Functionalization of PCL-Based Fiber Scaffolds with Different Sources of Calcium and Phosphate and Odontogenic Potential on Human Dental Pulp Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Incorporation of Inorganic Particles into Scaffolds
2.1.1. Synthesis of Fibrillar Scaffolds via Electrospinning
2.1.2. Morphological and Chemical Characterization
2.2. Bioactivity of the Fibrillar Scaffolds on Human Dental Pulp Cells (HDPCs)
2.2.1. Primary Culture of Human Dental Pulp Cells (HDPCs)
2.2.2. HDPC Biological Response in Contact with Scaffolds Incorporated into Different Inorganic Particles
2.2.3. Cell Adhesion and Viability
2.2.4. Mineralized Nodules Formation
2.3. Physical-Chemical Characterization and Odontogenic Differentiation Potential of PCL + CH Scaffolds
2.3.1. Degradation Profile of PCL + CH Scaffolds
2.3.2. Calcium Release by PCL-CH Scaffolds
2.3.3. Odontogenic Differentiation of HDPC in Contact with PCL + CH Scaffolds
2.4. Statistical Analysis
3. Results
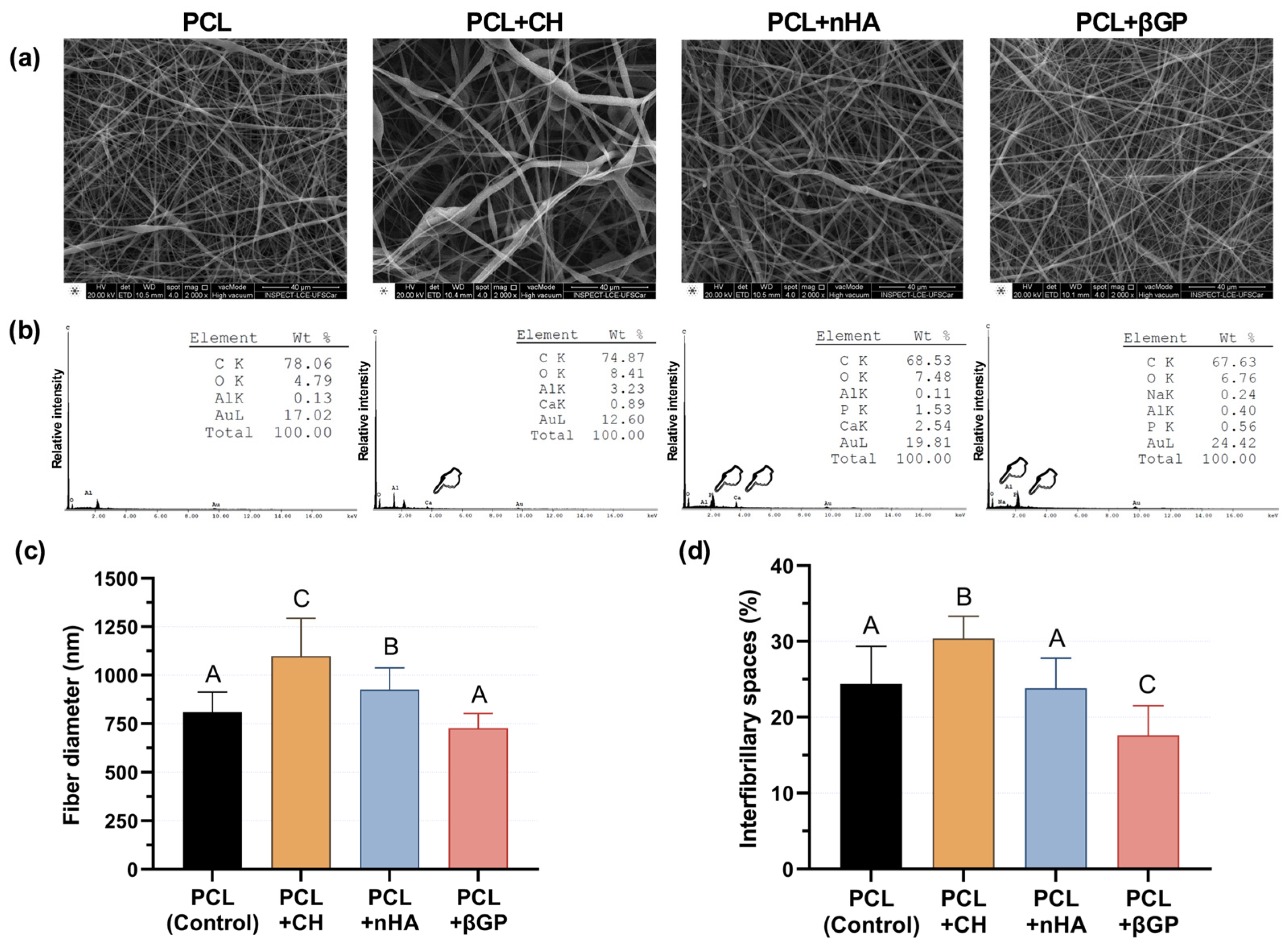
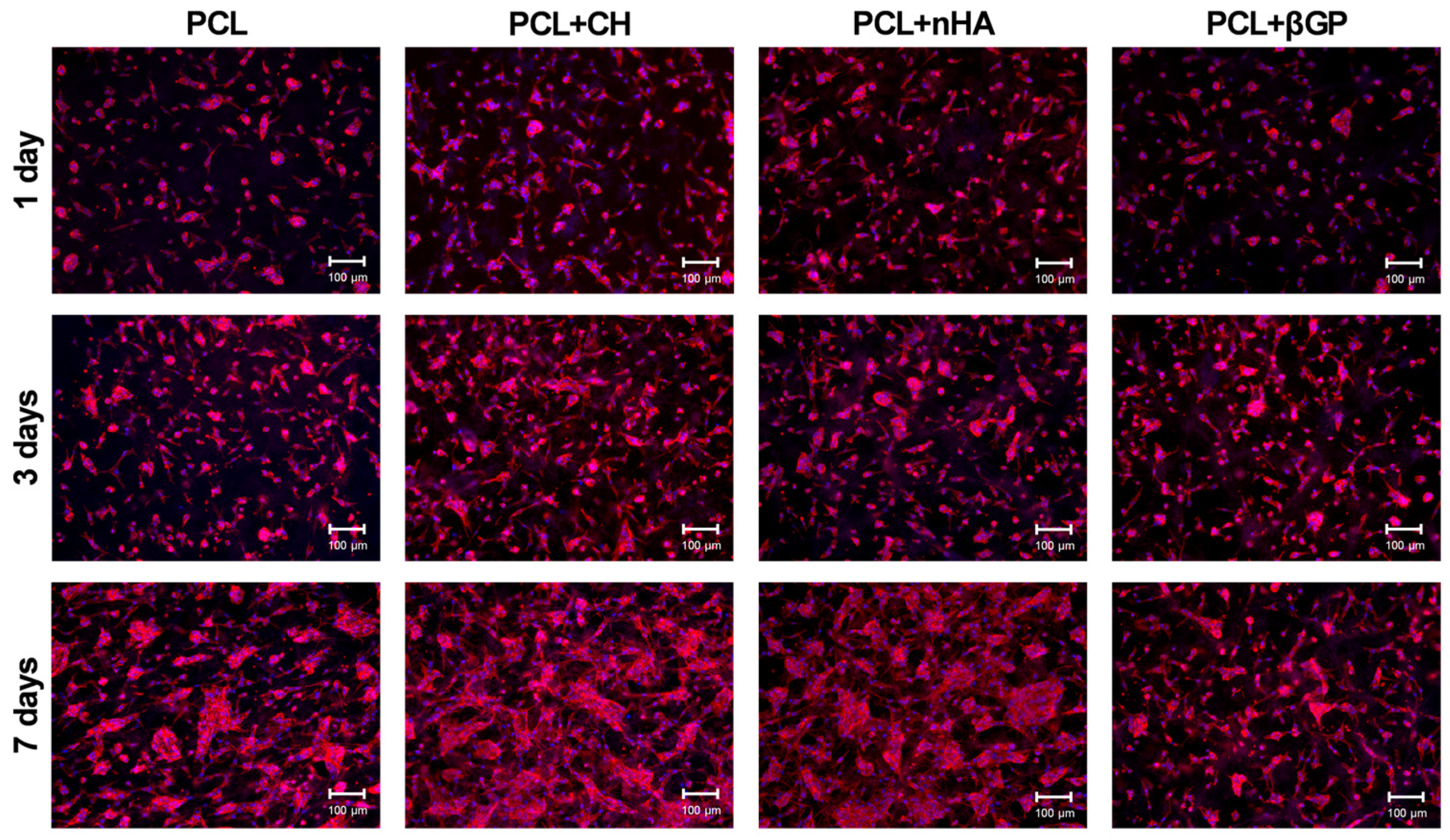
3.1. Incorporation of Inorganic Particles into Scaffolds and Evaluating Their Bioactivity on Human Dental Pulp Cells (HDPC)
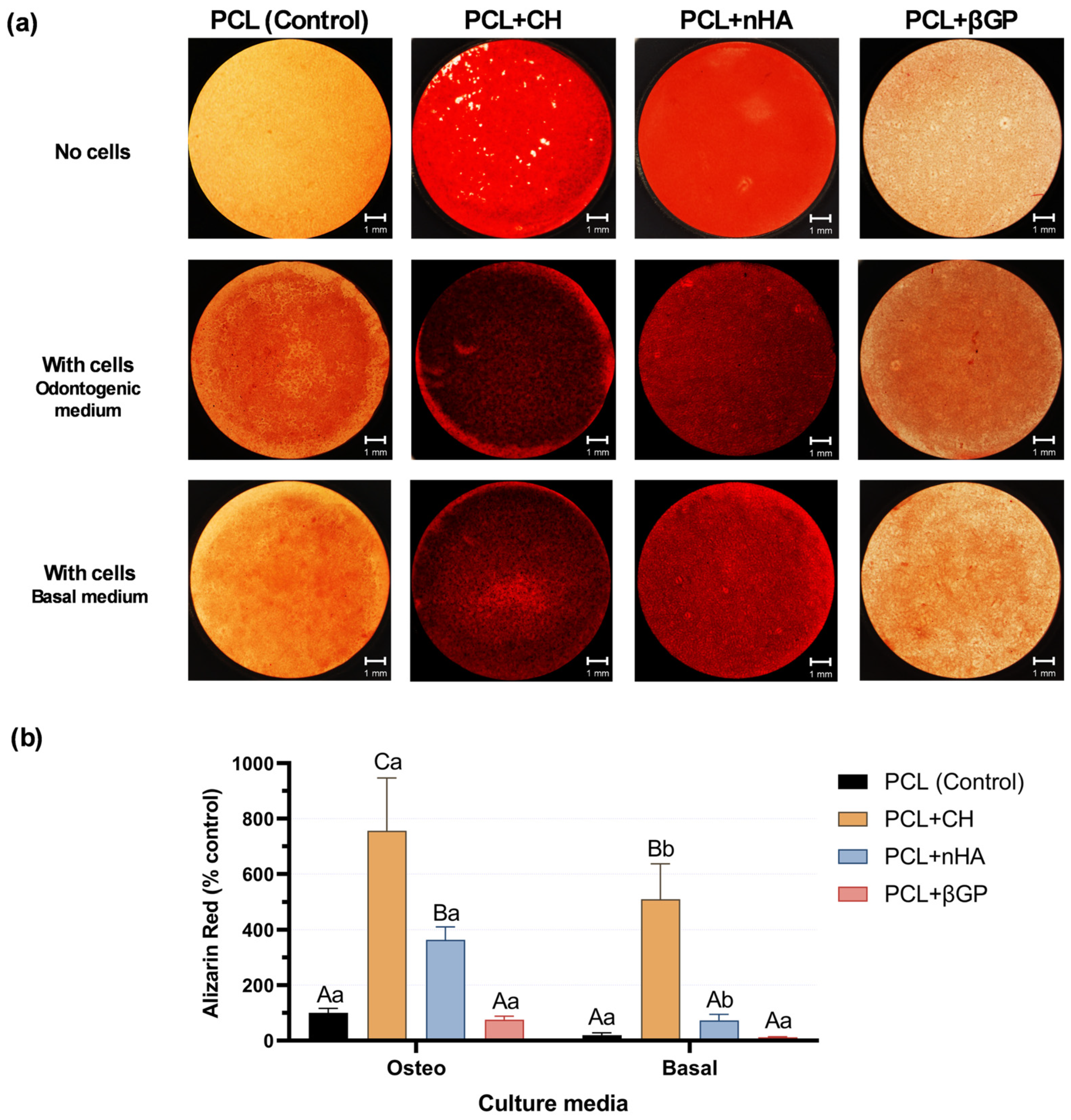
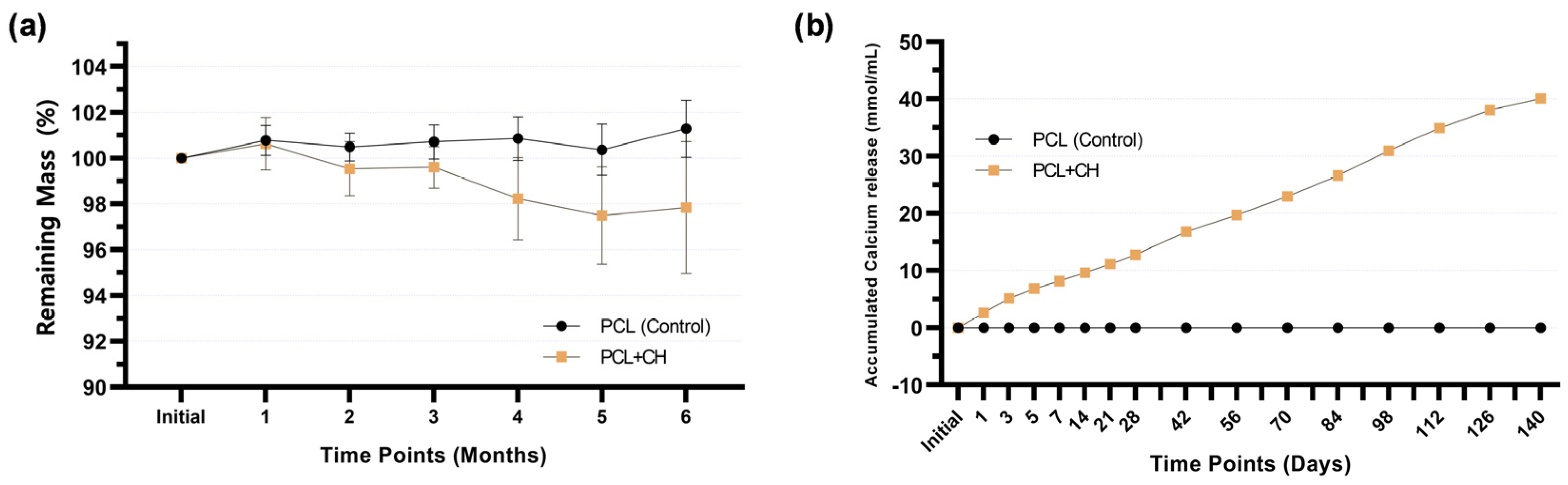
3.2. Physical–Chemical Characterization and Odontogenic Differentiation Potential of PCL + CH Scaffolds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moussa, D.G.; Aparicio, C. Present and Future of Tissue Engineering Scaffolds for Dentin-Pulp Complex Regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 58–75. [Google Scholar] [CrossRef]
- Simonsen, R.J. From Prevention to Therapy: Minimal Intervention with Sealants and Resin Restorative Materials. J. Dent. 2011, 39, S27–S33. [Google Scholar] [CrossRef]
- Hanna, S.N.; Alfayate, R.P.; Prichard, J. Vital Pulp Therapy an Insight over the Available Literature and Future Expectations. Eur. Endod. J. 2020, 5, 46–53. [Google Scholar] [CrossRef]
- Komabayashi, T.; Zhu, Q.; Eberhart, R.; Imai, Y. Current Status of Direct Pulp-Capping Materials for Permanent Teeth. Dent. Mater. J. 2016, 35, 1–12. [Google Scholar] [CrossRef] [PubMed]
- De Souza Costa, C.A.; Hebling, J.; Scheffel, D.L.S.; Soares, D.G.S.; Basso, F.G.; Ribeiro, A.P.D. Methods to Evaluate and Strategies to Improve the Biocompatibility of Dental Materials and Operative Techniques. Dent. Mater. 2014, 30, 769–784. [Google Scholar] [CrossRef]
- Graham, L.; Cooper, P.R.; Cassidy, N.; Nor, J.E.; Sloan, A.J.; Smith, A.J. The Effect of Calcium Hydroxide on Solubilisation of Bio-Active Dentine Matrix Components. Biomaterials 2006, 27, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Schröder, U. Effects of Calcium Hydroxide-Containing Pulp-Capping Agents on Pulp Cell Migration, Proliferation, and Differentiation. J. Dent. Res. 1985, 64, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L.; Cooper, P.R.; Smith, A.J. Can Interaction of Materials with the Dentin-Pulp Complex Contribute to Dentin Regeneration? Odontology 2010, 98, 2–14. [Google Scholar] [CrossRef]
- Goldberg, M.; Lacerda-Pinheiro, S.; Jegat, N.; Six, N.; Septier, D.; Priam, F.; Bonnefoix, M.; Tompkins, K.; Chardin, H.; Denbesten, P.; et al. The Impact of Bioactive Molecules to Stimulate Tooth Repair and Regeneration as Part of Restorative Dentistry. Dent. Clin. N. Am. 2006, 50, 277–298. [Google Scholar] [CrossRef]
- Mente, J.; Hufnagel, S.; Leo, M.; Michel, A.; Gehrig, H.; Panagidis, D.; Saure, D.; Pfefferle, T. Treatment Outcome of Mineral Trioxide Aggregate or Calcium Hydroxide Direct Pulp Capping: Long-Term Results. J. Endod. 2014, 40, 1746–1751. [Google Scholar] [CrossRef]
- Galler, K.M.; Widbiller, M. Cell-Free Approaches for Dental Pulp Tissue Engineering. J. Endod. 2020, 46, S143–S149. [Google Scholar] [CrossRef] [PubMed]
- Scheller, E.L.; Krebsbach, P.H.; Kohn, D.H. Tissue Engineering: State of the Art in Oral Rehabilitation. J. Oral Rehabil. 2009, 36, 368–389. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X. Biomimetic Materials for Tissue Engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, H.; Jin, X.; Hu, J.; Liu, X.; Ni, L.; Ma, P.X. The Effect of Scaffold Architecture on Odontogenic Differentiation of Human Dental Pulp Stem Cells. Biomaterials 2011, 32, 7822–7830. [Google Scholar] [CrossRef] [PubMed]
- Popovich, K.D.; Vagner, S.A.; Murashko, D.T.; Ten, G.N.; Ryabkin, D.I.; Savelyev, M.S.; Kitsyuk, E.P.; Gerasimenko, E.A.; Edelbekova, P.; Konovalov, A.N.; et al. Stability and Thrombogenicity Analysis of Collagen/Carbon Nanotube Nanocomposite Coatings Using a Reversible Microfluidic Device. Membranes 2023, 13, 403. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Luo, J.; Zhao, X.; Gao, J.; Xiong, J. Electrospun Homogeneous Silk Fibroin/Poly (ϵ-Caprolactone) Nanofibrous Scaffolds by Addition of Acetic Acid for Tissue Engineering. J. Biomater. Appl. 2016, 31, 421–437. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as Biomaterial for Bone Scaffolds: Review of Literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Liu, S.; He, Z.; Xu, G.; Xiao, X. Fabrication of Polycaprolactone Nanofibrous Scaffolds by Facile Phase Separation Approach. Mater. Sci. Eng. C 2014, 44, 201–208. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer—Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Soares, I.P.M.; Anselmi, C.; Kitagawa, F.A.; de Oliveira Ribeiro, R.A.; Leite, M.L.; de Souza Costa, C.A.; Hebling, J. Nano-Hydroxyapatite-Incorporated Polycaprolactone Nanofibrous Scaffold as a Dentin Tissue Engineering-Based Strategy for Vital Pulp Therapy. Dent. Mater. 2022, 38, 960–977. [Google Scholar] [CrossRef]
- Sunandhakumari, V.J.; Vidhyadharan, A.K.; Alim, A.; Kumar, D.; Ravindran, J.; Krishna, A.; Prasad, M. Fabrication and in Vitro Characterization of Bioactive Glass/Nano Hydroxyapatite Reinforced Electrospun Poly(ε-Caprolactone) Composite Membranes for Guided Tissue Regeneration. Bioengineering 2018, 5, 54. [Google Scholar] [CrossRef]
- Hwang, P.T.J.; Murdock, K.; Alexander, G.C.; Salaam, A.D.; Ng, J.I.; Lim, D.J.; Dean, D.; Jun, H.W. Poly(ε-Caprolactone)/Gelatin Composite Electrospun Scaffolds with Porous Crater-like Structures for Tissue Engineering. J. Biomed. Mater. Res.—Part A 2016, 104, 1017–1029. [Google Scholar] [CrossRef]
- Yang, X.; Yang, F.; Walboomers, X.F.; Bian, Z.; Fan, M.; Jansen, J.A. The Performance of Dental Pulp Stem Cells on Nanofibrous PCL/Gelatin/NHA Scaffolds. J. Biomed. Mater. Res.—Part A 2010, 93, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Yassen, G.H.; Platt, J.A.; Labban, N.; Windsor, L.J.; Spolnik, K.J.; Bressiani, A.H.A. A Novel Three-Dimensional Scaffold for Regenerative Endodontics: Materials and Biological Characterizations. J. Tissue Eng. Regen. Med. 2015, 9, E116–E123. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Yeung, A.W.K.; Zhang, C.; Tsoi, J.K.H. A Bibliometric Analysis of Electrospun Nanofibers for Dentistry. J. Funct. Biomater. 2022, 13, 90. [Google Scholar] [CrossRef]
- Bordini, E.A.F.; Cassiano, F.B.; Bronze-Uhle, E.S.; Alamo, L.; Hebling, J.; de Souza Costa, C.A.; Soares, D.G. Chitosan in Association with Osteogenic Factors as a Cell-Homing Platform for Dentin Regeneration: Analysis in a Pulp-in-a-Chip Model. Dent. Mater. 2022, 38, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.G.; Rosseto, H.L.; Scheffel, D.S.; Basso, F.G.; Huck, C.; Hebling, J.; de Souza Costa, C.A. Odontogenic Differentiation Potential of Human Dental Pulp Cells Cultured on a Calcium-Aluminate Enriched Chitosan-Collagen Scaffold. Clin. Oral Investig. 2017, 21, 2827–2839. [Google Scholar] [CrossRef]
- Roy, P.; Sailaja, R.R.N. Chitosan-Nanohydroxyapatite Composites: Mechanical, Thermal and Bio-Compatibility Studies. Int. J. Biol. Macromol. 2015, 73, 170–181. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, L.; Chen, W.; Liu, X.; Weir, M.D.; Xu, H.H.K. Stem Cells and Calcium Phosphate Cement Scaffolds for Bone Regeneration. J. Dent. Res. 2014, 93, 618–625. [Google Scholar] [CrossRef]
- Sun, B.; Ma, W.; Su, F.; Wang, Y.; Liu, J.; Wang, D.; Liu, H. The Osteogenic Differentiation of Dog Bone Marrow Mesenchymal Stem Cells in a Thermo-Sensitive Injectable Chitosan/Collagen/β-Glycerophosphate Hydrogel: In Vitro and in Vivo. J. Mater. Sci. Mater. Med. 2011, 22, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.K.; Zhao, L.; Weir, M.D. Stem Cell-Calcium Phosphate Constructs for Bone Engineering. J. Dent. Res. 2010, 89, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Nemoto, E.; Kanaya, S.; Hamaji, N.; Sato, H.; Shimauchi, H. Elevated Extracellular Calcium Increases Expression of Bone Morphogenetic Protein-2 Gene via a Calcium Channel and ERK Pathway in Human Dental Pulp Cells. Biochem. Biophys. Res. Commun. 2010, 394, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Okabe, T.; Sakamoto, M.; Takeuchi, H.; Matsushima, K. Effects of PH on Mineralization Ability of Human Dental Pulp Cells. J. Endod. 2006, 32, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Arthur, A.; Bartold, P.M.; Shi, S. A Method to Isolate and Culture Expand Human Dental Pulp Stem Cells. In Mesenchymal Stem Cell Assays and Applications; Springer: Berlin/Heidelberg, Germany, 2011; Volume 21, ISBN 9781607619987. [Google Scholar]
- Aeinehchi, M.; Eslami, B.; Ghanbariha, M.; Saffar, A.S. Mineral Trioxide Aggregate (MTA) and Calcium Hydroxide as Pulp-Capping Agents in Human Teeth: A Preliminary Report. Int. Endod. J. 2003, 36, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.N.R.; Duncan, H.F.; Pitt Ford, T.R.; Luder, H.U. Histological, Ultrastructural and Quantitative Investigations on the Response of Healthy Human Pulps to Experimental Capping with Mineral Trioxide Aggregate: A Randomized Controlled Trial. Int. Endod. J. 2008, 41, 128–150. [Google Scholar] [CrossRef]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium Phosphate Ceramics in Bone Tissue Engineering: A Review of Properties and Their Influence on Cell Behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef]
- Kostopoulos, V.; Kotrotsos, A.; Fouriki, K.; Kalarakis, A.; Portan, D. Fabrication and Characterization of Polyetherimide Electrospun Scaffolds Modified with Graphene Nano-Platelets and Hydroxyapatite Nano-Particles. Int. J. Mol. Sci. 2020, 21, 583. [Google Scholar] [CrossRef]
- Johannsen, K.; Rademacher, S. Modelling the Kinetics of Calcium Hydroxide Dissolution in Water. Acta Hydrochim. Hydrobiol. 1999, 27, 72–78. [Google Scholar] [CrossRef]
- Thomas, V.; Jagani, S.; Johnson, K.; Jose, M.V.; Dean, D.R.; Vohra, Y.K.; Nyairo, E. Electrospun Bioactive Nanocomposite Scaffolds of Polycaprolactone and Nanohydroxyapatite for Bone Tissue Engineering. J. Nanosci. Nanotechnol. 2006, 6, 487–493. [Google Scholar] [CrossRef]
- Leite, M.L.; Usberti, F.R.; Bordini, E.A.F.; Soares, D.G.; Hebling, J.; de Souza Costa, C.A. Synthesis and Characterization of Nanofibers Scaffolds and Their Biological. Rodyb 2019, 9, 9–15. [Google Scholar]
- Gupte, M.J.; Ma, P.X. Nanofibrous Scaffolds for Dental and Craniofacial Applications. J. Dent. Res. 2012, 91, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, C.; Mendes Soares, I.P.; Leite, M.L.; Kitagawa, F.A.; de Souza Costa, C.A.; Hebling, J. Cytocompatibility and Bioactivity of Calcium Hydroxide-Containing Nanofiber Scaffolds Loaded with Fibronectin for Dentin Tissue Engineering. Clin. Oral Investig. 2022, 26, 4031–4047. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.L.; Soares, D.G.; Anovazzi, G.; Mendes Soares, I.P.; Hebling, J.; de Souza Costa, C.A. Development of Fibronectin-Loaded Nanofiber Scaffolds for Guided Pulp Tissue Regeneration. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2021, 109, 1244–1258. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.K.; Lu, Q.Z.; Pei, R.; Ji, H.J.; Zhou, G.S.; Zhao, X.L.; Tang, R.K.; Zhang, M. The Effect of Extracellular Calcium and Inorganic Phosphate on the Growth and Osteogenic Differentiation of Mesenchymal Stem Cells in Vitro: Implication for Bone Tissue Engineering. Biomed. Mater. 2009, 4, 025004. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zheng, L.; Jiang, J.; Gui, J.; Zhang, L.; Huang, Y.; Chen, X.; Ji, J.; Fan, Y. Calcium Hydroxide–Induced Proliferation, Migration, Osteogenic Differentiation, and Mineralization via the Mitogen-Activated Protein Kinase Pathway in Human Dental Pulp Stem Cells. J. Endod. 2016, 42, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Tsapikouni, T.S.; Missirlis, Y.F. Protein-Material Interactions: From Micro-to-Nano Scale. Mater. Sci. Eng. B 2008, 152, 2–7. [Google Scholar] [CrossRef]
- Mizuno, M.; Banzai, Y. Calcium Ion Release from Calcium Hydroxide Stimulated Fibronectin Gene Expression in Dental Pulp Cells and the Differentiation of Dental Pulp Cells to Mineralized Tissue Forming Cells by Fibronectin. Int. Endod. J. 2008, 41, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Chuenjitkuntaworn, B.; Inrung, W.; Damrongsri, D.; Mekaapiruk, K.; Supaphol, P.; Pavasant, P. Polycaprolactone/Hydroxyapatite Composite Scaffolds: Preparation, Characterization, and in Vitro and in Vivo Biological Responses of Human Primary Bone Cells. J. Biomed. Mater. Res.—Part A 2010, 94, 241–251. [Google Scholar] [CrossRef]
- Kim, G.H.; Park, Y.D.; Lee, S.Y.; El-Fiqi, A.; Kim, J.J.; Lee, E.J.; Kim, H.W.; Kim, E.C. Odontogenic Stimulation of Human Dental Pulp Cells with Bioactive Nanocomposite Fiber. J. Biomater. Appl. 2015, 29, 854–866. [Google Scholar] [CrossRef]
- Kim, J.J.; Bae, W.J.; Kim, J.M.; Kim, J.J.; Lee, E.J.; Kim, H.W.; Kim, E.C. Mineralized Polycaprolactone Nanofibrous Matrix for Odontogenesis of Human Dental Pulp Cells. J. Biomater. Appl. 2014, 28, 1069–1078. [Google Scholar] [CrossRef]
- An, S.; Gao, Y.; Ling, J.; Wei, X.; Xiao, Y. Calcium Ions Promote Osteogenic Differentiation and Mineralization of Human Dental Pulp Cells: Implications for Pulp Capping Materials. J. Mater. Sci. Mater. Med. 2012, 23, 789–795. [Google Scholar] [CrossRef]
- Soares, D.G.; Bordini, E.A.F.; Cassiano, F.B.; Bronze-Uhle, E.S.; Pacheco, L.E.; Zabeo, G.; Hebling, J.; Lisboa-Filho, P.N.; Bottino, M.C.; de Souza Costa, C.A. Characterization of Novel Calcium Hydroxide-Mediated Highly Porous Chitosan-Calcium Scaffolds for Potential Application in Dentin Tissue Engineering. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2020, 108, 2546–2559. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Golub, E.E.; Forbes, E.; Tokuoka, T.; Shapiro, I.M. Mechanism of Action of β-Glycerophosphate on Bone Cell Mineralization. Calcif. Tissue Int. 1992, 51, 305–311. [Google Scholar] [CrossRef]
- Coelho, M.J.; Fernandes, M.H. Human Bone Cell Cultures in Biocompatibility Testing. Part II: Effect of Ascorbic Acid, β-Glycerophosphate and Dexamethasone on Osteoblastic Differentiation. Biomaterials 2000, 21, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, F.S.; Enderami, S.E.; Hadian, A.; Abazari, M.F.; Ardeshirylajimi, A.; Saburi, E.; Soleimanifar, F.; Nazemisalman, B. Efficient Osteogenic Differentiation of the Dental Pulp Stem Cells on β-Glycerophosphate Loaded Polycaprolactone/Polyethylene Oxide Blend Nanofibers. J. Cell. Physiol. 2019, 234, 13951–13958. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hu, H.; Guo, J.; Wang, Q.; Cao, J.; Wang, H.; Li, G.; Mao, J.; Zou, X.; Chen, D.; et al. Nano-Hydroxyapatite Modulates Osteoblast Differentiation through Autophagy Induction via MTOR Signaling Pathway. J. Biomed. Nanotechnol. 2019, 15, 405–415. [Google Scholar] [CrossRef]
- Khoshniat, S.; Bourgine, A.; Julien, M.; Petit, M.; Pilet, P.; Rouillon, T.; Masson, M.; Gatius, M.; Weiss, P.; Guicheux, J.; et al. Phosphate-Dependent Stimulation of MGP and OPN Expression in Osteoblasts via the ERK1/2 Pathway Is Modulated by Calcium. Bone 2011, 48, 894–902. [Google Scholar] [CrossRef]
- Jung, G.Y.; Park, Y.J.; Han, J.S. Effects of HA Released Calcium Ion on Osteoblast Differentiation. J. Mater. Sci. Mater. Med. 2010, 21, 1649–1654. [Google Scholar] [CrossRef]
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Structure, Composition and Mineralization Michel. Front. Biosci. 2011, 1, 711–735. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anselmi, C.; Mendes Soares, I.P.; Mota, R.L.M.; Leite, M.L.; Ribeiro, R.A.d.O.; Fernandes, L.d.O.; Bottino, M.C.; de Souza Costa, C.A.; Hebling, J. Functionalization of PCL-Based Fiber Scaffolds with Different Sources of Calcium and Phosphate and Odontogenic Potential on Human Dental Pulp Cells. J. Funct. Biomater. 2024, 15, 97. https://doi.org/10.3390/jfb15040097
Anselmi C, Mendes Soares IP, Mota RLM, Leite ML, Ribeiro RAdO, Fernandes LdO, Bottino MC, de Souza Costa CA, Hebling J. Functionalization of PCL-Based Fiber Scaffolds with Different Sources of Calcium and Phosphate and Odontogenic Potential on Human Dental Pulp Cells. Journal of Functional Biomaterials. 2024; 15(4):97. https://doi.org/10.3390/jfb15040097
Chicago/Turabian StyleAnselmi, Caroline, Igor Paulino Mendes Soares, Rafaella Lara Maia Mota, Maria Luísa Leite, Rafael Antonio de Oliveira Ribeiro, Lídia de Oliveira Fernandes, Marco C. Bottino, Carlos Alberto de Souza Costa, and Josimeri Hebling. 2024. "Functionalization of PCL-Based Fiber Scaffolds with Different Sources of Calcium and Phosphate and Odontogenic Potential on Human Dental Pulp Cells" Journal of Functional Biomaterials 15, no. 4: 97. https://doi.org/10.3390/jfb15040097
APA StyleAnselmi, C., Mendes Soares, I. P., Mota, R. L. M., Leite, M. L., Ribeiro, R. A. d. O., Fernandes, L. d. O., Bottino, M. C., de Souza Costa, C. A., & Hebling, J. (2024). Functionalization of PCL-Based Fiber Scaffolds with Different Sources of Calcium and Phosphate and Odontogenic Potential on Human Dental Pulp Cells. Journal of Functional Biomaterials, 15(4), 97. https://doi.org/10.3390/jfb15040097






