, S.C.; Wang, L. Exploring Natural Silk Protein Sericin for Regenerative Medicine: An Injectable, Photoluminescent, Cell-Adhesive 3D Hydrogel. Sci. Rep. 2014, 4, 7064. [Google Scholar] [CrossRef]
- Hu, D.; Xu, Z.; Hu, Z.; Hu, B.; Yang, M.; Zhu, L. pH-Triggered Charge-Reversal Silk Sericin-Based Nanoparticles for Enhanced Cellular Uptake and Doxorubicin Delivery. ACS Sustain. Chem. Eng. 2017, 5, 1638–1647. [Google Scholar] [CrossRef]
- Felgueiras, H.P.; Amorim, M.T.P. Functionalization of Electrospun Polymeric Wound Dressings with Antimicrobial Peptides. Colloids Surf. B Biointerfaces 2017, 156, 133–148. [Google Scholar] [CrossRef]
- Noosak, C.; Jantorn, P.; Meesane, J.; Voravuthikunchai, S.; Saeloh, D. Dual-Functional Bioactive Silk Sericin for Osteoblast Responses and Osteomyelitis Treatment. PLoS ONE 2022, 17, e0264795. [Google Scholar] [CrossRef]
- Ahmed, A.B.; Tahir, H.M.; Yousaf, M.S.; Munir, F.; Ali, S. Efficacy of Silk Sericin and Jasminum Grandiflorum L. Leaf Extract on Skin Injuries Induced by Burn in Mice. J. Burn Care Res. 2023, 44, 58–64. [Google Scholar] [CrossRef]
- Jiang, L.-B.; Ding, S.-L.; Ding, W.; Su, D.-H.; Zhang, F.-X.; Zhang, T.-W.; Yin, X.-F.; Xiao, L.; Li, Y.-L.; Yuan, F.-L.; et al. Injectable Sericin Based Nanocomposite Hydrogel for Multi-Modal Imaging-Guided Immunomodulatory Bone Regeneration. Chem. Eng. J. 2021, 418, 129323. [Google Scholar] [CrossRef]
- Veiga, A.; Castro, F.; Reis, C.C.; Sousa, A.; Oliveira, A.L.; Rocha, F. Hydroxyapatite/Sericin Composites: A Simple Synthesis Route under near-Physiological Conditions of Temperature and pH and Preliminary Study of the Effect of Sericin on the Biomineralization Process. Mater. Sci. Eng. C 2020, 108, 110400. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Wang, P.; Ma, Z.; Peng, L.; Guo, C.; Fu, Y.; Ding, L. Lupeol-Loaded Chitosan-Ag+ Nanoparticle/Sericin Hydrogel Accelerates Wound Healing and Effectively Inhibits Bacterial Infection. Int. J. Biol. Macromol. 2023, 243, 125310. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Omidi, M.; Chen, X. Development of the PVA/CS Nanofibers Containing Silk Protein Sericin as a Wound Dressing: In Vitro and in Vivo Assessment. Int. J. Biol. Macromol. 2020, 149, 513–521. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, C.; Zhu, Y.; Liu, W.; Li, H.; Wang, L.; Chen, W.; Wang, Z.; Wang, L. Lamprey-Teeth-Inspired Oriented Antibacterial Sericin Microneedles for Infected Wound Healing Improvement. Nano Lett. 2022, 22, 2702–2711. [Google Scholar] [CrossRef]
- Abdulghani, S.; Mitchell, G. Biomaterials for In Situ Tissue Regeneration: A Review. Biomolecules 2019, 9, 750. [Google Scholar] [CrossRef]
- Schäfer, S.; Smeets, R.; Köpf, M.; Drinic, A.; Kopp, A.; Kröger, N.; Hartjen, P.; Assaf, A.T.; Aavani, F.; Beikler, T.; et al. Antibacterial Properties of Functionalized Silk Fibroin and Sericin Membranes for Wound Healing Applications in Oral and Maxillofacial Surgery. Biomater. Adv. 2022, 135, 212740. [Google Scholar] [CrossRef]
- Li, Y.; Wu, T.; Zhang, G.; Fang, A.; Li, Y.; Wang, S.; Yan, H.; Liang, P.; Lian, J.; Zhang, Y. A Native Sericin Wound Dressing Spun Directly from Silkworms Enhances Wound Healing. Colloids Surf. B Biointerfaces 2023, 225, 113228. [Google Scholar] [CrossRef]
- Karthick, S.A.; Manjari, K.; Devi, M.G. Biocompatible and Bioactive PVA/Sericin/Chitosan Nanofibrous Wound Dressing Matrix. Appl. Surf. Sci. Adv. 2023, 13, 100362. [Google Scholar] [CrossRef]
- Bhoopathy, J.; Dharmalingam, S.; Sathyaraj, W.V.; Rajendran, S.; Rymbai, S.; Senthil, R.; Atchudan, R. Sericin/Human Placenta-Derived Extracellular Matrix Scaffolds for Cutaneous Wound Treatment—Preparation, Characterization, In Vitro and In Vivo Analyses. Pharmaceutics 2023, 15, 362. [Google Scholar] [CrossRef]
- Yuan, L.; Jiang, X.; Jiang, M.; Guo, Y.; Liu, Y.; Ming, P.; Li, S.; Zhou, P.; Cai, R.; Yu, K.; et al. Biocompatible Gellan Gum/Sericin Hydrogels Containing Halloysite@polydopamine Nanotubes with Hemostasis and Photothermal Antibacterial Properties for Promoting Infectious Wound Repair. Mater. Des. 2023, 227, 111744. [Google Scholar] [CrossRef]
- Li, S.; Jiang, M.; Zhang, Y.; Xie, X.; Li, W.; Ming, P.; Jiang, X.; Yang, B.; He, Y.; Chen, J.; et al. Multi-Functional Carboxymethyl Chitosan/Sericin Protein/Halloysite Composite Sponge with Efficient Antibacterial and Hemostatic Properties for Accelerating Wound Healing. Int. J. Biol. Macromol. 2023, 234, 123357. [Google Scholar] [CrossRef] [PubMed]
- Puspitasari, D.; Anwar, A.M.; Ananda, D.S.G.; Reza, G.; Jusuf, A.; Asri, L.A.T.W. Porous Sericin/PVA/Moringa Oleifera Hydrogels: Physical Properties and Hyperelastic Model. Procedia Struct. Integr. 2024, 52, 410–417. [Google Scholar] [CrossRef]
- Du, P.; Diao, L.; Lu, Y.; Liu, C.; Li, J.; Chen, Y.; Chen, J.; Lv, G.; Chen, X. Heparin-Based Sericin Hydrogel–Encapsulated Basic Fibroblast Growth Factor for in Vitro and in Vivo Skin Repair. Heliyon 2023, 9, e13554. [Google Scholar] [CrossRef]
- Valletregi, M. Calcium Phosphates as Substitution of Bone Tissues. Prog. Solid State Chem. 2004, 32, 1–31. [Google Scholar] [CrossRef]
- Cai, Y.; Jin, J.; Mei, D.; Xia, N.; Yao, J. Effect of Silk Sericin on Assembly of Hydroxyapatite Nanocrystals into Enamel Prism-like Structure. J. Mater. Chem. 2009, 19, 5751. [Google Scholar] [CrossRef]
- Veiga, A.; Castro, F.; Rocha, F.; Oliveira, A.L. Protein-Based Hydroxyapatite Materials: Tuning Composition toward Biomedical Applications. ACS Appl. Bio Mater. 2020, 3, 3441–3455. [Google Scholar] [CrossRef]
- Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef]
- Li, W.; Cai, Y.; Zhong, Q.; Yang, Y.; Kundu, S.C.; Yao, J. Silk Sericin Microcapsules with Hydroxyapatite Shells: Protection and Modification of Organic Microcapsules by Biomimetic Mineralization. J. Mater. Chem. B 2016, 4, 340–347. [Google Scholar] [CrossRef]
- Jia, F.; Liu, X.; Li, L.; Mallapragada, S.; Narasimhan, B.; Wang, Q. Multifunctional Nanoparticles for Targeted Delivery of Immune Activating and Cancer Therapeutic Agents. J. Control. Release 2013, 172, 1020–1034. [Google Scholar] [CrossRef]
- Ming, P.; Rao, P.; Wu, T.; Yang, J.; Lu, S.; Yang, B.; Xiao, J.; Tao, G. Biomimetic Design and Fabrication of Sericin-Hydroxyapatite Based Membranes With Osteogenic Activity for Periodontal Tissue Regeneration. Front. Bioeng. Biotechnol. 2022, 10, 899293. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Hou, K.; Chen, W.; Wang, Y.; Wang, R.; Tian, C.; Xu, S.; Ji, Y.; Yang, Q.; Zhao, P.; et al. Transgenic PDGF-BB/Sericin Hydrogel Supports for Cell Proliferation and Osteogenic Differentiation. Biomater. Sci. 2020, 8, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Deng, Y.; Xu, L.; Yang, C.; Zhu, Y.; Wang, G.; Wang, Z.; Wang, L. A Sericin/Graphene Oxide Composite Scaffold as a Biomimetic Extracellular Matrix for Structural and Functional Repair of Calvarial Bone. Theranostics 2020, 10, 741–756. [Google Scholar] [CrossRef] [PubMed]
- Griffanti, G.; McKee, M.D.; Nazhat, S.N. Mineralization of Bone Extracellular Matrix-like Scaffolds Fabricated as Silk Sericin-Functionalized Dense Collagen–Fibrin Hybrid Hydrogels. Pharmaceutics 2023, 15, 1087. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, H.; Qin, S.; Yang, C.; Lv, Q.; Wang, Z.; Wang, L. Antibacterial Sericin Cryogels Promote Hemostasis by Facilitating the Activation of Coagulation Pathway and Platelets. Adv. Healthc. Mater. 2022, 11, 2102717. [Google Scholar] [CrossRef]
- Liang, M.; Li, Z.; Gao, C.; Wang, F.; Chen, Z. Preparation and Characterization of Gelatin/Sericin/Carboxymethyl Chitosan Medical Tissue Glue. J. Appl. Biomater. Funct. Mater. 2018, 16, 97–106. [Google Scholar] [CrossRef]
- Liu, H.-X.; Yan, H.-L.; Jia, N.; Tang, S.; Cong, D.; Yang, B.; Li, Z.; Zhang, Y.; Esling, C.; Zhao, X.; et al. Machine-Learning-Assisted Discovery of Empirical Rule for Inherent Brittleness of Full Heusler Alloys. J. Mater. Sci. Technol. 2022, 131, 1–13. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Jin, Y.; Luo, Z.; Yang, W.; Xie, H.; Huang, K.; Wang, L. A Neuroprotective Sericin Hydrogel As an Effective Neuronal Cell Carrier for the Repair of Ischemic Stroke. ACS Appl. Mater. Interfaces 2015, 7, 24629–24640. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Song, Y.; Su, Q.; Xiaohalati, X.; Yang, W.; Xu, L.; Cai, B.; Wang, G.; Wang, Z.; et al. Injectable Silk Sericin Scaffolds with Programmable Shape-Memory Property and Neuro-Differentiation-Promoting Activity for Individualized Brain Repair of Severe Ischemic Stroke. Bioact. Mater. 2021, 6, 1988–1999. [Google Scholar] [CrossRef]
- Parker, B.J.; Rhodes, D.I.; O’Brien, C.M.; Rodda, A.E.; Cameron, N.R. Nerve Guidance Conduit Development for Primary Treatment of Peripheral Nerve Transection Injuries: A Commercial Perspective. Acta Biomater. 2021, 135, 64–86. [Google Scholar] [CrossRef]
- Xie, H.; Yang, W.; Chen, J.; Zhang, J.; Lu, X.; Zhao, X.; Huang, K.; Li, H.; Chang, P.; Wang, Z.; et al. A Silk Sericin/Silicone Nerve Guidance Conduit Promotes Regeneration of a Transected Sciatic Nerve. Adv. Healthc. Mater. 2015, 4, 2195–2205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, W.; Xie, H.; Wang, H.; Wang, J.; Su, Q.; Li, X.; Song, Y.; Wang, G.; Wang, L.; et al. Sericin Nerve Guidance Conduit Delivering Therapeutically Repurposed Clobetasol for Functional and Structural Regeneration of Transected Peripheral Nerves. ACS Biomater. Sci. Eng. 2019, 5, 1426–1439. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, W.; Xie, H.; Wang, J.; Zhang, L.; Wang, Z.; Wang, L. CNT/Sericin Conductive Nerve Guidance Conduit Promotes Functional Recovery of Transected Peripheral Nerve Injury in a Rat Model. ACS Appl. Mater. Interfaces 2020, 12, 36860–36872. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cheng, W.; Li, F.; Lin, F.; Wang, P.; Gao, X.; Peng, Y.; Liu, Y.; Zhang, H.; Chen, S.; et al. Ocular Factors of Fractal Dimension and Blood Vessel Tortuosity Derived From OCTA in a Healthy Chinese Population. Transl. Vis. Sci. Technol. 2022, 11, 1. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, C.; Zhang, J.; Sun, N.; Huang, K.; Li, H.; Wang, Z.; Huang, K.; Wang, L. An Injectable Silk Sericin Hydrogel Promotes Cardiac Functional Recovery after Ischemic Myocardial Infarction. Acta Biomater. 2016, 41, 210–223. [Google Scholar] [CrossRef]
- Chen, L.; Li, L.; Mo, Q.; Zhang, X.; Chen, C.; Wu, Y.; Zeng, X.; Deng, K.; Liu, N.; Zhu, P.; et al. An Injectable Gelatin/Sericin Hydrogel Loaded with Human Umbilical Cord Mesenchymal Stem Cells for the Treatment of Uterine Injury. Bioeng. Transl. Med. 2023, 8, e10328. [Google Scholar] [CrossRef]
- Guan, C.-Y.; Wang, F.; Zhang, L.; Sun, X.-C.; Zhang, D.; Wang, H.; Xia, H.-F.; Xia, Q.-Y.; Ma, X. Genetically Engineered FGF1-Sericin Hydrogel Material Treats Intrauterine Adhesion and Restores Fertility in Rat. Regen. Biomater. 2022, 9, rbac016. [Google Scholar] [CrossRef]
- Alcalá, A.C.; Contreras, M.A.; Cuevas-Juárez, E.; Ramírez, O.T.; Palomares, L.A. Effect of Sericin, a Silk Derived Protein, on the Amplification of Zika Virus in Insect and Mammalian Cell Cultures. J. Biotechnol. 2022, 353, 28–35. [Google Scholar] [CrossRef]
- Cao, T.-T.; Zhang, Y.-Q. The Potential of Silk Sericin Protein as a Serum Substitute or an Additive in Cell Culture and Cryopreservation. Amino Acids 2017, 49, 1029–1039. [Google Scholar] [CrossRef]
- Satrio, F.A.; Karja, N.W.K.; Setiadi, M.A.; Kaiin, E.M.; Gunawan, M.; Memili, E.; Purwantara, B. Improved Maturation Rate of Bovine Oocytes Following Sericin Supplementation in Collection and Maturation Media. Trop. Anim. Sci. J. 2022, 45, 24–29. [Google Scholar] [CrossRef]
- Satrio, F.A.; Karja, N.W.K.; Setiadi, M.A.; Kaiin, E.M.; Gunawan, M.; Memili, E.; Purwantara, B. Effect of Sericin Supplementation in Collection Medium on Bovine Oocyte Nuclear Maturation. IOP Conf. Ser. Earth Environ. Sci. 2020, 478, 012006. [Google Scholar] [CrossRef]
- Tian, H.; Qi, Q.; Yan, F.; Wang, C.; Hou, F.; Ren, W.; Zhang, L.; Hou, J. Enhancing the Developmental Competence of Prepubertal Lamb Oocytes by Supplementing the in Vitro Maturation Medium with Sericin and the Fibroblast Growth Factor 2—Leukemia Inhibitory Factor - Insulin-like Growth Factor 1 Combination. Theriogenology 2021, 159, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Aghaz, F.; Khazaei, M.; Vaisi-Raygani, A.; Bakhtiyari, M. Cryoprotective Effect of Sericin Supplementation in Freezing and Thawing Media on the Outcome of Cryopreservation in Human Sperm. Aging Male 2020, 23, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Z.; Utheim, T.P.; Moe, M.C.; Aass, H.C.D.; Sapkota, D.; Vallenari, E.M.; Eidet, J.R. The Silk Protein Sericin Promotes Viability of ARPE-19 and Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelial Cells in Vitro. Curr. Eye Res. 2021, 46, 504–514. [Google Scholar] [CrossRef]
- Banafshi, O.; Nasseri, S.; Farhadi, L.; Alasvand, M.; Khadem-Erfan, M.B.; Hosseini, J.; Miraki, S.; Fathi, F. The Effects of Supplemented Sericin on in Vitro Maturation and Preimplantation Development of Mouse Embryos: An Experimental Study. Int. J. Reprod. Biomed. IJRM 2021, 19, 921–928. [Google Scholar] [CrossRef]
- Ratchamak, R.; Ratsiri, T.; Kheawkanha, T.; Vongpralub, T.; Boonkum, W.; Chankitisakul, V. Evaluation of Cryopreserved Boar Semen after Supplementation Sericin Form Silkworm (Bombyx mori) in Semen Extender. Anim. Sci. J. 2020, 91, e13428. [Google Scholar] [CrossRef]
- Song, C.; Yang, Z.; Zhong, M.; Chen, Z. Sericin Protects against Diabetes-Induced Injuries in Sciatic Nerve and Related Nerve Cells. Neural Regen. Res. 2013, 8, 506–513. [Google Scholar] [CrossRef]
- Zhao, J.-G.; Wang, H.-Y.; Wei, Z.-G.; Zhang, Y.-Q. Therapeutic Effects of Ethanolic Extract from the Green Cocoon Shell of Silkworm Bombyx mori on Type 2 Diabetic Mice and Its Hypoglycaemic Mechanism. Toxicol. Res. 2019, 8, 407–420. [Google Scholar] [CrossRef]
- Wei, Z.-Z.; Weng, Y.-J.; Zhang, Y.-Q. Investigation of the Repairing Effect and Mechanism of Oral Degraded Sericin on Liver Injury in Type II Diabetic Rats. Biomolecules 2022, 12, 444. [Google Scholar] [CrossRef]
- Liu, D.; Chen, C.; Wang, D.; Chen, Z.; Song, C. Effect of Sericin on the p38MAPK Signaling Pathway and NLRP3 Inflammasome in the Kidney of Type 2 Diabetic Rats. Exp. Ther. Med. 2020, 20, 267. [Google Scholar] [CrossRef]
- Dong, X.; Zhao, S.-X.; Yin, X.-L.; Wang, H.-Y.; Wei, Z.-G.; Zhang, Y.-Q. Silk Sericin Has Significantly Hypoglycaemic Effect in Type 2 Diabetic Mice via Anti-Oxidation and Anti-Inflammation. Int. J. Biol. Macromol. 2020, 150, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Deori, M.; Devi, D.; Kumari, S.; Hazarika, A.; Kalita, H.; Sarma, R.; Devi, R. Antioxidant Effect of Sericin in Brain and Peripheral Tissues of Oxidative Stress Induced Hypercholesterolemic Rats. Front. Pharmacol. 2016, 7, 319. [Google Scholar] [CrossRef] [PubMed]
- Lapphanichayakool, P.; Sutheerawattananonda, M.; Limpeanchob, N. Hypocholesterolemic Effect of Sericin-Derived Oligopeptides in High-Cholesterol Fed Rats. J. Nat. Med. 2017, 71, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Ampawong, S.; Isarangkul, D.; Reamtong, O.; Aramwit, P. Adaptive Effect of Sericin on Hepatic Mitochondrial Conformation through Its Regulation of Apoptosis, Autophagy and Energy Maintenance: A Proteomics Approach. Sci. Rep. 2018, 8, 14943. [Google Scholar] [CrossRef] [PubMed]
- Rujimongkon, K.; Ampawong, S.; Isarangkul, D.; Reamtong, O.; Aramwit, P. Sericin-Mediated Improvement of Dysmorphic Cardiac Mitochondria from Hypercholesterolaemia Is Associated with Maintaining Mitochondrial Dynamics, Energy Production, and Mitochondrial Structure. Pharm. Biol. 2022, 60, 708–721. [Google Scholar] [CrossRef]
- Song, C.; Liu, D.; Yang, S.; Cheng, L.; Xing, E.; Chen, Z. Sericin Enhances the insulin-PI3K/AKT Signaling Pathway in the Liver of a Type 2 Diabetes Rat Model. Exp. Ther. Med. 2018, 16, 3345–3352. [Google Scholar] [CrossRef]
- Ampawong, S.; Isarangkul, D.; Aramwit, P. Sericin Ameliorated Dysmorphic Mitochondria in High-Cholesterol Diet/Streptozotocin Rat by Antioxidative Property. Exp. Biol. Med. 2017, 242, 411–421. [Google Scholar] [CrossRef]
- Cao, T.-T.; Zhang, Y.-Q. Processing and Characterization of Silk Sericin from Bombyx mori and Its Application in Biomaterials and Biomedicines. Mater. Sci. Eng. C 2016, 61, 940–952. [Google Scholar] [CrossRef]
- Sahu, N.; Pal, S.; Sapru, S.; Kundu, J.; Talukdar, S.; Singh, N.I.; Yao, J.; Kundu, S.C. Non-Mulberry and Mulberry Silk Protein Sericins as Potential Media Supplement for Animal Cell Culture. BioMed Res. Int. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- He, M.; Hu, H.; Wang, P.; Fu, H.; Yuan, J.; Wang, Q.; Fan, X. Preparation of a Bio-Composite of Sericin-g-PMMA via HRP-Mediated Graft Copolymerization. Int. J. Biol. Macromol. 2018, 117, 323–330. [Google Scholar] [CrossRef]
- Hu, H.; Wang, L.; Xu, B.; Wang, P.; Yuan, J.; Yu, Y.; Wang, Q. Construction of a Composite Hydrogel of Silk Sericin via Horseradish Peroxidase-catalyzed Graft Polymerization of poly-PEGDMA. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2643–2655. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, C.; Pizzichini, M.; Spadoni, M.; Zeddita, G. Treatment of Waste Water from Silk Degumming Processes for Protein Recovery and Water Reuse. Desalination 1996, 105, 1–9. [Google Scholar] [CrossRef]
- Fatahian, R.; Fatahian, A.; Fatahian, E.; Fatahian, H. A Critical Review on Application of Silk Sericin and Its Mechanical Properties in Various Industries. J. Res. Appl. Mech. Eng. JRAME 2021, 9, JRAME21. [Google Scholar] [CrossRef]
- Belhaj Khalifa, I.; Ladhari, N.; Touay, M. Application of Sericin to Modify Textile Supports. J. Text. Inst. 2012, 103, 370–377. [Google Scholar] [CrossRef]
- Bhandari, B.; Singh, S.S.J.; Rose, N.M. Effect of Sericin Treatment Conditions on Dye Abilty of Cotton Fabric. J. Appl. Nat. Sci. 2018, 10, 102–106. [Google Scholar] [CrossRef]
- Anis, P.; Toprak, T.; Kutlu, E. Sericin Assisted Eco-Friendly Reactive Dyeing for Cotton Fabric. Cellulose 2019, 26, 6317–6331. [Google Scholar] [CrossRef]
- Gupta, D.; Chaudhary, H.; Gupta, C. Sericin-Based Polyester Textile for Medical Applications. J. Text. Inst. 2015, 106, 366–376. [Google Scholar] [CrossRef]
- Giovannelli, L.; Milanesi, A.; Ugazio, E.; Fracchia, L.; Segale, L. Effect of Methyl–β–Cyclodextrin and Trehalose on the Freeze–Drying and Spray–Drying of Sericin for Cosmetic Purposes. Pharmaceuticals 2021, 14, 262. [Google Scholar] [CrossRef]
- Wen, Q.; Zhang, L.; Chen, Y.; Su, Y.; Yu, J.; Chen, P.; Zheng, T. Novel Applications of Silk Proteins Based on Their Interactions with Metal Ions. Sustainability 2023, 15, 16053. [Google Scholar] [CrossRef]
- Adel, A.M.; Ibrahim, A.A.; El-Shafei, A.M.; Al-Shemy, M.T. Inclusion Complex of Clove Oil with Chitosan/β-Cyclodextrin Citrate/Oxidized Nanocellulose Biocomposite for Active Food Packaging. Food Packag. Shelf Life 2019, 20, 100307. [Google Scholar] [CrossRef]
- Kabir, J.; Kore, V.; Tawade, S. Application of Edible Coatings on Fruits and Vegetables. Imp. J. Interdiscip. Res. IJIR 2016, 3, 591–603. [Google Scholar]
- Rajput, S.K.; Singh, M.K. Sericin—A Unique Biomaterial. IOSR J. Polym. Text. Eng. 2015, 2, 29–35. [Google Scholar] [CrossRef]
- World Population Prospects 2019: Highlights; United Nations: New York, NY, USA, 2019; ISBN 978-92-1-148316-1.
- Building a Common Vision for Sustainable Food and Agriculture: Principles and Approaches; FAO, Ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; ISBN 978-92-5-108471-7. [Google Scholar]
- Pontoniere, P. Food Lasts 1 Week Longer with This Edible Silk Coating. proto.life. 2022. Available online: https://proto.life/2022/06/food-lasts-1-week-longer-with-this-edible-silk-coating/ (accessed on 10 October 2024).
- Low, J.T.; Yusoff, N.I.S.M.; Othman, N.; Wong, T.; Wahit, M.U. Silk Fibroin-based Films in Food Packaging Applications: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2253–2273. [Google Scholar] [CrossRef] [PubMed]
- Sam, S.T.; Nuradibah, M.A.; Chin, K.M.; Hani, N. Current Application and Challenges on Packaging Industry Based on Natural Polymer Blending. In Natural Polymers; Olatunji, O., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 163–184. ISBN 978-3-319-26412-7. [Google Scholar]
- Tarangini, K.; Kavi, P.; Jagajjanani Rao, K. Application of Sericin-based Edible Coating Material for Postharvest Shelf-life Extension and Preservation of Tomatoes. eFood 2022, 3, e36. [Google Scholar] [CrossRef]
- Wang, J.; Shang, J.; Ren, F.; Leng, X. Study of the Physical Properties of Whey Protein: Sericin Protein-Blended Edible Films. Eur. Food Res. Technol. 2010, 231, 109–116. [Google Scholar] [CrossRef]
- Kwak, H.W.; Lee, H.; Lee, M.E.; Jin, H.-J. Facile and Green Fabrication of Silk Sericin Films Reinforced with Bamboo-Derived Cellulose Nanofibrils. J. Clean. Prod. 2018, 200, 1034–1042. [Google Scholar] [CrossRef]
- Barajas-Gamboa, J.A.; Serpa-Guerra, A.M.; Restrepo-Osorio, A.; Álvarez-López, C. Aplicaciones de La Sericina: Una Proteina Globular Proveniente de La Seda. Ing. Compet. 2016, 18, 193. [Google Scholar] [CrossRef]
- Oh, S.; Park, J.; Nam, J.; Hyun, Y.; Jin, H.-J.; Kwak, H.W. Antioxidant and UV-Blocking Glucose-Crosslinked Sericin Films with Enhanced Structural Integrity. React. Funct. Polym. 2021, 165, 104942. [Google Scholar] [CrossRef]
- Meerasri, J.; Chollakup, R.; Sothornvit, R. Factors Affecting Sericin Hydrolysis and Application of Sericin Hydrolysate in Sericin Films. RSC Adv. 2022, 12, 28441–28450. [Google Scholar] [CrossRef]
- Ghosh, S.; Rao, R.S.; Nambiar, K.S.; Haragannavar, V.C.; Augustine, D.; Sowmya, S.V. Sericin, a Dietary Additive: Mini Review. J. Med. Radiol. Pathol. Surg. 2017, 4, 13–17. [Google Scholar] [CrossRef]
- Mei, S.; Fu, B.; Su, X.; Chen, H.; Lin, H.; Zheng, Z.; Dai, C.; Yang, D.-P. Developing Silk Sericin-Based and Carbon Dots Reinforced Bio-Nanocomposite Films and Potential Application to Litchi Fruit. LWT 2022, 164, 113630. [Google Scholar] [CrossRef]
- Sothornvit, R.; Chollakup, R. Properties of Sericin–Glucomannan Composite Films. Int. J. Food Sci. Technol. 2009, 44, 1395–1400. [Google Scholar] [CrossRef]
- Lamboni, L.; Li, Y.; Liu, J.; Yang, G. Silk Sericin-Functionalized Bacterial Cellulose as a Potential Wound-Healing Biomaterial. Biomacromolecules 2016, 17, 3076–3084. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Kim, M.K.; Kwak, H.W.; Lee, J.Y.; Kim, M.H.; Lee, K.H. The Role of Glycerol and Water in Flexible Silk Sericin Film. Int. J. Biol. Macromol. 2016, 82, 945–951. [Google Scholar] [CrossRef]
- Li, W.; Huang, Z.; Cai, R.; Yang, W.; He, H.; Wang, Y. Rational Design of Ag/ZnO Hybrid Nanoparticles on Sericin/Agarose Composite Film for Enhanced Antimicrobial Applications. Int. J. Mol. Sci. 2021, 22, 105. [Google Scholar] [CrossRef]
- Sheng, J.Y.; Xu, J.; Zhuang, Y.; Sun, D.Q.; Xing, T.L.; Chen, G.Q. Study on the Application of Sericin in Cosmetics. Adv. Mater. Res. 2013, 796, 416–423. [Google Scholar] [CrossRef]
- Singh, A.K.; Mukhopadhyay, S. Synthesis and Application of Sericin/β-Cyclodextrin Material for Skincare Textile. IARJSET 2023, 10, 186–202. [Google Scholar] [CrossRef]
- Wang, W.-H.; Lin, W.-S.; Shih, C.-H.; Chen, C.-Y.; Kuo, S.-H.; Li, W.-L.; Lin, Y.-S. Functionality of Silk Cocoon (Bombyx mori L.) Sericin Extracts Obtained through High-Temperature Hydrothermal Method. Materials 2021, 14, 5314. [Google Scholar] [CrossRef]
- Kim, H.; Lim, Y.; Park, J.-H.; Cho, Y. Dietary Silk Protein, Sericin, Improves Epidermal Hydration with Increased Levels of Filaggrins and Free Amino Acids in NC/Nga Mice. Br. J. Nutr. 2012, 108, 1726–1735. [Google Scholar] [CrossRef]
- Fact.MR—Sericin Market by Form (Solid, Liquid), by Application (Personal Care & Cosmetics, Pharmaceuticals, Others), by Region (North America, Latin America, Europe)—Global Market Insights 2022–2032. Available online: https://www.factmr.com/report/3248/sericin-market (accessed on 5 February 2024).
- Products That Contain SERICIN || Skin Deep® Cosmetics Database. Available online: http://www.ewg.org/skindeep/browse/ingredients/705884-SERICIN/?ingredient_id=705884-SERICIN&page=5 (accessed on 24 April 2024).
- Crema Giorno con Sericina di Seta. Available online: https://www.imersa.it/it/products/crema-viso-giorno-con-sericina-di-seta/ (accessed on 24 April 2024).
- Fondonatura—Natural Hair Care. Available online: https://www.fondonatura.it/ (accessed on 24 April 2024).
- SERICA—Integral Sericin 10% with Diamond Powder—J AND C Cosmetici. Available online: https://jandc-cosmetici.com/en/ (accessed on 10 October 2024).
- Elhacham, E.; Ben-Uri, L.; Grozovski, J.; Bar-On, Y.M.; Milo, R. Global Human-Made Mass Exceeds All Living Biomass. Nature 2020, 588, 442–444. [Google Scholar] [CrossRef]
- Seo, S.-J.; Das, G.; Shin, H.-S.; Patra, J.K. Silk Sericin Protein Materials: Characteristics and Applications in Food-Sector Industries. Int. J. Mol. Sci. 2023, 24, 4951. [Google Scholar] [CrossRef] [PubMed]
- Porta, R.; Sabbah, M.; Di Pierro, P. Bio-Based Materials for Packaging. Int. J. Mol. Sci. 2022, 23, 3611. [Google Scholar] [CrossRef] [PubMed]
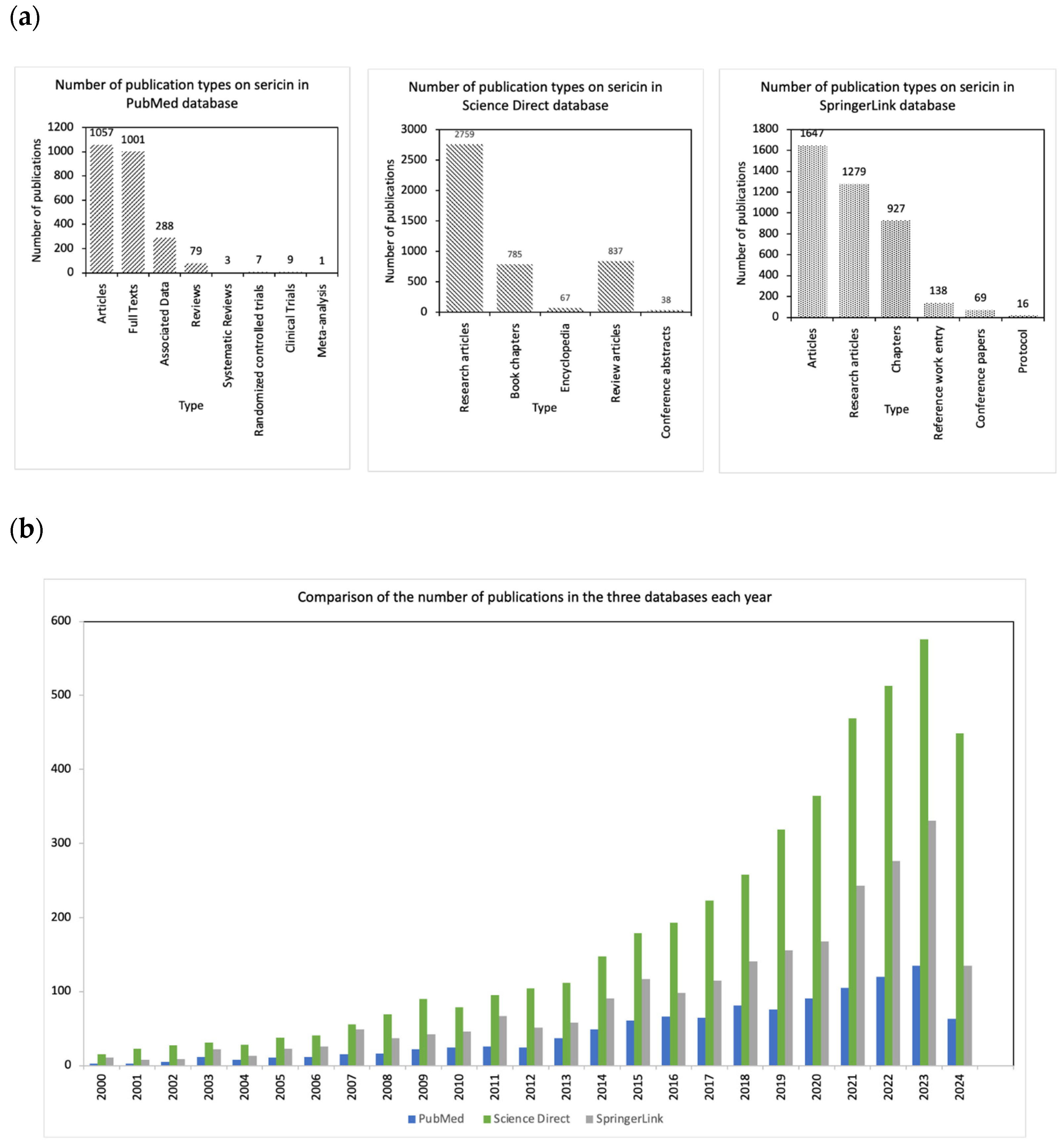
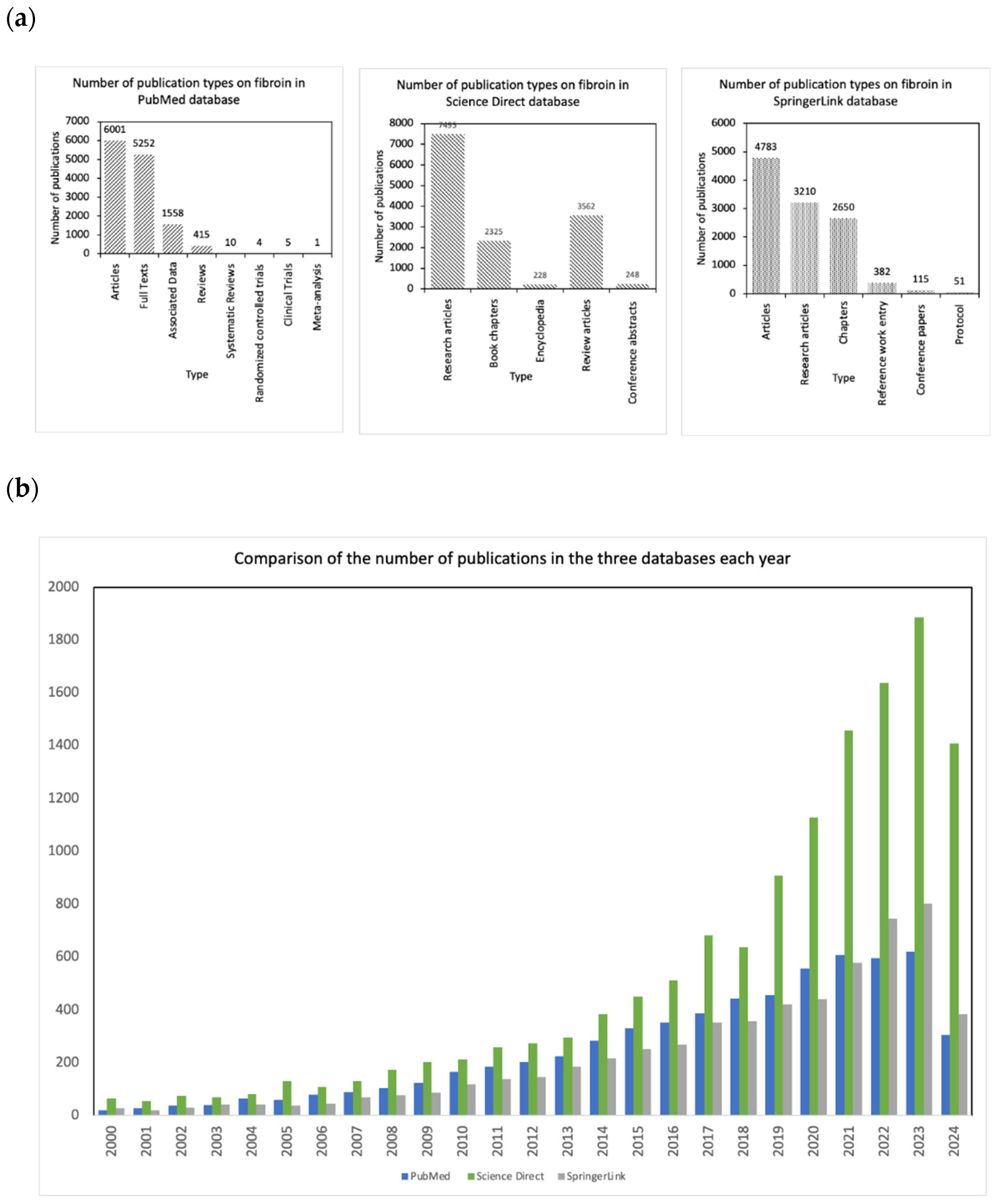

| Libraries | PubMed | Science Direct | SpringerLink | ||
|---|---|---|---|---|---|
| Articles | 1057 | Research articles | 2759 | Articles | 1647 |
| Full Texts | 1001 | Book chapters | 785 | Research articles | 1279 |
| Associated Data | 288 | Encyclopedia | 67 | Chapters | 927 |
| Reviews | 79 | Review articles | 837 | Reference work entry | 138 |
| Systematic Reviews | 3 | Conference abstracts | 38 | Conference papers | 69 |
| Randomized controlled trials | 7 | - | - | Protocol | 16 |
| Clinical Trials | 9 | - | - | - | - |
| Meta-analysis | 1 | - | - | - | - |
| Libraries | PubMed | Science Direct | SpringerLink | ||
|---|---|---|---|---|---|
| Articles | 6001 | Research articles | 7495 | Articles | 4783 |
| Full Texts | 5252 | Book chapters | 2325 | Research articles | 3210 |
| Associated Data | 1558 | Encyclopedia | 228 | Chapters | 2650 |
| Reviews | 415 | Review articles | 3562 | Reference work entry | 382 |
| Systematic Reviews | 10 | Conference abstracts | 248 | Conference papers | 115 |
| Randomized controlled trials | 4 | - | - | Protocol | 51 |
| Clinical Trials | 5 | - | - | - | - |
| Meta-analysis | 1 | - | - | - | - |
| Degumming Method | Approach | Advantages | Disadvantages |
|---|---|---|---|
| Conventional | Soaps and Alkalis |
|
|
| Chemical | Alkaline solutions |
|
|
| Acidic solutions |
|
| |
| Urea (with or without mercaptoethanol) |
|
| |
| Biological | Proteolytic enzymes |
|
|
| Thermal | Boiling |
|
|
| HTHP (autoclave) |
|
| |
| Modern | Infrared |
|
|
| Microwave |
|
| |
| Ultrasound |
|
| |
| Steam treatment |
|
| |
| CO2 supercritical fluid |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aad, R.; Dragojlov, I.; Vesentini, S. Sericin Protein: Structure, Properties, and Applications. J. Funct. Biomater. 2024, 15, 322. https://doi.org/10.3390/jfb15110322
Aad R, Dragojlov I, Vesentini S. Sericin Protein: Structure, Properties, and Applications. Journal of Functional Biomaterials. 2024; 15(11):322. https://doi.org/10.3390/jfb15110322
Chicago/Turabian StyleAad, Rony, Ivana Dragojlov, and Simone Vesentini. 2024. "Sericin Protein: Structure, Properties, and Applications" Journal of Functional Biomaterials 15, no. 11: 322. https://doi.org/10.3390/jfb15110322
APA StyleAad, R., Dragojlov, I., & Vesentini, S. (2024). Sericin Protein: Structure, Properties, and Applications. Journal of Functional Biomaterials, 15(11), 322. https://doi.org/10.3390/jfb15110322







