The Impact of Ultrashort Pulse Laser Structuring of Metals on In-Vitro Cell Adhesion of Keratinocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Pretreatment
2.2. Micro-Structuring Using Ultrashort Laser Pulses
2.3. Surface Characterization
2.4. Cell Culture
2.5. Cell Adhesion
2.6. Morphology and Spreading of Keratinocytes
2.7. Actin Cytoskeleton Organization
2.8. Cell Viability
2.9. Statistical Analysis
3. Results
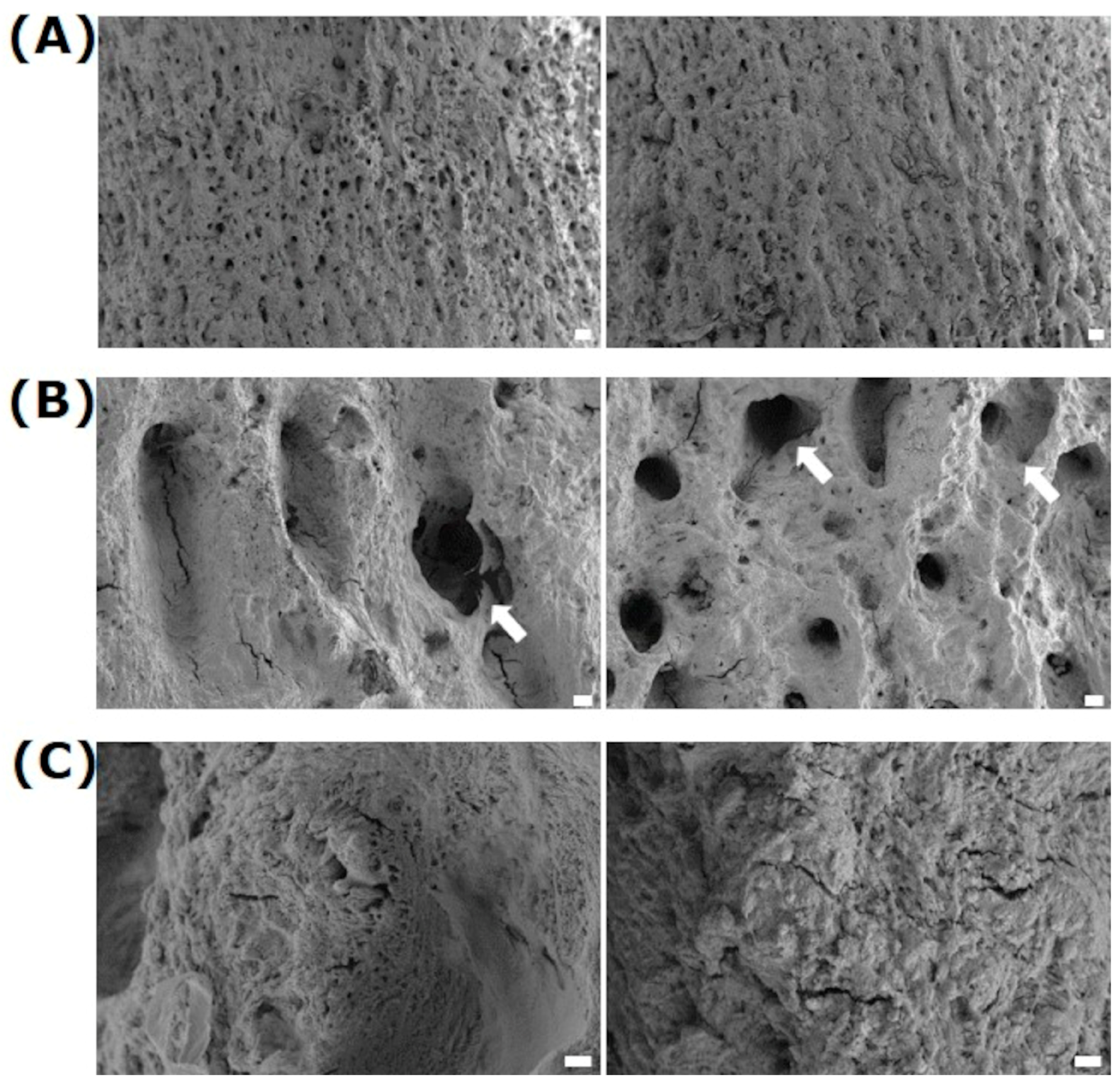
3.1. Surface Characterization
3.2. Cell Response
3.2.1. Cellular Adhesion
3.2.2. Cell Spreading
3.2.3. Cell Viability
3.2.4. Cell Morphology and Actin Cytoskeleton
3.2.5. Cell Growth after 7 Days
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Structure | LSM | Ø Dimple (µm) | Depth (µm) | Dimples per mm2 | Contact Angle (°) |
|---|---|---|---|---|---|
| A (polished reference, Ref) |  | - | - | - | 58.9 (steel) 80.6 (Ti6Al4V) |
| B (nanostructure, LIPSS) |  | - | - | - | 66.7 (steel) 8.5 (TiAl4V5) |
| C (microstructure, periodic dimples, 42/mm2) |  | 50 | 20 | 42 | 60.9 (steel) 62.2 (Ti6Al4V) |
| D (microstructure, periodic dimples, 81/mm2) |  | 50 | 20 | 81 | 68.9 (steel) 43.8 (Ti6Al4V) |
| E (micro- and nanostructure, periodic dimples (42/mm2) + LIPSS) |  | 50 | 20 | 42 | 71.55 (steel) 22.2 (Ti6Al4V) |
| F (micro- and nanostructure, periodic dimples (81/mm2) + LIPSS) |  | 50 | 20 | 81 | 78.9 (steel) 42.5 (Ti6Al4V) |
| G (micro-dimple cluster, Ø 35µm) |  | 35 | 10 | 80 | 72.8 (steel) 40.8 (Ti6Al4V) |
| H (microstructure, dimple cluster, Ø 20 µm; DC20) |  | 20 | 10 | 80 | 62.9 (steel) 40.5 (Ti6Al4V) |
| I (microstructure, dimple cluster (Ø 35 µm) + LIPSS) |  | 35 | 10 | 80 | 94.6 (steel) 28.9 (Ti6Al4V) |
| J (microstructure, periodic dimples (Ø 20 µm) + LIPSS, DC20 + LIPSS) |  | 20 | 10 | 80 | 98.7 (steel) 28.2 (Ti6Al4V) |
| K (microstructure, periodic dimples (400/mm2) + LIPSS) |  | 50 | 10 | 400 | 87.8 (steel) 26.4 (Ti6Al4V) |
Appendix B

References
- Kani, K.K.; Porrino, J.A.; Chew, F.S. External fixators: Looking beyond the hardware maze. Skelet. Radiol. 2020, 49, 359–374. [Google Scholar] [CrossRef]
- Hadeed, A.; Werntz, R.L.; Varacallo, M. External Fixation Principles and Overview. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547694/ (accessed on 4 August 2023).
- Arveladze, S.; Moriarty, F.; Jennison, T. The Influence of Pin Material and Coatings on the Incidence of Pin Site Infection after External Fixation. J. Limb Lengthening Reconstr. 2022, 8 (Suppl. S1), S16–S23. [Google Scholar] [CrossRef]
- Nakhaei, M.; Jirofti, N.; Ebrahimzadeh, M.H.; Moradi, A. Different methods of hydroxyapatite-based coatings on external fixator pin with high adhesion approach. Plasma Process. Polym. 2023, 20, e2200219. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, C.; Feng, Y.; Li, D.; Ai, T.; Huang, Y.; Chen, X.; Huang, L.; Tan, J. Osteoimmunomodulatory effects of biomaterial modification strategies on macrophage polarization and bone regeneration. Regen. Biomater. 2020, 7, 233–245. [Google Scholar] [CrossRef]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Kortram, K.; Bezstarosti, H.; Metsemakers, W.-J.; Raschke, M.J.; Van Lieshout, E.M.M.; Verhofstad, M.H.J. Risk factors for infectious complications after open fractures; a systematic review and meta-analysis. Int. Orthop. 2017, 41, 1965–1982. [Google Scholar] [CrossRef]
- Yuan, Z.Z.; Yang, Z.; Liu, Q.; Liu, Y.M. Complications following open reduction and internal fixation versus external fixation in treating unstable distal radius fractures: Grading the evidence through a meta-analysis. Orthop. Traumatol. Surg. Res. 2018, 104, 95–103. [Google Scholar] [CrossRef]
- Collinge, C.A.; Goll, G.; Seligson, D.; Easley, K.J. Pin tract infections: Silver vs uncoated pins. Orthopedics 1994, 17, 445–448. [Google Scholar] [CrossRef]
- Shirai, T.; Tsuchiya, H.; Shimizu, T.; Ohtani, K.; Zen, Y.; Tomita, K. Prevention of pin tract infection with titanium-copper alloys. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 373–380. [Google Scholar] [CrossRef]
- Brennan, S.A.; Ní Fhoghlú, C.; Devitt, B.; O’mahony, F.; Brabazon, D.; Walsh, A. Silver nanoparticles and their orthopaedic applications. Bone Jt. J. 2015, 97, 582–589. [Google Scholar] [CrossRef]
- Scarano, A.; Piattelli, M.; Vrespa, G.; Caputi, S.; Piattelli, A. Bacterial adhesion on titanium nitride-coated and uncoated implants: An in vivo human study. J. Oral Implantol. 2003, 29, 80–85. [Google Scholar] [CrossRef]
- Teixeira, A.I.; Abrams, G.A.; Bertics, P.J.; Murphy, C.J.; Nealey, P.F. Epithelial contact guidance on well-defined micro-and nanostructured substrates. J. Cell Sci. 2003, 116, 1881. [Google Scholar] [CrossRef]
- Pendegrass, C.J.; Goodship, A.E.; Price, J.S.; Blunn, G.W. Nature’s answer to breaching the skin barrier: An innovative development for amputees. J. Anat. 2006, 209, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Suttie, J.M. Histological studies of pedicle skin formation and its transformation to antler velvet in red deer (Cervus elaphus). Anat. Rec. 2000, 260, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Rychly, J.; Nebe, B. Cell-material interaction. BioNanoMaterials. 2013, 14, 153–160. [Google Scholar] [CrossRef]
- Staehlke, S.; Koertge, A.; Nebe, B. Intracellular calcium dynamics in dependence on topographical features of titanium. Biomaterials 2015, 46, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Ruoslathi, E.; Pierschbacher, M.D. New perspectives in cell adhesion: RGD and integrins. Science 1987, 238, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.; Raghavan, S. A skin-depth analysis of integrins: Role of the integrin network in health and disease. Cell Commun. Adhes. 2013, 20, 155–169. [Google Scholar] [CrossRef]
- Lim, J.Y.; Donahue, H.J. Cell sensing and response to micro-and nanostructured surfaces produced by chemical and topographic patterning. Tissue Eng. 2007, 13, 1879–1891. [Google Scholar] [CrossRef]
- Anselme, K.; Ponche, A.; Bigerelle, M. Relative influence of surface topography and surface chemistry on cell response to bone implant materials. Part 2: Biological aspects. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2010, 224, 1487–1507. [Google Scholar] [CrossRef]
- Schnell, G.; Staehlke, S.; Duenow, U.; Nebe, J.B.; Seitz, H. Femtosecond laser nano/micro textured Ti6Al4V surfaces–effect on wetting and MG-63 cell adhesion. Materials 2019, 12, 2210. [Google Scholar] [CrossRef]
- Kietzig, A.-M.; Hatzikiriakos, S.G.; Englezos, P. Patterned superhydrophobic metallic surfaces. Langmuir 2009, 25, 4821–4827. [Google Scholar] [CrossRef]
- Long, J.; Zhong, M.; Zhang, H.; Fan, P. Superhydrophilicity to superhydrophobicity transition of picosecond laser microstructured aluminum in ambient air. J. Colloid Interface Sci. 2015, 441, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Raimbault, O.; Benayoun, S.; Anselme, K.; Mauclair, C.; Bourgade, T.; Kietzig, A.-M.; Girard-Lauriault, P.-L.; Valette, S.; Donnet, C. The effects of femtosecond laser-textured Ti-6Al-4V on wettability and cell response. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Staehlke, S.; Haack, F.; Waldner, A.-C.; Koczan, D.; Moerke, C.; Mueller, P.; Uhrmacher, A.M.; Nebe, J.B. ROS Dependent Wnt/β-Catenin Pathway and Its Regulation on Defined Micro-Pillars—A Combined In Vitro and In Silico Study. Cells 2020, 9, 1784. [Google Scholar] [CrossRef] [PubMed]
- Gristina, A.G.; Naylor, P.; Myrvik, Q. Infections from biomaterials and implants: A race for the surface. Med. Prog. Technol. 1988, 14, 205–224. [Google Scholar] [PubMed]
- Gallo, J.; Holinka, M.; Moucha, C.S. Antibacterial surface treatment for orthopaedic implants. Int. J. Mol. Sci. 2014, 15, 13849–13880. [Google Scholar] [CrossRef] [PubMed]
- Stratakis, E.; Ranella, A.; Fotakis, C. Biomimetic micro/nanostructured functional surfaces for microfluidic and tissue engineering applications. Biomicrofluidics 2011, 5, 13411. [Google Scholar] [CrossRef]
- Tsibidis, G.D.; Stratakis, E. Ultrafast Laser Biomimetic Micro-/Nanostructuring. In Ultrafast Laser Nanostructuring; Springer Series in Optical Sciences; Stoian, R., Bonse, J., Eds.; Springer: Cham, Switzerland, 2023; Volume 239. [Google Scholar] [CrossRef]
- Borenstein, J.T.; Weinberg, E.J.; Orrick, B.K.; Sundback, C.; Kaazempur-Mofrad, M.R.; Vacanti, J.P. Microfabrication of three-dimensional engineered scaffolds. Tissue Eng. 2007, 13, 1837–1844. [Google Scholar] [CrossRef]
- Martinez, E.; Engel, E.; Planell, J.; Samitier, J. Effects of artificial micro-and nano-structured surfaces on cell behaviour. Ann. Anat. Anat. Anz. 2009, 191, 126–135. [Google Scholar] [CrossRef]
- Variola, F.; Yi, J.H.; Richert, L.; Wuest, J.D.; Rosei, F.; Nanci, A. Tailoring the surface properties of Ti6Al4V by controlled chemical oxidation. Biomaterials 2008, 29, 1285–1298. [Google Scholar] [CrossRef]
- Backes, L.T.; Oldorf, P.; Peters, R.; Wendlandt, R.; Schnell, G.; Schulz, A.P. Study of the tribological properties of surface structures using ultrashort laser pulses to reduce wear in endoprosthetics. J. Orthop. Surg. Res. 2020, 15, 205. [Google Scholar] [CrossRef] [PubMed]
- Drescher, P.; Oldorf, P.; Dreier, T.; Schnell, G.; Peters, R.; Seitz, H. Ring-Shaped Surface Microstructures for Improved Lubrication Performance of Joint Prostheses. Lubricants 2020, 8, 45. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immorta-lized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Staehlke, S.; Oster, P.; Seemann, S.; Kruse, F.; Brief, J.; Nebe, B. Laser Structured Dental Zirconium for Soft Tissue Cell Occupation-Importance of Wettability Modulation. Materials 2022, 15, 732. [Google Scholar] [CrossRef]
- Van Kooten, T.G.; Klein, C.L.; Wagner, M.; Kirkpatrick, C.J. Focal adhesions and assessment of cytotoxicity. J. Biomed. Mater. Res. 1999, 46, 33–43. [Google Scholar] [CrossRef]
- Kierdorf, U.; Kierdorf, H.; Schultz, M. The macroscopic and microscopic structure of double-head antlers and pedicle bone of cervidae (Mammalia, Artiodactyla). Ann. Anat. Anat. Anz. 1994, 176, 251–257. [Google Scholar] [CrossRef]
- Pendegrass, C.J.; Goodship, A.E.; Blunn, G.W. Development of a soft tissue seal around bone-anchored transcutaneous amputation prostheses. Biomaterials 2006, 27, 4183–4191. [Google Scholar] [CrossRef]
- Pospiech, P.T.; Wendlandt, R.; Aschoff, H.H.; Ziegert, S.; Schulz, A.P. Quality of life of persons with transfemoral amputation: Comparison of socket prostheses and osseointegrated prostheses. Prosthet. Orthot. Int. 2021, 45, 20–25. [Google Scholar] [CrossRef]
- Örgel, M.; Schwarze, F.; Graulich, T.; Krettek, C.; Weidemann, F.; Aschoff, H.H.; Winkelmann, M.; Ranker, A. Comparison of functional outcome and patient satisfaction between patients with socket prosthesis and patients treated with transcutaneous osseointegrated prosthetic systems (TOPS) after transfemoral amputation. Eur. J. Trauma Emerg. Surg. 2022, 48, 4867–4876. [Google Scholar] [CrossRef]
- Pendegrass, C.J.; Oddy, M.J.; Sundar, S.; Cannon, S.R.; Goodship, A.E.; Blunn, G.W. The novel use of resorbable Vicryl mesh for in vivo tendon reconstruction to a metal prosthesis. J. Bone Jt. Surg. Br. 2006, 88, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Chimutengwende-Gordon, M.; Pendegrass, C.; Blunn, G. Enhancing the soft tissue seal around intraosseous transcutaneous amputation prostheses using silanized fibronectin titanium alloy. Biomed. Mater. 2011, 6, 025008. [Google Scholar] [CrossRef] [PubMed]
- Chimutengwende-Gordon, M.; Pendegrass, C.; Bayston, R.; Blunn, G. Preventing infection of osseointegrated transcutaneous implants: Incorporation of silver into preconditioned fibronectin-functionalized hydroxyapatite coatings suppresses Staphylococcus aureus colonization while promoting viable fibroblast growth in vitro. Biointerphases 2014, 9, 031010. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.H. External fixators and minimally invasive osteosynthesis in small animal veterinary medicine. Vet. Clin. North. Am. Small Anim. Pract. 2012, 42, 913–934. [Google Scholar] [CrossRef] [PubMed]
- Bible, J.E.; Mir, H.R. External Fixation: Principles and Applications. J. Am. Acad. Orthop. Surg. 2015, 23, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Bigerelle, M.; Anselme, K. Statistical correlation between cell adhesion and proliferation on biocompatible metallic materials. J. Biomed. Mater. Res. A 2005, 72, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.; Oliveira, V.; Serro, A.; Zouani, O.; Almeida, A.; Durrieu, M.-C.; Vilar, R. Ultrafast laser texturing of Ti-6Al-4V surfaces for biomedical applications. In Proceedings of the International Congress on Applications of Lasers & Electro-Optics, Orlando, FL, USA, 14–18 October 2013. [Google Scholar] [CrossRef]
- Miller, P.R.; Aggarwal, R.; Doraiswamy, A.; Lin, Y.; Lee, Y.-S.; Narayan, J. Laser Micromachining for Biomedical Applications. JOM 2009, 61, 35–40. [Google Scholar] [CrossRef]
- Cunha, A.; Zouani, O.F.; Plawinski, L.; Botelho do Rego, A.M.; Almeida, A.; Vilar, R.; Durrieu, M.C. Human mesenchymal stem cell behavior on femtosecond laser-textured Ti-6Al-4V surfaces. Nanomedicine 2015, 10, 725–739. [Google Scholar] [CrossRef]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Biggs, M.J.P. Focal adhesion in osteoneogenesis. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2010, 224, 1441–1453. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Andersson, A.S.; Bäckhed, F.; von Euler, A.; Richter-Dahlfors, A.; Sutherland, D.; Kasemo, B. Nanoscale features influence epithelial cell morphology and cytokine production. Biomaterials 2003, 24, 3427–3436. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Hansen, J.C.; Siedlecki, C.A.; Hengstebeck, R.W.; Cheng, J.; Winograd, N.; Donahue, H.J. Osteoblast Adhesion on Poly(l-lactic Acid)/Polystyrene Demixed Thin Film Blends: Effect of Nanotopography, Surface Chemistry, and Wettability. Biomacromolecules 2005, 6, 3319–3327. [Google Scholar] [CrossRef] [PubMed]
- Lüthen, F.; Lange, R.; Becker, P.; Rychly, J.; Beck, U.; Nebe, J.G. The influence of surface roughness of titanium on beta1- and beta3-integrin adhesion and the organization of fibronectin in human osteoblastic cells. Biomaterials 2005, 26, 2423–2440. [Google Scholar] [CrossRef]
- Esser, L.; Springer, R.; Dreissen, G.; Lövenich, L.; Konrad, J.; Hampe, N.; Merkel, R.; Hoffmann, B.; Noetzel, E. Elastomeric Pillar Cages Modulate Actomyosin Contractility of Epithelial Microtissues by Substrate Stiffness and Topography. Cells 2023, 2, 1256. [Google Scholar] [CrossRef] [PubMed]
- Finke, B.; Luethen, F.; Schroeder, K.; Mueller, P.; Bergemann, C.; Frant, M.; Ohl, A.; Nebe, J.B. The effect of positively charged plasma polymerization on initial osteoblastic focal adhesion on titanium surfaces. Biomaterials 2007, 28, 4521–4534. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Daraio, C.; Chen, L.H.; Pisanic, T.R.; Fiñones, R.R.; Jin, S. Significantly accelerated osteoblast cell growth on aligned TiO2 nanotubes. J. Biomed. Mater. Res. A 2006, 78, 97–103. [Google Scholar] [CrossRef]
- Dorkhan, M.; Yücel-Lindberg, T.; Hall, J.; Svensäter, G.; Davies, J.R. Adherence of human oral keratinocytes and gingival fibroblasts to nano-structured titanium surfaces. BMC Oral Health 2014, 14, 75. [Google Scholar] [CrossRef]
- Cunha, W.; Carvalho, O.; Henriques, B.; Silva, F.S.; Özcan, M.; Souza, J.C.M. Surface modification of zirconia dental implants by laser texturing. Lasers Med. Sci. 2022, 37, 77–93. [Google Scholar] [CrossRef]
- Martínez-Calderon, M.; Manso-Silván, M.; Rodríguez, A.; Gómez-Aranzadi, M.; García-Ruiz, J.P.; Olaizola, S.M.; Martín-Palma, R.J. Surface micro- and nano-texturing of stainless steel by femtosecond laser for the control of cell migration. Sci. Rep. 2016, 6, 36296. [Google Scholar] [CrossRef] [PubMed]
- Butkus, S.; Jukna, V.; Kažukauskas, E.; Svirksas, Ž.; Paipulas, D.; Sirutkaitis, V. High-Contrast Marking of Stainless-Steel Using Bursts of Femtosecond Laser Pulses. Micromachines 2023, 14, 194. [Google Scholar] [CrossRef] [PubMed]
- Wilson, V.G. Growth and differentiation of HaCaT keratinocytes. Methods Mol. Biol. 2014, 1195, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, Y.; Yang, L.; Shi, Z.; Yang, P.; Cheng, G. In Vitro Bioactivity and Biocompatibility of Bio-Inspired Ti-6Al-4V Alloy Surfaces Modified by Combined Laser Micro/Nano Structuring. Molecules 2020, 25, 1494. [Google Scholar] [CrossRef]
- Dumas, V.; Rattner, A.; Vico, L.; Audouard, E.; Dumas, J.C.; Naisson, P.; Bertrand, P. Multiscale grooved titanium processed with femtosecond laser influences mesenchymal stem cell morphology, adhesion, and matrix organization. J. Biomed. Mater. Res. A 2012, 100, 3108–3116. [Google Scholar] [CrossRef]
- Dumas, V.; Guignandon, A.; Vico, L.; Mauclair, C.; Zapata, X.; Linossier, M.T.; Bouleftour, W.; Granier, J.; Peyroche, S.; Dumas, J.C.; et al. Femtosecond laser nano/micro patterning of titanium influences mesenchymal stem cell adhesion and commitment. Biomed. Mater. 2015, 10, 055002. [Google Scholar] [CrossRef]
- Ng, I.C.; Pawijit, P.; Tan, J.; Yu, H. Anatomy and Physiology for Biomaterials Research and Development; Narayan, R., Ed.; Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 225–236. ISBN 9780128051443. [Google Scholar] [CrossRef]
- Senthil, R.; Kavukcu, S.B.; Vedakumari, W.S. Cellulose based biopolymer nanoscaffold: A possible biomedical applications. Int. J. Biol. Macromol. 2023, 246, 125656. [Google Scholar] [CrossRef] [PubMed]
- Löffek, S.; Hurskainen, T.; Jackow, J.; Sigloch, F.C.; Schilling, O.; Tasanen, K.; Bruckner-Tuderman, L.; Franzke, C.W. Transmembrane collagen XVII modulates integrin dependent keratinocyte migration via PI3K/Rac1 signaling. PLoS ONE 2014, 9, e87263. [Google Scholar] [CrossRef] [PubMed]
- Jacków, J.; Löffek, S.; Nyström, A.; Bruckner-Tuderman, L.; Franzke, C.W. Collagen XVII Shedding Suppresses Re-Epithelialization by Directing Keratinocyte Migration and Dampening mTOR Signaling. J. Investig. Dermatol. 2016, 136, 1031–1041. [Google Scholar] [CrossRef]
- Cecato, R.C.; Martinez, E.F.; Benfatti, C.A.M. Analysis of the Viability and Morphology of Gingival Cells on Materials Used in Novel Prosthetic Components: In Vitro Study. J. Contemp. Dent. Pract. 2022, 23, 22–30. [Google Scholar] [CrossRef]
- Du, C.; Wang, C.; Zhang, T.; Zheng, L. Antibacterial Performance of Zr-BMG, Stainless Steel, and Titanium Alloy with Laser-Induced Periodic Surface Structures. ACS Appl. Bio Mater. 2022, 5, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.; Elie, A.M.; Plawinski, L.; Serro, A.P.; do Rego, A.M.B.; Almeida, A.; Vilar, R. Femtosecond laser surface texturing of titanium as a method to reduce the adhesion of Staphylococcus aureus and biofilm formation. Appl. Surf. Sci. 2016, 360, 485–493. [Google Scholar] [CrossRef]
- Grase, L.; Onufrijevs, P.; Rezevska, D.; Racenis, K.; Skadins, I.; Karosas, J.; Gecys, P.; Iesalnieks, M.; Pludons, A.; Kroica, J.; et al. Effect of Femtosecond Laser-Irradiated Titanium Plates on Enhanced Antibacterial Activity and Preservation of Bacteriophage Stability. Nanomaterials 2023, 13, 2032. [Google Scholar] [CrossRef] [PubMed]
- Sych, K.; Nold, S.P.; Pfeilschifter, J.; Vutukuri, R.; Meisterknecht, J.; Wittig, I.; Frank, S.; Goren, I. Expression of PKM2 in wound keratinocytes is coupled to angiogenesis during skin repair in vivo and in HaCaT keratinocytes in vitro. J. Mol. Med. 2023, 101, 151–169. [Google Scholar] [CrossRef] [PubMed]







| Structure | Ø Dimple (µm) | Depth (µm) | Dimples per mm2 |
|---|---|---|---|
| Ref (polished) | - | - | - |
| LIPSS (nanostructure) | - | - | - |
| DC20 (micro-dimple cluster) | 20 | 10 | 80 |
| DC20 + LIPSS (micro-dimple + nanostructure) | 20 | 10 | 80 |
| Structure | Contact Angle, θ [°] |
|---|---|
| Ref (polished) | 58.9 (steel) 80.6 (Ti6Al4V) |
| LIPSS (nanostructure) | 66.7 (steel) 8.5 (Ti6Al4V) |
| DC20 (micro-dimple cluster) | 62.9 (steel) 40.5 (Ti6Al4V) |
| DC20 + LIPSS (micro-dimple + nanostructure) | 98.7 (steel) 28.2 (Ti6Al4V) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staehlke, S.; Barth, T.; Muench, M.; Schroeter, J.; Wendlandt, R.; Oldorf, P.; Peters, R.; Nebe, B.; Schulz, A.-P. The Impact of Ultrashort Pulse Laser Structuring of Metals on In-Vitro Cell Adhesion of Keratinocytes. J. Funct. Biomater. 2024, 15, 34. https://doi.org/10.3390/jfb15020034
Staehlke S, Barth T, Muench M, Schroeter J, Wendlandt R, Oldorf P, Peters R, Nebe B, Schulz A-P. The Impact of Ultrashort Pulse Laser Structuring of Metals on In-Vitro Cell Adhesion of Keratinocytes. Journal of Functional Biomaterials. 2024; 15(2):34. https://doi.org/10.3390/jfb15020034
Chicago/Turabian StyleStaehlke, Susanne, Tobias Barth, Matthias Muench, Joerg Schroeter, Robert Wendlandt, Paul Oldorf, Rigo Peters, Barbara Nebe, and Arndt-Peter Schulz. 2024. "The Impact of Ultrashort Pulse Laser Structuring of Metals on In-Vitro Cell Adhesion of Keratinocytes" Journal of Functional Biomaterials 15, no. 2: 34. https://doi.org/10.3390/jfb15020034
APA StyleStaehlke, S., Barth, T., Muench, M., Schroeter, J., Wendlandt, R., Oldorf, P., Peters, R., Nebe, B., & Schulz, A.-P. (2024). The Impact of Ultrashort Pulse Laser Structuring of Metals on In-Vitro Cell Adhesion of Keratinocytes. Journal of Functional Biomaterials, 15(2), 34. https://doi.org/10.3390/jfb15020034








