Analysis of Gingival Fibroblasts Behaviour in the Presence of 3D-Printed versus Milled Methacrylate-Based Dental Resins—Do We Have a Winner?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Additive Manufacturing (3D Printing)
2.2. Subtracting Manufacturing
2.3. Specimens Finalization
2.4. Cell Culture
2.5. Cell Viability Assay
2.6. Griess Assay
2.7. Lactate Dehydrogenase (LDH) Assay
2.8. Fluorescence Staining Assays
2.9. MMP-2 Assay
2.10. Statistical Analysis
3. Results
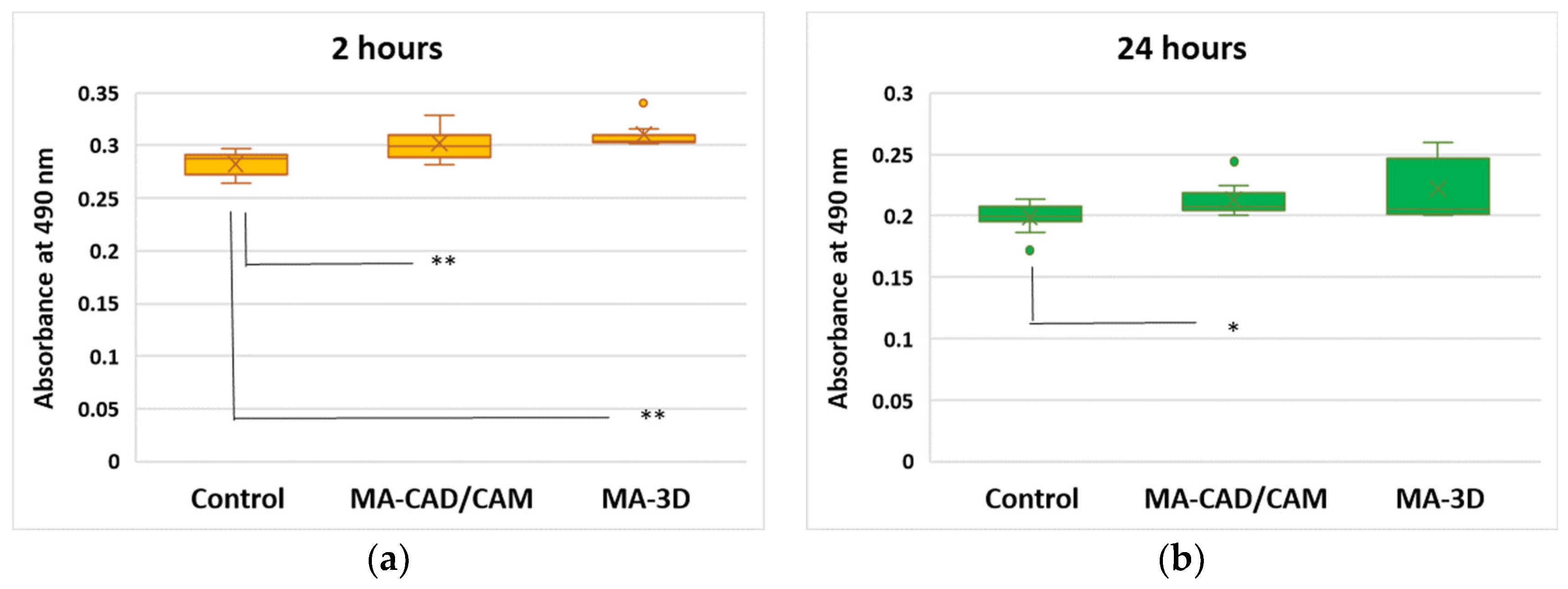
3.1. Cell Viability Analysis
3.2. Oxidative Stress, Apoptosis and Autophagy Evaluation
3.3. Analysis of MMP-2 Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Fouzan, A.F.; Al-Mejrad, L.A.; Albarrag, A.M. Adherence of Candida to complete denture surfaces in vitro: A comparison of conventional and CAD/CAM complete dentures. J. Adv. Prosthodont. 2017, 9, 402–408. [Google Scholar] [CrossRef]
- Al-Dwairi, Z.N.; Tahboub, K.Y.; Baba, N.Z.; Goodacre, C.J. A comparison of the flexural and impact strengths and flexural Modulus of CAD/CAM and conventional heat-cured polymethyl methacrylate (MA). J. Prosthodont. 2020, 29, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Hussain, B.; Thieu, M.K.L.; Johnsen, G.F.; Reseland, J.E.; Haugen, H.J. Can CAD/CAM resin blocks be considered as substitute for conventional resins? Dent. Mater. 2017, 33, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Vichi, A.; Balestra, D.; Scotti, N.; Louca, C.; Paolone, G. Translucency of CAD/CAM and 3D Printable Composite Materials for Permanent Dental Restorations. Polymers 2023, 15, 1443. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.; Li, N.; Cui, J.; Karlin, S.; Rohr, N.; Sharma, N.; Thieringer, F.M. Biomimetic, mussel-inspired surface modification of 3D-printed biodegradable polylactic acid scaffolds with nano-hydroxyapatite for bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 989729. [Google Scholar] [CrossRef] [PubMed]
- Modgill, V.; Balas, B.; Chi, M.; Honigmann, P.; Thieringer, F.M.; Sharma, N. Knowledge domain and innovation trends concerning medical 3D printing for craniomaxillofacial surgery applications: A 30-year bibliometric and visualized analysis. Craniomaxillofacial Res. Innov. 2023, 8, 1–14. [Google Scholar] [CrossRef]
- Steinmassl, P.A.; Wiedemair, V.; Huck, C.; Klaunzer, F.; Steinmassl, O.; Grunert, I.; Dumfahrt, H. Do CAD/CAM dentures really release less monomer than conventional dentures? Clinic. Oral Investig. 2017, 21, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Alifui-Segbaya, F.; Bowman, J.; White, A.R.; George, R.; Fidan, I. Characterization of the double bond conversion of acrylic resins for 3D printing of dental prostheses. Compend. Contin. Educ. Dent. 2019, 40, e7–e11. [Google Scholar] [PubMed]
- Wedekind, L.; Güth, J.F.; Schweiger, J.; Kollmuss, M.; Reichl, F.X.; Edelhoff, D.; Högg, C. Elution behavior of a 3D-printed, milled and conventional resin-based occlusal splint material. Dent. Mater. 2021, 37, 701–710. [Google Scholar] [CrossRef]
- Bürgers, R.; Schubert, A.; Müller, J.; Krohn, S.; Rödiger, M.; Leha, A.; Wassmann, T. Cytotoxicity of 3D-printed, milled, and conventional oral splint resins to L929 cells and human gingival fibroblasts. Clin. Exp. Dent. Res. 2022, 8, 650–657. [Google Scholar] [CrossRef]
- Ansteinsson, V.; Solhaug, A.; Samuelsen, J.T.; Holme, J.A.; Dahl, J.E. DNA-damage, cell-cycle arrest and apoptosis induced in BEAS-2B cells by 2-hydroxyethyl methacrylate (HEMA). Mutat. Res. 2011, 723, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Bartoloni, J.A.; Murchison, D.F.; Wofford, D.T.; Sarkar, N.K. Degree of conversion in denture base materials for varied polymerization techniques. J. Oral Rehabil. 2000, 27, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.L.; de Gee, A.J. Light-curing units, polymerization, and clinical implications. J. Adhes. Dent. 2000, 2, 167–173. [Google Scholar] [PubMed]
- Bural, C.; Aktaş, E.; Deniz, G.; Ünlüçerçi, Y.; Kızılcan, N.; Bayraktar, G. Effect of post-polymerization heat-treatments on degree of conversion, leaching residual MA and in vitro cytotoxicity of autopolymerizing acrylic repair resin. Dent. Mater. 2011, 27, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Nik, T.H.; Shahroudi, A.S.; Eraghihzadeh, Z.; Aghajani, F. Comparison of residual monomer loss from cold-cure orthodontic acrylic resins processed by different polymerization techniques. J. Orthod. 2014, 41, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.C.; Bains, M.E. The detection and estimation of residual monomer in polymethyl methacrylate. J. Dent. Res. 1956, 35, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Marigo, L.; Nocca, G.; Fiorenzano, G.; Callà, C.; Castagnola, R.; Cordaro, M.; Paolone, G.; Sauro, S. Influences of different air-inhibition coatings on monomer release, microhardness, and color stability of two composite materials. Biomed. Res. Int. 2019, 2019, 4240264. [Google Scholar] [CrossRef] [PubMed]
- Yourtee, D.M.; Smith, R.E.; Russo, K.A.; Burmaster, S.; Cannon, J.M.; Eick, J.D.; Kostoryz, E.L. The stability of methacrylate biomaterials when enzyme challenged: Kinetic and systematic evaluations. J. Biomed. Mater. Res. 2001, 57, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Willershausen, B.; Callaway, A.; Ernst, C.P.; Stender, E. The influence of oral bacteria on the surfaces of resin-based dental restorative materials—An in vitro study. Int. Dent. J. 1999, 49, 231–239. [Google Scholar] [CrossRef]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef]
- Stanislawski, L.; Soheili-Majd, E.; Perianin, A.; Goldberg, M. Dental restorative biomaterials induce glutathione depletion in cultured human gingival fibroblast: Protective effect of N-acetyl cysteine. J. Biomed. Mater. Res. 2000, 51, 469–474. [Google Scholar] [CrossRef]
- Samuelsen, J.T.; Dahl, J.E.; Karlsson, S.; Morisbak, E.; Becher, R. Apoptosis induced by the monomers HEMA and TEGDMA involves formation of ROS and differential activation of the MAP-kinases p38, JNK and ERK. Dent. Mater. 2007, 23, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Kuzet, S.E.; Gaggioli, C. Fibroblast activation in cancer: When seed fertilizes soil. Cell Tissue Res. 2016, 365, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Kowalczuk, M. Intrinsically biocompatible polymer systems. Polymers 2020, 12, 272. [Google Scholar] [CrossRef] [PubMed]
- Williams, D. Revisiting the definition of biocompatibility. Med. Device Technol. 2003, 14, 10–13. [Google Scholar] [PubMed]
- Ratner, B.D. A pore way to heal and regenerate: 21st century thinking on biocompatibility. Regen. Biomater. 2016, 3, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Kolahreez, D.; Ramakrishna, S.; Williams, D. Key terminology in biomaterials and biocompatibility. Curr. Opin. Biomed. Eng. 2019, 10, 45–50. [Google Scholar] [CrossRef]
- ISO 10993–5:2009; Biological Evaluation of Medical Splint—Part 5: Tests for In Vitro Cytotoxicity. International Standards Organization: Geneva, Switzerland, 2009.
- Javaid, M.; Haleem, A. Current status and applications of additive manufacturing in dentistry: A literature-based review. J. Oral Biol. Craniofac. Res. 2019, 9, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Poiana, I.R.; Dobre, R.; Popescu, R.I.; Pituru, S.M.; Bucur, A. Utility of Cone-Beam Computed Tomography in the Detection of Low Bone Mass-A Systematic Review. J. Clin. Med. 2023, 12, 5890. [Google Scholar] [CrossRef]
- Markovic, J.; Mora, N.J.; Broseta, A.M.; Gimeno, A.; de-la-Concepción, N.; Viña, J.; Pallardó, F.V. The depletion of nuclear glutathione impairs cell proliferation in 3t3 fibroblasts. PLoS ONE 2009, 4, e6413. [Google Scholar] [CrossRef]
- Ziegler, U.; Groscurth, P. Morphological features of cell death. News Physiol. Sci. 2004, 19, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Schweikl, H.; Spagnuolo, G.; Schmalz, G. Genetic and cellular toxicology of dental resin monomers. J. Dent. Res. 2006, 85, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Bakopoulou, A.; Papadopoulos, T.; Garefis, P. Molecular toxicology of substances released from resin-based dental restorative materials. Int. J. Mol. Sci. 2009, 10, 3861–3899. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Q.; Wogan, G.N. Nitric oxide as a modulator of apoptosis. Cancer Lett. 2005, 226, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Pan, Y.; Wang, M.; Wang, Y.; Lin, H.; Jiang, L.; Lin, D.; Cheng, H. Comparative analysis of leaching residual monomer and biological effects of four types of conventional and CAD/CAM dental polymers: An in vitro study. Clin. Oral Investig. 2022, 26, 2887–2898. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Mariño, G.; Levine, B. Autophagy and the integrated stress response. Mol. Cell 2010, 40, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Ornatowski, W.; Lu, Q.; Yegambaram, M.; Garcia, A.E.; Zemskov, E.A.; Maltepe, E.; Fineman, J.R.; Wang, T.; Black, S.M. Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biol. 2020, 36, 101679. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.M. Caspases: The executioners of apoptosis. Biochem. J. 1997, 326, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Taghizadeh, S.; Kaviani, E.; Vakili, O.; Taheri-Anganeh, M.; Tahamtan, M.; Savardashtaki, A. Caspase-3: Structure, function, and biotechnological aspects. Biotechnol. Appl. Biochem. 2022, 69, 1633–1645. [Google Scholar] [CrossRef]
- Teti, G.; Orsini, G.; Salvatore, V.; Focaroli, S.; Mazzotti, M.C.; Ruggeri, A.; Mattioli-Belmonte, M.; Falconi, M. HEMA but not TEGDMA induces autophagy in human gingival fibroblasts. Front. Physiol. 2015, 6, 275. [Google Scholar] [CrossRef]
- Galluzzi, L.; Pietrocola, F.; Levine, B.; Kroemer, G. Metabolic control of autophagy. Cell 2014, 159, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Yorimitsu, T.; Klionsky, D.J. Autophagy: Molecular machinery for self-eating. Cell Death Differ. 2005, 12, 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Hirata, N.; Tanaka, T.; Suizu, F.; Nakajima, H.; Chiorini, J.A. Autophagy as a modulator of cell death machinery. Cell Death Dis. 2020, 11, 517. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tan, J.; Miao, Y.; Lei, P.; Zhang, Q. The dual role of autophagy under hypoxia-involvement of interaction between autophagy and apoptosis. Apoptosis 2015, 20, 769–777. [Google Scholar] [CrossRef]
- Diomede, F.; Tripodi, D.; Trubiani, O.; Pizzicannella, J. HEMA effects on autophagy mechanism in human dental pulp stem cells. Materials 2019, 12, 2285. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef] [PubMed]
- Tokar, T.; Ulicny, J. The mathematical model of the Bcl-2 family mediated MOMP regulation can perform a non-trivial pattern recognition. PLoS ONE 2013, 8, e81861. [Google Scholar] [CrossRef] [PubMed]
- Stanislawski, L.; Lefeuvre, M.; Bourd, K.; Soheili-Majd, E.; Goldberg, M.; Périanin, A. TEGDMA-induced toxicity in human fibroblasts is associated with early and drastic glutathione depletion with subsequent production of oxygen reactive species. J. Biomed. Mater. Res. A. 2003, 66, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Guo, M.K.; Kasten, F.H.; Chang, M.C.; Huang, G.F.; Wang, Y.L.; Wang, R.S.; Jeng, J.H. Stimulation of glutathione depletion, ROS production and cell cycle arrest of dental pulp cells and gingival epithelial cells by HEMA. Biomaterials 2005, 26, 745–753. [Google Scholar] [CrossRef]
- Hanks, C.T.; Strawn, S.E.; Wataha, J.C.; Craig, R.G. Cytotoxic effects of resin components on cultured mammalian fibroblasts. J. Dent. Res. 1991, 70, 1450–1455. [Google Scholar] [CrossRef]
- Yoshii, E. Cytotoxic effects of acrylates and methacrylates: Relationships of monomer structures and cytotoxicity. J. Biomed. Mater. Res. 1997, 37, 517–524. [Google Scholar] [CrossRef]
- Spagnuolo, G.; Galler, K.; Schmalz, G.; Cosentino, C.; Rengo, S.; Schweikl, H. Inhibition of phosphatidylinositol 3-kinase amplifies TEGDMA-induced apoptosis in primary human pulp cells. J. Dent. Res. 2004, 83, 703–707. [Google Scholar] [CrossRef]
- Spagnuolo, G.; Mauro, C.; Leonardi, A.; Santillo, M.; Paterno, R.; Schweikl, H.; Avvedimento, E.V.; Rengo, S. NF-kappaB protection against apoptosis induced by HEMA. J. Dent. Res. 2004, 83, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Ansteinsson, V.; Kopperud, H.B.; Morisbak, E.; Samuelsen, J.T. Cell toxicity of methacrylate monomers-the role of glutathione adduct formation. J. Biomed. Mater. Res. A. 2013, 101, 3504–3510. [Google Scholar] [CrossRef] [PubMed]
- Nicolae, I.; Tampa, M.; Mitran, C.; Ene, C.D.; Mitran, M.; Matei, C.; Muşetescu, A.; Pițuru, S.; Pop, C.S.; Georgescu, S.R. Gamma-glutamyl transpeptidase alteration as a biomarker of oxidative stress in patients with human papillomavirus lesions following topical treatment with sinecatechins. Farmacia 2017, 65, 4. [Google Scholar]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, Y.; Wang, C.; Liu, Y. Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death Dis. 2018, 9, 753. [Google Scholar] [CrossRef] [PubMed]
- Mancilla, H.; Maldonado, R.; Cereceda, K.; Villarroel-Espíndola, F.; Montes de Oca, M.; Angulo, C.; Castro, M.A.; Slebe, J.C.; Vera, J.C.; Lavandero, S.; et al. Glutathione depletion induces spermatogonial cell autophagy. J. Cell. Biochem. 2015, 116, 2283–2292. [Google Scholar] [CrossRef]
- Chang, P.C.; Lang, N.P.; Giannobile, W.V. Evaluation of functional dynamics during osseointegration and regeneration associated with oral implants. Clin. Oral Implants Res. 2010, 21, 1–12. [Google Scholar] [CrossRef]
- Sapna, G.; Gokul, S.; Bagri-Manjrekar, K. Matrix metalloproteinases and periodontal diseases. Oral Dis. 2014, 20, 538–550. [Google Scholar] [CrossRef]
- Fernandez-Patron, C.; Zouki, C.; Whittal, R.; Chan, J.S.; Davidge, S.T.; Filep, J.G. Matrix metalloproteinases regulate neutrophil-endothelial cell adhesion through generation of endothelin-1[1-32]. FASEB J. 2001, 15, 2230–2240. [Google Scholar] [CrossRef] [PubMed]
- Lundahl, J.; Sköld, C.M.; Halldén, G.; Hallgren, M.; Eklund, A. Monocyte and neutrophil adhesion to matrix proteins is selectively enhanced in the presence of inflammatory mediators. Scand. J. Immunol. 1996, 44, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Renò, F.; Traina, V.; Cannas, M. Adsorption of matrix metalloproteinases onto biomedical polymers: A new aspect in biological acceptance. J. Biomater. Sci. Polym. Ed. 2008, 19, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of biomaterial-cell interactions by adsorbed proteins: A review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]






| Tested Sample | Characteristics | Manufacturer | Lot Number |
|---|---|---|---|
| MA-based 3D-printed | Very tough, non-irritant, odourless resin. Colour A1–A2 according to Vita scale. | Dental Sand A1–A2 by HARZ Labs LLC, (Moscow, Russian Federation) Official Dealer: 3D Printing Zone SRL, (Bucharest, Romania) | Dental A1–A2 Lot 107 production date: february 2020 |
| MA-based CAD/CAM | Monolayer, shade B1 according to Vita scale. Ultimate flexural strengh ≧ 65 MPa, water absorbtion ≦ 32 μg/mm3. | HUGE PMMA BLOCK for CAD/CAM, MedNet EC-REP GmbH (Muenster, Germany) | LOT 200220010, Expiration date: 19 February 2025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saramet, V.; Stan, M.S.; Ripszky Totan, A.; Țâncu, A.M.C.; Voicu-Balasea, B.; Enasescu, D.S.; Rus-Hrincu, F.; Imre, M. Analysis of Gingival Fibroblasts Behaviour in the Presence of 3D-Printed versus Milled Methacrylate-Based Dental Resins—Do We Have a Winner? J. Funct. Biomater. 2024, 15, 147. https://doi.org/10.3390/jfb15060147
Saramet V, Stan MS, Ripszky Totan A, Țâncu AMC, Voicu-Balasea B, Enasescu DS, Rus-Hrincu F, Imre M. Analysis of Gingival Fibroblasts Behaviour in the Presence of 3D-Printed versus Milled Methacrylate-Based Dental Resins—Do We Have a Winner? Journal of Functional Biomaterials. 2024; 15(6):147. https://doi.org/10.3390/jfb15060147
Chicago/Turabian StyleSaramet, Veaceslav, Miruna S. Stan, Alexandra Ripszky Totan, Ana Maria Cristina Țâncu, Bianca Voicu-Balasea, Dan Sebastian Enasescu, Florentina Rus-Hrincu, and Marina Imre. 2024. "Analysis of Gingival Fibroblasts Behaviour in the Presence of 3D-Printed versus Milled Methacrylate-Based Dental Resins—Do We Have a Winner?" Journal of Functional Biomaterials 15, no. 6: 147. https://doi.org/10.3390/jfb15060147





