Advancements in Biomedical Applications of Calcium Phosphate Glass and Glass-Based Devices—A Review
Abstract
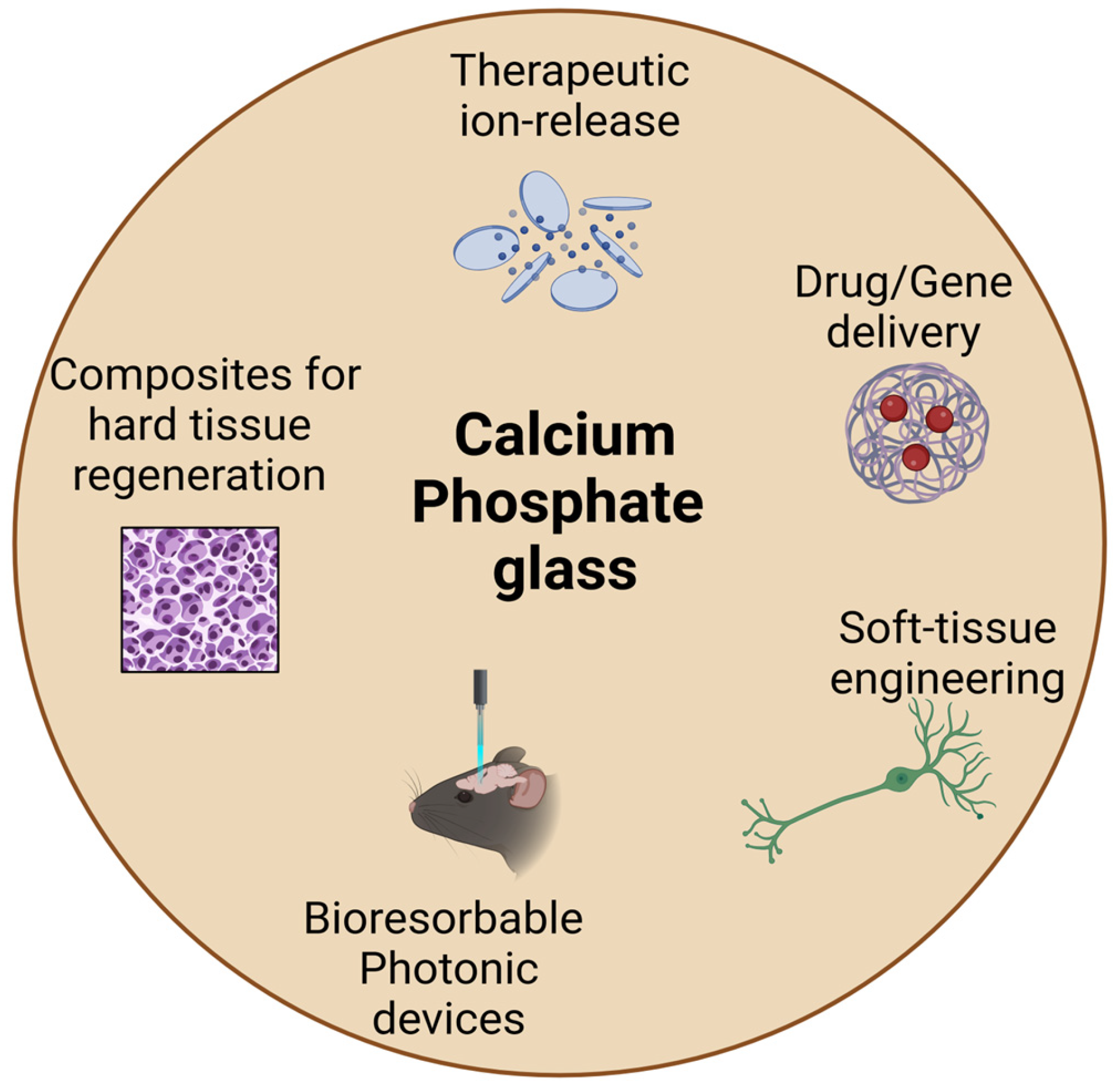
:1. Introduction
2. Methodology
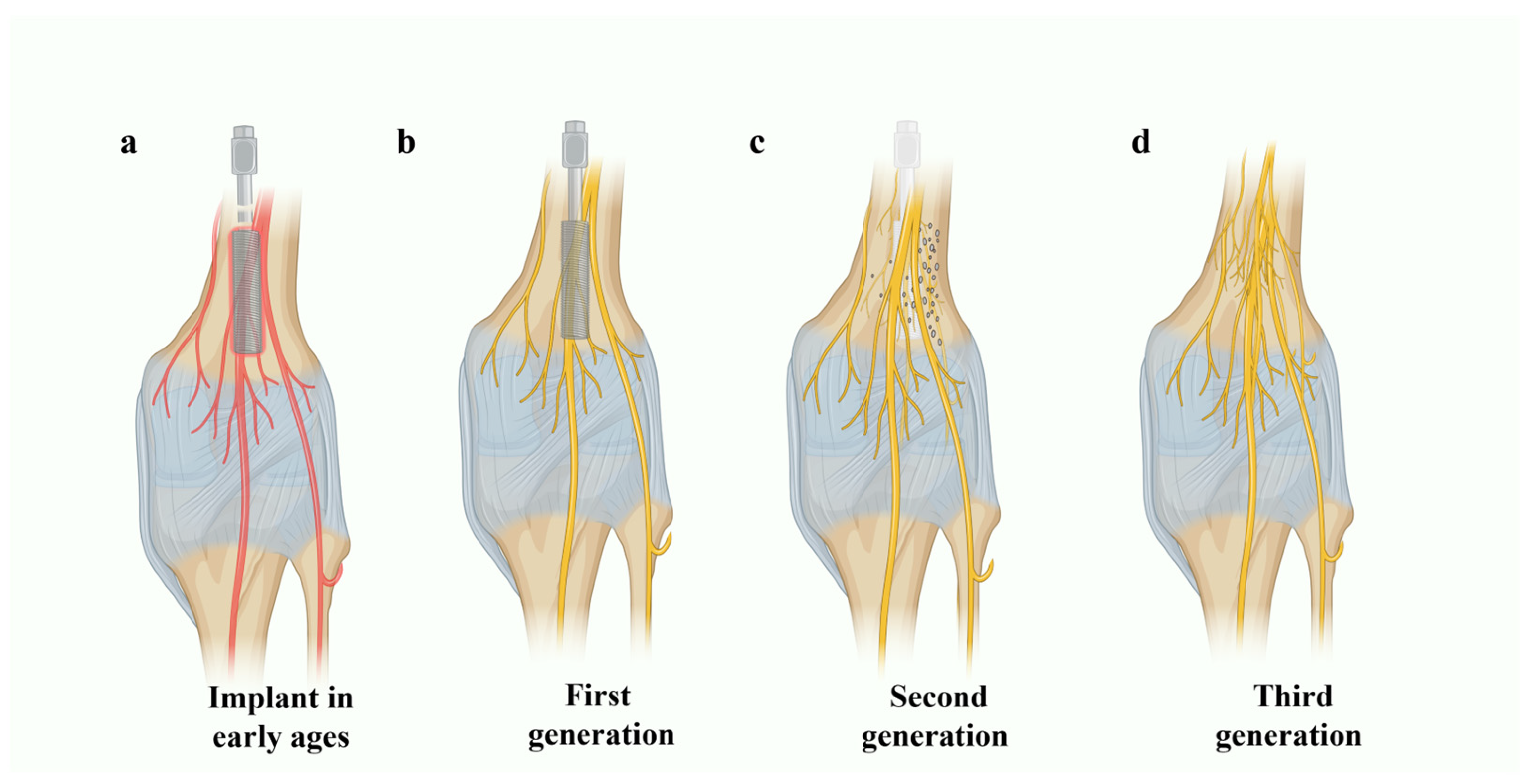
3. CaP Glass in Second- and Third-Generation Biomaterials
3.1. CaP Glass as Reinforcing Agents
3.1.1. CaP Glass-Based PCL Reinforcement
3.1.2. CaP Glass-Based PLA Reinforcement
3.1.3. CaP Glass-Based PAA Reinforcement
3.1.4. Phosphate Fiber–Collagen Composites
3.2. Dopants in Phosphate Glass Systems and Ion Releasing
3.2.1. Effect of Doping on Glass Solubility
3.2.2. Antibacterial Potential of Doped CaP Glass
3.2.3. Doped CaP Glass in Dental Applications
3.2.4. Doped CaP Glass in Veterinary Applications
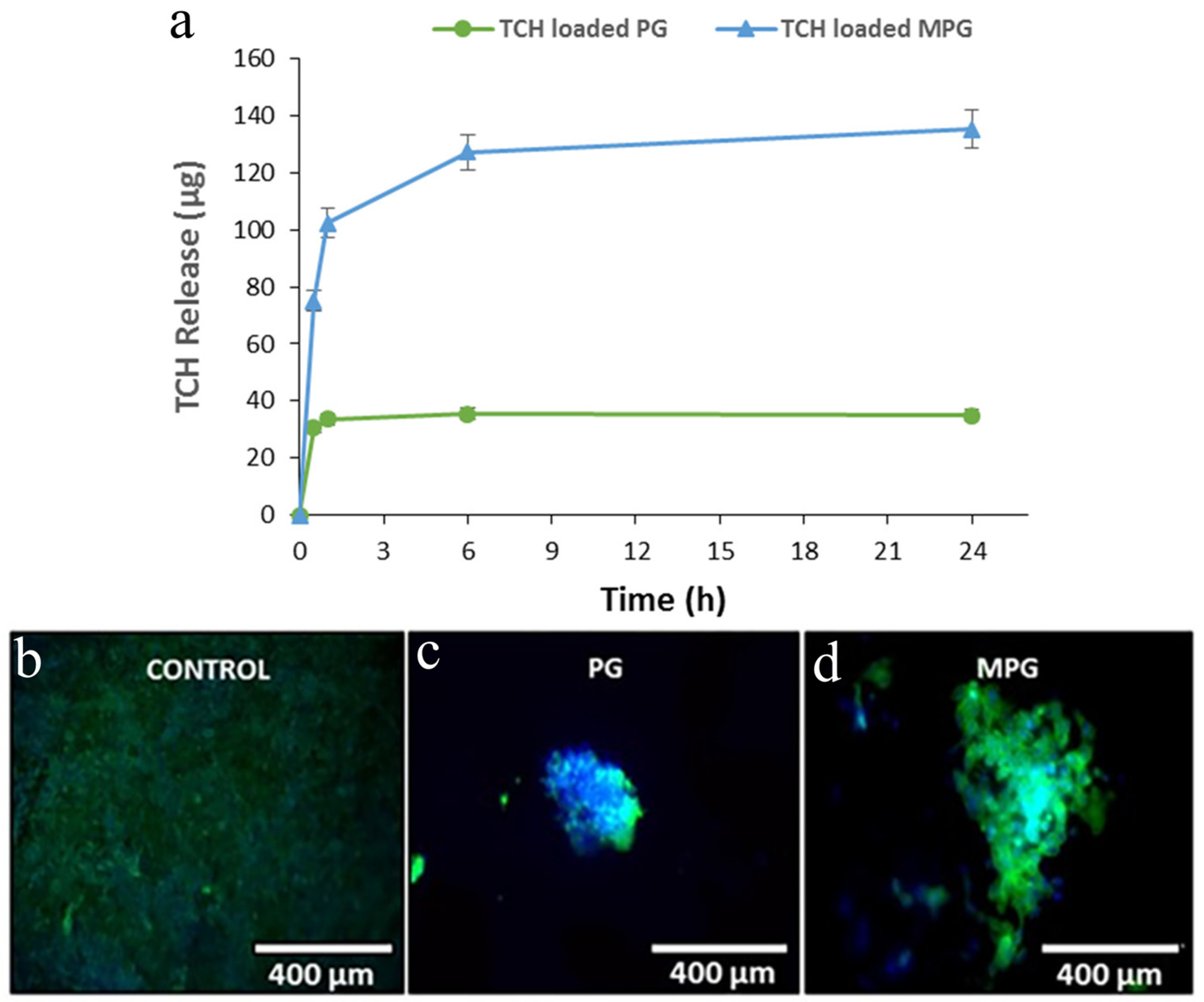
3.3. CaP Glass-Based Drug- and Gene-Delivery Systems
3.4. CaP Glass in Soft-Tissue Engineering
4. CaP Glass towards the Use in the Fourth Generation of Biomaterials
4.1. Optical Quality CaP Glass-Based Optical Fibers
4.2. Optical Quality CaP Multifunctional Devices
5. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huebsch, N.; Mooney, D.J. Inspiration and application in the evolution of biomaterials. Nature 2009, 462, 426–432. [Google Scholar] [CrossRef]
- Musculoskeletal Key. Available online: https://musculoskeletalkey.com/the-history-of-fracture-treatment/ (accessed on 10 November 2023).
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef]
- Battafarano, G.; Rossi, M.; De Martino, V.; Marampon, F.; Borro, L.; Secinaro, A.; Del Fattore, A. Strategies for bone regeneration: From graft to tissue engineering. Int. J. Mol. Sci. 2021, 22, 1128. [Google Scholar] [CrossRef]
- Christopher, S. (Ed.) The Chemistry of Inorganic Biomaterials; Royal Society of Chemistry: London, UK, 2021; Volume 7. [Google Scholar]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic biomaterials: Current challenges and opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Kalirajan, C.; Dukle, A.; Nathanael, A.J.; Oh, T.-H.; Manivasagam, G. A critical review on polymeric biomaterials for biomedical applications. Polymers 2021, 13, 3015. [Google Scholar] [CrossRef]
- Hench, L.L.; Thompson, I. Twenty-first century challenges for biomaterials. J. R. Soc. Interface 2010, 7 (Suppl. S4), S379–S391. [Google Scholar] [CrossRef]
- Hench, L.L.; Polak, J.M. Third-generation biomedical materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Wilson, J. Surface-active biomaterials. Science 1984, 226, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Wilson, J. Bioactive materials. MRS Online Proc. Libr. 1985, 55, 65. [Google Scholar] [CrossRef]
- Hench, L.L.; Ethridge, E.C. Biomaterials: An Interfacial Approach; Academic Press: Cambridge, MA, USA, 1982. [Google Scholar]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Lin, S.T.; Krebs, S.L.; Kadiyala, S.; Leong, K.W.; LaCourse, W.C.; Kumar, B. Development of bioabsorbable glass fibres. Biomaterials 1994, 15, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Michiardi, A.; Castano, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive glasses: Where are we and where are we going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Bongio, M.; Van Den Beucken, J.J.; Leeuwenburgh, S.C.; Jansen, J.A. Development of bone substitute materials: From ‘biocompatible’to ‘instructive’. J. Mater. Chem. 2010, 20, 8747–8759. [Google Scholar] [CrossRef]
- Ahmed, I.; Collins, C.; Lewis, M.; Olsen, I.; Knowles, J. Processing, characterisation and biocompatibility of iron-phosphate glass fibres for tissue engineering. Biomaterials 2004, 25, 3223–3232. [Google Scholar] [CrossRef]
- Salih, V.; Franks, K.; James, M.; Hastings, G.W.; Knowles, J.C.; Olsen, I. Development of soluble glasses for biomedical use Part II: The biological response of human osteoblast cell lines to phosphate-based soluble glasses. J. Mater. Sci. Mater. Med. 2000, 11, 615–620. [Google Scholar] [CrossRef]
- Navarro, M.; del Valle, S.; Martínez, S.; Zeppetelli, S.; Ambrosio, L.; Planell, J.A.; Ginebra, M.P. New macroporous calcium phosphate glass ceramic for guided bone regeneration. Biomaterials 2004, 25, 4233–4241. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Taylor, T.D.; Tian, Y.; Latour, R.A., Jr. Real-time dissolution measurement of sized and unsized calcium phosphate glass fibers. J. Biomed. Mater. Res. 1999, 48, 833–840. [Google Scholar] [CrossRef]
- Rajendran, V.; Rajkumar, G.; Aravindan, S.; Saravanakumar, B. Analysis of Physical Properties and Hydroxyapatite Precipitation In Vitro of TiO2-Containing Phosphate-Based Glass Systems. J. Am. Ceram. Soc. 2010, 93, 4053–4060. [Google Scholar] [CrossRef]
- Lakhkar, N.J.; Park, J.H.; Mordan, N.J.; Salih, V.; Wall, I.B.; Kim, H.W.; King, S.P.; Hanna, J.V.; Martin, R.A.; Addison, O.; et al. Titanium phosphate glass microspheres for bone tissue engineering. Acta Biomater. 2012, 8, 4181–4190. [Google Scholar] [CrossRef]
- Ning, C.; Zhou, L.; Tan, G. Fourth-generation biomedical materials. Mater. Today 2016, 19, 2–3. [Google Scholar] [CrossRef]
- Best, S.M.; Porter, A.E.; Thian, E.S.; Huang, J. Bioceramics: Past, present and for the future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327. [Google Scholar] [CrossRef]
- Andreu-Perez, J.; Leff, D.R.; Ip, H.M.D.; Yang, G.-Z. From wearable sensors to smart implants-–toward pervasive and personalized healthcare. IEEE Trans. Biomed. Eng. 2015, 62, 2750–2762. [Google Scholar] [CrossRef] [PubMed]
- Lepry, W.C.; Nazhat, S.N. A review of phosphate and borate sol–gel glasses for biomedical applications. Adv. NanoBiomed Res. 2021, 1, 2000055. [Google Scholar] [CrossRef]
- Blencke, B.A.; Brömer, H.; Deutscher, K.K. Compatibility and long-term stability of glass–ceramic implants. J. Biomed. Mater. Res. 1978, 12, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H.; Taki, Y.; Abe, Y. Implantation of new calcium phosphate glass-ceramics. J. Dent. Res. 1977, 56, 1260. [Google Scholar] [CrossRef] [PubMed]
- Graves, G.A., Jr.; Kumar, B. Bioabsorbable Glass Fibers for Use in the Reinforcement of Bioabsorbable Polymers for Bone Fixation Devices and Artificial Ligaments. European Patent Office Publ. of Application with Search Report EP19860901605, 10 February 1986. [Google Scholar]
- Burnie, J.; Gilchrist, T.; Duff, S.R.I.; Drake, C.F.; Harding, N.G.L.; Malcolm, A.J. Controlled release glasses (CRG) for biomedical uses. Biomaterials 1981, 2, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.P.; Abe, Y.; Hosono, H.; De Groot, K. Different calcium phosphate bioglass ceramics implanted in rabbit cortical bone. An interface study. Biomaterials 1984, 5, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Davies, J.E. The in vitro response of osteoblasts to bioactive glass. Biomaterials 1987, 8, 275–284. [Google Scholar] [CrossRef]
- Kokubo, T. Bioactive glass ceramics: Properties and applications. Biomaterials 1991, 12, 155–163. [Google Scholar] [CrossRef]
- Brink, M.; Turunen, T.; Happonen, R.P.; Yli-Urpo, A. Compositional dependence of bioactivity of glasses in the system Na2O-K2O-MgO-CaO-B2O3-P2O5-SiO2. J. Biomed. Mater. Res. 1997, 37, 114–121. [Google Scholar] [CrossRef]
- Salinas, A.J.; Roman, J.; Vallet-Regi, M.; Oliveira, J.M.; Correia, R.N.; Fernandes, M.H. In vitro bioactivity of glass and glass-ceramics of the 3CaO· P2O5–CaO·SiO2–CaO·MgO·2SiO2 system. Biomaterials 2000, 21, 251–257. [Google Scholar] [CrossRef]
- Moghadasi, K.; Isa, M.S.M.; Ariffin, M.A.; Jamil, M.Z.M.; Raja, S.; Wu, B.; Yamani, M.; Bin Muhamad, M.R.; Yusof, F.; Jamaludin, M.F.; et al. A review on biomedical implant materials and the effect of friction stir based techniques on their mechanical and tribological properties. J. Mater. Res. Technol. 2022, 17, 1054–1121. [Google Scholar] [CrossRef]
- Jin, S.; Xia, X.; Huang, J.; Yuan, C.; Zuo, Y.; Li, Y.; Li, J. Recent advances in PLGA-based biomaterials for bone tissue regeneration. Acta Biomater. 2021, 127, 56–79. [Google Scholar] [CrossRef]
- Felfel, R.M.; Ahmed, I.; Parsons, A.J.; Harper, L.T.; Rudd, C.D. Initial mechanical properties of phosphate-glass fibre-reinforced rods for use as resorbable intramedullary nails. J. Mater. Sci. 2012, 47, 4884–4894. [Google Scholar] [CrossRef]
- Sglavo, V.M.; Pugliese, D.; Sartori, F.; Boetti, N.G.; Ceci-Ginistrelli, E.; Franco, G.; Milanese, D. Mechanical properties of resorbable calcium-phosphate glass optical fiber and capillaries. J. Alloys Compd. 2019, 778, 410–417. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, R.L.; Brocchini, S.; Knowles, J.C. Effect of glass composition on the degradation properties and ion release characteristics of phosphate glass—Polycaprolactone composites. Biomaterials 2005, 26, 2209–2218. [Google Scholar] [CrossRef] [PubMed]
- Alani, A.; Knowles, J.C.; Chrzanowski, W.; Ng, Y.L.; Gulabivala, K. Ion release characteristics, precipitate formation and sealing ability of a phosphate glass–polycaprolactone-based composite for use as a root canal obturation material. Dent. Mater. 2009, 25, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Scotchford, C.A.; Shataheri, M.; Chen, P.S.; Evans, M.; Parsons, A.J.; Aitchison, G.A.; Efeoglu, C.A.; Burke, J.L.; Vikram, A.; Fisher, S.E.; et al. Repair of calvarial defects in rats by prefabricated, degradable, long fibre composite implants. J. Biomed. Mater. Res. Part A 2011, 96, 230–238. [Google Scholar] [CrossRef]
- Felfel, R.M.; Ahmed, I.; Parsons, A.J.; Rudd, C.D. Bioresorbable screws reinforced with phosphate glass fibre: Manufacturing and mechanical property characterisation. J. Mech. Behav. Biomed. Mater. 2013, 17, 76–88. [Google Scholar] [CrossRef]
- Furko, M.; Horváth, Z.E.; Czömpöly, O.; Balázsi, K.; Balázsi, C. Biominerals Added Bioresorbable Calcium Phosphate Loaded Biopolymer Composites. Int. J. Mol. Sci. 2022, 23, 15737. [Google Scholar] [CrossRef] [PubMed]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Brauer, D.S.; Rüssel, C.; Vogt, S.; Weisser, J.; Schnabelrauch, M. Degradable phosphate glass fiber reinforced polymer matrices: Mechanical properties and cell response. J. Mater. Sci. Mater. Med. 2008, 19, 121–127. [Google Scholar] [CrossRef]
- Ahmed, I.; Cronin, P.S.; Neel, E.A.A.; Parsons, A.J.; Knowles, J.C.; Rudd, C.D. Retention of mechanical properties and cytocompatibility of a phosphate-based glass fiber/polylactic acid composite. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Saloumi, N.; Daki, I.; El Bouchti, M.; Oumam, M.; Manoun, B.; Yousfi, M.; Hannache, H.; Cherkaoui, O. Development and Characterization of Phosphate Glass Fibers and Their Application in the Reinforcement of Polyester Matrix Composites. Materials 2022, 15, 7601. [Google Scholar] [CrossRef]
- Nazhat, S.N.; Abou Neel, E.A.; Kidane, A.; Ahmed, I.; Hope, C.; Kershaw, M.; Lee, P.D.; Stride, E.; Saffari, N.; Knowles, J.C.; et al. Controlled microchannelling in dense collagen scaffolds by soluble phosphate glass fibers. Biomacromolecules 2007, 8, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Ahmed, I.; Parsons, A.J.; Harper, L.; Scotchford, C.A.; Scammell, B.E.; Rudd, C.D. Influence of screw holes and gamma sterilization on properties of phosphate glass fiber-reinforced composite bone plates. J. Biomater. Appl. 2013, 27, 990–1002. [Google Scholar] [CrossRef]
- Dalei, G.; Das, S. Polyacrylic acid-based drug delivery systems: A comprehensive review on the state-of-art. J. Drug Deliv. Sci. Technol. 2022, 78, 103988. [Google Scholar] [CrossRef]
- Li, F.; Xing, Q.; Han, Y.; Li, Y.; Wang, W.; Perera, T.S.H.; Dai, H. Ultrasonically assisted preparation of poly (acrylic acid)/calcium phosphate hybrid nanogels as pH-responsive drug carriers. Mater. Sci. Eng. C 2017, 80, 688–697. [Google Scholar] [CrossRef]
- Chen, Q.; Jing, J.; Qi, H.; Ahmed, I.; Yang, H.; Liu, X.; Lu, T.L.; Boccaccini, A.R. Electric field-assisted orientation of short phosphate glass fibers on stainless steel for biomedical applications. ACS Appl. Mater. Interfaces 2018, 10, 11529–11538. [Google Scholar] [CrossRef]
- Sharifi, E.; Azami, M.; Kajbafzadeh, A.-M.; Moztarzadeh, F.; Faridi-Majidi, R.; Shamousi, A.; Karimi, R.; Ai, J. Preparation of a biomimetic composite scaffold from gelatin/collagen and bioactive glass fibers for bone tissue engineering. Mater. Sci. Eng. C 2016, 59, 533–541. [Google Scholar] [CrossRef]
- Francis, S. Evonik Launches Composite Polymers for Enhancing Performance of Bone Fixation Devices. Available online: https://www.compositesworld.com/news/evonik-launches-new-product-to-enhance-performance-of-bone-fixation-devices (accessed on 15 November 2023).
- Ahmed, I.; Lewis, M.; Olsen, I.; Knowles, J.C. Phosphate glasses for tissue engineering: Part 1. Processing and characterisation of a ternary-based P2O5–CaO–Na2O glass system. Biomaterials 2004, 25, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Burling, L.D. Novel Phosphate Glasses for Bone Regeneration Applications. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2005. [Google Scholar]
- Franks, K.; Salih, V.; Knowles, J.C.; Olsen, I. The effect of MgO on the solubility behavior and cell proliferation in a quaternary soluble phosphate-based glass system. J. Mater. Sci. Mater. Med. 2002, 13, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Bunker, B.C.; Arnold, G.W.; Wilder, J.A. Phosphate glass dissolution in aqueous solutions. J. Non-Cryst. Solids 1984, 64, 291–316. [Google Scholar] [CrossRef]
- Delahaye, F.; Montagne, L.; Palavit, G.; Touray, J.C.; Baillif, P. Acid dissolution of sodium–calcium metaphosphate glasses. J. Non-Cryst. Solids 1998, 242, 25–32. [Google Scholar] [CrossRef]
- Massera, J.; Bourhis, K.; Petit, L.; Couzi, M.; Hupa, L.; Hupa, M.; Videau, J.; Cardinal, T. Effect of the glass composition on the chemical durability of zinc-phosphate-based glasses in aqueous solutions. J. Phys. Chem. Solids 2013, 74, 121–127. [Google Scholar] [CrossRef]
- Lapa, A.; Cresswell, M.; Jackson, P.; Boccaccini, A.R. Phosphate glass fibres with therapeutic ions release capability—A review. Adv. Appl. Ceram. 2019, 119, 1–14. [Google Scholar] [CrossRef]
- Patel, U.; Moss, R.M.; Hossain, K.M.Z.; Kennedy, A.R.; Barney, E.R.; Ahmed, I.; Hannon, A.C. Structural and physico-chemical analysis of calcium/strontium substituted, near-invert phosphate based glasses for biomedical applications. Acta Biomater. 2017, 60, 109–127. [Google Scholar] [CrossRef]
- Neel, E.A.; Ahmed, I.; Pratten, J.; Nazhat, S.; Knowles, J. Characterisation of antibacterial copper releasing degradable phosphate glass fibres. Biomaterials 2005, 26, 2247–2254. [Google Scholar] [CrossRef]
- Ahmed, I.; Parsons, A.; Jones, A.; Walker, G.; Scotchford, C.; Rudd, C. Cytocompatibility and effect of increasing MgO content in a range of quaternary invert phosphate-based glasses. J. Biomater. Appl. 2010, 24, 555–575. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, N.; Hasan, M.S.; Parsons, A.J.; Furniss, D.; Scotchford, C.A.; Ahmed, I.; Rudd, C.D. Effect of boron addition on the thermal, degradation, and cytocompatibility properties of phosphate-based glasses. BioMed Res. Int. 2013, 2013, 902427. [Google Scholar] [CrossRef] [PubMed]
- Andreadis, G.A.; Toumba, K.J.; Curzon, M.E.J. Slow-release flouride glass devices: In vivo flouride release and retention of the devices in children. Eur. Arch. Paediatr. Dent. 2006, 7, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, G.; Dhivya, V.; Mahalaxmi, S.; Rajkumar, K.; Sathishkumar, G.K.; Karpagam, R. Influence of fluoride for enhancing bioactivity onto phosphate based glasses. J. Non-Cryst. Solids 2018, 493, 108–118. [Google Scholar] [CrossRef]
- Raja, F.N.; Worthington, T.; Isaacs, M.A.; Rana, K.S.; Martin, R.A. The antimicrobial efficacy of zinc doped phosphate-based glass for treating catheter associated urinary tract infections. Mater. Sci. Eng. C 2019, 103, 109868. [Google Scholar] [CrossRef] [PubMed]
- Novajra, G.; Lousteau, J.; Milanese, D.; Vitale-Brovarone, C. Resorbable hollow phosphate glass fibres as controlled release systems for biomedical applications. Mater. Lett. 2013, 99, 125–127. [Google Scholar] [CrossRef]
- Shah, R.; Sinanan, A.C.; Knowles, J.C.; Hunt, N.P.; Lewis, M.P. Craniofacial muscle engineering using a 3-dimensional phosphate glass fibre construct. Biomaterials 2005, 26, 1497–1505. [Google Scholar] [CrossRef]
- Ahmed, I.; Ready, D.; Wilson, M.; Knowles, J.C. Antimicrobial effect of silver-doped phosphate-based glasses. J. Biomed. Mater. Res. Part A 2006, 79, 618–626. [Google Scholar] [CrossRef]
- Islam, T.; Hossain, K.M.Z.; Sharmin, N.; Parsons, A.J.; Ahmed, I. Effect of magnesium content on bioactivity of near invert phosphate-based glasses. Int. J. Appl. Glass Sci. 2017, 8, 391–402. [Google Scholar] [CrossRef]
- Dias, A.G.; Gibson, I.R.; Santos, J.D.; Lopes, M.A. Physicochemical degradation studies of calcium phosphate glass ceramic in the CaO–P2O5–MgO–TiO2 system. Acta Biomater. 2007, 3, 263–269. [Google Scholar] [CrossRef]
- Foroutan, F.; De Leeuw, N.H.; Martin, R.A.; Palmer, G.; Owens, G.J.; Kim, H.W.; Knowles, J.C. Novel sol–gel preparation of (P2 O5) 0.4–(CaO) 0.25–(Na2O) X–(TiO2)(0.35−X) bioresorbable glasses (X = 0.05, 0.1, and 0.15). J. Sol-Gel Sci. Technol. 2015, 73, 434–442. [Google Scholar] [CrossRef]
- Pickup, D.M.; Abou Neel, E.A.; Moss, R.M.; Wetherall, K.M.; Guerry, P.; Smith, M.E.; Knowles, J.C.; Newport, R.J. Ti K-edge XANES study of the local environment of titanium in bioresorbable TiO2–CaO–Na2O–P2O5 glasses. J. Mater. Sci. Mater. Med. 2008, 19, 1681–1685. [Google Scholar] [CrossRef]
- Li, J.; Cai, S.; Xu, G.; Li, X.; Zhang, W.; Zhang, Z. In vitro biocompatibility study of calcium phosphate glass ceramic scaffolds with different trace element doping. Mater. Sci. Eng. C 2012, 32, 356–363. [Google Scholar] [CrossRef]
- Lakhkar, N.J.; Lee, I.-H.; Kim, H.-W.; Salih, V.; Wall, I.B.; Knowles, J.C. Knowles. Bone formation controlled by biologically relevant inorganic ions: Role and controlled delivery from phosphate-based glasses. Adv. Drug Deliv. Rev. 2013, 65, 405–420. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, T.N.; Smith, J.D.; Thomas, J.D.; Drake, C.F. Copper molluscicides for control of schistosomiasis. 1. Effect of inorganic complexes on toxicity. Environ. Sci. Technol. 1989, 23, 1102–1106. [Google Scholar] [CrossRef]
- Mulligan, A.M.; Wilson, M.; Knowles, J.C. The effect of increasing copper content in phosphate-based glasses on biofilms of Streptococcus sanguis. Biomaterials 2003, 24, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, A.M.; Wilson, M.; Knowles, J.C. Effect of increasing silver content in phosphate-based glasses on biofilms of Streptococcus sanguis. J. Biomed. Mater. Res. Part A 2003, 67, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, F.; McGuire, J.; Gupta, P.; Nikolaou, A.; Kyffin, B.A.; Kelly, N.L.; Hanna, J.V.; Gutierrez-Merino, J.; Knowles, J.C.; Baek, S.Y.; et al. Antibacterial copper-doped calcium phosphate glasses for bone tissue regeneration. ACS Biomater. Sci. Eng. 2019, 5, 6054–6062. [Google Scholar] [CrossRef]
- Restivo, E.; Pugliese, D.; Gallichi-Nottiani, D.; Sammartino, J.C.; Bloise, N.; Peluso, E.; Percivalle, E.; Janner, D.; Milanese, D.; Visai, L. Effect of Low Copper Doping on the Optical, Cytocompatible, Antibacterial, and SARS-CoV-2 Trapping Properties of Calcium Phosphate Glasses. ACS Omega 2023, 8, 42264–42274. [Google Scholar] [CrossRef]
- Pickup, D.M.; Valappil, S.P.; Moss, R.M.; Twyman, H.L.; Guerry, P.; Smith, M.E.; Wilson, M.; Knowles, J.C.; Newport, R.J. Preparation, structural characterisation and antibacterial properties of Ga-doped sol–gel phosphate-based glass. J. Mater. Sci. 2009, 44, 1858–1867. [Google Scholar] [CrossRef]
- Foroutan, F.; Kyffin, B.A.; Abrahams, I.; Knowles, J.C.; Sogne, E.; Falqui, A.; Carta, D. Mesoporous strontium-doped phosphate-based sol-gel glasses for biomedical applications. Front. Chem. 2020, 8, 249. [Google Scholar] [CrossRef]
- Łapa, A.; Cresswell, M.; Campbell, I.; Jackson, P.; Goldmann, W.H.; Detsch, R.; Parsons, A.; Ahmed, I.; Boccaccini, A.R. Ga and Ce ion-doped phosphate glass fibres with antibacterial properties and their composite for wound healing applications. J. Mater. Chem. B 2019, 7, 6981–6993. [Google Scholar] [CrossRef]
- Foroutan, F.; Nikolaou, A.; Kyffin, B.A.; Elliott, R.M.; Felipe-Sotelo, M.; Gutierrez-Merino, J.; Carta, D. Multifunctional phosphate-based glass fibres prepared via electrospinning of coacervate precursors: Controlled delivery, biocompatibility and antibacterial activity. Materialia 2020, 14, 100939. [Google Scholar] [CrossRef]
- Mneimne, M.; Hill, R.G.; Bushby, A.J.; Brauer, D.S. High phosphate content significantly increases apatite formation of fluoride-containing bioactive glasses. Acta Biomater. 2011, 7, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- BioMin. Available online: https://biomin.co.uk/professional-information/bioactive-glasses (accessed on 17 November 2023).
- Al Qaysi, M.; Walters, N.J.; Foroutan, F.; Owens, G.J.; Kim, H.W.; Shah, R.; Knowles, J.C. Strontium-and calcium-containing, titanium-stabilised phosphate-based glasses with prolonged degradation for orthopaedic tissue engineering. J. Biomater. Appl. 2015, 30, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.F.; Allen, W.M. The use of controlled-release glass for the controlled delivery of bioactive materials. Biochem. Soc. Trans. 1985, 13, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.C. Phosphate based glasses for biomedical applications. J. Mater. Chem. 2003, 13, 2395–2401. [Google Scholar] [CrossRef]
- Bose, S.; Fielding, G.; Tarafder, S.; Bandyopadhyay, A. Trace element doping in calcium phosphate ceramics to understand osteogenesis and angiogenesis. Trends Biotechnol. 2013, 31, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Ignjatović, N.; Ajduković, Z.; Savić, V.; Najman, S.; Mihailović, D.; Vasiljević, P.; Stojanović, Z.; Uskoković, V.; Uskoković, D. Nanoparticles of cobalt-substituted hydroxyapatite in regeneration of mandibular osteoporotic bones. J. Mater. Sci. Mater. Med. 2013, 24, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Pickup, D.M.; Newport, R.J.; Knowles, J.C. Sol–gel phosphate-based glass for drug delivery applications. J. Biomater. Appl. 2012, 26, 613–622. [Google Scholar] [CrossRef]
- Foroutan, F.; Kyffin, B.A.; Abrahams, I.; Corrias, A.; Gupta, P.; Velliou, E.; Knowles, J.C.; Carta, D. Mesoporous phosphate-based glasses prepared via Sol–Gel. ACS Biomater. Sci. Eng. 2020, 6, 1428–1437. [Google Scholar] [CrossRef]
- Sun, R.; Åhlén, M.; Tai, C.W.; Bajnóczi, É.G.; de Kleijne, F.; Ferraz, N.; Persson, I.; Strømme, M.; Cheung, O. Highly porous amorphous calcium phosphate for drug delivery and bio-medical applications. Nanomaterials 2019, 10, 20. [Google Scholar] [CrossRef]
- Islam, M.T.; Felfel, R.M.; Abou Neel, E.A.; Grant, D.M.; Ahmed, I.; Hossain, K.M. Bioactive calcium phosphate–based glasses and ceramics and their biomedical applications: A review. J. Tissue Eng. 2017, 8, 2041731417719170. [Google Scholar] [CrossRef]
- Baino, F.; Vitale-Brovarone, C. Three-dimensional glass-derived scaffolds for bone tissue engineering: Current trends and forecasts for the future. J. Biomed. Mater. Res. Part A 2011, 97, 514–535. [Google Scholar] [CrossRef] [PubMed]
- Joo, N.Y.; Knowles, J.C.; Lee, G.S.; Kim, J.W.; Kim, H.W.; Son, Y.J.; Hyun, J.K. Effects of phosphate glass fiber–collagen scaffolds on functional recovery of completely transected rat spinal cords. Acta Biomater. 2012, 8, 1802–1812. [Google Scholar] [CrossRef]
- Kim, Y.P.; Lee, G.S.; Kim, J.W.; Kim, M.S.; Ahn, H.S.; Lim, J.Y.; Kim, H.W.; Son, Y.J.; Knowles, J.C.; Hyun, J.K. Phosphate glass fibres promote neurite outgrowth and early regeneration in a peripheral nerve injury model. J. Tissue Eng. Regen. Med. 2015, 9, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Vitale-Brovarone, C.; Novajra, G.; Lousteau, J.; Milanese, D.; Raimondo, S.; Fornaro, M. Phosphate glass fibres and their role in neuronal polarization and axonal growth direction. Acta Biomater. 2012, 8, 1125–1136. [Google Scholar] [CrossRef]
- Kargozar, S.; Hamzehlou, S.; Baino, F. Can bioactive glasses be useful to accelerate the healing of epithelial tissues? Mater. Sci. Eng. C 2019, 97, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Naseri, S.; Lepry, W.C.; Nazhat, S.N. Bioactive glasses in wound healing: Hope or hype? J. Mater. Chem. B 2017, 5, 6167–6174. [Google Scholar] [CrossRef]
- Pirhonen, E.; Moimas, L.; Haapanen, J. Porous bioactive 3-D glass fiber scaffolds for tissue engineering applications manufactured by sintering technique. Key Eng. Mater. 2002, 240, 237–240. [Google Scholar] [CrossRef]
- Vitale-Brovarone, C.; Ciapetti, G.; Leonardi, E.; Baldini, N.; Bretcanu, O.; Verné, E.; Baino, F. Resorbable glass–ceramic phosphate-based scaffolds for bone tissue engineering: Synthesis, properties, and in vitro effects on human marrow stromal cells. J. Biomater. Appl. 2011, 26, 465–489. [Google Scholar] [CrossRef]
- Cai, S.; Xu, G.H.; Yu, X.Z.; Zhang, W.J.; Xiao, Z.Y.; Yao, K.D. Fabrication and biological characteristics of β-tricalcium phosphate porous ceramic scaffolds reinforced with calcium phosphate glass. J. Mater. Sci. Mater. Med. 2009, 20, 351–358. [Google Scholar] [CrossRef]
- Karp, J.M.; Langer, R. Development and therapeutic applications of advanced biomaterials. Curr. Opin. Biotechnol. 2007, 18, 454–459. [Google Scholar] [CrossRef]
- Amukarimi, S.; Ramakrishna, S.; Mozafari, M. Smart biomaterials—A proposed definition and overview of the field. Curr. Opin. Biomed. Eng. 2021, 19, 100311. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F. Advanced glass-ceramic materials for biomedical applications. J. Bone Rep. Recomm. 2017, 3, 97–108. [Google Scholar] [CrossRef]
- Gierej, A.; Geernaert, T.; Van Vlierberghe, S.; Dubruel, P.; Thienpont, H.; Berghmans, F. Challenges in the fabrication of biodegradable and implantable optical fibers for biomedical applications. Materials 2021, 14, 1972. [Google Scholar] [CrossRef] [PubMed]
- Elbashar, Y.H.; Saeed, A.; Moslem, S.S. Spectroscopic analysis of copper calcium phosphate glasses matrix. Nonlinear Opt. Quantum Opt. 2016, 48, 41–48. [Google Scholar]
- Lee, E.T.Y.; Taylor, E.R.M. Optical and thermal properties of binary calcium phosphate and barium phosphate glasses. Opt. Mater. 2006, 28, 200–206. [Google Scholar] [CrossRef]
- Ceci-Ginistrelli, E.; Pugliese, D.; Boetti, N.G.; Novajra, G.; Ambrosone, A.; Lousteau, J.; Vitale-Brovarone, C.; Abrate, S.; Milanese, D. Novel biocompatible and resorbable UV-transparent phosphate glass based optical fiber. Opt. Mater. Express 2016, 6, 2040–2051. [Google Scholar] [CrossRef]
- Podrazký, O.; Peterka, P.; Kašík, I.; Vytykáčová, S.; Proboštová, J.; Mrázek, J.; Kuneš, M.; Závalová, V.; Radochová, V.; Lyutakov, O.; et al. In vivo testing of a bioresorbable phosphate-based optical fiber. J. Biophotonics 2019, 12, e201800397. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, D.; Konstantaki, M.; Konidakis, I.; Ceci-Ginistrelli, E.; Boetti, N.G.; Milanese, D.; Pissadakis, S. Bioresorbable optical fiber Bragg gratings. Opt. Lett. 2018, 43, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Luo, W.; Nazempour, R.; Tan, D.; Ding, H.; Zhang, K.; Yin, L.; Guan, J.; Sheng, X. Implantable and biodegradable poly (l-lactic acid) fibers for optical neural interfaces. Adv. Opt. Mater. 2018, 6, 1700941. [Google Scholar] [CrossRef]
- Farajikhah, S.; Runge, A.F.; Boumelhem, B.B.; Rukhlenko, I.D.; Stefani, A.; Sayyar, S.; Innis, P.C.; Fraser, S.T.; Fleming, S.; Large, M.C. Thermally drawn biodegradable fibers with tailored topography for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, M.; Zhang, Y.; Xu, Y.; Zhang, Y.; Yang, X.; Zhang, J.; Yang, J.; Yuan, L. Spider silk-based tapered optical fiber for humidity sensing based on multimode interference. Sens. Actuators A Phys. 2020, 313, 112179. [Google Scholar] [CrossRef]
- Gallichi-Nottiani, D.; Pugliese, D.; Giovanna Boetti, N.; Milanese, D.; Janner, D. Toward the fabrication of extruded microstructured bioresorbable phosphate glass optical fibers. Int. J. Appl. Glass Sci. 2020, 11, 632–640. [Google Scholar] [CrossRef]
- Rizi, S.H.; Boetti, N.G.; Pugliese, D.; Janner, D. Phosphate glass-based microstructured optical fibers with hole and core for biomedical applications. Opt. Mater. 2022, 131, 112644. [Google Scholar] [CrossRef]
- Peterka, P.; Pugliese, D.; Jiříčková, B.; Boetti, N.G.; Turčičová, H.; Mirza, I.; Borodkin, A.; Milanese, D. High-power laser testing of calcium-phosphate-based bioresorbable optical fibers. Opt. Mater. Express 2021, 11, 2049–2058. [Google Scholar] [CrossRef]
- Theodosiou, A.; Pugliese, D.; Ceci-Ginistrelli, E.; Boetti, N.G.; Janner, D.; Milanese, D.; Kalli, K. Femtosecond laser written plane-by-plane Bragg grating sensors in bioresorbable phosphate optical fibres. J. Light. Technol. 2019, 37, 2363–2369. [Google Scholar] [CrossRef]
- Di Sieno, L.; Boetti, N.G.; Dalla Mora, A.; Pugliese, D.; Farina, A.; Konugolu Venkata Sekar, S.; Ceci-Ginistrelli, E.; Janner, D.; Pifferi, A.; Milanese, D. Towards the use of bioresorbable fibers in time-domain diffuse optics. J. Biophotonics 2018, 11, e201600275. [Google Scholar] [CrossRef]
- Pandayil, J.T.; Rizi, S.H.; Russo, S.; Boetti, N.G.; Pugliese, D.; Janner, D. Towards a novel bi-functional bioresorbable micro-structured optical fiber for theranostic applications. In Proceedings of the European Conference on Biomedical Optics, Munich, Germany, 25–29 June 2023; Optica Publishing Group: Washington, DC, USA, 2023; p. 126271N. [Google Scholar]





| Flexural Strength (MPa) | Elastic Modulus (GPa) | Reference | |
|---|---|---|---|
| Cortical bone tissue | 70–150 | 7–30 | [6] |
| Ti and Ti alloys (metals) | 960–1100 | 105–125 | [37] |
| PLA (polymer) | 90 | 3.8 | [39] |
| Phosphate glass fiber | 366 | 51 | [40] |
| Author And Year of Study | Glass Composition | In Vitro Tests | In Vivo Tests | Main Results |
|---|---|---|---|---|
| L.L. Hench, 1971 Ref: [13] |
|
|
|
|
| B.A. Blencke, 1976 Ref: [28] |
|
|
|
|
| Hisao Fukui, 1977 Ref: [29] |
|
|
|
|
| J. Burnie, T. Glichrist, 1981 Ref: [31] |
|
|
|
|
| Graves, Jr., 1986 Ref: [30] |
|
|
|
|
| Composite | Fiber Composition | Mechanical Property | Application | Ref |
|---|---|---|---|---|
| PLA-PBG fiber composites | 50 P2O5-40CaO-5Na2O-5Fe2O3 | Y: 5 GPa F.S: 90 MPa | Bone fracture-fixation devices | [49] |
| Methacrylate- modified oligolactide-PBG fiber | 35 P2O5–27.5 CaO–9.5 MgO–22.5 Na2O–5.5 TiO2 | Y: 16 GPa F.S:115 MPa | Bone-fixation devices | [48] |
| PLA-PGF (UD) | 40P2O5−24MgO−16CaO−15Na2O−4Fe2O3 | Maximum flexural load PLA: 200 N PLA-PGF: 400 N | Bioresorbable screws | [45] |
| PLA and PBG | 50P2O5–40CaO–5Na2O–5Fe2O3 | Y: 18 Gpa F.S: 190 Mpa | Intramedullary rods | [39] |
| 40P2O5–24MgO–16CaO–16Na2O–4Fe2O | Y: 26 Gpa F.S: 240 Mpa | |||
| Polyester–PGF | 52P2O5-24CaO-5K2O-13MgO-1TiO2-5Fe2O3 | Y: 3 GPa F.S: 43.8 MPa | Orthopedic implants | [50] |
| Dopant | Effect on Phosphate Glass | Fiber Composition | Biomedical Application | Ref |
| Iron (Substitute of Na2O) | Increased Tg and chemical durability, and decreased dissolution rate | P2O5 Na2O CaO Fe2O3 | Better cell adherence and proliferation | [18] |
| Copper (Substitute of Na2O) | Increased Tg and durability, and decreased dissolution rate | P2O5 Na2O CaO CuO | Antibacterial and wound healing | [66] |
| Magnesium (Substitute of Na2O) | Increased Tg and chemical durability, and decreased dissolution rate | P2O5 CaO Na2O MgO | Polymer reinforcement and bone repair | [67] |
| Strontium (Substitute of CaO) | Slight decrease in Tg and a significant decrease in Tm | P2O5 CaO Na2O MgO SrO | Osteoporosis | [65] |
| Boron (Substitute of Na2O) | Increased Tg, Tm, and Tc, and decreased dissolution rate | P2O5 B2O3 CaO MgO Na2O | Bone fixation and growth | [68] |
| Fluoride (Substitute of CaO) | Lowered Tg and Tx, and slight decrease in mechanical properties | P2O5 CaO Na2O CaF2 | Oral healthcare | [69,70] |
| Zinc (Substitute of CaO) | Increased mechanical and reduced degradation properties | P2O5 Na2OCaO ZnO | Treats catheter-associated urinary tract infections | [71] |
| Titanium (Substitute of Na2O) | Increased Tg and Tx and glass stability | P2O5 CaO Na2O TiO2 | Bone-binding application (increased hydroxy apatite formation) | [22] |
| Potassium | Higher viscosity and glass durability | P2O5 CaO Na2O SiO2 K2O TiO2 | Fibers for nerve tissue regeneration and controlled-release systems | [72] |
| Aluminum | Increased durability | P2O5 Al2O3 ZnO | Muscle engineering (craniofacial) | [73] |
| Silver (Substitute of Na2O) | Decreased degradation | P2O5 CaO Na2O Ag | Antibacterial property | [74] |
| Material | Fabrication Technique | Fiber Type | Optical Loss | Reference |
|---|---|---|---|---|
| Spider silk | Native spider silk directly woven by spiders | Unclad fiber | 10 dB/cm at VIS | |
| Cellulose | Core was produced using dry-jet wet spinning in a water bath as a coagulant. Cladding produced by coating the core with cellulose acetate dissolved in acetone | Core–cladding fiber | 10 dB/cm at 750–1350 nm | [113] |
| Agarose | Boiled agar solution poured into the glass mold tube with rods, cooled down, and released after solidification | Structured fiber Core: 0.64 mm Clad: 2.5 mm Airholes: 0.5 mm | 3.23 dB/cm at 633 nm | |
| PLLA | Thermal drawing process of PLLA crystalline powders melts at 220 °C | Unclad fiber | 1.6 dB/cm at 473 nm 4.8 dB/cm after 40 days of soaking in water | [119] |
| PCL | Thermal drawing of the preform in the drawing tower | Unclad solid-core and grooved fibers | 1.5 dB/cm at 635 nm in PBS | |
| PCL | Fiber draw tower | PCL capillary | 2 dB/cm at 635 nm over 21 days immersion in PBS | [120] |
| Wrapped spider silk on tapered SMF-28 | Heating of SMF and followed by wrapping the silk over the biconical fiber shape | Tapered commercial silica fiber with silk wrapping | −32 dB at 1360–1390 nm (measured by Optical Spectrum Analyzer) | [121] |
| Phosphate glass fiber | Rod in tube technique | Core–cladding MMF Core–cladding SMF | 0.019 dB/cm at 1300 nm 0.047 db/cm at 633 nm | [116] |
| Phosphate glass fiber | Stacking of the extruded tube within the extruded capillary, followed by thermal drawing | Microstructured | - | [122] |
| Phosphate glass fiber | Stack and draw | Core–clad for light delivery and channel for drug delivery | 0.024 dB/cm at 1300 nm | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandayil, J.T.; Boetti, N.G.; Janner, D. Advancements in Biomedical Applications of Calcium Phosphate Glass and Glass-Based Devices—A Review. J. Funct. Biomater. 2024, 15, 79. https://doi.org/10.3390/jfb15030079
Pandayil JT, Boetti NG, Janner D. Advancements in Biomedical Applications of Calcium Phosphate Glass and Glass-Based Devices—A Review. Journal of Functional Biomaterials. 2024; 15(3):79. https://doi.org/10.3390/jfb15030079
Chicago/Turabian StylePandayil, Jawad T., Nadia G. Boetti, and Davide Janner. 2024. "Advancements in Biomedical Applications of Calcium Phosphate Glass and Glass-Based Devices—A Review" Journal of Functional Biomaterials 15, no. 3: 79. https://doi.org/10.3390/jfb15030079
APA StylePandayil, J. T., Boetti, N. G., & Janner, D. (2024). Advancements in Biomedical Applications of Calcium Phosphate Glass and Glass-Based Devices—A Review. Journal of Functional Biomaterials, 15(3), 79. https://doi.org/10.3390/jfb15030079








