A Novel Graphene-Based Nanomaterial for the Development of a Pelvic Implant to Treat Pelvic Organ Prolapse
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hastalex Polymer
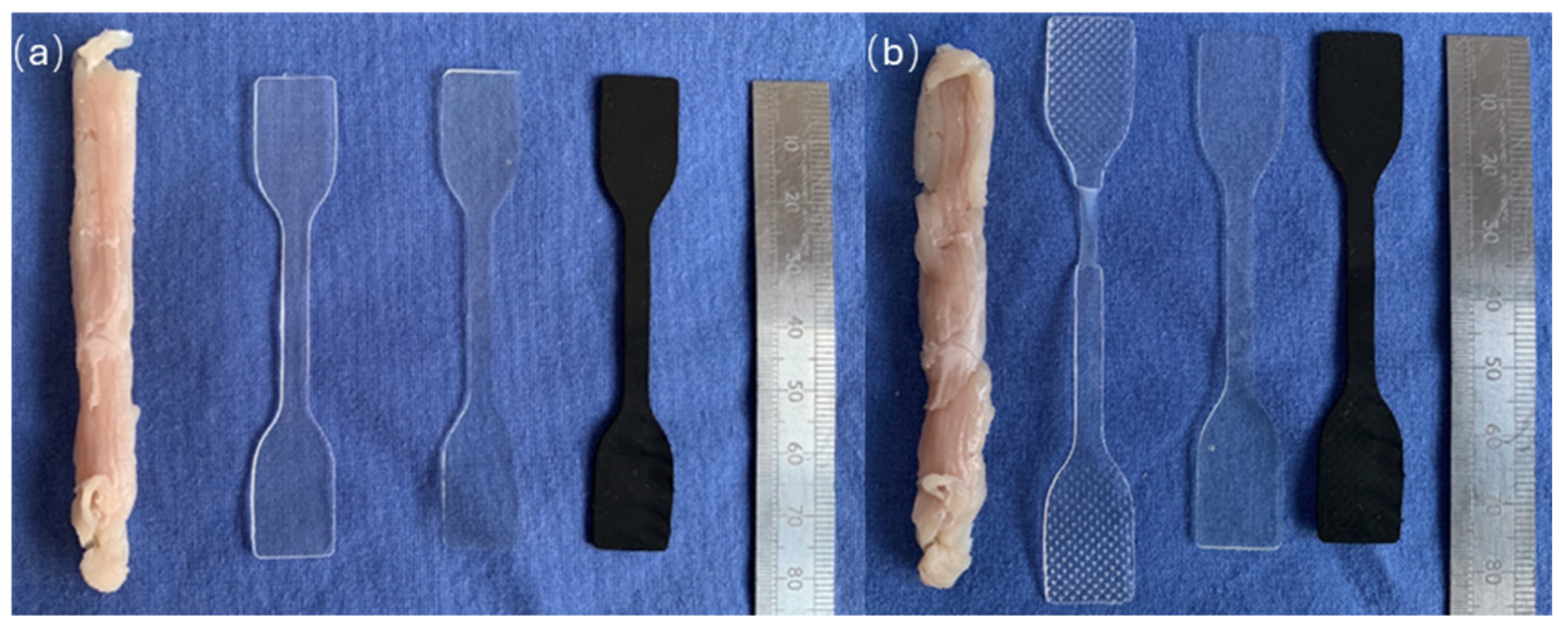
2.3. Preparation of Materials for Investigation
2.4. Surface Chemistry Structure Using ATR-FTIR
2.5. Mechanical Properties
2.5.1. Uniaxial Tensile Test
2.5.2. Elastic Test of Deformation
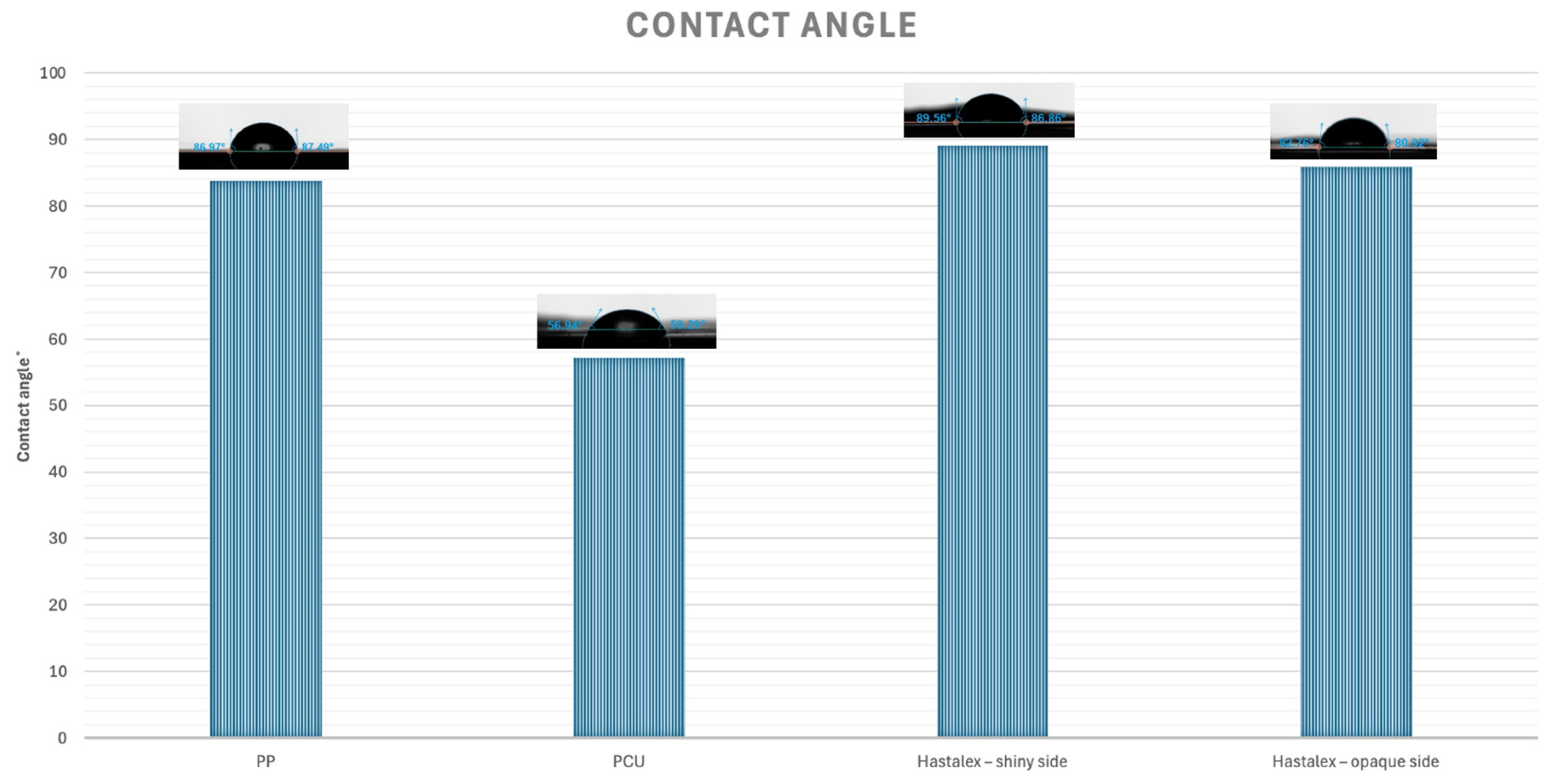
2.6. Assessment of Hydrophilicity of the Material
2.7. Assessment of Surface Topography
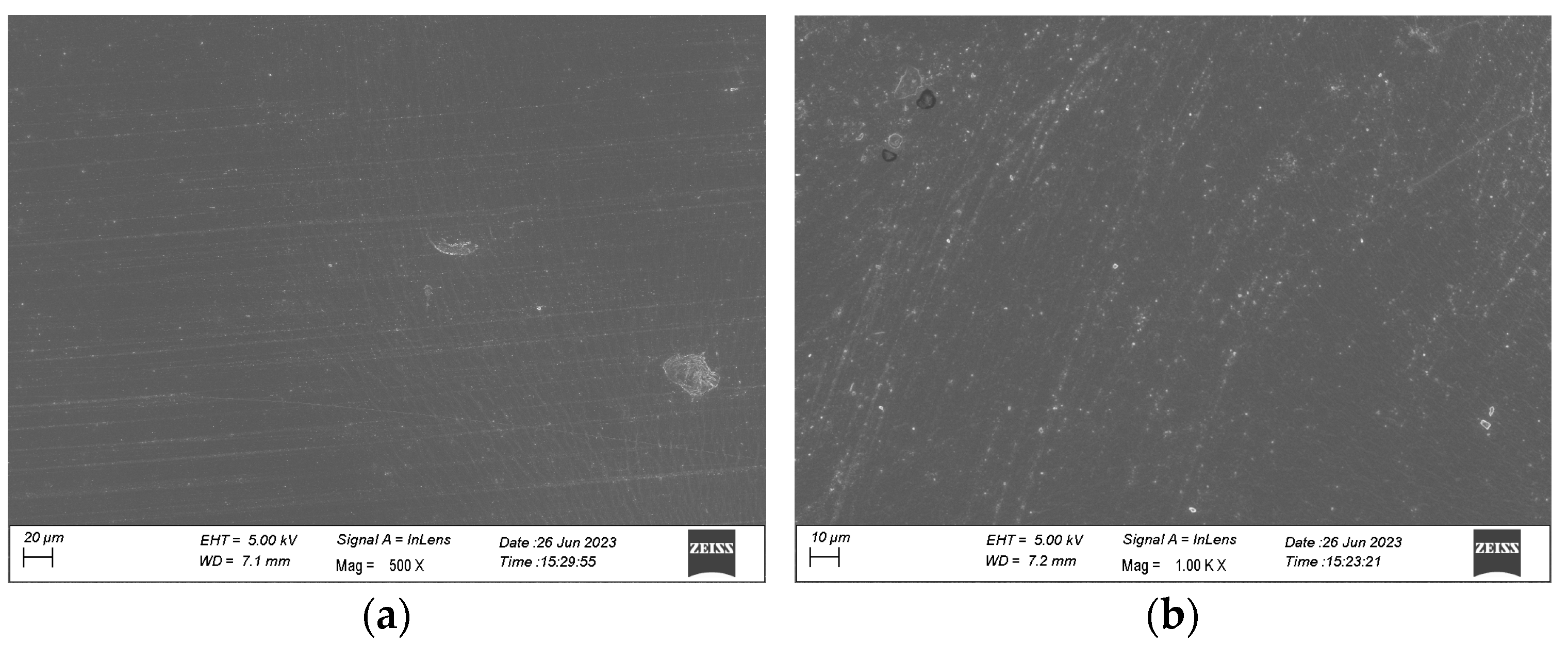
2.7.1. Scanning Electron Microscope (SEM)
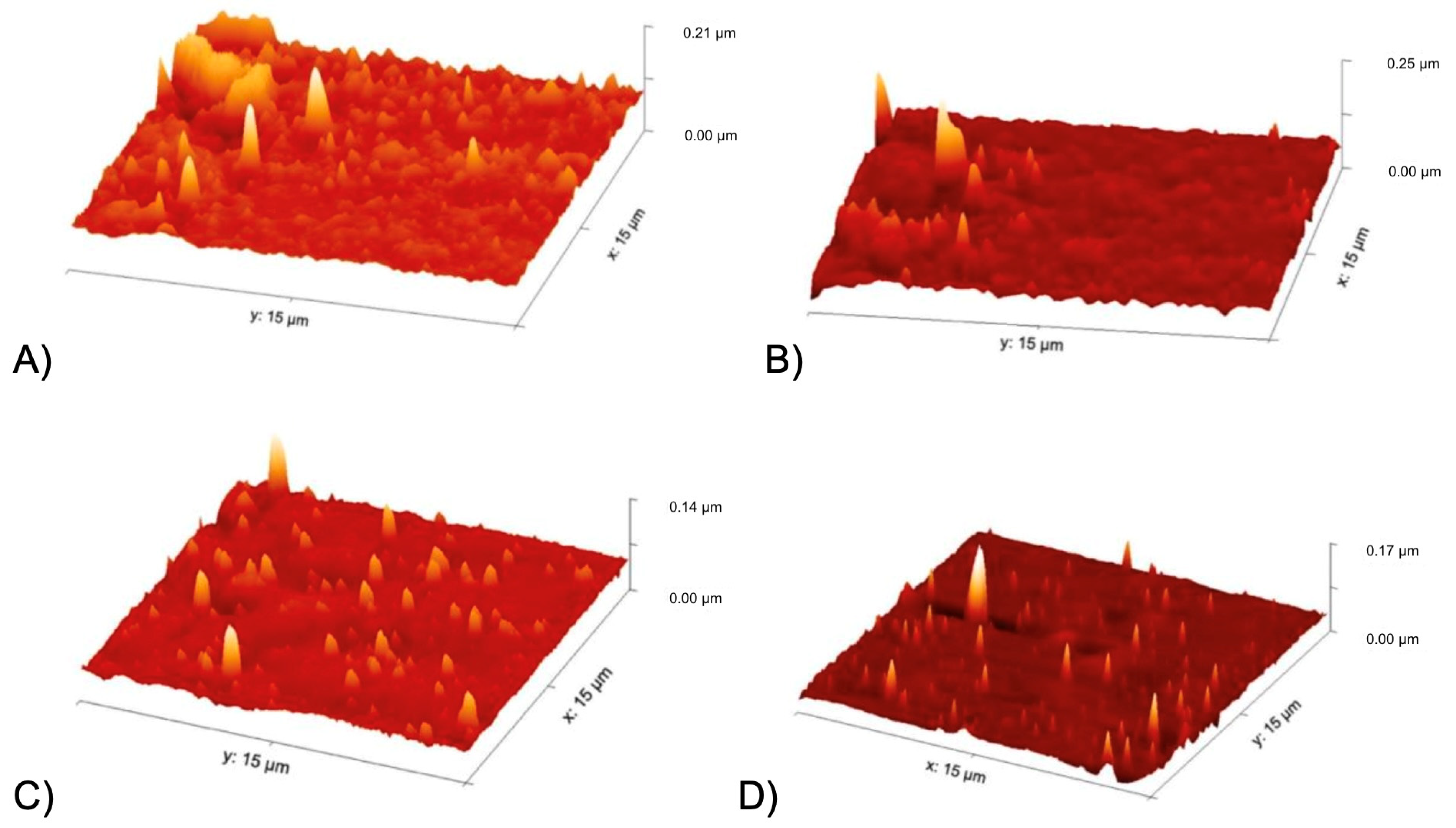
2.7.2. Atomic Force Microscope (AFM)
2.8. Statistical Analysis
3. Results
3.1. Surface Chemistry Structure Using ATR-FTIR
3.2. Mechanical Properties
3.2.1. Uniaxial Tensile Test
3.2.2. Elastic Test of Deformation
3.3. Assessment of Hydrophilicity of the Material
3.4. Assessment of Surface Topography
3.4.1. Scanning Electron Microscope (SEM)
3.4.2. Atomic Force Microscope (AFM)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jelovsek, J.E.; Maher, C.; Barber, M.D. Pelvic Organ Prolapse. Lancet 2007, 369, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Jelovsek, J.E.; Barber, M.D.; Brubaker, L.; Norton, P.; Gantz, M.; Richter, H.E.; Weidner, A.; Menefee, S.; Schaffer, J.; Pugh, N.; et al. Effect of Uterosacral Ligament Suspension vs Sacrospinous Ligament Fixation With or Without Perioperative Behavioral Therapy for Pelvic Organ Vaginal Prolapse on Surgical Outcomes and Prolapse Symptoms at 5 Years in the OPTIMAL Randomized Clinical Trial. JAMA 2018, 319, 1554–1565. [Google Scholar] [CrossRef] [PubMed]
- Seifalian, A.; Basma, Z.; Digesu, A.; Khullar, V. Polypropylene Pelvic Mesh: What Went Wrong and What Will Be of the Future? Biomedicines 2023, 11, 741. [Google Scholar] [CrossRef] [PubMed]
- Seifalian, A.; Digesu, A.; Khullar, V. The Use of Animal Models in Preclinical Investigations for the Development of a Surgical Mesh for Pelvic Organ Prolapse. Int. Urogynecol. J. 2024, 35, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Ghulam, A.N.; dos Santos, O.A.L.; Hazeem, L.; Pizzorno Backx, B.; Bououdina, M.; Bellucci, S. Graphene Oxide (GO) Materials—Applications and Toxicity on Living Organisms and Environment. J. Funct. Biomater. 2022, 13, 77. [Google Scholar] [CrossRef]
- Sheshmani, S.; Amini, R. Preparation and Characterization of Some Graphene Based Nanocomposite Materials. Carbohydr. Polym. 2013, 95, 348–359. [Google Scholar] [CrossRef]
- Ding, X.; Liu, H.; Fan, Y. Graphene-Based Materials in Regenerative Medicine. Adv. Healthc. Mater. 2015, 4, 1451–1468. [Google Scholar] [CrossRef]
- Zare, P.; Aleemardani, M.; Seifalian, A.; Bagher, Z.; Seifalian, A.M. Graphene Oxide: Opportunities and Challenges in Biomedicine. Nanomaterials 2021, 11, 1083. [Google Scholar] [CrossRef]
- Ovcharenko, E.A.; Seifalian, A.; Rezvova, M.A.; Klyshnikov, K.Y.; Glushkova, T.V.V.; Akenteva, T.N.; Antonova, L.V.V.; Velikanova, E.A.; Chernonosova, V.S.; Shevelev, G.Y.; et al. A New Nanocomposite Copolymer Based on Functionalised Graphene Oxide for Development of Heart Valves. Sci. Rep. 2020, 10, 5271. [Google Scholar] [CrossRef]
- Aleemardani, M.; Zare, P.; Seifalian, A.; Bagher, Z.; Seifalian, A.M. Graphene-Based Materials Prove to Be a Promising Candidate for Nerve Regeneration Following Peripheral Nerve Injury. Biomedicines 2022, 10, 73. [Google Scholar] [CrossRef]
- Shariati, A.; Hosseini, S.M.; Chegini, Z.; Seifalian, A.; Arabestani, M.R. Graphene-Based Materials for Inhibition of Wound Infection and Accelerating Wound Healing. Biomed. Pharmacother. 2023, 158, 114184. [Google Scholar] [CrossRef] [PubMed]
- Seifalian, A.; Hancock, S. Composite Material and Its Method of Production 2017. U.S. Patent 11993711, 28 May 2024. [Google Scholar]
- Slepičková Kasálková, N.; Rimpelová, S.; Vacek, C.; Fajstavr, D.; Švorčík, V.; Sajdl, P.; Slepička, P. Surface Activation of Hastalex by Vacuum Argon Plasma for Cytocompatibility Enhancement. Heliyon 2024, 10, e27816. [Google Scholar] [CrossRef] [PubMed]
- ISO 527; Plastics-Determination of Tensile Properties. International Organization for Standardization: Geneva, Switzerland, 2021.
- Mardina, Z.; Venezuela, J.; Maher, C.; Shi, Z.; Dargusch, M.S.; Atrens, A. Design, Mechanical and Degradation Requirements of Biodegradable Metal Mesh for Pelvic Floor Reconstruction. Biomater. Sci. 2022, 10, 3371–3392. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, L.; He, L.; Lin, W.; Yu, B.; Yu, X.; Lin, Y. Roles and Mechanisms of Biomechanical-Biochemical Coupling in Pelvic Organ Prolapse. Front. Med. 2024, 11, 1303044. [Google Scholar] [CrossRef]
- Gasparotto, M.; Bellet, P.; Scapin, G.; Busetto, R.; Rampazzo, C.; Vitiello, L.; Shah, D.I.; Filippini, F. 3D Printed Graphene-PLA Scaffolds Promote Cell Alignment and Differentiation. Int. J. Mol. Sci. 2022, 23, 1736. [Google Scholar] [CrossRef]
- Hajduga, M.B.; Bobinski, R.; Dutka, M.; Bujok, J.; Cwiertnia, M.; Pajak, C.; Kurowska, A.; Rajzer, I. The Influence of Graphene Content on the Antibacterial Properties of Polycaprolactone. Int. J. Mol. Sci. 2022, 23, 10899. [Google Scholar] [CrossRef]
- Jo, S.B.; Erdenebileg, U.; Dashnyam, K.; Jin, G.-Z.; Cha, J.-R.; El-Fiqi, A.; Knowles, J.C.; Patel, K.D.; Lee, H.-H.; Lee, J.-H.; et al. Nano-Graphene Oxide/Polyurethane Nanofibers: Mechanically Flexible and Myogenic Stimulating Matrix for Skeletal Tissue Engineering. J. Tissue Eng. 2020, 11, 2041731419900424. [Google Scholar] [CrossRef]
- Shahid, U.; Chen, Z.; Maher, C. Sacrocolpopexy: The Way I Do It. Int. Urogynecol. J. 2024. [Google Scholar] [CrossRef]
- Nayyer, L.; Birchall, M.; Seifalian, A.M.; Jell, G. Design and Development of Nanocomposite Scaffolds for Auricular Reconstruction. Nanomedicine 2014, 10, 235–246. [Google Scholar] [CrossRef]
- Mori da Cunha, M.G.M.C.; Mackova, K.; Hympanova, L.H.; Bortolini, M.A.T.; Deprest, J. Animal Models for Pelvic Organ Prolapse: Systematic Review. Int. Urogynecol. J. 2021, 32, 1331–1344. [Google Scholar] [CrossRef]
- Lei, L.; Song, Y.; Chen, R. Biomechanical Properties of Prolapsed Vaginal Tissue in Pre- and Postmenopausal Women. Int. Urogynecol. J. 2007, 18, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.; Lopes Silva-Filho, A.; Rodrigues Maciel da Fonseca, A.M.; Santos, A.; Santos, L.; Mascarenhas, T.; Natal Jorge, R.M.; Ferreira, A.J.M. Biomechanical Properties of Vaginal Tissue in Women with Pelvic Organ Prolapse. Gynecol. Obstet. Investig. 2013, 75, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Knight, K.M.; King, G.E.; Palcsey, S.L.; Suda, A.; Liang, R.; Moalli, P.A. Mesh Deformation: A Mechanism Underlying Polypropylene Prolapse Mesh Complications in Vivo. Acta. Biomater. 2022, 148, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.C.; Routzong, M.R.; Abramowitch, S.D.; Rostaminia, G. Effect of Squeeze, Cough, and Strain on Dynamic Urethral Function in Nulligravid Asymptomatic Women: A Cross-Sectional Cohort Study. Urogynecology 2023, 29, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Daniels, C.; Horning, A.; Phillips, A.; Massote, D.V.P.; Liang, L.; Bullard, Z.; Sumpter, B.G.; Meunier, V. Elastic, Plastic, and Fracture Mechanisms in Graphene Materials. J. Phys. Condens. Mat. 2015, 27, 373002. [Google Scholar] [CrossRef]
- Lourenço, B.N.; Marchioli, G.; Song, W.; Reis, R.L.; van Blitterswijk, C.A.; Karperien, M.; van Apeldoorn, A.; Mano, J.F. Wettability Influences Cell Behavior on Superhydrophobic Surfaces with Different Topographies. Biointerphases 2012, 7, 46. [Google Scholar] [CrossRef]
- Metwally, S.; Stachewicz, U. Surface Potential and Charges Impact on Cell Responses on Biomaterials Interfaces for Medical Applications. Mat. Sci. Eng. C. 2019, 104, 109883. [Google Scholar] [CrossRef]
- Llopis-Hernández, V.; Rico, P.; Ballester-Beltrán, J.; Moratal, D.; Salmerón-Sánchez, M. Role of Surface Chemistry in Protein Remodeling at the Cell-Material Interface. PLoS. ONE 2011, 6, e19610. [Google Scholar] [CrossRef]
- Fan, H.; Guo, Z. Bioinspired Surfaces with Wettability: Biomolecule Adhesion Behaviors. Biomater. Sci. 2020, 8, 1502–1535. [Google Scholar] [CrossRef]
- Ngandu Mpoyi, E.; Cantini, M.; Reynolds, P.M.; Gadegaard, N.; Dalby, M.J.; Salmerón-Sánchez, M. Protein Adsorption as a Key Mediator in the Nanotopographical Control of Cell Behavior. Acs. Nano. 2016, 10, 6638–6647. [Google Scholar] [CrossRef]
- Al-Azzam, N.; Alazzam, A. Micropatterning of Cells via Adjusting Surface Wettability Using Plasma Treatment and Graphene Oxide Deposition. PLoS ONE 2022, 17, e0269914. [Google Scholar] [CrossRef] [PubMed]
- Pitchai, M.S.; Ipe, D.S.; Hamlet, S. The Effect of Titanium Surface Topography on Adherent Macrophage Integrin and Cytokine Expression. J. Funct. Biomater. 2023, 14, 211. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-C.; Siedlecki, C.A. Surface Texturing and Combinatorial Approaches to Improve Biocompatibility of Implanted Biomaterials. Front. Phys. 2022, 10, 994438. [Google Scholar] [CrossRef] [PubMed]



| Material | Tensile at 100% Strain (N/mm2) | Tensile at 200% Strain (N/mm2) | Tensile at 300% Strain (N/mm2) | Maximum Tensile Strength at Break (N/mm2) | Elongation at Break (%) | Young’s Modulus (kPa) |
|---|---|---|---|---|---|---|
| Hastalex | 3.3 ± 0.3 | 7.0 ± 0.5 | 13.0 ± 0.6 | 58.0 ± 1.0 | 701.0 ± 14.8 | 78.5 ± 12.0 |
| PP | 15.3 ± 0.8 | 15.3 ± 0.5 | 15.1 ± 0.7 | 25.2 ± 1.2 | 652.0 ± 4.0 | 5011.4 ± 343.9 |
| PCU | 2.4 ± 0.3 | 4.4 ± 0.5 | 8.1 ± 1.0 | 23.3 ± 1.9 | 531.8 ± 7.9 | 71.3 ± 8.6 |
| Muscle | 21.8 ± 5.9 | 26.3 ± 6.1 | 39.2 ± 6.3 | 40.6 ± 6.8 | 392.5 ± 48.1 | 728.0 ± 226.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seifalian, A.; Digesu, A.; Khullar, V. A Novel Graphene-Based Nanomaterial for the Development of a Pelvic Implant to Treat Pelvic Organ Prolapse. J. Funct. Biomater. 2024, 15, 351. https://doi.org/10.3390/jfb15110351
Seifalian A, Digesu A, Khullar V. A Novel Graphene-Based Nanomaterial for the Development of a Pelvic Implant to Treat Pelvic Organ Prolapse. Journal of Functional Biomaterials. 2024; 15(11):351. https://doi.org/10.3390/jfb15110351
Chicago/Turabian StyleSeifalian, Amelia, Alex Digesu, and Vik Khullar. 2024. "A Novel Graphene-Based Nanomaterial for the Development of a Pelvic Implant to Treat Pelvic Organ Prolapse" Journal of Functional Biomaterials 15, no. 11: 351. https://doi.org/10.3390/jfb15110351
APA StyleSeifalian, A., Digesu, A., & Khullar, V. (2024). A Novel Graphene-Based Nanomaterial for the Development of a Pelvic Implant to Treat Pelvic Organ Prolapse. Journal of Functional Biomaterials, 15(11), 351. https://doi.org/10.3390/jfb15110351






