Bone Marrow-Derived Mesenchymal Stem Cell-Laden Nanocomposite Scaffolds Enhance Bone Regeneration in Rabbit Critical-Size Segmental Bone Defect Model
Abstract
:1. Introduction
2. Materials and Method
2.1. Experimental Animals
2.2. Isolation, Culture, and Expansion of Bone Marrow-Derived Mesenchymal Stem Cells
2.3. Preparation of Nano-Scaffolds
2.4. Stem Cell Loading into Nano-Biomaterial Constructs
2.5. Rabbit Radius Critical-Sized Defect Creation
2.6. Experimental Design and Treatment Protocol
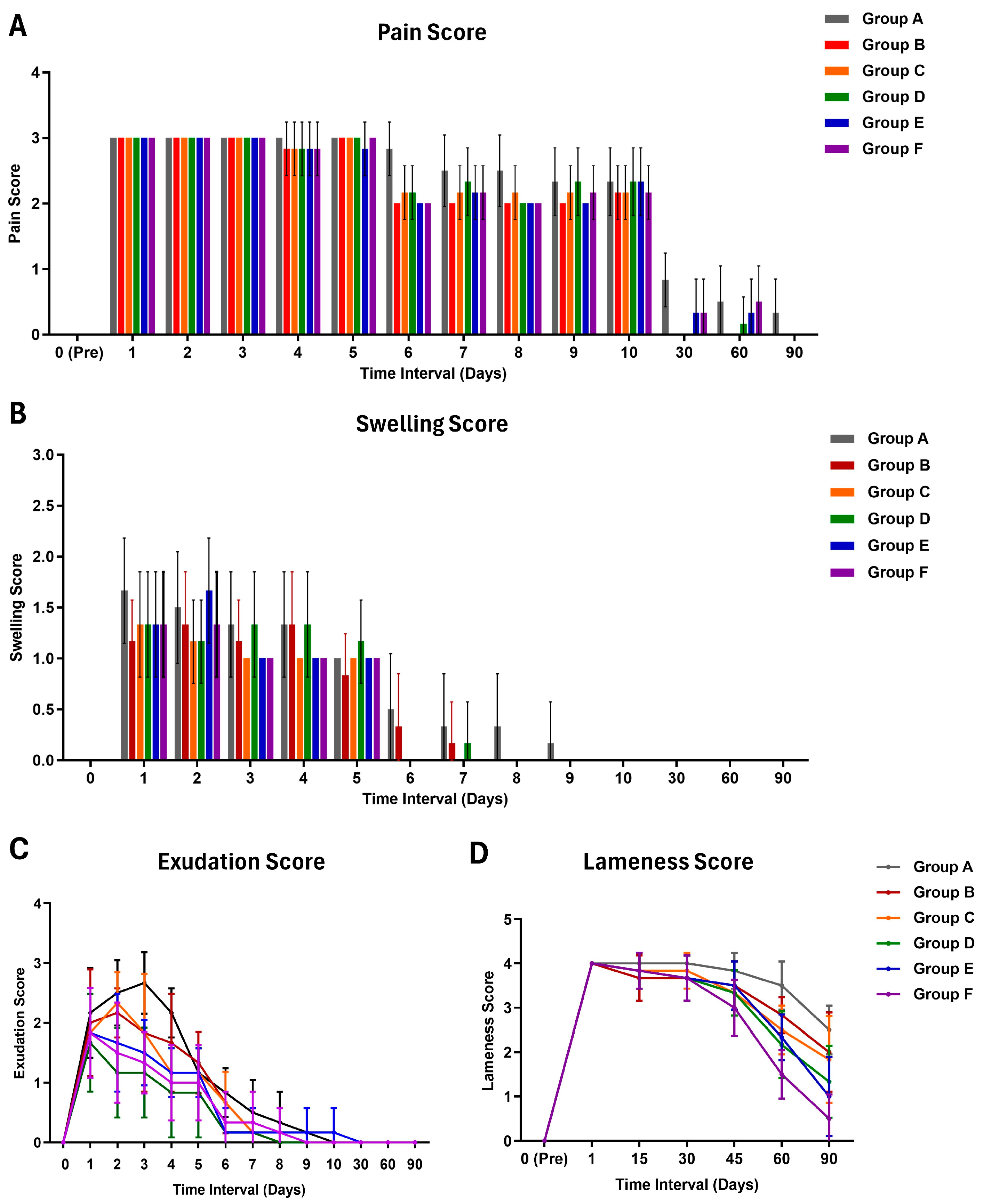
2.7. Clinical Observations
2.8. Gait and Mobility Analysis
2.9. Serum Biochemical Parameters
2.10. Radiographic Analysis
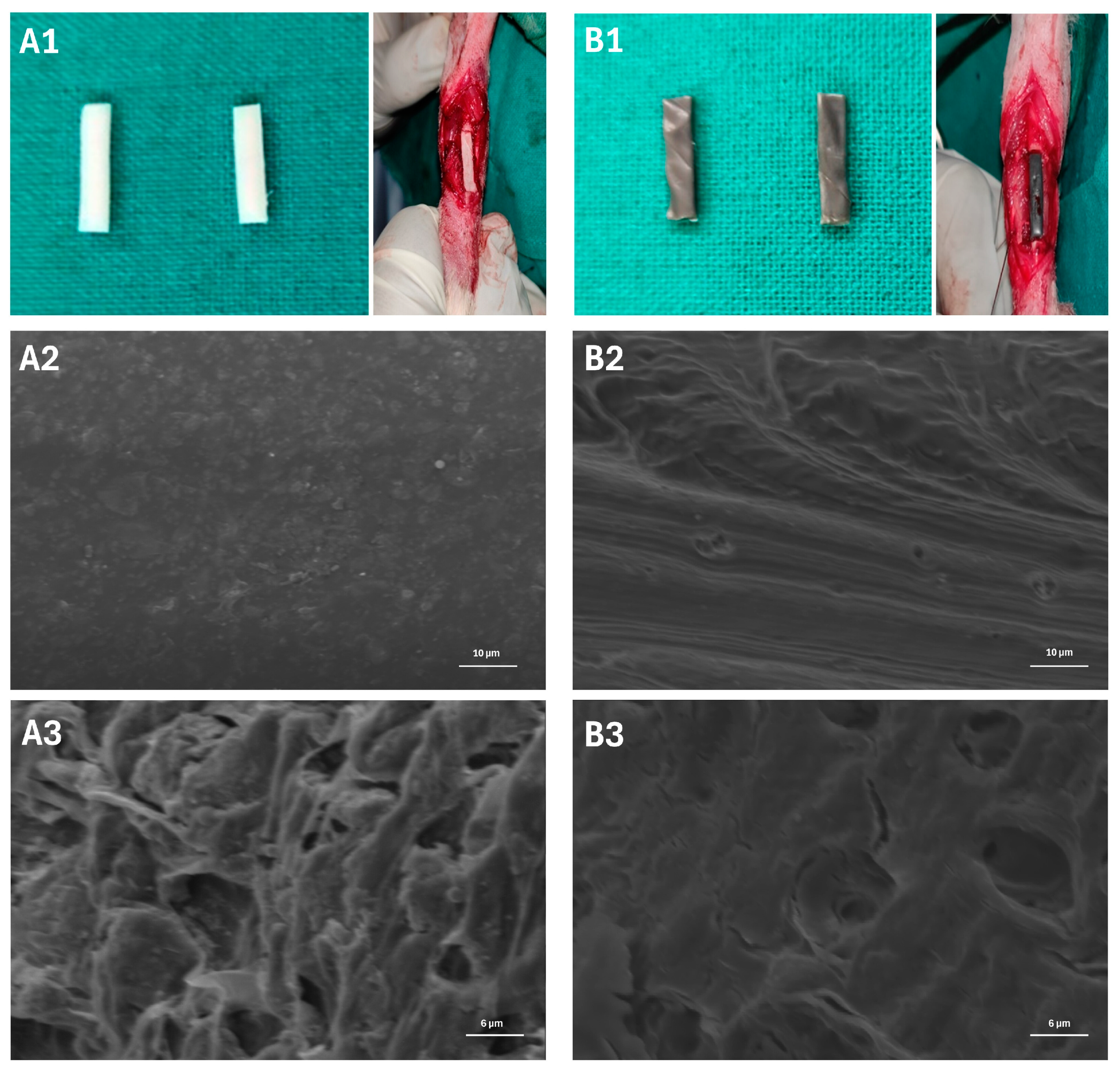
2.11. Scanning Electron Microscopic (SEM) Analysis
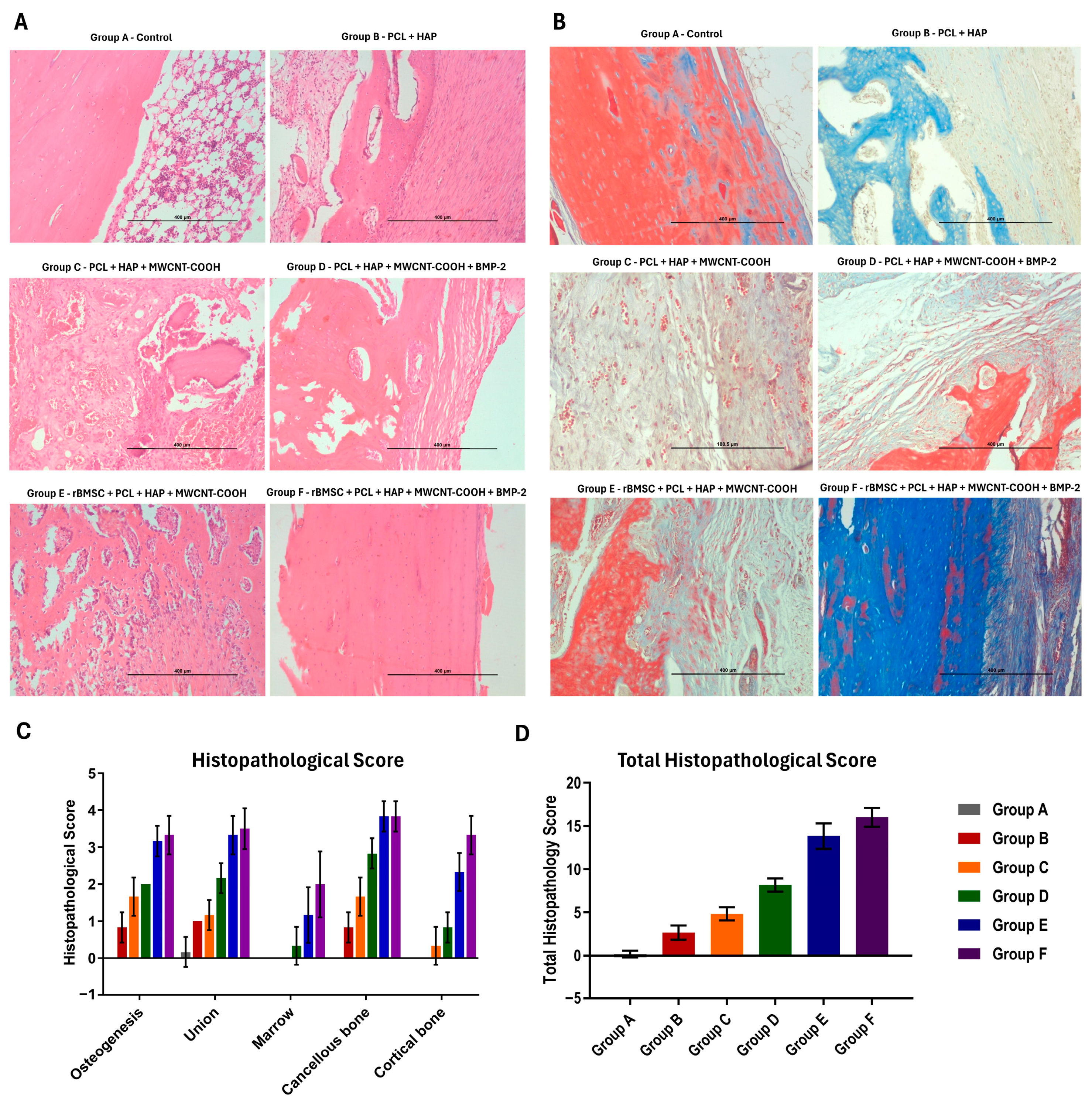
2.12. Histopathological Analysis
2.13. Statistical Analysis
3. Results
3.1. Surface Morphology of the Scaffold
3.2. Clinical Findings
3.3. Impact on Gait and Mobility
3.4. Serum Biochemical Changes
3.5. Radiographic Findings
3.6. Scanning Electron Microscopy
3.7. Histopathological Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stetzer, K.; Cooper, G.; Gassner, R.; Kapucu, R.; Mundell, R.; Mooney, M.P. Effects of Fixation Type and Guided Tissue Regeneration on Maxillary Osteotomy Healing in Rabbits. J. Oral. Maxillofac. Surg. 2002, 60, 427–436; discussion 436–437. [Google Scholar] [CrossRef]
- Hollinger, J.O.; Kleinschmidt, J.C. The Critical Size Defect as an Experimental Model to Test Bone Repair Materials. J. Craniofac. Surg. 1990, 1, 60–68. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, Osteoconduction and Osseointegration. Eur. Spine J. 2001, 10 (Suppl. S2), S96–S101. [Google Scholar] [CrossRef]
- Pathak, R.; Amarpal, A.H.; Kinjavdekar, P.; Pawde, A.M.; Rashmi, M.T.; Sharma, N.; Dhama, K. Bone Tissue Engineering: Latest Trends and Future Perspectives. Adv. Anim Vet. Sci. 2015, 3, 9–22. [Google Scholar] [CrossRef]
- Pape, H.C.; Evans, A.; Kobbe, P. Autologous Bone Graft: Properties and Techniques. J. Orthop. Trauma 2010, 24 (Suppl. S1), S36–S40. [Google Scholar] [CrossRef]
- Maiti, S.K.; Singh, G.R.; Mogha, I.V. Bone Allografts: A Review. Indian J. Vet. Surg. 2002, 23, 1–11. [Google Scholar]
- Dang, M.; Saunders, L.; Niu, X.; Fan, Y.; Ma, P.X. Biomimetic Delivery of Signals for Bone Tissue Engineering. Bone Res. 2018, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.K.; Singh, G.R. Ceramic Biomaterials in Reconstructive Surgery: A Review. Indian J. Vet. Surg. 2003, 24, 1–10. [Google Scholar]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ushida, T.; Tateishi, T. Scaffold Design for Tissue Engineering. Macromol. Biosci. 2002, 2, 67–77. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D Bioactive Composite Scaffolds for Bone Tissue Engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, J.R.; Low, S.; Choon, A.T.; Kumar, A.B.; Ramakrishna, S. Nanobioengineered Electrospun Composite Nanofibers and Osteoblasts for Bone Regeneration. Artif. Organs 2008, 32, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Dzenis, Y. Material Science. Spinning Continuous Fibers for Nanotechnology. Science 2004, 304, 1917–1919. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Zhu, J.; Deng, D.; Jin, S.; Li, J.; Man, Y. Enhanced Osteogenesis and Angiogenesis by PCL/Chitosan/Sr-Doped Calcium Phosphate Electrospun Nanocomposite Membrane for Guided Bone Regeneration. J. Biomater. Sci. Polym. Ed. 2019, 30, 1505–1522. [Google Scholar] [CrossRef]
- Zhou, X.; Pan, Y.; Liu, R.; Luo, X.; Zeng, X.; Zhi, D.; Li, J.; Cheng, Q.; Huang, Z.; Zhang, H.; et al. Biocompatibility and Biodegradation Properties of Polycaprolactone/Polydioxanone Composite Scaffolds Prepared by Blend or Co-Electrospinning. J. Bioact. Compat. Polym. 2019, 34, 115–130. [Google Scholar] [CrossRef]
- Campiglio, C.E.; Marcolin, C.; Draghi, L. Electrospun ECM Macromolecules as Biomimetic Scaffold for Regenerative Medicine: Challenges for Preserving Conformation and Bioactivity. AIMS Mater. Sci. 2017, 4, 638–669. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Taha, M.; Mousa, H.M.; Bartnikowski, M.; Hassan, M.L.; Dewidar, M.; Ivanovski, S. Engineering of Electrically-Conductive Poly(ε-Caprolactone)/ Multi-Walled Carbon Nanotubes Composite Nanofibers for Tissue Engineering Applications. Ceram. Int. 2019, 45, 15736–15740. [Google Scholar] [CrossRef]
- Domingos, M.; Gloria, A.; Coelho, J.; Bartolo, P.; Ciurana, J. Three-Dimensional Printed Bone Scaffolds: The Role of Nano/Micro-Hydroxyapatite Particles on the Adhesion and Differentiation of Human Mesenchymal Stem Cells. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2017, 231, 555–564. [Google Scholar] [CrossRef]
- Gandhimathi, C.; Venugopal, J.; Ravichandran, R.; Sundarrajan, S.; Suganya, S.; Ramakrishna, S. Mimicking Nanofibrous Hybrid Bone Substitute for Mesenchymal Stem Cells Differentiation into Osteogenesis. Macromol. Biosci. 2013, 13, 696–706. [Google Scholar] [CrossRef]
- Rumiński, S.; Ostrowska, B.; Jaroszewicz, J.; Skirecki, T.; Włodarski, K.; Święszkowski, W.; Lewandowska-Szumieł, M. Three-Dimensional Printed Polycaprolactone-Based Scaffolds Provide an Advantageous Environment for Osteogenic Differentiation of Human Adipose-Derived Stem Cells. J. Tissue Eng. Regen Med. 2018, 12, e473–e485. [Google Scholar] [CrossRef]
- Huang, B.; Vyas, C.; Byun, J.J.; El-Newehy, M.; Huang, Z.; Bártolo, P. Aligned Multi-Walled Carbon Nanotubes with Nanohydroxyapatite in a 3D Printed Polycaprolactone Scaffold Stimulates Osteogenic Differentiation. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110374. [Google Scholar] [CrossRef]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.M.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions-A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef]
- Banu, S.A.; Pawde, A.M.; Sharun, K.; Kalaiselvan, E.; Shivaramu, S.; Mathesh, K.; Chandra, V.; Kumar, R.; Maiti, S.K.; Verma, M.R.; et al. Evaluation of Bone Marrow-Derived Mesenchymal Stem Cells with Eggshell Membrane for Full-Thickness Wound Healing in a Rabbit Model. Cell Tissue Bank 2023, 1–16. [Google Scholar] [CrossRef]
- Al Shehadat, S.; Gorduysus, M.O.; Hamid, S.S.A.; Abdullah, N.A.; Samsudin, A.R.; Ahmad, A. Optimization of Scanning Electron Microscope Technique for Amniotic Membrane Investigation: A Preliminary Study. Eur. J. Dent. 2018, 12, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.K.; Shivakumar, M.U.; Mohan, D.; Kumar, N.; Singh, K.P. Mesenchymal Stem Cells of Different Origin-Seeded Bioceramic Construct in Regeneration of Bone Defect in Rabbit. Tissue Eng. Regen. Med. 2018, 15, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Omlor, G.W.; Kleinschmidt, K.; Gantz, S.; Speicher, A.; Guehring, T.; Richter, W. Increased Bone Formation in a Rabbit Long-Bone Defect Model after Single Local and Single Systemic Application of Erythropoietin. Acta. Orthop. 2016, 87, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Committee for the Purpose of Control and Supervision on Experiments on Animals. CPCSEA Guidelines for Laboratory Animal Facility. Indian J. Pharmacol. 2003, 35, 257–274. [Google Scholar]
- Ravindran, N.A.; Maiti, S.K.; Palakkara, S.; Kritaniya, D.; Mahan, T.; Kumar, N. In Vitro Osteoinduction Potential of a Novel Silica Coated Hydroxyapatite Bioscaffold Seeded with Rabbit Mesenchymal Stem Cell. J. Stem. Cell Res. Ther. 2016, 1, 00009. [Google Scholar]
- Stasiak, K.L.; Maul, D.; French, E.; Hellyer, P.W.; Vande Woude, S. Species-Specific Assessment of Pain in Laboratory Animals. Contemp. Top Lab. Anim. Sci. 2003, 42, 13–20. [Google Scholar] [PubMed]
- Barnhart, M.D.; Maritato, K.; Schankereli, K.; Wotton, H.; Naber, S. Evaluation of an Intra-Articular Synthetic Ligament for Treatment of Cranial Cruciate Ligament Disease in Dogs: A Six-Month Prospective Clinical Trial. Vet. Comp. Orthop. Traumatol. 2016, 29, 491–498. [Google Scholar] [CrossRef]
- Wilkinson, M.J.; Menz, H.B.; Raspovic, A. The Measurement of Gait Parameters from Footprints. Foot 1995, 5, 84–90. [Google Scholar] [CrossRef]
- Yang, C.Y.; Simmons, D.J.; Lozano, R. The Healing of Grafts Combining Freeze-Dried and Demineralized Allogeneic Bone in Rabbits. Clin. Orthop. Relat. Res. 1994, 298, 286–295. [Google Scholar] [CrossRef]
- Eltokhey, H.M.; Zahran, D.H. Evaluation of the Effect of Omega 3 Fatty Acid (N- 3) on Socket Healing in Orchiectomized Rats. J. Am. Sci. 2011, 7, 263–271. [Google Scholar]
- Nnaji, T.; Kene, R.; Chah, K.; Udegbunam, S.; Ogbanya, K.; Okpe, G. Histopathological Evaluation of the Osteogenic Activity of Autologous Platelet-Rich Plasma in Experimentally Induced Ulna Defect in Dogs. Comp. Clin. Pathol. 2015, 24, 1593–1597. [Google Scholar] [CrossRef]
- Lane, J.M.; Sandhu, H.S. Current Approaches to Experimental Bone Grafting. Orthop. Clin. N. Am. 1987, 18, 213–225. [Google Scholar] [CrossRef]
- Heiple, K.G.; Goldberg, V.M.; Powell, A.E.; Bos, G.D.; Zika, J.M. Biology of Cancellous Bone Grafts. Orthop. Clin. N. Am. 1987, 18, 179–185. [Google Scholar] [CrossRef]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma. 2017, 31 (Suppl. S5), S20–S22. [Google Scholar] [CrossRef]
- Stewart, S. Fracture Non-Union: A Review of Clinical Challenges and Future Research Needs. Malays. Orthop. J. 2019, 13, 1–10. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363. [Google Scholar] [CrossRef]
- Nuss, K.M.R.; Rechenberg, B. von Biocompatibility Issues with Modern Implants in Bone-A Review for Clinical Orthopedics. Open Orthop. J. 2008, 2, 66. [Google Scholar] [CrossRef]
- Kim, T.; See, C.W.; Li, X.; Zhu, D. Orthopedic Implants and Devices for Bone Fractures and Defects: Past, Present and Perspective. Eng. Regen. 2020, 1, 6–18. [Google Scholar] [CrossRef]
- Riester, O.; Borgolte, M.; Csuk, R.; Deigner, H.-P. Challenges in Bone Tissue Regeneration: Stem Cell Therapy, Biofunctionality and Antimicrobial Properties of Novel Materials and Its Evolution. Int. J. Mol. Sci. 2020, 22, 192. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, C.; Hellwarth, P.B.; Bao, X. Biomaterials for Stem Cell Engineering and Biomanufacturing. Bioact. Mater. 2019, 4, 366–379. [Google Scholar] [CrossRef]
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of Critical-Sized Bone Defects: Clinical and Tissue Engineering Perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362. [Google Scholar] [CrossRef]
- Piacentini, F.; Ceglia, M.J.; Bettini, L.; Bianco, S.; Buzzi, R.; Campanacci, D.A. Induced Membrane Technique Using Enriched Bone Grafts for Treatment of Posttraumatic Segmental Long Bone Defects. J. Orthop. Traumatol. 2019, 20, 13. [Google Scholar] [CrossRef]
- Szwed-Georgiou, A.; Płociński, P.; Kupikowska-Stobba, B.; Urbaniak, M.M.; Rusek-Wala, P.; Szustakiewicz, K.; Piszko, P.; Krupa, A.; Biernat, M.; Gazińska, M.; et al. Bioactive Materials for Bone Regeneration: Biomolecules and Delivery Systems. ACS Biomater. Sci. Eng. 2023, 9, 5222–5254. [Google Scholar] [CrossRef]
- Ohtsuki, C.; Kamitakahara, M.; Miyazaki, T. Bioactive Ceramic-Based Materials with Designed Reactivity for Bone Tissue Regeneration. J. R. Soc. Interface 2009, 6, S349–S360. [Google Scholar] [CrossRef]
- BaoLin, G.; MA, P.X. Synthetic Biodegradable Functional Polymers for Tissue Engineering: A Brief Review. Sci. China Chem. 2014, 57, 490–500. [Google Scholar]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Jafari, M.; Paknejad, Z.; Rad, M.R.; Motamedian, S.R.; Eghbal, M.J.; Nadjmi, N.; Khojasteh, A. Polymeric Scaffolds in Tissue Engineering: A Literature Review. J. Biomed Mater. Res. B Appl. Biomater. 2017, 105, 431–459. [Google Scholar] [CrossRef]
- Dirauf, M.; Muljajew, I.; Weber, C.; Schubert, U.S. Recent Advances in Degradable Synthetic Polymers for Biomedical Applications—Beyond Polyesters. Prog. Polym. Sci. 2022, 129, 101547. [Google Scholar] [CrossRef]
- Zulkifli, M.Z.A.; Nordin, D.; Shaari, N.; Kamarudin, S.K. Overview of Electrospinning for Tissue Engineering Applications. Polymers 2023, 15, 2418. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Anjum, S.; Rahman, F.; Pandey, P.; Arya, D.K.; Alam, M.; Rajinikanth, P.S.; Ao, Q. Electrospun Biomimetic Nanofibrous Scaffolds: A Promising Prospect for Bone Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 9206. [Google Scholar] [CrossRef]
- Yan, X.; Yao, H.; Luo, J.; Li, Z.; Wei, J. Functionalization of Electrospun Nanofiber for Bone Tissue Engineering. Polymers 2022, 14, 2940. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of Electrospinning Technique for Biomedical Applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef]
- Shar, A.; Shar, A.; Joung, D. Carbon Nanotube Nanocomposite Scaffolds: Advances in Fabrication and Applications for Tissue Regeneration and Cancer Therapy. Front. Bioeng. Biotechnol. 2023, 11, 1299166. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Pham, N.T.; Dinh, T.T.M.; Vu, T.T.; Nguyen, H.S.; Tran, L.D. Electrodeposition of Hydroxyapatite-Multiwalled Carbon Nanotube Nanocomposite on Ti6Al4V. Adv. Polym. Technol. 2020, 2020, e8639687. [Google Scholar] [CrossRef]
- Zeng, H.-L.; Gao, C.; Yan, D.-Y. Poly(ϵ-Caprolactone)-Functionalized Carbon Nanotubes and Their Biodegradation Properties. Adv. Funct. Mater. 2006, 16, 812–818. [Google Scholar] [CrossRef]
- Song, L. Calcium and Bone Metabolism Indices. Adv. Clin. Chem. 2017, 82, 1–46. [Google Scholar] [CrossRef]
- Li, G.; Bouxsein, M.L.; Luppen, C.; Li, X.J.; Wood, M.; Seeherman, H.J.; Wozney, J.M.; Simpson, H. Bone Consolidation Is Enhanced by rhBMP-2 in a Rabbit Model of Distraction Osteogenesis. J. Orthop. Res. 2002, 20, 779–788. [Google Scholar] [CrossRef]
- Fischer, V.; Haffner-Luntzer, M.; Prystaz, K.; Vom Scheidt, A.; Busse, B.; Schinke, T.; Amling, M.; Ignatius, A. Calcium and Vitamin-D Deficiency Marginally Impairs Fracture Healing but Aggravates Posttraumatic Bone Loss in Osteoporotic Mice. Sci. Rep. 2017, 7, 7223. [Google Scholar] [CrossRef]
- Heubel, B.; Nohe, A. The Role of BMP Signaling in Osteoclast Regulation. J. Dev. Biol. 2021, 9, 24. [Google Scholar] [CrossRef]
- Yassine, K.A.; Mokhtar, B.; Houari, H.; Karim, A.; Mohamed, M. Repair of Segmental Radial Defect with Autologous Bone Marrow Aspirate and Hydroxyapatite in Rabbit Radius: A Clinical and Radiographic Evaluation. Vet. World 2017, 10, 752–757. [Google Scholar] [CrossRef]
- Scarfì, S. Use of Bone Morphogenetic Proteins in Mesenchymal Stem Cell Stimulation of Cartilage and Bone Repair. World J. Stem. Cells 2016, 8, 1–12. [Google Scholar] [CrossRef]
- Du, Z.; Feng, X.; Cao, G.; She, Z.; Tan, R.; Aifantis, K.E.; Zhang, R.; Li, X. The Effect of Carbon Nanotubes on Osteogenic Functions of Adipose-Derived Mesenchymal Stem Cells in Vitro and Bone Formation in Vivo Compared with That of Nano-Hydroxyapatite and the Possible Mechanism. Bioact. Mater. 2021, 6, 333–345. [Google Scholar] [CrossRef]
- Mori, H.; Ogura, Y.; Enomoto, K.; Hara, M.; Maurstad, G.; Stokke, B.T.; Kitamura, S. Dense Carbon-Nanotube Coating Scaffolds Stimulate Osteogenic Differentiation of Mesenchymal Stem Cells. PLoS ONE 2020, 15, e0225589. [Google Scholar] [CrossRef]
- Minto, B.W.; Sprada, A.G.; Gonçalves Neto, J.A.; de Alcântara, B.M.; Rocha, T.A.S.D.S.; Hespanha, A.C.V.; Quarterone, C.; Sartori, M.D.R.; Hataka, A.; Uscategui, R.A.R.; et al. Three-Dimensional Printed Poly (L-Lactide) and Hydroxyapatite Composite for Reconstruction of Critical Bone Defect in Rabbits. Acta Cir. Bras. 2021, 36, e360404. [Google Scholar] [CrossRef]
- Usui, Y.; Aoki, K.; Narita, N.; Murakami, N.; Nakamura, I.; Nakamura, K.; Ishigaki, N.; Yamazaki, H.; Horiuchi, H.; Kato, H.; et al. Carbon Nanotubes with High Bone-Tissue Compatibility and Bone-Formation Acceleration Effects. Small 2008, 4, 240–246. [Google Scholar] [CrossRef]
- Khan, A.A.; Mirza, E.H.; Syed, J.; Al-Khureif, A.; Mehmood, A.; Vallittu, P.K.; Alfotawi, R. Single and Multi-Walled Carbon Nanotube Fillers in Poly (Methyl Methacrylate)-Based Implant Material. J. Biomater. Tissue Eng. 2017, 7, 798–806. [Google Scholar] [CrossRef]
- Wen, X.-X.; Xu, C.; Wang, F.-Q.; Feng, Y.-F.; Zhao, X.; Yan, Y.-B.; Lei, W. Temporal Changes of Microarchitectural and Mechanical Parameters of Cancellous Bone in the Osteoporotic Rabbit. Biomed Res. Int. 2015, 2015, 263434. [Google Scholar] [CrossRef]
- E Silva, E.P.; Huang, B.; Helaehil, J.V.; Nalesso, P.R.L.; Bagne, L.; De Oliveira, M.A.; Albiazetti, G.C.C.; Aldalbahi, A.; El-Newehy, M.; Santamaria-Jr, M.; et al. In Vivo Study of Conductive 3D Printed PCL/MWCNTs Scaffolds with Electrical Stimulation for Bone Tissue Engineering. Bio-Des. Manuf. 2021, 4, 190–202. [Google Scholar] [CrossRef]
- Namgung, S.; Kim, T.; Baik, K.Y.; Lee, M.; Nam, J.; Hong, S. Fibronectin–Carbon-Nanotube Hybrid Nanostructures for Controlled Cell Growth. Small 2011, 7, 56–61. [Google Scholar] [CrossRef]
- Narita, N.; Kobayashi, Y.; Nakamura, H.; Maeda, K.; Ishihara, A.; Mizoguchi, T.; Usui, Y.; Aoki, K.; Simizu, M.; Kato, H.; et al. Multiwalled Carbon Nanotubes Specifically Inhibit Osteoclast Differentiation and Function. Nano. Lett. 2009, 9, 1406–1413. [Google Scholar] [CrossRef]
- Shimizu, M.; Kobayashi, Y.; Mizoguchi, T.; Nakamura, H.; Kawahara, I.; Narita, N.; Usui, Y.; Aoki, K.; Hara, K.; Haniu, H.; et al. Carbon Nanotubes Induce Bone Calcification by Bidirectional Interaction with Osteoblasts. Adv. Mater. 2012, 24, 2176–2185. [Google Scholar] [CrossRef]
- Tonelli, F.M.P.; Santos, A.K.; Gomes, K.N.; Lorençon, E.; Guatimosim, S.; Ladeira, L.O.; Resende, R.R. Carbon Nanotube Interaction with Extracellular Matrix Proteins Producing Scaffolds for Tissue Engineering. Int. J. Nanomed. 2012, 7, 4511–4529. [Google Scholar] [CrossRef]
- Valiani, A.; Hashemibeni, B.; Esfandiary, E.; Ansar, M.M.; Kazemi, M.; Esmaeili, N. Study of Carbon Nano-Tubes Effects on the Chondrogenesis of Human Adipose Derived Stem Cells in Alginate Scaffold. Int. J. Prev. Med. 2014, 5, 825–834. [Google Scholar]
- Yu, Y.Y.; Lieu, S.; Lu, C.; Colnot, C. Bone Morphogenetic Protein 2 Stimulates Endochondral Ossification by Regulating Periosteal Cell Fate during Bone Repair. Bone 2010, 47, 65–73. [Google Scholar] [CrossRef]
- Valente, F.L.; Reis, E.C.C.; Sepúlveda, R.V.; Ochoa, C.C.R.; Santos, L.C.; Corsini, C.M.; Borges, A.P.B. Hydroxyapatite, Polycaprolactone and Alendronate Composites for Bone Regeneration in Rabbits’ Olecranon: Histological Features. Arq. Bras. Med. Veterinária Zootec. 2016, 68, 543–547. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as Biomaterial for Bone Scaffolds: Review of Literature. J. Oral. Biol. Craniofac. Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Singh, B.N.; Veeresh, V.; Mallick, S.P.; Jain, Y.; Sinha, S.; Rastogi, A.; Srivastava, P. Design and Evaluation of Chitosan/Chondroitin Sulfate/Nano-Bioglass Based Composite Scaffold for Bone Tissue Engineering. Int. J. Biol. Macromol. 2019, 133, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.S.; Broggini, N.; Hjørting-Hansen, E.; Schenk, R.; Buser, D. Bone Healing and Graft Resorption of Autograft, Anorganic Bovine Bone and Beta-Tricalcium Phosphate. A Histologic and Histomorphometric Study in the Mandibles of Minipigs. Clin. Oral. Implant. Res. 2006, 17, 237–243. [Google Scholar] [CrossRef]
- Eggli, P.S.; Müller, W.; Schenk, R.K. Porous Hydroxyapatite and Tricalcium Phosphate Cylinders with Two Different Pore Size Ranges Implanted in the Cancellous Bone of Rabbits. A Comparative Histomorphometric and Histologic Study of Bony Ingrowth and Implant Substitution. Clin. Orthop. Relat. Res. 1988, 232, 127–138. [Google Scholar] [CrossRef]
- Mohammed, E.E.A.; Beherei, H.H.; El-Zawahry, M.; Farrag, A.R.H.; Kholoussi, N.; Helwa, I.; Gaber, K.; Allam, M.A.; Mabrouk, M.; Aleem, A.K.A. Combination of Human Amniotic Fluid Derived-Mesenchymal Stem Cells and Nano-Hydroxyapatite Scaffold Enhances Bone Regeneration. Open Access. Maced. J. Med. Sci. 2019, 7, 2739–2750. [Google Scholar] [CrossRef]



| Group | Animals | Treatment Protocol | Specimen Collection |
|---|---|---|---|
| A (Control) | 6 | External coaptation-splinting (ECS) | Day 90 PO |
| B | 6 | Polycaprolactone (PCL) + Hydroxyapatite (HAP) + ECS | Day 90 PO |
| C | 6 | PCL + HAP + MWCNT-COOH + ECS | Day 90 PO |
| D | 6 | PCL + HAP + MWCNT-COOH + Bone Morphogenetic Protein 2 (BMP-2) + ECS | Day 90 PO |
| E | 6 | PCL + HAP + MWCNT-COOH + rBMSC + ECS | Day 90 PO |
| F | 6 | PCL + HAP + MWCNT-COOH + rBMSC + BMP-2 + ECS | Day 90 PO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalaiselvan, E.; Maiti, S.K.; Shivaramu, S.; Banu, S.A.; Sharun, K.; Mohan, D.; Palakkara, S.; Bag, S.; Sahoo, M.; Ramalingam, S.; et al. Bone Marrow-Derived Mesenchymal Stem Cell-Laden Nanocomposite Scaffolds Enhance Bone Regeneration in Rabbit Critical-Size Segmental Bone Defect Model. J. Funct. Biomater. 2024, 15, 66. https://doi.org/10.3390/jfb15030066
Kalaiselvan E, Maiti SK, Shivaramu S, Banu SA, Sharun K, Mohan D, Palakkara S, Bag S, Sahoo M, Ramalingam S, et al. Bone Marrow-Derived Mesenchymal Stem Cell-Laden Nanocomposite Scaffolds Enhance Bone Regeneration in Rabbit Critical-Size Segmental Bone Defect Model. Journal of Functional Biomaterials. 2024; 15(3):66. https://doi.org/10.3390/jfb15030066
Chicago/Turabian StyleKalaiselvan, Elangovan, Swapan Kumar Maiti, Shivaraju Shivaramu, Shajahan Amitha Banu, Khan Sharun, Divya Mohan, Sangeetha Palakkara, Sadhan Bag, Monalisa Sahoo, Suresh Ramalingam, and et al. 2024. "Bone Marrow-Derived Mesenchymal Stem Cell-Laden Nanocomposite Scaffolds Enhance Bone Regeneration in Rabbit Critical-Size Segmental Bone Defect Model" Journal of Functional Biomaterials 15, no. 3: 66. https://doi.org/10.3390/jfb15030066
APA StyleKalaiselvan, E., Maiti, S. K., Shivaramu, S., Banu, S. A., Sharun, K., Mohan, D., Palakkara, S., Bag, S., Sahoo, M., Ramalingam, S., & Hescheler, J. (2024). Bone Marrow-Derived Mesenchymal Stem Cell-Laden Nanocomposite Scaffolds Enhance Bone Regeneration in Rabbit Critical-Size Segmental Bone Defect Model. Journal of Functional Biomaterials, 15(3), 66. https://doi.org/10.3390/jfb15030066








