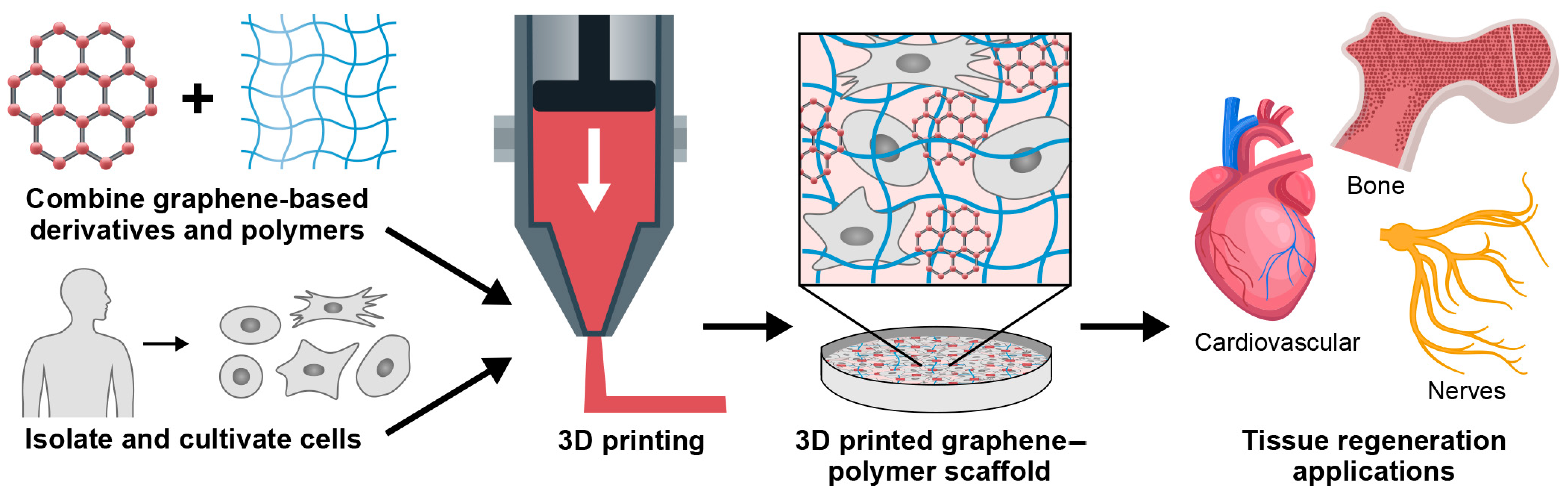
Graphene in 3D Bioprinting
Abstract
:1. Introduction
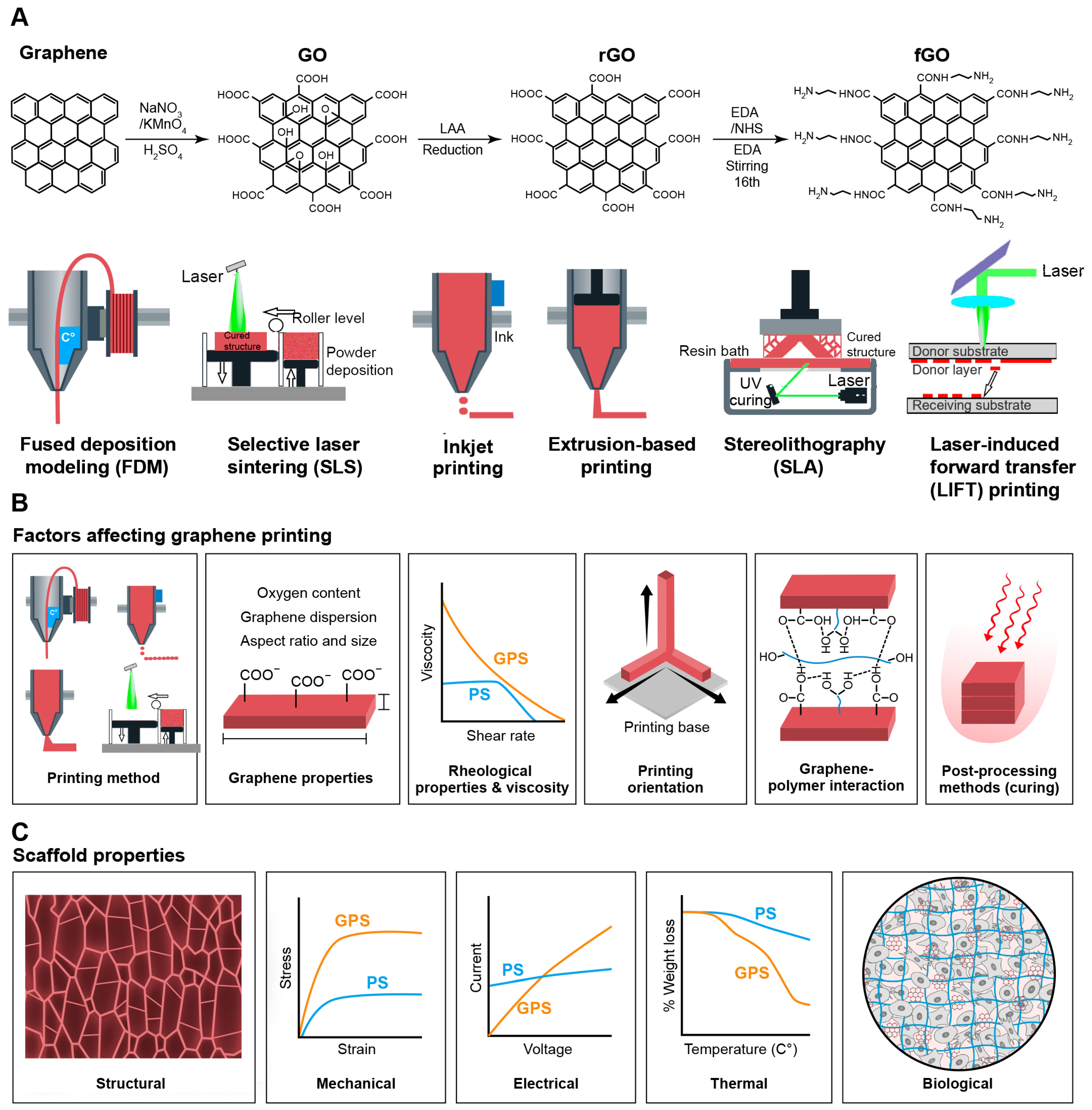
2. Graphene and Graphene-Based Materials for 3D Printing
2.1. Graphene
2.2. Graphene Oxide (GO)
2.3. Reduced Graphene Oxide (rGO)
2.4. Functionalized Graphene Oxide (fGO)
3. The Role of Graphene-Based Material Properties in 3D Printing
3.1. Graphene Sheet’s Aspect Ratio
3.2. Graphene-Polymer Interactions
3.3. Oxygen Content
3.4. Graphene Dispersion
3.5. Rheological Property and Viscosity
3.6. Printing Orientation
3.7. Post-Processing Methods
| Material | 3D Printing Method | Tissue Engineering Application | Concerns/Challenges | Refs. |
|---|---|---|---|---|
| Graphene | ||||
| Graphene/PLA | FDM | Bone, Cardiovascular Neural |
| [57,58,143] |
| Graphene/PCL | Extrusion-based 3D printing | Bone |
| [144] |
| Graphene/PEEK | SLS | Bone |
| [63] |
| Graphene/PLG | Inkjet 3D printing | Bone |
| [67,145] |
| Graphene | Extrusion-based 3D printing | Multiple applications |
| [66] |
| Graphene/GelMA | Extrusion-based 3D printing | Neural |
| [146] |
| Graphene oxide (GO) | ||||
| GO | FDM | Multiple applications |
| [58,84,85,86] |
| GO/HAP/PLLA | SLS | Bone |
| [88] |
| GO/GP | Extrusion-based 3D printing | Biomedical applications |
| [19,89] |
| GO/fibrin | Extrusion-based 3D printing | Bone |
| [147] |
| GO/Col aerogel | Extrusion-based 3D printing | Bone |
| [148] |
| PCL/GO/Ag/Arg | Extrusion-based 3D printing | Skin |
| [149] |
| GO/Au/Chitosan | Extrusion-based 3D printing | Cardiovascular |
| [150] |
| Reduced graphene oxide (rGO) | ||||
| rGO | Extrusion-based 3D printing | Cardiovascular |
| [151] |
| rGO | SLA | Neural |
| [97] |
| rGO/Zn | SLS | Bone |
| [98] |
| rGO/PCL | Extrusion0based 3D printing | Skin |
| [99] |
| rGO/ Isabgol | Extrusion-based 3D printing | Skin |
| [152]. |
| rGO/PEA/Chitosan | Extrusion-based 3D printing | Cardiovascular |
| [153] |
| Functionalized graphene oxide (fGO) | ||||
| fGO | SLS | Bone |
| [107] |
| rGO/ Mxene/Hydrogel | Extrusion-based 3D printing | Neural |
| [154] |
| fGO/Fe3O4/ Polymer | Extrusion-based 3D printing | Biomedical applications |
| [104] |
4. Evaluation of 3D Printed Graphene-Incorporated Polymeric Scaffolds
4.1. Microstructural and Mechanical Properties Analysis
4.2. Thermal and Electrical Properties
4.3. Biocompatibility
5. Applications of 3D Printed Graphene-Based Material in Tissue Engineering
5.1. Hard Tissue Engineering
5.2. Soft Tissue Engineering
5.2.1. Nerve Tissue Engineering
5.2.2. Cardiovascular Tissue Engineering
6. Challenges and Future Directions in the 3D Printing of Graphene-Based Scaffolds
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| CAD | Computer-aided design |
| FDM | Fused deposition modeling |
| SLA | Stereolithography |
| SLS | Selective laser sintering |
| GO | Graphene oxide |
| rGO | Reduced graphene oxide |
| fGO | Functionalized graphene oxide |
| CNTs | Carbon nanotubes |
| GOxNP | Graphite nanoplatelets |
| PVA | Polyvinyl alcohol |
| PLA | Polylactic acid |
| PLLA | Poly-L-lactic acid |
| PU | Polyurethane |
| PLG | Polylactide-co-glycolide |
| PCL | Polycaprolactone |
| HAP | Hydroxyapatite |
| PEA | Poly ester amide |
| hMSCs | Human mesenchymal stem cells |
| GP | Geopolymer |
| PEEK | Polyether ether ketone |
| PEG | Polyethylene glycol |
| FGP | Fe3O4 functionalized graphene polymer |
| GMFs | Graphene microflakes |
| HPC | Hydroxypropyl cellulose |
| NPs | Nanoparticles |
| ABS | Acrylonitrile butadiene styrene |
| OCGs | Oxygen-containing groups |
| hNSCs | Human neural stem cells |
| LBLC | Layer-by-layer casting |
| GelMA | Gelatin methacryloyl |
| LIFT | Laser-induced forward transfer. |
| SEM | Scanning electron microscope |
| AFM | Atomic force microscopy |
| KPFM | Kelvin probe force microscopy |
| TGA | Thermogravimetric |
| DSC | Differential scanning calorimetric |
| DMA | Dynamic mechanical analysis |
References
- Deliormanlı, A.M.; Atmaca, H. Effect of pore architecture on the mesenchymal stem cell responses to graphene/polycaprolactone scaffolds prepared by solvent casting and robocasting. J. Porous Mater. 2020, 27, 49–61. [Google Scholar] [CrossRef]
- Sivashankari, P.; Krishna Kumar, K.; Devendiran, M.; Prabaharan, M. Graphene oxide-reinforced pectin/chitosan polyelectrolyte complex scaffolds. J. Biomater. Sci. Polym. Ed. 2021, 32, 2246–2266. [Google Scholar] [CrossRef] [PubMed]
- Sayyar, S.; Officer, D.L.; Wallace, G.G. Fabrication of 3D structures from graphene-based biocomposites. J. Mater. Chem. B 2017, 5, 3462–3482. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.G.; Muthoosamy, K.; Manickam, S.; Hilal-Alnaqbi, A. Graphene-based 3D scaffolds in tissue engineering: Fabrication, applications, and future scope in liver tissue engineering. Int. J. Nanomed. 2019, 14, 5753. [Google Scholar]
- Bahraminasab, M. Challenges on optimization of 3D-printed bone scaffolds. BioMedical Eng. OnLine 2020, 19, 69. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, D.; Loke, G.; Tafel, I.; Park, S.; Chiang, P.H.; Fink, Y.; Anikeeva, P. Scalable fabrication of porous microchannel nerve guidance scaffolds with complex geometries. Adv. Mater. 2019, 31, 1902021. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, X.; Li, X.; Chen, Z.; Zhou, C.; Zhou, Q.; Chen, Y. Recent progress in biomimetic additive manufacturing technology: From materials to functional structures. Adv. Mater. 2018, 30, 1706539. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Jambhulkar, S.; Ravichandran, D.; Zhu, Y.; Kakarla, M.; Nian, Q.; Azeredo, B.; Chen, X.; Jin, K.; Vernon, B. 3D printing-enabled nanoparticle alignment: A review of mechanisms and applications. Small 2021, 17, 2100817. [Google Scholar] [CrossRef]
- Kumar, V.; Kaur, H.; Kumari, A.; Hooda, G.; Garg, V.; Dureja, H. Drug delivery and testing via 3D printing. Bioprinting 2023, 36, e00298. [Google Scholar] [CrossRef]
- Banga, H.K.; Kalra, P.; Belokar, R.M.; Kumar, R. Design and fabrication of prosthetic and orthotic product by 3D printing. In Prosthetics and Orthotics; IntechOpen: London, UK, 2020. [Google Scholar]
- Bücking, T.M.; Hill, E.R.; Robertson, J.L.; Maneas, E.; Plumb, A.A.; Nikitichev, D.I. From medical imaging data to 3D printed anatomical models. PLoS ONE 2017, 12, e0178540. [Google Scholar] [CrossRef]
- Rengier, F.; Mehndiratta, A.; Von Tengg-Kobligk, H.; Zechmann, C.M.; Unterhinninghofen, R.; Kauczor, H.-U.; Giesel, F.L. 3D printing based on imaging data: Review of medical applications. Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 335–341. [Google Scholar] [CrossRef]
- Giannopoulos, A.A.; Mitsouras, D.; Yoo, S.-J.; Liu, P.P.; Chatzizisis, Y.S.; Rybicki, F.J. Applications of 3D printing in cardiovascular diseases. Nat. Rev. Cardiol. 2016, 13, 701–718. [Google Scholar] [CrossRef]
- Mohanavel, V.; Ali, K.A.; Ranganathan, K.; Jeffrey, J.A.; Ravikumar, M.; Rajkumar, S. The roles and applications of additive manufacturing in the aerospace and automobile sector. Mater. Today Proc. 2021, 47, 405–409. [Google Scholar] [CrossRef]
- Bozkurt, Y.; Karayel, E. 3D printing technology; methods, biomedical applications, future opportunities and trends. J. Mater. Res. Technol. 2021, 14, 1430–1450. [Google Scholar] [CrossRef]
- Salmi, M. Additive manufacturing processes in medical applications. Materials 2021, 14, 191. [Google Scholar] [CrossRef]
- Weng, T.; Zhang, W.; Xia, Y.; Wu, P.; Yang, M.; Jin, R.; Xia, S.; Wang, J.; You, C.; Han, C. 3D bioprinting for skin tissue engineering: Current status and perspectives. J. Tissue Eng. 2021, 12, 20417314211028574. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Ju, Y.; Hu, Y.; Xie, X.; Fang, B.; Lei, L. Emerging 3D bioprinting applications in plastic surgery. Biomater. Res. 2023, 27, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Zhou, G.-X.; He, P.-G.; Yang, Z.-H.; Jia, D.-C. 3D printing strong and conductive geo-polymer nanocomposite structures modified by graphene oxide. Carbon 2017, 117, 421–426. [Google Scholar] [CrossRef]
- Schöbel, L.; Boccaccini, A.R. A review of glycosaminoglycan-modified electrically conductive polymers for biomedical applications. Acta Biomater. 2023, 169, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Jia, Z.; Xu, X.; Zhu, D.; Zheng, Y. Design, printing, and engineering of regenerative biomaterials for personalized bone healthcare. Prog. Mater. Sci. 2023, 134, 101072. [Google Scholar] [CrossRef]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and shape fidelity of bioinks in 3D bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Chang, S.Y.; Gan, S.W.; Ma, S.; Lu, W.F.; Yen, C.-C. Nanocomposite bioinks for 3D bioprinting. Acta Biomater. 2022, 151, 45–69. [Google Scholar] [CrossRef] [PubMed]
- Ashammakhi, N.; Ahadian, S.; Xu, C.; Montazerian, H.; Ko, H.; Nasiri, R.; Barros, N.; Khademhosseini, A. Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs. Mater. Today Bio 2019, 1, 100008. [Google Scholar] [CrossRef] [PubMed]
- Itapu, B.M.; Jayatissa, A.H. A review in graphene/polymer composites. Chem. Sci. Int. J. 2018, 23, 1–16. [Google Scholar] [CrossRef]
- Palmieri, V.; Spirito, M.D.; Papi, M. Graphene-based scaffolds for tissue engineering and photothermal therapy. Nanomedicine 2020, 15, 1411–1417. [Google Scholar] [CrossRef]
- Mantecón-Oria, M.; Tapia, O.; Lafarga, M.; Berciano, M.T.; Munuera, J.M.; Villar-Rodil, S.; Paredes, J.I.; Rivero, M.J.; Diban, N.; Urtiaga, A. Influence of the properties of different graphene-based nanomaterials dispersed in polycaprolactone membranes on astrocytic differentiation. Sci. Rep. 2022, 12, 13408. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, J.; Wu, B.; Liu, Z.; Li, M.; Wang, X.; Tang, P.; Wang, Z. Graphene and its derivatives for bone tissue engineering: In vitro and in vivo evaluation of graphene-based scaffolds, membranes and coatings. Front. Bioeng. Biotechnol. 2021, 9, 734688. [Google Scholar] [CrossRef]
- Maleki, M.; Zarezadeh, R.; Nouri, M.; Sadigh, A.R.; Pouremamali, F.; Asemi, Z.; Kafil, H.S.; Alemi, F.; Yousefi, B. Graphene oxide: A promising material for regenerative medicine and tissue engineering. Biomol. Concepts 2020, 11, 182–200. [Google Scholar] [CrossRef]
- Kumar, S.; Parekh, S.H. Linking graphene-based material physicochemical properties with molecular adsorption, structure and cell fate. Commun. Chem. 2020, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Belaid, H.; Nagarajan, S.; Teyssier, C.; Barou, C.; Barés, J.; Balme, S.; Garay, H.; Huon, V.; Cornu, D.; Cavailles, V. Development of new biocompatible 3D printed graphene oxide-based scaffolds. Mater. Sci. Eng. C 2020, 110, 110595. [Google Scholar] [CrossRef]
- Rastogi, S.K.; Raghavan, G.; Yang, G.; Cohen-Karni, T. Effect of graphene on nonneuronal and neuronal cell viability and stress. Nano Lett. 2017, 17, 3297–3301. [Google Scholar] [CrossRef]
- Clarissa, W.H.-Y.; Chia, C.H.; Zakaria, S.; Evyan, Y.C.-Y. Recent advancement in 3-D printing: Nanocomposites with added functionality. Prog. Addit. Manuf. 2021, 7, 325–350. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Fan, Z.; Wang, W.; Wang, B.; Guo, Z. Ink-based 3D printing technologies for graphene-based materials: A review. Adv. Compos. Hybrid Mater. 2019, 2, 1–33. [Google Scholar] [CrossRef]
- Magne, T.M.; de Oliveira Vieira, T.; Alencar, L.M.R.; Junior, F.F.M.; Gemini-Piperni, S.; Carneiro, S.V.; Fechine, L.M.; Freire, R.M.; Golokhvast, K.; Metrangolo, P. Graphene and its derivatives: Understanding the main chemical and medicinal chemistry roles for biomedical applications. J. Nanostruct. Chem. 2022, 12, 693–727. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Klopocinska, A.; Horvat, K.; Abdel Hamid, Z. Graphene-based nanocomposites: Synthesis, mechanical properties, and characterizations. Polymers 2021, 13, 2869. [Google Scholar] [CrossRef] [PubMed]
- Farahani, R.D.; Dubé, M.; Therriault, D. Three-dimensional printing of multifunctional nanocomposites: Manufacturing techniques and applications. Adv. Mater. 2016, 28, 5794–5821. [Google Scholar] [CrossRef]
- Bei, H.P.; Yang, Y.; Zhang, Q.; Tian, Y.; Luo, X.; Yang, M.; Zhao, X. Graphene-based nanocomposites for neural tissue engineering. Molecules 2019, 24, 658. [Google Scholar] [CrossRef]
- Bellier, N.; Baipaywad, P.; Ryu, N.; Lee, J.Y.; Park, H. Recent biomedical advancements in graphene oxide-and reduced graphene oxide-based nanocomposite nanocarriers. Biomater. Res. 2022, 26, 65. [Google Scholar] [CrossRef]
- Birenboim, M.; Nadiv, R.; Alatawna, A.; Buzaglo, M.; Schahar, G.; Lee, J.; Kim, G.; Peled, A.; Regev, O. Reinforcement and workability aspects of graphene-oxide-reinforced cement nanocomposites. Compos. Part. B Eng. 2019, 161, 68–76. [Google Scholar] [CrossRef]
- Jeong, J.H.; Kang, S.; Kim, N.; Joshi, R.; Lee, G.-H. Recent trends in covalent functionalization of 2D materials. Phys. Chem. Chem. Phys. 2022, 24, 10684–10711. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Ryzhkov, S.A.; Kirilenko, D.A.; Ulin, N.V.; Baidakova, M.V.; Shnitov, V.V.; Pavlov, S.I.; Chumakov, R.G.; Stolyarova, D.Y.; Besedina, N.A. From graphene oxide towards aminated graphene: Facile synthesis, its structure and electronic properties. Sci. Rep. 2020, 10, 6902. [Google Scholar] [CrossRef]
- Zhi, L.; Müllen, K. A bottom-up approach from molecular nanographenes to unconventional carbon materials. J. Mater. Chem. 2008, 18, 1472–1484. [Google Scholar] [CrossRef]
- Munuera, J.M.; Paredes, J.I.; Enterria, M.; Pagan, A.; Villar-Rodil, S.; Pereira, M.F.R.; Martins, J.I.; Figueiredo, J.L.; Cenis, J.L.; Martinez-Alonso, A.; et al. Electrochemical Exfoliation of Graphite in Aqueous Sodium Halide Electrolytes toward Low Oxygen Content Graphene for Energy and Environmental Applications. ACS Appl. Mater. Interfaces 2017, 9, 24085–24099. [Google Scholar] [CrossRef] [PubMed]
- Shinde, D.B.; Brenker, J.; Easton, C.D.; Tabor, R.F.; Neild, A.; Majumder, M. Shear Assisted Electrochemical Exfoliation of Graphite to Graphene. Langmuir 2016, 32, 3552–3559. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wu, D.; Feng, X.; Mullen, K. Bottom-up fabrication of photoluminescent graphene quantum dots with uniform morphology. J. Am. Chem. Soc. 2011, 133, 15221–15223. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.; Abdelkader, A.M.; Alonso, C.; Andrieux-Ledier, A.; Arenal, R.; Azpeitia, J.; Balakrishnan, N.; Banszerus, L.; Barjon, J.; Bartali, R. Production and processing of graphene and related materials. 2D Mater. 2020, 7, 022001. [Google Scholar] [CrossRef]
- Minati, L.; Torrengo, S.; Maniglio, D.; Migliaresi, C.; Speranza, G. Luminescent graphene quantum dots from oxidized multi-walled carbon nanotubes. Mater. Chem. Phys. 2012, 137, 12–16. [Google Scholar] [CrossRef]
- Chua, C.K.; Sofer, Z.; Simek, P.; Jankovsky, O.; Klimova, K.; Bakardjieva, S.; Hrdlickova Kuckova, S.; Pumera, M. Synthesis of strongly fluorescent graphene quantum dots by cage-opening buckminsterfullerene. ACS Nano 2015, 9, 2548–2555. [Google Scholar] [CrossRef]
- Jakus, A.E.; Shah, R.N. Multi and mixed 3 D-printing of graphene-hydroxyapatite hybrid materials for complex tissue engineering. J. Biomed. Mater. Res. Part A 2017, 105, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Xigong, L.; Weiyi, D.; Jingen, H.; Shuo, W.; Xiangjin, L.; Junsong, W. Potential use of 3D-printed graphene oxide scaffold for construction of the cartilage layer. J. Nanobiotechnol. 2020, 18, 97. [Google Scholar] [CrossRef]
- Hussain, S.; Maktedar, S.S. Structural, functional and mechanical performance of advanced graphene-based composite hydrogels. Results Chem. 2023, 6, 101029. [Google Scholar] [CrossRef]
- Yang, R.; Zhou, J.; Yang, C.; Qiu, L.; Cheng, H. Recent progress in 3d printing of 2d material-based macrostructures. Adv. Mater. Technol. 2020, 5, 1901066. [Google Scholar] [CrossRef]
- Biru, E.I.; Necolau, M.I.; Zainea, A.; Iovu, H. Graphene Oxide–Protein-Based Scaffolds for Tissue Engineering: Recent Advances and Applications. Polymers 2022, 14, 1032. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Li, D.; Jiang, W.; Gu, Z.; Wang, X.; Zhang, Z.; Sun, Z. 3D printable graphene composite. Sci. Rep. 2015, 5, 11181. [Google Scholar] [CrossRef]
- Prashantha, K.; Roger, F. Multifunctional properties of 3D printed poly (lactic acid)/graphene nanocomposites by fused deposition modeling. J. Macromol. Sci. Part A 2017, 54, 24–29. [Google Scholar] [CrossRef]
- de Armentia, S.L.; Fernández-Villamarín, S.; Ballesteros, Y.; Del Real, J.; Dunne, N.; Paz, E. 3D Printing of a Graphene-Modified Photopolymer Using Stereolithography for Biomedical Applications: A Study of the Polymerization Reaction. Int. J. Bioprint. 2022, 8, 503. [Google Scholar] [CrossRef]
- Korhonen, H.; Sinh, L.H.; Luong, N.D.; Lehtinen, P.; Verho, T.; Partanen, J.; Seppälä, J. Fabrication of graphene-based 3D structures by stereolithography. Phys. Status Solidi 2016, 213, 982–985. [Google Scholar] [CrossRef]
- Boschetto, A.; Bottini, L.; Macera, L.; Veniali, F. Post-processing of complex SLM parts by barrel finishing. Appl. Sci. 2020, 10, 1382. [Google Scholar] [CrossRef]
- Phillips, T.; Ricker, T.; Fish, S.; Beaman, J. Design of a laser control system with continuously variable power and its application in additive manufacturing. Addit. Manuf. 2020, 34, 101173. [Google Scholar] [CrossRef]
- Feng, P.; Jia, J.; Peng, S.; Yang, W.; Bin, S.; Shuai, C. Graphene oxide-driven interfacial coupling in laser 3D printed PEEK/PVA scaffolds for bone regeneration. Virtual Phys. Prototyp. 2020, 15, 211–226. [Google Scholar] [CrossRef]
- Mohan, D.; Sajab, M.S.; Bakarudin, S.B.; Roslan, R.; Kaco, H. 3D Printed Polyurethane Reinforced Graphene Nanoplatelets. In Materials Science Forum; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2021; pp. 47–52. [Google Scholar]
- Chen, B.; Berretta, S.; Evans, K.; Smith, K.; Ghita, O. A primary study into graphene/polyether ether ketone (PEEK) nanocomposite for laser sintering. Appl. Surf. Sci. 2018, 428, 1018–1028. [Google Scholar] [CrossRef]
- Jakus, A.E.; Secor, E.B.; Rutz, A.L.; Jordan, S.W.; Hersam, M.C.; Shah, R.N. Three-dimensional printing of high-content graphene scaffolds for electronic and biomedical applications. ACS Nano 2015, 9, 4636–4648. [Google Scholar] [CrossRef]
- Das, S.R.; Nian, Q.; Cargill, A.A.; Hondred, J.A.; Ding, S.; Saei, M.; Cheng, G.J.; Claussen, J.C. 3D nanostructured inkjet printed graphene via UV-pulsed laser irradiation enables paper-based electronics and electrochemical devices. Nanoscale 2016, 8, 15870–15879. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.J.; Park, H.B. Effect of hydrogen peroxide on properties of graphene oxide in Hummers method. Carbon 2019, 141, 515–522. [Google Scholar] [CrossRef]
- Gebreegziabher, G.; Asemahegne, A.; Ayele, D.; Dhakshnamoorthy, M.; Kumar, A. One-step synthesis and characterization of reduced graphene oxide using chemical exfoliation method. Mater. Today Chem. 2019, 12, 233–239. [Google Scholar] [CrossRef]
- Zhang, T.-Y.; Zhang, D. Aqueous colloids of graphene oxide nanosheets by exfoliation of graphite oxide without ultrasonication. Bull. Mater. Sci. 2011, 34, 25–28. [Google Scholar] [CrossRef]
- Shao, G.; Lu, Y.; Wu, F.; Yang, C.; Zeng, F.; Wu, Q. Graphene oxide: The mechanisms of oxidation and exfoliation. J. Mater. Sci. 2012, 47, 4400–4409. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, J.; Huang, Y.; Ma, Y.; Wang, Y.; Chen, Y. Size-controlled synthesis of graphene oxide sheets on a large scale using chemical exfoliation. Carbon 2009, 47, 3365–3368. [Google Scholar] [CrossRef]
- Motiee, E.-S.; Karbasi, S.; Bidram, E.; Sheikholeslam, M. Investigation of physical, mechanical and biological properties of polyhydroxybutyrate-chitosan/graphene oxide nanocomposite scaffolds for bone tissue engineering applications. Int. J. Biol. Macromol. 2023, 247, 125593. [Google Scholar] [CrossRef] [PubMed]
- Challa, A.A.; Saha, N.; Szewczyk, P.K.; Karbowniczek, J.E.; Stachewicz, U.; Ngwabebhoh, F.A.; Saha, P. Graphene oxide produced from spent coffee grounds in electrospun cellulose acetate scaffolds for tissue engineering applications. Mater. Today Commun. 2023, 35, 105974. [Google Scholar] [CrossRef]
- Lee, E.A.; Kwak, S.-Y.; Yang, J.-K.; Lee, Y.-S.; Kim, J.-H.; Kim, H.D.; Hwang, N.S. Graphene oxide film guided skeletal muscle differentiation. Mater. Sci. Eng. C 2021, 126, 112174. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Thirupathi, R.; Rao, L.P.; Atreya, H.S. Unraveling the dynamic nature of protein–graphene oxide interactions. RSC Adv. 2016, 6, 52539–52548. [Google Scholar] [CrossRef]
- Arnold, A.M.; Holt, B.D.; Tang, C.; Sydlik, S.A. Phosphate modified graphene oxide: Long–term biodegradation and cytocompatibility. Carbon 2019, 154, 342–349. [Google Scholar] [CrossRef]
- Nuncira, J.; Seara, L.M.; Sinisterra, R.D.; Caliman, V.; Silva, G.G. Long-term colloidal stability of graphene oxide aqueous nanofluids. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 407–417. [Google Scholar] [CrossRef]
- Holt, B.D.; Arnold, A.M.; Sydlik, S.A. In it for the long haul: The cytocompatibility of aged graphene oxide and its degradation products. Adv. Healthc. Mater. 2016, 5, 3056–3066. [Google Scholar] [CrossRef]
- Vlăsceanu, G.M.; Iovu, H.; Ioniţă, M. Graphene inks for the 3D printing of cell culture scaffolds and related molecular arrays. Compos. Part B Eng. 2019, 162, 712–723. [Google Scholar] [CrossRef]
- Jouibary, Y.M.; Rezvanpour, A.; Akbari, B. Fabrication of polycaprolactone/collagen scaffolds reinforced by graphene oxide for tissue engineering applications. Mater. Lett. 2022, 322, 132477. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Crook, J.M.; Wallace, G.G. 3D printing of cytocompatible graphene/alginate scaffolds for mimetic tissue constructs. Front. Bioeng. Biotechnol. 2020, 8, 824. [Google Scholar] [CrossRef]
- Valencia, C.; Valencia, C.H.; Zuluaga, F.; Valencia, M.E.; Mina, J.H.; Grande-Tovar, C.D. Synthesis and application of scaffolds of chitosan-graphene oxide by the freeze-drying method for tissue regeneration. Molecules 2018, 23, 2651. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Smith, D.E. Anisotropic mechanical properties of oriented carbon fiber filled polymer composites produced with fused filament fabrication. Addit. Manuf. 2017, 18, 84–94. [Google Scholar] [CrossRef]
- Mogan, J.; Sandanamsamy, L.; Halim, N.; Harun, W.; Kadirgama, K.; Ramasamy, D. A review of FDM and graphene-based polymer composite. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Pekan, Malaysia, 19–20 January 2021; p. 012032. [Google Scholar]
- Dul, S.; Fambri, L.; Pegoretti, A. Fused deposition modelling with ABS–graphene nanocomposites. Compos. Part A Appl. Sci. Manuf. 2016, 85, 181–191. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef]
- Shuai, C.; Peng, B.; Feng, P.; Yu, L.; Lai, R.; Min, A. In situ synthesis of hydroxyapatite nanorods on graphene oxide nanosheets and their reinforcement in biopolymer scaffold. J. Adv. Res. 2022, 35, 13–24. [Google Scholar] [CrossRef]
- Lee, H.; Yoo, J.M.; Ponnusamy, N.K.; Nam, S.Y. 3D-printed hydroxyapatite/gelatin bone scaffolds reinforced with graphene oxide: Optimized fabrication and mechanical characterization. Ceram. Int. 2022, 48, 10155–10163. [Google Scholar] [CrossRef]
- Shen, X.; Chu, M.; Hariri, F.; Vedula, G.; Naguib, H.E. Binder jetting fabrication of highly flexible and electrically conductive graphene/PVOH composites. Addit. Manuf. 2020, 36, 101565. [Google Scholar] [CrossRef]
- Lesiak, B.; Trykowski, G.; Tóth, J.; Biniak, S.; Kövér, L.; Rangam, N.; Stobinski, L.; Malolepszy, A. Chemical and structural properties of reduced graphene oxide—Dependence on the reducing agent. J. Mater. Sci. 2021, 56, 3738–3754. [Google Scholar] [CrossRef]
- Kondratowicz, I.; Żelechowska, K.; Sadowski, W. Optimization of graphene oxide synthesis and its reduction. In Proceedings of the Nanoplasmonics, Nano-Optics, Nanocomposites, and Surface Studies: Selected Proceedings of the Second FP7 Conference and the Third International Summer School Nanotechnology: From Fundamental Research to Innovations, Yaremche-Lviv, Ukraine, 23–30 August 2014; pp. 467–484. [Google Scholar]
- Shahdeo, D.; Roberts, A.; Abbineni, N.; Gandhi, S. Graphene based sensors. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; Volume 91, pp. 175–199. [Google Scholar]
- Shin, S.R.; Zihlmann, C.; Akbari, M.; Assawes, P.; Cheung, L.; Zhang, K.; Manoharan, V.; Zhang, Y.S.; Yüksekkaya, M.; Wan, K.T. Reduced graphene oxide-gelMA hybrid hydrogels as scaffolds for cardiac tissue engineering. Small 2016, 12, 3677–3689. [Google Scholar] [CrossRef]
- Norahan, M.H.; Amroon, M.; Ghahremanzadeh, R.; Rabiee, N.; Baheiraei, N. Reduced graphene oxide: Osteogenic potential for bone tissue engineering. IET Nanobiotechnol. 2019, 13, 720–725. [Google Scholar] [CrossRef]
- Sieradzka, M.; Fabia, J.; Biniaś, D.; Graczyk, T.; Fryczkowski, R. High-impact polystyrene reinforced with reduced graphene oxide as a filament for fused filament fabrication 3D printing. Materials 2021, 14, 7008. [Google Scholar] [CrossRef]
- Ajiteru, O.; Sultan, M.T.; Lee, Y.J.; Seo, Y.B.; Hong, H.; Lee, J.S.; Lee, H.; Suh, Y.J.; Ju, H.W.; Lee, O.J. A 3D printable electroconductive biocomposite bioink based on silk fibroin-conjugated graphene oxide. Nano Lett. 2020, 20, 6873–6883. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheng, Y.; Peng, S.; Xu, L.; He, C.; Qi, F.; Zhao, M.; Shuai, C. Microstructure evolution and texture tailoring of reduced graphene oxide reinforced Zn scaffold. Bioact. Mater. 2021, 6, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- Seyedsalehi, A.; Daneshmandi, L.; Barajaa, M.; Riordan, J.; Laurencin, C.T. Fabrication and characterization of mechanically competent 3D printed polycaprolactone-reduced graphene oxide scaffolds. Sci. Rep. 2020, 10, 22210. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Patil, R.; Dubey, S.K.; Bahadur, P. Graphene nanosheets as reinforcement and cell-instructive material in soft tissue scaffolds. Adv. Colloid Interface Sci. 2020, 281, 102167. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhang, X. Chemical Functionalization of Graphene Nanoplatelets with Hydroxyl, Amino, and Carboxylic Terminal Groups. Chemistry 2021, 3, 873–888. [Google Scholar] [CrossRef]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Chatterjee, K. Poly (Ethylene glycol) functionalized graphene oxide in tissue engineering: A review on recent advances. Int. J. Nanomed. 2020, 15, 5991. [Google Scholar] [CrossRef]
- Wajahat, M.; Kim, J.H.; Ahn, J.; Lee, S.; Bae, J.; Pyo, J.; Seol, S.K. 3D printing of Fe3O4 functionalized graphene-polymer (FGP) composite microarchitectures. Carbon 2020, 167, 278–284. [Google Scholar] [CrossRef]
- Yang, C.; Xu, J.; Xing, Y.; Hao, S.; Ren, Z. Covalent polymer functionalized graphene oxide/poly (ether ether ketone) composites for fused deposition modeling: Improved mechanical and tribological performance. RSC Adv. 2020, 10, 25685–25695. [Google Scholar] [CrossRef]
- Palaganas, J.O.; Palaganas, N.B.; Ramos, L.J.I.; David, C.P.C. 3D printing of covalent functionalized graphene oxide nanocomposite via stereolithography. ACS Appl. Mater. Interfaces 2019, 11, 46034–46043. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, F.; Shuai, Y.; Peng, S.; Chen, S.; Deng, Y.; Feng, P. Silicon dioxide nanoparticles decorated on graphene oxide nanosheets and their application in poly (L-lactic acid) scaffold. J. Adv. Res. 2022, 48, 175–190. [Google Scholar] [CrossRef] [PubMed]
- De Leon, A.C.; Chen, Q.; Palaganas, N.B.; Palaganas, J.O.; Manapat, J.; Advincula, R.C. High performance polymer nanocomposites for additive manufacturing applications. React. Funct. Polym. 2016, 103, 141–155. [Google Scholar] [CrossRef]
- Wang, W.; Caetano, G.F.; Chiang, W.-H.; Braz, A.L.; Blaker, J.J.; Frade, M.A.C.; Bartolo, P.J.D.S. Morphological, mechanical and biological assessment of PCL/pristine graphene scaffolds for bone regeneration. Int. J. Bioprint. 2016, 2, 95–104. [Google Scholar] [CrossRef]
- Tarani, E.; Chrysafi, I.; Kállay-Menyhárd, A.; Pavlidou, E.; Kehagias, T.; Bikiaris, D.N.; Vourlias, G.; Chrissafis, K. Influence of graphene platelet aspect ratio on the mechanical properties of HDPE nanocomposites: Microscopic observation and micromechanical modeling. Polymers 2020, 12, 1719. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hu, N.; Han, M.; Atobe, S.; Ning, H.; Liu, Y.; Wu, L. Investigation of interfacial mechanical properties of graphene-polymer nanocomposites. Mol. Simul. 2016, 42, 1165–1170. [Google Scholar] [CrossRef]
- Pandele, A.M.; Dinescu, S.; Costache, M.; Vasile, E.; Obreja, C.; Iovu, H.; Ionita, M. Preparation and in vitro, bulk, and surface investigation of chitosan/graphene oxide composite films. Polym. Compos. 2013, 34, 2116–2124. [Google Scholar] [CrossRef]
- Chong, H.; Hinder, S.; Taylor, A. Graphene nanoplatelet-modified epoxy: Effect of aspect ratio and surface functionality on mechanical properties and toughening mechanisms. J. Mater. Sci. 2016, 51, 8764–8790. [Google Scholar] [CrossRef]
- Govindaraj, P.; Sokolova, A.; Salim, N.; Juodkazis, S.; Fuss, F.K.; Fox, B.; Hameed, N. Distribution states of graphene in polymer nanocomposites: A review. Compos. Part B Eng. 2021, 226, 109353. [Google Scholar] [CrossRef]
- Singh, R.; Sandhu, G.S.; Penna, R.; Farina, I. Investigations for thermal and electrical conductivity of ABS-graphene blended prototypes. Materials 2017, 10, 881. [Google Scholar] [CrossRef]
- Potts, J.R.; Lee, S.H.; Alam, T.M.; An, J.; Stoller, M.D.; Piner, R.D.; Ruoff, R.S. Thermomechanical properties of chemically modified graphene/poly (methyl methacrylate) composites made by in situ polymerization. Carbon 2011, 49, 2615–2623. [Google Scholar] [CrossRef]
- Terrones, M.; Martín, O.; González, M.; Pozuelo, J.; Serrano, B.; Cabanelas, J.C.; Vega-Díaz, S.M.; Baselga, J. Interphases in graphene polymer-based nanocomposites: Achievements and challenges. Adv. Mater. 2011, 23, 5302–5310. [Google Scholar] [CrossRef] [PubMed]
- Morimune-Moriya, S.; Goto, T.; Nishino, T. Effect of aspect ratio of graphene oxide on properties of poly (vinyl alcohol) nanocomposites. Nanocomposites 2019, 5, 84–93. [Google Scholar] [CrossRef]
- Chen, Q.; Mangadlao, J.D.; Wallat, J.; De Leon, A.; Pokorski, J.K.; Advincula, R.C. 3D printing biocompatible polyurethane/poly (lactic acid)/graphene oxide nanocomposites: Anisotropic properties. ACS Appl. Mater. Interfaces 2017, 9, 4015–4023. [Google Scholar] [CrossRef] [PubMed]
- Galashev, A.E.e.; Rakhmanova, O.R. Mechanical and thermal stability of graphene and graphene-based materials. Phys. Uspekhi 2014, 57, 970. [Google Scholar] [CrossRef]
- Fang, M.; Long, J.; Zhao, W.; Wang, L.; Chen, G. pH-responsive chitosan-mediated graphene dispersions. Langmuir 2010, 26, 16771–16774. [Google Scholar] [CrossRef]
- Girao, A.F.; Sousa, J.; Dominguez-Bajo, A.; Gonzalez-Mayorga, A.; Bdikin, I.; Pujades-Otero, E.; Casan-Pastor, N.; Hortigüela, M.a.J.s.; Otero-Irurueta, G.; Completo, A. 3D reduced graphene oxide scaffolds with a combinatorial fibrous-porous architecture for neural tissue engineering. ACS Appl. Mater. Interfaces 2020, 12, 38962–38975. [Google Scholar] [CrossRef]
- Sakhakarmy, M.; Tian, S.; Raymond, L.; Xiong, G.; Chen, J.; Jin, Y. Printability study of self-supporting graphene oxide-laponite nanocomposites for 3D printing applications. Int. J. Adv. Manuf. Technol. 2021, 114, 343–355. [Google Scholar] [CrossRef]
- Li, C.; Adamcik, J.; Mezzenga, R. Biodegradable nanocomposites of amyloid fibrils and graphene with shape-memory and enzyme-sensing properties. Nat. Nanotechnol. 2012, 7, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Miura, Y.; Macosko, C.W. Graphene/polyurethane nanocomposites for improved gas barrier and electrical conductivity. Chem. Mater. 2010, 22, 3441–3450. [Google Scholar] [CrossRef]
- Luo, J.; Yang, L.; Sun, D.; Gao, Z.; Jiao, K.; Zhang, J. Graphene Oxide “Surfactant”-Directed Tunable Concentration of Graphene Dispersion. Small 2020, 16, 2003426. [Google Scholar] [CrossRef] [PubMed]
- McCoy, T.M.; Pottage, M.J.; Tabor, R.F. Graphene oxide-stabilized oil-in-water emulsions: pH-controlled dispersion and flocculation. J. Phys. Chem. C 2014, 118, 4529–4535. [Google Scholar] [CrossRef]
- Zhao, W.; Sugunan, A.; Gillgren, T.; Larsson, J.A.; Zhang, Z.-B.; Zhang, S.-L.; Nordgren, N.; Sommertune, J.; Ahniyaz, A. Surfactant-free stabilization of aqueous graphene dispersions using starch as a dispersing agent. ACS Omega 2021, 6, 12050–12062. [Google Scholar] [CrossRef] [PubMed]
- Bordes, E.; Morcos, B.; Bourgogne, D.; Andanson, J.-M.; Bussière, P.-O.; Santini, C.C.; Benayad, A.; Costa Gomes, M.; Pádua, A.A. Corrigendum: Dispersion and Stabilization of Exfoliated Graphene in Ionic Liquids. Front. Chem. 2020, 8, 556. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.S.; Dutta, N.K.; Choudhury, N.R. Poly (ionic liquid)-stabilized graphene nanoinks for scalable 3D printing of graphene aerogels. ACS Appl. Nano Mater. 2020, 3, 11608–11619. [Google Scholar] [CrossRef]
- Kim, J.; Choi, Y.J.; Gal, C.W.; Park, H.; Yoon, S.Y.; Yun, H.s. Effect of dispersants on structural integrity of 3D printed ceramics. Int. J. Appl. Ceram. Technol. 2022, 19, 968–978. [Google Scholar] [CrossRef]
- Haney, R.; Tran, P.; Trigg, E.B.; Koerner, H.; Dickens, T.; Ramakrishnan, S. Printability and performance of 3D conductive graphite structures. Addit. Manuf. 2021, 37, 101618. [Google Scholar] [CrossRef]
- Borode, A.O.; Ahmed, N.A.; Olubambi, P.A.; Sharifpur, M.; Meyer, J.P. Effect of various surfactants on the viscosity, thermal and electrical conductivity of graphene nanoplatelets Nanofluid. Int. J. Thermophys. 2021, 42, 158. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Lin, L. Rheological study on 3D printability of alginate hydrogel and effect of graphene oxide. Int. J. Bioprint. 2016, 2, 54–66. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.; Maniruzzaman, M. Rheological and dielectric behavior of 3D-printable chitosan/graphene oxide hydrogels. ACS Biomater. Sci. Eng. 2019, 6, 88–99. [Google Scholar] [CrossRef]
- Unkovskiy, A.; Bui, P.H.-B.; Schille, C.; Geis-Gerstorfer, J.; Huettig, F.; Spintzyk, S. Objects build orientation, positioning, and curing influence dimensional accuracy and flexural properties of stereolithographically printed resin. Dent. Mater. 2018, 34, e324–e333. [Google Scholar] [CrossRef]
- Lai, C.Q.; Markandan, K.; Luo, B.; Lam, Y.C.; Chung, W.C.; Chidambaram, A. Viscoelastic and high strain rate response of anisotropic graphene-polymer nanocomposites fabricated with stereolithographic 3D printing. Addit. Manuf. 2021, 37, 101721. [Google Scholar] [CrossRef]
- Ravi, P.; Chepelev, L.; Lawera, N.; Haque, K.M.A.; Chen, V.C.; Ali, A.; Rybicki, F.J. A systematic evaluation of medical 3D printing accuracy of multi-pathological anatomical models for surgical planning manufactured in elastic and rigid material using desktop inverted vat photopolymerization. Med. Phys. 2021, 48, 3223–3233. [Google Scholar] [CrossRef]
- Pagac, M.; Hajnys, J.; Ma, Q.-P.; Jancar, L.; Jansa, J.; Stefek, P.; Mesicek, J. A review of vat photopolymerization technology: Materials, applications, challenges, and future trends of 3d printing. Polymers 2021, 13, 598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Zhang, Y.; Huangfu, H.; Yang, Y.; Qin, Q.; Zhang, Y.; Zhou, Y. 3D printed reduced graphene oxide-GelMA hybrid hydrogel scaffolds for potential neuralized bone regeneration. J. Mater. Chem. B 2023, 11, 1288–1301. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Helal, E.; Gutierrez, G.; Moghimian, N.; Madinehei, M.; David, E.; Samara, M.; Demarquette, N. A review on graphene’s light stabilizing effects for reduced photodegradation of polymers. Crystals 2020, 11, 3. [Google Scholar] [CrossRef]
- Chiulan, I.; Voicu, Ş.I.; Batalu, D. The use of graphene and its Derivatives for the development of polymer matrix composites by stereolithographic 3D printing. Appl. Sci. 2022, 12, 3521. [Google Scholar] [CrossRef]
- Gasparotto, M.; Bellet, P.; Scapin, G.; Busetto, R.; Rampazzo, C.; Vitiello, L.; Shah, D.I.; Filippini, F. 3D printed graphene-PLA scaffolds promote cell alignment and differentiation. Int. J. Mol. Sci. 2022, 23, 1736. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, W.; Bártolo, P. Novel poly (ε-caprolactone)/graphene scaffolds for bone cancer treatment and bone regeneration. 3D Print. Addit. Manuf. 2020, 7, 222–229. [Google Scholar] [CrossRef]
- Sui, Y.; Zorman, C.A. Inkjet printing of metal structures for electrochemical sensor applications. J. Electrochem. Soc. 2020, 167, 037571. [Google Scholar] [CrossRef]
- Huang, Q.; Cai, Y.; Zhang, X.; Liu, J.; Liu, Z.; Li, B.; Wong, H.; Xu, F.; Sheng, L.; Sun, D. Aligned graphene mesh-supported double network natural hydrogel conduit loaded with netrin-1 for peripheral nerve regeneration. ACS Appl. Mater. Interfaces 2021, 13, 112–122. [Google Scholar] [CrossRef] [PubMed]
- La, W.-G.; Jung, M.-J.; Yoon, J.-K.; Bhang, S.H.; Jang, H.-K.; Lee, T.-J.; Yoon, H.-H.; Shin, J.-Y.; Kim, B.-S. Bone morphogenetic protein-2 for bone regeneration–Dose reduction through graphene oxide-based delivery. Carbon 2014, 78, 428–438. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, C.; Mou, S.; Li, J.; Zhou, M.; Zeng, Y.; Luo, C.; Sun, J.; Wang, Z.; Xu, W. Biocompatible graphene oxide–collagen composite aerogel for enhanced stiffness and in situ bone regeneration. Mater. Sci. Eng. C 2019, 105, 110137. [Google Scholar] [CrossRef] [PubMed]
- Shahmoradi, S.; Golzar, H.; Hashemi, M.; Mansouri, V.; Omidi, M.; Yazdian, F.; Yadegari, A.; Tayebi, L. Optimizing the nanostructure of graphene oxide/silver/arginine for effective wound healing. Nanotechnology 2018, 29, 475101. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Sareen, N.; Abu-El-Rub, E.; Ashour, H.; Sequiera, G.L.; Ammar, H.I.; Gopinath, V.; Shamaa, A.A.; Sayed, S.S.E.; Moudgil, M. Graphene oxide-gold nanosheets containing chitosan scaffold improves ventricular contractility and function after implantation into infarcted heart. Sci. Rep. 2018, 8, 15069. [Google Scholar] [CrossRef] [PubMed]
- Stone, H.; Lin, S.; Mequanint, K. Preparation and characterization of electrospun rGO-poly (ester amide) conductive scaffolds. Mater. Sci. Eng. C 2019, 98, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, P.; Kannan, R.; Ramachandran, B.; Moorthy, G.; Suguna, L.; Muthuvijayan, V. Development of reduced graphene oxide (rGO)-isabgol nanocomposite dressings for enhanced vascularization and accelerated wound healing in normal and diabetic rats. J. Colloid Interface Sci. 2018, 517, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Aghaei-Ghareh-Bolagh, B.; Gao, X.; Nikkhah, M.; Jung, S.M.; Dolatshahi-Pirouz, A.; Kim, S.B.; Kim, S.M.; Dokmeci, M.R.; Tang, X. Layer-by-layer assembly of 3D tissue constructs with functionalized graphene. Adv. Funct. Mater. 2014, 24, 6136–6144. [Google Scholar] [CrossRef]
- Wychowaniec, J.K.; Litowczenko, J.; Tadyszak, K.; Natu, V.; Aparicio, C.; Peplińska, B.; Barsoum, M.W.; Otyepka, M.; Scheibe, B. Unique cellular network formation guided by heterostructures based on reduced graphene oxide-Ti3C2Tx MXene hydrogels. Acta Biomater. 2020, 115, 104–115. [Google Scholar] [CrossRef]
- Wu, Y.; An, C.; Guo, Y. 3D Printed Graphene and Graphene/Polymer Composites for Multifunctional Applications. Materials 2023, 16, 5681. [Google Scholar] [CrossRef]
- Markandan, K.; Lai, C.Q. Enhanced mechanical properties of 3D printed graphene-polymer composite lattices at very low graphene concentrations. Compos. Part A Appl. Sci. Manuf. 2020, 129, 105726. [Google Scholar] [CrossRef]
- Vatani, M.; Zare, Y.; Gharib, N.; Rhee, K.Y.; Park, S.-J. Simulating of effective conductivity for graphene–polymer nanocomposites. Sci. Rep. 2023, 13, 5907. [Google Scholar] [CrossRef]
- Solìs Moré, Y.; Panella, G.; Fioravanti, G.; Perrozzi, F.; Passacantando, M.; Giansanti, F.; Ardini, M.; Ottaviano, L.; Cimini, A.; Peniche, C. Biocompatibility of composites based on chitosan, apatite, and graphene oxide for tissue applications. J. Biomed. Mater. Res. Part A 2018, 106, 1585–1594. [Google Scholar] [CrossRef]
- Patil, R.; Bahadur, P.; Tiwari, S. Dispersed graphene materials of biomedical interest and their toxicological consequences. Adv. Colloid Interface Sci. 2020, 275, 102051. [Google Scholar] [CrossRef]
- Gao, F.; Hu, Y.; Li, G.; Liu, S.; Quan, L.; Yang, Z.; Wei, Y.; Pan, C. Layer-by-layer deposition of bioactive layers on magnesium alloy stent materials to improve corrosion resistance and biocompatibility. Bioact. Mater. 2020, 5, 611–623. [Google Scholar] [CrossRef]
- Andrews, J.P.; Joshi, S.S.; Tzolos, E.; Syed, M.B.; Cuthbert, H.; Crica, L.E.; Lozano, N.; Okwelogu, E.; Raftis, J.B.; Bruce, L. First-in-human controlled inhalation of thin graphene oxide nanosheets to study acute cardiorespiratory responses. Nat. Nanotechnol. 2024, 1–10. [Google Scholar] [CrossRef]
- Kenry, W.C.L.; Loh, K.P.; Lim, C.T. When stem cells meet graphene: Opportunities and challenges in regenerative medicine. Biomaterials 2018, 155, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Li, Y.-C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, V.; Lattanzi, W.; Perini, G.; Augello, A.; Papi, M.; De Spirito, M. 3D-printed graphene for bone reconstruction. 2D Mater. 2020, 7, 022004. [Google Scholar] [CrossRef]
- Xie, H.; Cao, T.; Gomes, J.V.; Neto, A.H.C.; Rosa, V. Two and three-dimensional graphene substrates to magnify osteogenic differentiation of periodontal ligament stem cells. Carbon 2015, 93, 266–275. [Google Scholar] [CrossRef]
- Hermenean, A.; Codreanu, A.; Herman, H.; Balta, C.; Rosu, M.; Mihali, C.V.; Ivan, A.; Dinescu, S.; Ionita, M.; Costache, M. Chitosan-graphene oxide 3D scaffolds as promising tools for bone regeneration in critical-size mouse calvarial defects. Sci. Rep. 2017, 7, 16641. [Google Scholar] [CrossRef]
- Daneshmandi, L.; Holt, B.D.; Arnold, A.M.; Laurencin, C.T.; Sydlik, S.A. Ultra-low binder content 3D printed calcium phosphate graphene scaffolds as resorbable, osteoinductive matrices that support bone formation in vivo. Sci. Rep. 2022, 12, 6960. [Google Scholar] [CrossRef]
- Qin, W.; Li, C.; Liu, C.; Wu, S.; Liu, J.; Ma, J.; Chen, W.; Zhao, H.; Zhao, X. 3D printed biocompatible graphene oxide, attapulgite, and collagen composite scaffolds for bone regeneration. J. Biomater. Appl. 2022, 36, 1838–1851. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Caetano, G.; Ambler, W.S.; Blaker, J.J.; Frade, M.A.; Mandal, P.; Diver, C.; Bártolo, P. Enhancing the hydrophilicity and cell attachment of 3D printed PCL/graphene scaffolds for bone tissue engineering. Materials 2016, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Sahafnejad-Mohammadi, I.; Rahmati, S.; Najmoddin, N.; Bodaghi, M. Biomimetic polycaprolactone-graphene oxide composites for 3D printing bone scaffolds. Macromol. Mater. Eng. 2023, 308, 2200558. [Google Scholar] [CrossRef]
- Alazab, M.H.; Abouelgeit, S.A.; Aboushelib, M.N. Histomorphometric evaluation of 3D printed graphene oxide-enriched poly (ε-caprolactone) scaffolds for bone regeneration. Heliyon 2023, 9, e15844. [Google Scholar] [CrossRef]
- Karthic, M.; Chockalingam, K.; Vignesh, C.; Nagarajan, K. Characterization of 3D Printed graphene reinforced PLA scaffold for bone regeneration application. Emerg. Mater. Res. 2023, 12, 382–394. [Google Scholar]
- Seok, J.M.; Choe, G.; Lee, S.J.; Yoon, M.-A.; Kim, K.-S.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Lee, K.; Park, S.A. Enhanced three-dimensional printing scaffold for osteogenesis using a mussel-inspired graphene oxide coating. Mater. Des. 2021, 209, 109941. [Google Scholar] [CrossRef]
- Zhu, S.; Yao, L.; Pan, C.; Tian, J.; Li, L.; Luo, B.; Zhou, C.; Lu, L. 3D printed gellan gum/graphene oxide scaffold for tumor therapy and bone reconstruction. Compos. Sci. Technol. 2021, 208, 108763. [Google Scholar] [CrossRef]
- Park, S.Y.; Park, J.; Sim, S.H.; Sung, M.G.; Kim, K.S.; Hong, B.H.; Hong, S. Enhanced differentiation of human neural stem cells into neurons on graphene. Adv. Mater. 2011, 23, H263–H267. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, S.; Xiao, M.; Qian, F.; He, Z.; Li, D.; Zhang, X.; Li, H.; Yang, X.; Wang, M. Accelerating bioelectric functional development of neural stem cells by graphene coupling: Implications for neural interfacing with conductive materials. Biomaterials 2016, 106, 193–204. [Google Scholar] [CrossRef]
- Fang, Q.; Zhang, Y.; Chen, X.; Li, H.; Cheng, L.; Zhu, W.; Zhang, Z.; Tang, M.; Liu, W.; Wang, H. Three-dimensional graphene enhances neural stem cell proliferation through metabolic regulation. Front. Bioeng. Biotechnol. 2020, 7, 436. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Thaharah, S.; Zhang, S.; Lu, W.F.; Fuh, J.Y.H. 3D-printed PCL/rGO conductive scaffolds for peripheral nerve injury repair. Artif. Organs 2019, 43, 515–523. [Google Scholar] [CrossRef]
- Qian, Y.; Zhao, X.; Han, Q.; Chen, W.; Li, H.; Yuan, W. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat. Commun. 2018, 9, 323. [Google Scholar] [CrossRef]
- Magaz, A.; Li, X.; Gough, J.E.; Blaker, J.J. Graphene oxide and electroactive reduced graphene oxide-based composite fibrous scaffolds for engineering excitable nerve tissue. Mater. Sci. Eng. C 2021, 119, 111632. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, D.; Wang, Z.; Zhang, Z.; Xia, Y.; Xue, H.; Liu, Y. Preparation of an electrically conductive graphene oxide/chitosan scaffold for cardiac tissue engineering. Appl. Biochem. Biotechnol. 2019, 188, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Karimi Hajishoreh, N.; Baheiraei, N.; Naderi, N.; Salehnia, M. Reduced graphene oxide facilitates biocompatibility of alginate for cardiac repair. J. Bioact. Compat. Polym. 2020, 35, 363–377. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, S.; Sharma, L.K. Role of graphene in biomedical applications. Mater. Today Proc. 2022, 63, 542–546. [Google Scholar] [CrossRef]
- Jara, A.D.; Betemariam, A.; Woldetinsae, G.; Kim, J.Y. Purification, application and current market trend of natural graphite: A review. Int. J. Min. Sci. Technol. 2019, 29, 671–689. [Google Scholar] [CrossRef]
- Fernández-Pradas, J.M.; Serra, P. Laser-induced forward transfer: A method for printing functional inks. Crystals 2020, 10, 651. [Google Scholar] [CrossRef]
- Florian, C.; Serra, P. Printing via laser-induced forward transfer and the future of digital manufacturing. Materials 2023, 16, 698. [Google Scholar] [CrossRef]
- Paula, K.T.; Santos, M.V.; Facure, M.H.; Andrade, M.B.; Araujo, F.L.; Correa, D.S.; Ribeiro, S.J.; Mendonca, C.R. Laser patterning and induced reduction of graphene oxide functionalized silk fibroin. Opt. Mater. 2020, 99, 109540. [Google Scholar] [CrossRef]
- Vasanthakumar, A.; Rejeeth, C.; Vivek, R.; Ponraj, T.; Jayaraman, K.; Anandasadagopan, S.K.; Vinayaga Moorthi, P. Design of bio-graphene-based multifunctional nanocomposites exhibits intracellular drug delivery in cervical cancer treatment. ACS Appl. Bio Mater. 2022, 5, 2956–2964. [Google Scholar] [CrossRef] [PubMed]
- Loukelis, K.; Helal, Z.A.; Mikos, A.G.; Chatzinikolaidou, M. Nanocomposite bioprinting for tissue engineering applications. Gels 2023, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Gul, J.Z.; Sajid, M.; Choi, K.H. Retraction: 3D printed highly flexible strain sensor based on TPU–graphene composite for feedback from high speed robotic applications. J. Mater. Chem. C 2020, 8, 2597. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, R.; Alimperti, S. Graphene in 3D Bioprinting. J. Funct. Biomater. 2024, 15, 82. https://doi.org/10.3390/jfb15040082
Patil R, Alimperti S. Graphene in 3D Bioprinting. Journal of Functional Biomaterials. 2024; 15(4):82. https://doi.org/10.3390/jfb15040082
Chicago/Turabian StylePatil, Rahul, and Stella Alimperti. 2024. "Graphene in 3D Bioprinting" Journal of Functional Biomaterials 15, no. 4: 82. https://doi.org/10.3390/jfb15040082
APA StylePatil, R., & Alimperti, S. (2024). Graphene in 3D Bioprinting. Journal of Functional Biomaterials, 15(4), 82. https://doi.org/10.3390/jfb15040082








