Abstract
Endogenous peptides, particularly those with post-translational modifications, are increasingly being studied as biomarkers for diagnosing various diseases. However, they are weakly ionizable, have a low abundance in biological samples, and may be interfered with by high levels of proteins, peptides, and other macromolecular impurities, resulting in a high limit of detection and insufficient amounts of post-translationally modified peptides in real biological samples to be examined. Therefore, separation and enrichment are necessary before analyzing these biomarkers using mass spectrometry. Mesoporous materials have regular adjustable pores that can eliminate large proteins and impurities, and their large specific surface area can bind more target peptides, but this may result in the partial loss or destruction of target peptides during centrifugal separation. On the other hand, magnetic mesoporous materials can be used to separate the target using an external magnetic field, which improves the separation efficiency and yield. Core–shell magnetic mesoporous materials are widely utilized for peptide separation and enrichment due to their biocompatibility, efficient enrichment capability, and excellent recoverability. This paper provides a review of the latest progress in core–shell magnetic mesoporous materials for enriching glycopeptides and phosphopeptides and compares their enrichment performance with different types of functionalization methods.
1. Introduction
Peptides are biological macromolecules formed by the dehydration condensation of amino acids. They serve as the basic unit of protein composition and play a crucial role in regulating various physiological processes in organisms [1]. Additionally, peptides are involved in mineral binding [2,3], antibacterial activity [4], antihypertensive effects [5,6], antithrombotic activity [7], and antioxidant activity [8,9]. For instance, angiotensin II is an 8-peptide that increases blood pressure by narrowing small arteries throughout the body. Inhibiting angiotensin, however, can reduce blood pressure [5]. Post-translationally modified peptides and proteins, such as phosphorylated or glycosylated [10] peptides and proteins, play important roles in intercellular signaling [11] and neuromodulation [12]. However, abnormal post-translationally modified peptides and proteins may cause diseases such as Alzheimer’s disease [13], cancer [14], and cardiovascular disease [15]. Endogenous post-translationally modified peptides and proteins have been shown to be potential biomarkers of human physiology and pathology. Therefore, in-depth studies of these peptides can help understand the pathology of human disease and provide reliable information for disease diagnosis.
In recent years, the development of fast-scanning high-resolution mass spectrometry (HRMS) has provided a more reliable, efficient, and rapid method for analyzing small-molecule peptides [16]. This technology has become increasingly popular due to its ability to accurately identify and quantify compounds in complex samples. However, the concentration of endogenous peptides in real biological samples is extremely low, making them difficult to ionize. Additionally, they are often susceptible to interference from high-abundance macromolecular proteins, buffer salts, and surface-active substances introduced by pretreatment [17]. Furthermore, post-translationally modified endogenous peptides are dynamic in nature [18,19], which greatly limits their analysis by means of mass spectrometry. Therefore, it is necessary to selectively enrich low-abundance peptides and post-translationally modified peptides before mass spectrometry analysis [20].
General methods for separating and enriching post-translationally modified peptides include membrane separation, organic solvent precipitation, molecular imprinting and magnetic solid-phase extraction (MSPE). The membrane separation method is based on the selective transport of membranes to achieve the separation and enrichment of different components [21]. Meanwhile, the organic solvent precipitation method utilizes organic solvents to reduce the dielectric constant of water, resulting in a decrease in hydrophobic interactions between macromolecular proteins and an increase in electrostatic interactions, leading to aggregation and precipitation; the post-translationally modified peptides are then dissolved in organic solvent [22]. Molecular imprinting involves pre-polymerization of a template molecule with a functional monomer using a cross-linking agent. Removal of the template molecule results in a molecularly imprinted polymer that can specifically recognize and selectively bind to a target molecule [23]. The MSPE separates the target from interfering substances by exploiting the difference in force strengths between them on the extractant [24]. Membrane separation has the limitations of easy clogging and unstable recovery. The molecular weight range of target peptides obtained by organic solvent precipitation is unclear, and some target peptides may be co-precipitated with proteins. Molecular imprinting, on the other hand, has the advantage of high specificity, but it is not suitable for global analysis of endogenous peptides. MSPE has become the most commonly applied method for enriching endogenous peptides due to its simple operation, high extraction efficiency, low organic solvent consumption, and easy surface functionalization [24]. This method allows for the efficient separation of the magnetic adsorbent and the target peptide from the sample solution using an external magnetic field. The target peptide can then be obtained through elution, thus avoiding loss and denaturation caused by centrifugation. Some of the target peptides may co-precipitate with proteins, which represents a significant cause of poor recovery during centrifugation. One study demonstrated that peptide recoveries obtained by ultrafiltration and organic solvent precipitation were approximately half of those obtained by solid-phase extraction [25]. Magnetic materials such as iron (Fe), cobalt (Co), nickel (Ni), and their alloys are known to be biotoxic. Magnetite (Fe3O4) has good biocompatibility [26] and superb dispersibility in aqueous solutions and can reach adsorption equilibrium quickly. The magnetic properties of Fe3O4 are generated by the spin magnetic moments of Fe2+ and Fe3+ ions. The reduction in the Fe3O4 particle size from bulk to nanoscale reduces the number of exchange-coupled spins that spontaneously resist magnetic reorientation, a phenomenon that leads to superparamagnetic behavior. Furthermore, an increase in temperature also leads to superparamagnetic behavior [27]. It should be noted that under extreme conditions, Fe3O4 magnetic nanomaterials (Fe3O4 MNPs) may be less stable [28], which limits the application of this advanced material. A composite material with a magnetic core and a mesoporous shell exhibits both an improved stability of Fe3O4 MNPs and strong magnetic properties. Mesoporous materials are a type of porous materials with pore sizes ranging from 2 to 50 nm. They can effectively filter out large proteins and other bulky impurities in samples [29]. These materials have several advantages, including a large specific surface area, adjustable pore sizes, modifiable inner walls, and good stability. As a result, they are highly suitable for immobilizing and separating enzymes, proteins, and small-molecule peptides [30]. Fe3O4 belongs to spinel nanomaterials, which exhibit, among other characteristics, engineering defects that can be useful for tuning these kinds of materials for functionalization [31]. Fe3O4 MNPs have numerous hydroxyl functional groups on their surface [32], which are easily modifiable and covalently connected to the mesoporous shell. This shell not only provides mechanical strength but also enhances the stability of the Fe3O4 MNPs wrapped in it, making them highly promising for the enrichment of low-abundance peptides [33,34,35].
Mesoporous silica is a material with highly ordered pores, an easy functionalized surface, and good biocompatibility. Covalent organic frameworks (COFs) are porous crystalline materials consisting of organic monomers connected by covalent bonds with two- or three-dimensional structures. They have a low density, a controllable pore size, good stability, easy surface modification, and a high surface area. COFs have broad potential for adsorbing and enriching low-abundance post-translationally modified peptides [36]. Metal–organic frameworks (MOFs) are porous materials made up of metal ions/clusters and organic ligands. They have similar surface properties to COFs. However, MOFs have a large number of unsaturated metal sites, making them excellent adsorbent materials for enriching post-translationally modified peptides, especially phosphopeptides, in biological samples [37]. This paper presents a review of recent advances in the synthesis and modification strategies of core–shell magnetic mesoporous composites for enriching post-translationally modified peptides over the past five years. The composites discussed include magnetic mesoporous silica, magnetic COFs, and magnetic MOFs.
2. Physical and Chemical Properties of Core–Shell Magnetic Mesoporous Materials and Characterization Techniques
2.1. Physical Properties and Characterization Techniques of Core–Shell Magnetic Mesoporous Materials
The shape and size of core–shell magnetic mesoporous materials are typically quantified via transmission electron microscopy or swept electron microscopy. Iron oxide nanoparticles are categorized as ultra-small superparamagnetic iron oxides, superparamagnetic iron oxides, or micron-sized iron oxides according to their particle size, which is less than 50 nm, approximately 50–150 nm, or approximately 1 μm, respectively [38]. The magnetic properties of core–shell magnetic mesoporous materials are typically slightly inferior to those of Fe3O4MNPs. This is due to the fact that the outer layer of Fe3O4MNPs is encapsulated by a mesoporous shell, and their saturation magnetic values are generally determined using a vibrating sample magnetometer. The pore size of the mesoporous shell determines the molecular weight range of the adsorbed peptide, while the surface area and pore volume of the magnetic mesoporous material determine its ability to load peptides. The pore size, pore volume, and surface area of the magnetic mesoporous materials are determined by N2 adsorption–desorption isotherms [39].
2.2. Chemical Properties and Characterization Techniques of Core–Shell Magnetic Mesoporous Materials
In order to efficiently enrich post-translationally modified peptides, the surface of magnetic mesoporous materials is modified with compounds that can specifically bind to the post-translationally modified peptides. Infrared spectroscopy and Raman spectroscopy are based on spectral signals radiated by vibrations or rotations of chemical bonds and lattices, which can be used to determine the composition of the magnetic mesoporous material. These techniques are employed to ascertain whether the mesoporous shells and modified compounds are successfully bound to the magnetic cores [40]. X-ray photoelectron spectroscopy (XPS) can provide the elemental composition of the magnetic mesoporous material, thereby offering confirmatory information about the successful modification of the magnetic material [41]. X-ray diffraction (XRD) is employed to analyze the diffraction spectra of X-ray stimulated materials. Typically, a wide-angle XRD pattern indicates the presence of compound magnetic solids. A low-angle XRD pattern shows the pore structure of porous magnetic materials [42]. Thermogravimetric analysis is mainly used to determine the composition of the material and to predict its thermal stability. Nevertheless, when employing magnetic mesoporous materials to enrich endogenous peptides, which are frequently dispersed in solution, the zeta potential represents a means of characterizing the stability of the magnetic mesoporous material in solution. Zeta potential is the potential of colloidal particles moving under an electric field in the sliding/shear plane, and the greater the absolute value of the zeta potential, the more stable the system [43].
3. Progress in Magnetic Mesoporous Materials for Glycopeptide Enrichment—Strategies Based on Modification of Hydrophilic Compounds
Glycosylation is the process of attaching sugars to proteins through glycosyltransferases. It is the most common post-translational modification in the human body, accounting for about 50% of protein modifications [44]. The two main modes of glycosylation are O-linked and N-linked glycosylation. O-linked glycosylation mainly occurs on the hydroxyl groups of serine, threonine, hydroxylysine, and hydroxyproline. N-linked glycosylation mainly occurs on the amide group of aspartic acid, the α-amino group of N-terminal amino acids, and the ω-amino group of lysine or arginine. Glycosylation is a complex process that adds structural complexity to proteins. Identifying glycosylated proteins or peptides is a significant challenge. This subsection summarizes the advances and processes in the strategy of selective enrichment of glycopeptides based on functionalized magnetic mesoporous materials.
3.1. Magnetic Mesoporous Silica Materials for Glycopeptide Enrichment
Mesoporous silica is commonly used in composites coated with magnetic nanoparticles due to its high specific surface area, uniform and adjustable pores, easy surface functionalization, and good stability. Based on its size exclusion effect and good magnetism, the step of enriching low-abundance peptides and low-molecular-weight proteins from complex biological matrices becomes simple, fast, and efficient.
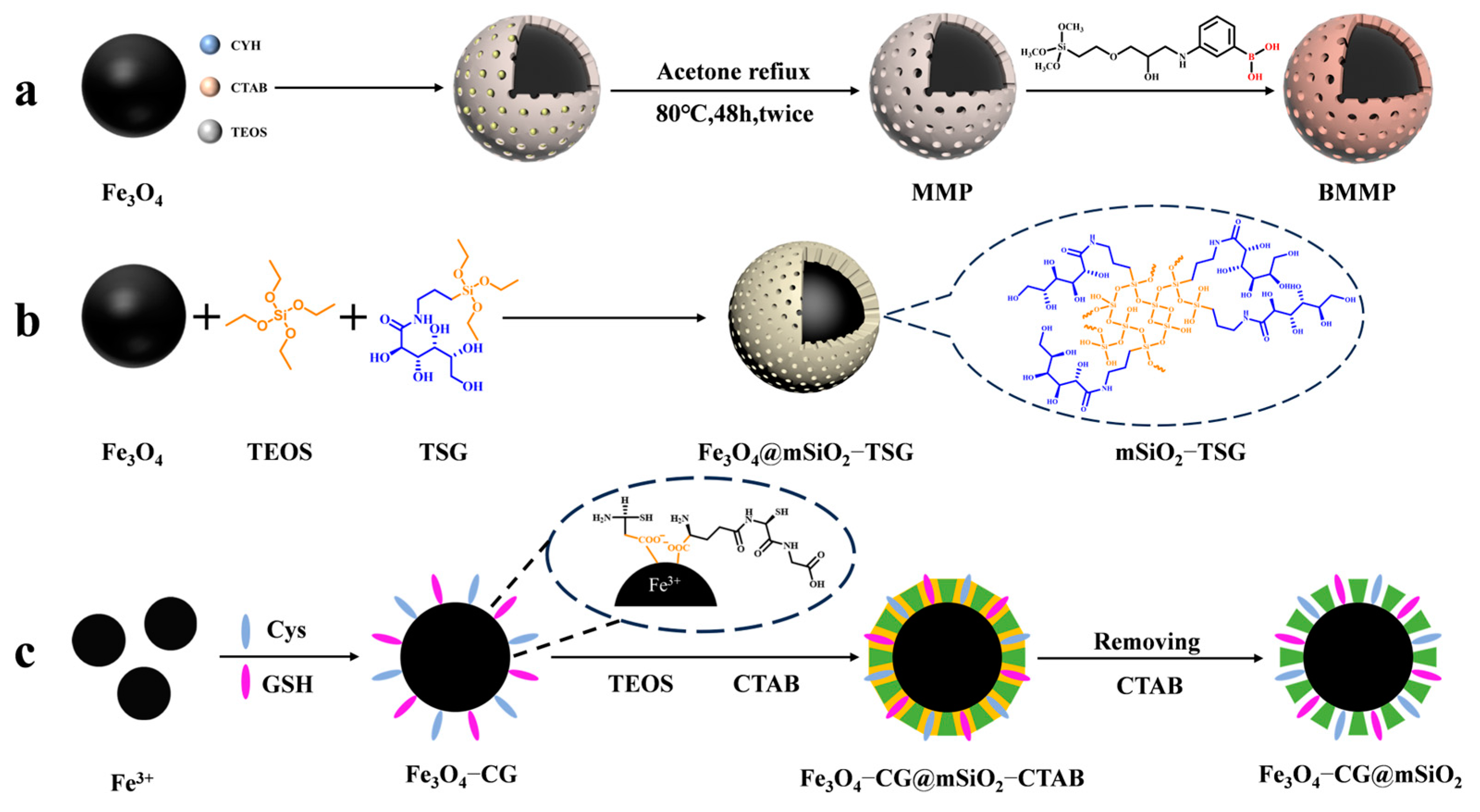
In recent years, the enrichment and identification of peptides in organisms have shifted towards glycosylated peptides; as one of the most important post-translational modifications, glycopeptides carry rich biological information. A large number of studies have shown that glycopeptides carry specific diagnostic information for certain diseases, especially cancers. Thus, the study of glycopeptides can improve the methods for diagnosing diseases in humans. However, glycopeptides have a large spatial structure, and the small pore size (2~4 nm) of mesoporous silica in general does not allow some larger glycopeptides to enter. Yang et al. [45] designed a magnetic mesoporous silica material modified with boric acid (shown in Figure 1a). They expanded the pore size of the mesoporous layer to 10 nm in the presence of cyclohexane and functionalized the surface with boric acid to enhance the material’s hydrophilicity. The material achieved multimodal global enrichment of glycopeptides by utilizing the reversible binding of boric acid to cis-diols. Under alkaline conditions, boric acid can form a stable cyclic ester with cis-diols. This ester hydrolyzes in acidic environments, allowing for glycopeptide-specific binding and elution. Boric acid chemistry can yield intact glycopeptides, without altering their structures, but the different affinities between boric acid and different glycopeptide chains limit the generalizability of the method.
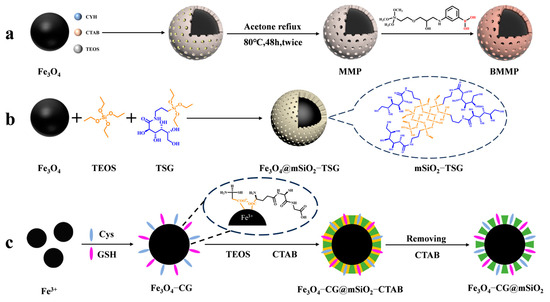
Figure 1.
Magnetic mesoporous silica materials for enrichment of glycopeptides, the synthesis steps of BMMP, Fe3O4@mSiO2−TSG, and Fe3O4@mSiO2−TSG are represented by processes (a), (b), and (c), respectively.
In contrast, hydrophilic interaction chromatography (HILIC) has gained increasing attention due to its excellent enrichment ability and mild enrichment conditions. Glycopeptides possess strong hydrophilicity due to the polyhydroxyl structure of the glycosyl part, while non-glycopeptides are less hydrophilic. Based on these differences, HILIC can obtain the intact glycopeptide structure and enrich all types of glycopeptides without bias. Chen et al. [46] reported core–shell magnetic mesoporous silica microspheres modified by L-cysteine, denoted as L-Cys-Fe3O4@mSiO2. A large amount of L-cysteine was modified within the vertically aligned apertures utilizing Fe-S bonding. This modification improved the hydrophilicity of the material, enabling it to have a strong recognition and adsorption ability. While the hydrophilicity of the material can enhance the efficiency of glycopeptide enrichment, the process of functionalizing the modification on the surface of core–shell magnetic nanomaterials is typically cumbersome. Xu et al. [47] reported a one-step synthesis of strongly hydrophilic magnetic mesoporous silica nanomaterials modified with N-(3-triethoxysilylpropyl) lucosamido-amido (TSG), denoted as Fe3O4@mSiO2-TSG (shown in Figure 1b and Figure 2(1a–1c)). The sol–gel method was used to carry out this procedure, with tetraethyl orthosilicate (TEOS) and TSG serving as the silica source and CTAB as the surfactant templating agent. The TSG-functionalized silica layer was then deposited on Fe3O4 spheres, and then the CTAB was removed through heating and refluxing to obtain Fe3O4@mSiO2-TSG. The synthesis steps were greatly simplified by this method. The detection limit for the selective enrichment of glycopeptides in the HRP digests was 0.1 f·mol·μL−1, and the maximum adsorption capacity was 160 mg/g. The material can be reused up to eight times. Even when the molar ratio of glycoprotein ovalbumin to HRP digest was 1:5000, the mass spectra after enrichment were still dominated by glycopeptide peaks and protein peaks were not detected, indicating good selectivity. After enriching with Fe3O4@mSiO2-TSG, we identified 162 glycopeptides corresponding to 71 glycoproteins in healthy human serum. In serum from breast cancer patients, we identified 156 glycopeptides corresponding to 70 glycoproteins.
While the selectivity of HILIC is interfered with by hydrophilic peptides that do not contain glycans, leading to a decrease in selectivity for glycopeptides, HILIC is being developed in the direction of superhydrophilic materials with stronger hydrophilicity to improve its selection of glycopeptides. Yi et al. [48] designed a superhydrophilic magnetic mesoporous silica microspheres modified with reduced glutathione (GSH) and L−cysteine (Cys), named Fe3O4−CG@mSiO2 (shown in Figure 1c). Superhydrophilic Fe3O4−CG nanospheres were first synthesized by Fe-S interactions, and then a mesoporous silica shell was modified on the surface of Fe3O4−CG via the surfactant template method, with CTAB as a structure-directing agent to give it the property of specifically adsorbing glycopeptides by excluding large proteins. Fe3O4−CG@mSiO2 nanospheres were enriched with a high sensitivity and selectivity and could be reused ten times with an average recovery of 108.6 ± 5.5%. For the selective enrichment of glycopeptides in HRP digests, the detection limit was 5 a·mol·μL−1. BSA protein was used as an interfering agent to verify the selectivity of Fe3O4−CG@mSiO2 nanospheres, and when the molar ratio of BSA protein to HRP digest reached 1:10,000, Fe3O4-CG@mSiO2 nanospheres could still enrich seven glycopeptides. In addition, 156 glycopeptides from 64 human serum exosomal proteins were detected using Fe3O4−CG@mSiO2.
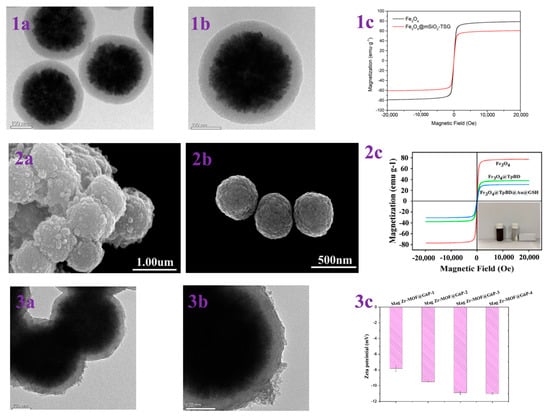
Figure 2.
(1a,1b) TEM images of Fe3O4@mSiO2-TSG; (1c) magnetic hysteresis curves of Fe3O4 and Fe3O4@mSiO2-TSG. Reprinted with permission from Ref. [47]. Copyright 2024 Elsevier. (2a,2b) SEM of Fe3O4@TpBD@Au@GSH and Fe3O4; (2c) VSM analysis of Fe3O4, Fe3O4@TpBD and Fe3O4@TpBD@Au@GSH. Reprinted with permission from Ref. [49]. Copyright 2024 Elsevier. (3a,3b) TEM images of Mag Zr-MOF@G6P; (3c) zeta potentials of Mag Zr-MOF@G6P−1/2/3/4. Reprinted with permission from Ref. [50]. Copyright 2024 Springer Nature.
Figure 2.
(1a,1b) TEM images of Fe3O4@mSiO2-TSG; (1c) magnetic hysteresis curves of Fe3O4 and Fe3O4@mSiO2-TSG. Reprinted with permission from Ref. [47]. Copyright 2024 Elsevier. (2a,2b) SEM of Fe3O4@TpBD@Au@GSH and Fe3O4; (2c) VSM analysis of Fe3O4, Fe3O4@TpBD and Fe3O4@TpBD@Au@GSH. Reprinted with permission from Ref. [49]. Copyright 2024 Elsevier. (3a,3b) TEM images of Mag Zr-MOF@G6P; (3c) zeta potentials of Mag Zr-MOF@G6P−1/2/3/4. Reprinted with permission from Ref. [50]. Copyright 2024 Springer Nature.
Functionalized magnetic mesoporous silica materials exhibit a high selectivity and adsorption capacity during the enrichment step before mass spectrometry characterization of glycopeptides. When modifying magnetic mesoporous materials, it is important to consider not only the hydrophilicity of the material, which is central to the HILIC strategy, but also the complexity of the material synthesis method and its cost.
3.2. Magnetic COF Materials for Glycopeptide Enrichment
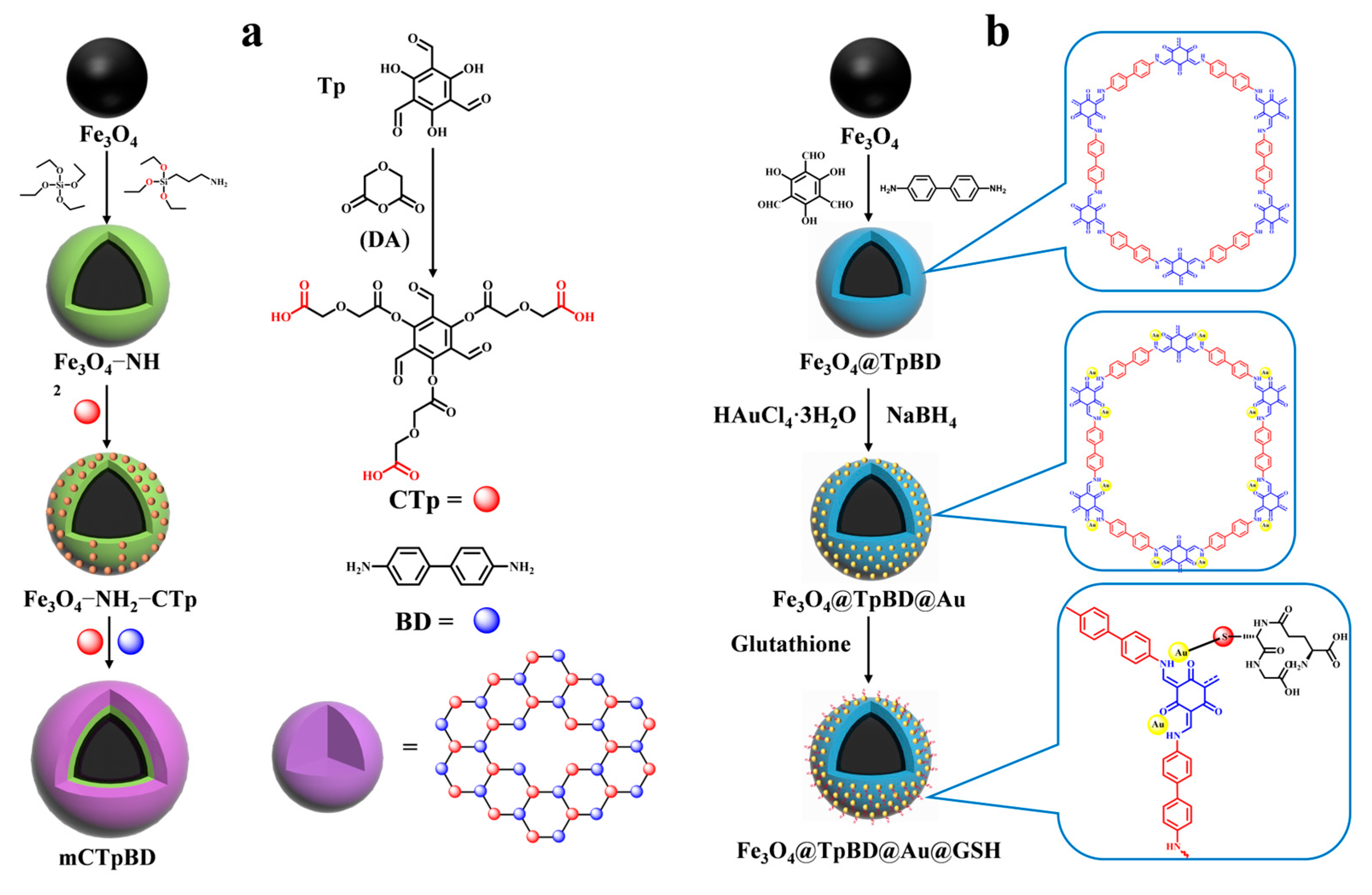
Various modified covalent organic frameworks (COFs) [51] have been reported for peptide enrichment since the first successful synthesis of COFs. The following methods are employed in the preparation of magnetic COFs: the Schiff base condensation method, the template-controlled precipitation polymerization method, the co-precipitation method, the microwave-enhanced high-temperature ionization thermophoresis method, and the solvent thermophoresis method. Among the aforementioned methods, the template-controlled precipitation polymerization method employs carboxyl groups to uniformly disperse the magnetic nanoparticles within the synthesis system, thereby ensuring the formation of imide-based COFs on the surface of the magnetic nanoparticles. This method enables the synthesis of magnetic COFs with core–shell structures under mild conditions, obviating the necessity for cumbersome reaction steps. Furthermore, it represents a more economical and straightforward approach to the synthesis of magnetic COFs [52]. However, general COFs with a low hydrophilicity are less effective for enriching glycosylated peptides with a high hydrophilicity. Therefore, it is necessary to enhance the hydrophilicity of COFs to improve their enrichment efficiency of glycosylated peptides. There are two ways to achieve this. The first is modifying the functional groups with hydrophilic properties on the monomers of the synthesized COFs, as demonstrated by Wu et al. [53]. COF shells were synthesized using carboxyl-modified 1,3,5-tricarboxy-mesityltriphenol (CTp) and p-diaminobiphenyl as the monomers on amine-amidated Fe3O4 (as shown in Figure 3a). This resulted in the successful enrichment of glycopeptides from the saliva of both healthy individuals and patients with inflammatory bowel disease in magnetic COF hydrophilic composites (mCTpBD). The detection limit of mCTpBD for glycopeptides was as low as 0.5 f·mol·μL−1 (HRP standard protein digest) based on the modification of carboxyl groups. Additionally, 28, 32, and 49 glycopeptides were detected in three healthy human saliva samples, while 27, 39, and 40 glycopeptides were detected in three patient saliva samples. The particle size exclusion effect was 1/1000 (HRP digestion product/BSA protein, w/w).
Figure 3.
Magnetic covalent organic framework materials for enrichment of glycopeptides, the synthesis steps of mCpBD and Fe3O4@TpBD@Au@GSH are represented by processes (a) and (b), respectively.
While modifying hydrophilic functional groups on monomers may lead to the reaction of the introduced groups with other functional groups, causing a change in the structure of the covalent organic framework, an alternative method to improve the hydrophilicity of COFs is to modify the hydrophilic groups after synthesis. This approach avoids the aforementioned scenario. Su et al. [49] synthesized glutathione-modified dual hydrophilic magnetic COF composites (Fe3O4@TpBD@Au@GSH) using a two-step post-synthesis modification method with 2,4,6-trihydroxybenzene-1,3,5- tricarbaldehyde (Tp) and benzidine (BD) as monomers to form the COF layer (shown in Figure 2(2a–2c) and Figure 3b). Glutathione was then grafted onto the surface of TpBD using Au-S bonds. Due to the hydrophilicity of TpBD and the superhydrophilicity of glutathione, Fe3O4@TpBD@Au@GSH exhibited a low sensitivity of 0.1 f·mol·μL−1 for standard glycopeptides (HRP tryptophan digest), a maximum loading of 160 mg/g for glycopeptides, good immunity to interference from large proteins (1:2000, HRP tryptophan digest/bovine serum albumin, M/M), and an excellent selectivity (1:2000, HRP tryptic digest/bovine serum albumin tryptic digest, M/M). A total of 492 glycopeptides and 160 glycopeptides were detected in 5 μL of human serum and saliva, corresponding to 134 and 64 glycoproteins, respectively, which demonstrated an excellent performance in the enrichment of endogenous glycopeptides. However, the post-synthetic modification steps are relatively cumbersome and costly. Therefore, there is a need to develop simpler and more cost-effective synthetic methods for wider application.
3.3. Magnetic MOF Materials for Glycopeptide Enrichment
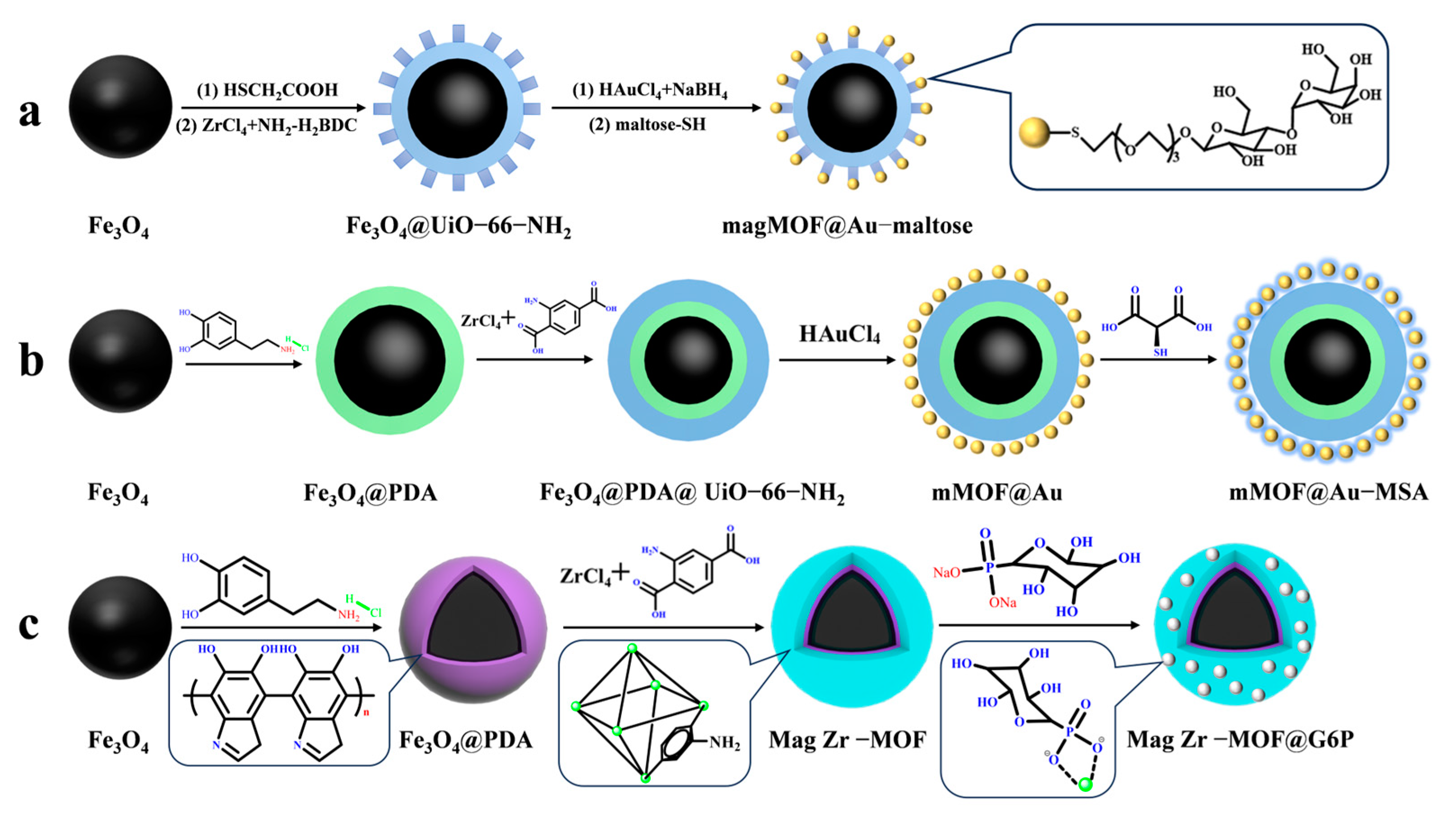
Magnetic MOF composites modified with hydrophilic molecules are widely used for enriching low-abundance glycopeptides due to their efficient enrichment performance, non-destructive separation, and excellent reproducibility. This makes them a suitable choice for glycopeptide enrichment. UiO-66-NH2 has been shown to be stable in humid, high-temperature, and acidic chemical environments, while also being biocompatible. A study by Lu et al. [54] supports these findings. Mag MOF@Au–maltose (shown in Figure 4a) was used to immobilize hydrophilic maltose onto magnetic MOF composites, with the Au-S bond serving as the connecting bond. This composite demonstrated an excellent glycopeptide enrichment performance, with a low detection limit of 10 f·mol, a high recovery rate of over 83.3%, and a large binding capacity of 83 μg/mg. In 1 μL of human serum, 113 glycopeptides with 123 unique N-glycosylation sites were detected, corresponding to 46 different glycoproteins.
Figure 4.
Magnetic metal–organic framework materials for enrichment of glycopeptides, the synthesis steps of magMOF@Au−maltose, mMOF@Au−MSA and Mag Zr−MOF@G6P are represented by processes (a), (b) and (c), respectively.
Similarly, Hu et al. [55] used highly hydrophilic mercaptosuccinic acid (MSA) attached to the surface of magnetic UiO-66-NH2 modified with gold nanoparticles using a Au-S bond as a bridge to obtain mMOF@Au-MSA (shown in Figure 4b). The MOF’s large surface area provided a large number of binding sites for mercaptosuccinic acid, resulting in an enhanced detection limit of glycopeptides of 0.5 f·mol·μL−1 (HRP digest) and an increased enrichment capacity of 100 mg/g. In 2 μL of breast serum from breast cancer patients, 307 unique glycopeptides from 96 glycoproteins were detected. This may be due to the smaller spatial site resistance of mercaptosuccinic acid compared to maltose, which makes it more favorable for glycopeptides to enter the pores of MOFs. While noble metal nanoparticles have a tendency to aggregate, leading to clustering of functional groups and higher costs, which makes them impractical for many applications, this group [50] designed a hydrophilic glucose-6-phosphate (G6P)-modified magnetic UiO-66-NH2 material. Mag Zr-MOF@G6P was used to analyze N-glycopeptides in the urine of healthy people and kidney cancer patients by utilizing the coordination between Zr4+ and phosphate groups present in UiO-66-NH2 as a bridge. The structure of Mag Zr-MOF@G6P is shown in Figure 2(3a–3c) and Figure 4c. A total of 123 N-glycopeptides with 104 glycosylation sites and 111 N-glycopeptides with 97 glycosylation sites, corresponding to 78 and 76 glycoproteins, respectively, were detected from the urine of renal cancer patients and healthy subjects. These findings suggest that the detected glycoproteins are significantly associated with cancer bioprocesses. Table 1 presents the properties of the core-shell magnetic mesoporous materials discussed in the text, which are employed for the enrichment of glycopeptides.

Table 1.
A representative selection of magnetic mesoporous materials that have been employed in the enrichment of glycopeptides over the past five years.
Overall, the boronic-acid-method-based affinity strategy can obtain intact glycopeptides without destroying their structure. This is undoubtedly useful for exploiting the bioinformation carried by glycopeptides. However, its different affinities for different glycan chains limit its generalizability. Hydrophilic interaction chromatography (HILIC) enables the complete characterization of glycopeptide structures and provides highly specific enrichment of glycopeptides based on their hydrophilicity. Nevertheless, the HILIC strategy is not without its limitations, particularly in terms of achieving highly selective enrichment. For instance, some HILIC materials are capable of enriching neutral or positively charged glycopeptides, but are less effective at enriching negatively charged glycopeptides [56]. Additionally, the introduction of positively charged metal ions can enhance the binding ability of HILIC materials to negatively charged glycopeptides. However, it is important to note that HILIC materials may also bind some hydrophilic non-glycopeptides and other hydrophilic substances. HILIC can be combined with other strategies, such as lectins and IMAC, with the aim of increasing the density of hydrophilic groups on the immobilized material and thereby improving the detection sensitivity and loading capacity. Furthermore, the functional groups and metal ions present on the surface of COFs and MOFs provide numerous binding sites for hydrophilic molecules. Secondly, the structural diversity of COFs and MOFs allows for the exploration of additional functions, and self-assembled COFs and MOFs can be specifically modified for glycopeptides with varying properties. This characteristic allows for a wider range of applications in enriching glycopeptides, potentially leading to significant advancements in the field.
4. Progress in Magnetic Mesoporous Materials for Phosphopeptide Enrichment
Protein phosphorylation is mediated by kinases and phosphatases, which alter the protein conformation and charge distribution, thereby changing protein–protein interactions and enzyme activities. This process directs biological processes such as cell growth, division, differentiation, apoptosis, and signaling [57]. It occurs predominantly on serine and threonine residues. Reversible phosphorylation is a dynamic process [58] that occurs extensively in eukaryotic cells. Its occurrence is typically rare, and abnormal phosphorylation has been linked to certain diseases [59]. For instance, serum endogenous peptide levels have been proposed as biomarkers for cancer. Zhai et al. [60] employed zirconium arsenate-modified magnetic nanoparticles (ZrAs-Fe3O4@SiO2) to enrich and quantify four phosphopeptides in serum and observed that the levels of two of the phosphopeptides in the serum of colorectal patients were significantly reduced in comparison to healthy controls. The expression levels of these two phosphopeptides may represent the expression and activity of proteases, kinases, and phosphatases in individual serum, and maintain the dynamic balance of these enzymes. Therefore, it was possible to identify serum phosphopeptides as biomarkers for cancer. Direct detection of low-ionization phosphopeptides is not possible. Therefore, the study of methods to enrich and identify phosphopeptides has garnered significant attention. This subsection summarizes recent research progress on strategies for enriching phosphopeptides.
4.1. Magnetic Mesoporous Silica Materials for Phosphopeptide Enrichment
This enrichment is based on the chelation of metal ions with phosphate groups, following the immobilized metal ion affinity chromatography strategy (IMAC) and metal oxide affinity chromatography strategy (MOAC). The section describes the advances in the enrichment of phosphopeptides using magnetic mesoporous silica materials functionalized with metal ions and metal oxides.
4.1.1. Strategies for Immobilized Metal ion Affinity Chromatography
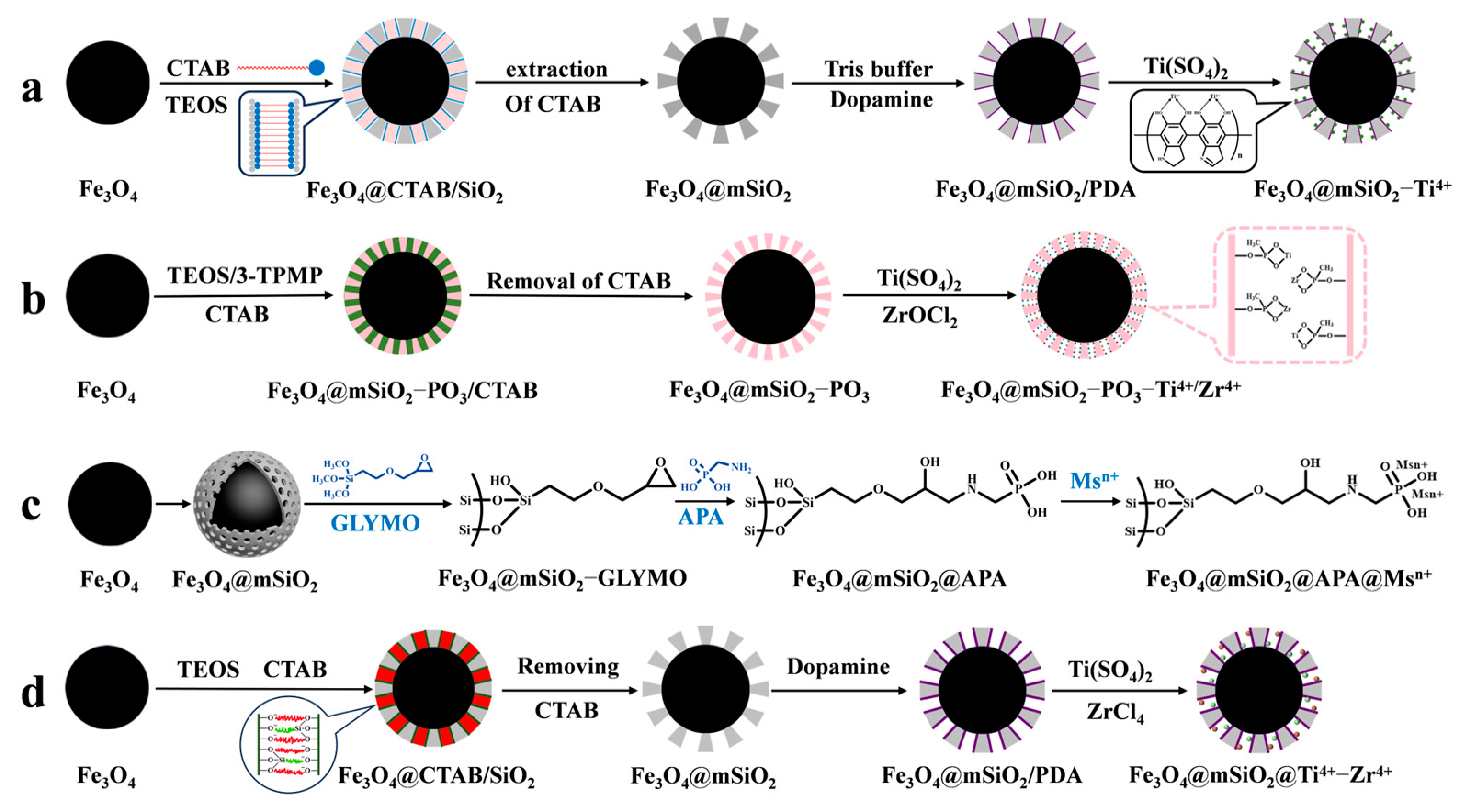
IMAC material is composed of three parts: metal ions for binding phosphopeptides, chelating ligands for immobilizing metal ions, and a matrix. The principle of enriching phosphopeptides with IMAC material is based on the ligand chelating between metal ions and phosphate groups. Different metal ions have varying affinities and coordination numbers for phosphate groups, so the selection of metal ions determines the efficiency of IMAC for phosphopeptide enrichment. As an example, Yao et al. [61] used polydopamine as a coupling agent to immobilize Ti4+ on the surface of magnetic mesoporous silica. This was conducted to enrich phosphopeptides in human serum and saliva before mass spectrometry analysis in order to reduce interference from biological macromolecules such as non-phosphopeptides (as shown in Figure 5a). Traditional metal ions, such as Cu2+, Fe3+, In3+, Ga3+, and Ti4+, have been extensively studied and are well established. However, their weak binding with phosphate groups results in the incomplete enrichment of phosphopeptides in organisms. To achieve a more comprehensive enrichment of phosphopeptides, alternative methods must be explored. Studies have shown that Ti4+, Ce4+, and Fe3+ have a greater tendency to enrich monophosphate peptides, while Nb5+, Zr4+, Ga3+, Y3+, and In3+ have a greater tendency to enrich polyphosphate peptides [62]. IMAC materials with single metal ions are not effective in comprehensively enriching and identifying phosphopeptides. It has been demonstrated that iron ions tend to bind acidic phosphopeptides, while titanium ions tend to bind basic phosphopeptides. Consequently, the enrichment of phosphopeptides in biological samples is not as comprehensive as might be expected from a single metal ion. However, the use of bimetallic ion MIAC material compensates for this [63]. In recent years, there has been a trend towards developing bimetallic IMAC materials that combine the advantages of different ions to achieve more comprehensive phosphopeptide enrichment. Fang et al. [64] introduced phosphates in the formation of mesoporous silica via a one-pot synthesis (as shown in Figure 5b). They then immobilized Ti4+ and Zr4+ on the magnetic mesoporous silica surface using phosphate, resulting in Fe3O4@mSiO2-PO3-Ti4+/Zr4+. The strong coordination between phosphate and metal ions was used as a chelating agent for the IMAC material, which greatly reduced the loss of metal ions. The (Fe3O4@mSiO2-PO3-Ti4+/Zr4+) material had a limit of detection of 1 f mol uL−1 (β-casein digest) and exhibited good selectivity for phosphopeptides (1:500, β-casein: BSA, m/m). In healthy saliva samples, 25 phosphopeptides were detected, while in gastritis saliva samples, 85 phosphopeptides were detected.
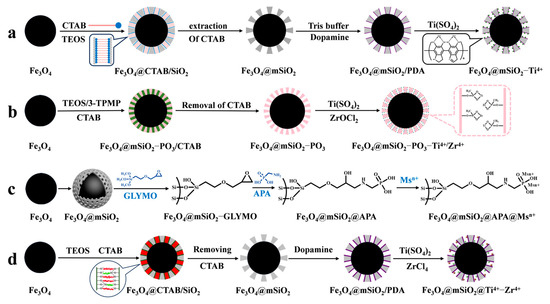
Figure 5.
Magnetic mesoporous silica materials functionalized with metal ions, the synthesis steps of Fe3O4@mSiO2−Ti4+, Fe3O4@mSiO2−PO3−Ti4+/Zr4+, Fe3O4@mSiO2@APA@Msn+ and Fe3O4@mSiO2@Ti4+−Zr4+ are represented by processes (a), (b), (c) and (d), respectively.
However, the chelating ligands utilized to immobilize metal ions can also impact the enrichment ability of IMAC materials. Common chelating ligands, such as iminodiacetic acid (IDA) and nitrilotriacetic acid (NTA), coordinate with metal ions through their carboxyl groups. However, this coordination is weak, leading to the loss of metal ions during elution of phosphopeptides under acidic conditions. Additionally, these ligands tend to bind with acidic peptides, reducing the selectivity for phosphopeptides. Liu et al. [65] developed a new chelating agent, aminomethylphosphonic acid, to bind Ti4+ and Nb5+ immobilized on a magnetic mesoporous silica matrix (Fe3O4@mSiO2@APA@Ti4+/Nb5+). This allowed for the comprehensive enrichment and identification of phosphopeptides in organisms, as shown in Figure 5c. Fe3O4@mSiO2@APA@Ti4+/Nb5+ exhibited a high sensitivity (0.05 f·mol·uL−1 β-casein digest) and good selectivity (β-casein to BSA mass ratio of 1:1500), as well as a high loading capacity of phosphopeptides (330 μg/mg). The polydopamine chelator, which has gained attention in recent years, possesses amino and hydroxyl bifunctional groups. This not only results in a strong chelating effect with metal ions but also provides good hydrophilicity, super adhesion, and biocompatibility. Hu et al. [66] modified magnetic mesoporous silica with metal ions Ti4+ and Zr4+ to obtain Fe3O4@mSiO2@Ti4+-Zr4+ affinity probes (shown in Figure 5d). These probes were used for the selective enrichment of endogenous phosphopeptides in human saliva. The mesoporous material with superparamagnetism, ordered mesoporous channels, and bimetallic ions affinity sites was obtained by first synthesizing Fe3O4@mSiO2 using the sol–gel method. Next, polydopamine was coated onto the surface of Fe3O4@mSiO2, and finally, Ti4+ and Zr4+ were grafted onto the polydopamine. The limit of detection was 0.1 f mol μL−1 for β-casein digest, and it was found to have a selectivity of 1:1000:1000 (β-casein digest/BAS protein/α-casein). The affinity probes, Fe3O4@mSiO2@Ti4+-Zr4+, were able to capture 95 single-phosphate peptides and 9 polyphosphate peptides from human saliva. The probes can be reused up to five times.
4.1.2. Strategies for Metal Oxide Affinity Chromatography
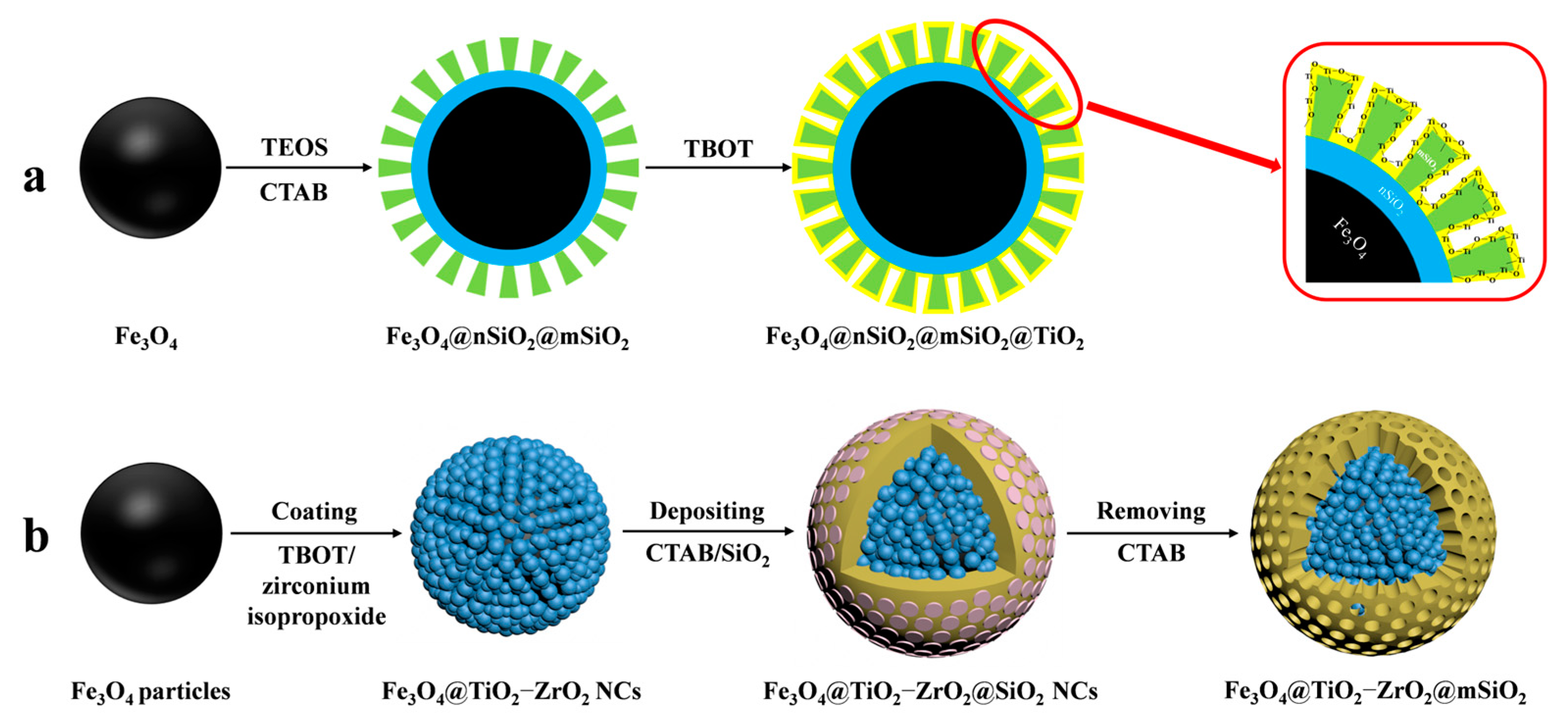
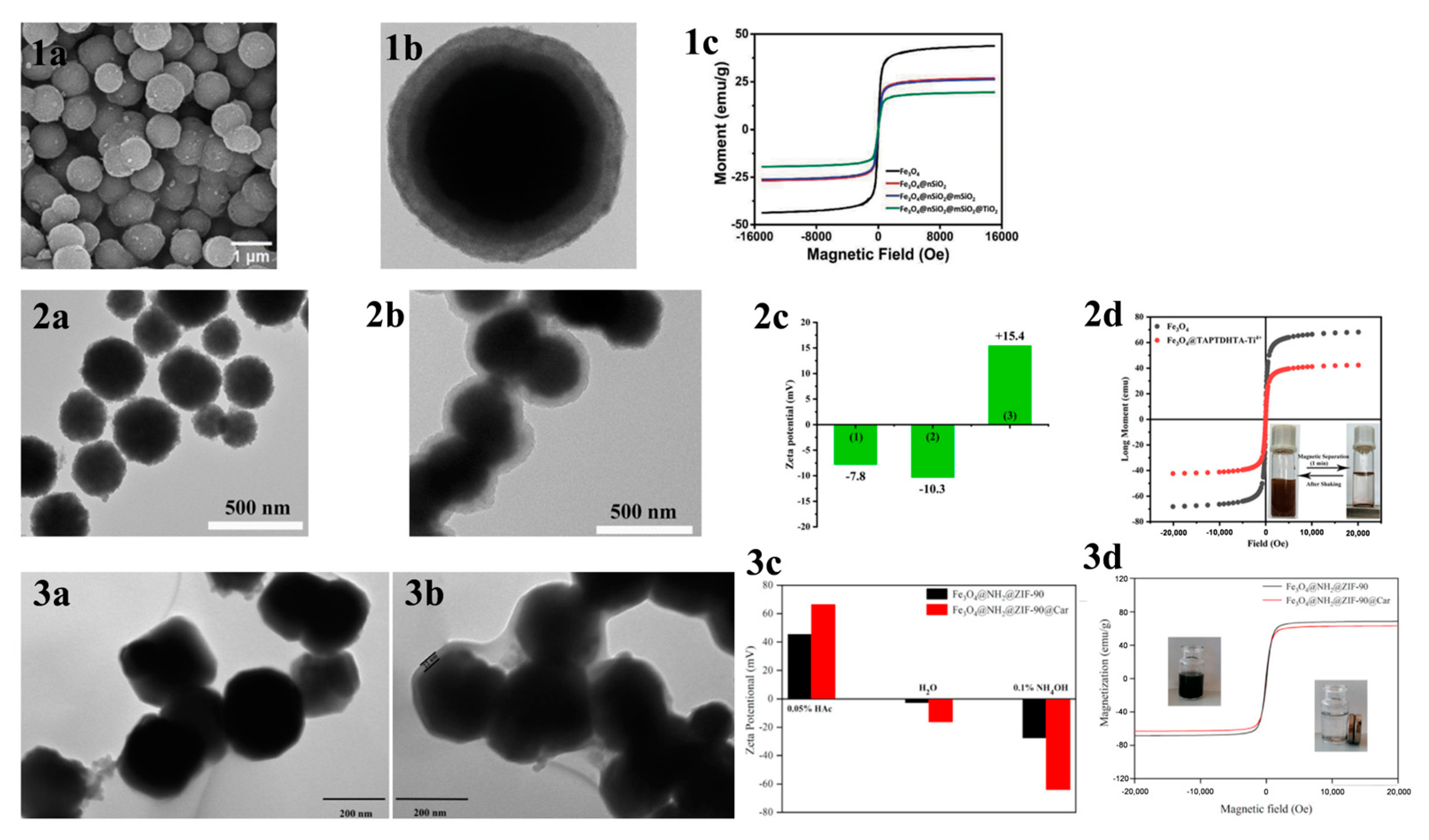
MOAC materials have an affinity for phosphopeptides due to the chelation of metal ions with phosphate groups. In acidic conditions, metal oxides bind to phosphate groups on peptides via bridging bidentate chelation. In contrast, under alkaline conditions, captured phosphopeptides elute via reversible Lewis acid–base interactions. The differing pKa values of various metal oxides result in different binding capacities of different metal oxides to phosphopeptides. The kinetic mechanism of the adsorption of phosphopeptides by metal oxides is not well understood due to the complex physicochemical properties of their surfaces [67]. In contrast to IMAC, MOAC materials immobilize metal ions on their surface in the form of oxides. This prevents the loss of metal ions that can occur in the IMAC method and allows for direct modification of the material surface without the need for chelating agents. Gao et al. [68] synthesized Fe3O4@nSiO2@mSiO2@TiO2, a titanium-dioxide-modified magnetic mesoporous material (shown in Figure 6a and Figure 7(1a–1c)). They also synthesized three mesoporous layers with different pore sizes (12.51 nm, 6.38 nm, and 1.82 nm) as a trypsin reactor by controlling the appropriate experimental conditions. The results indicate that the mesoporous layer with a pore size of 6.38 nm has a larger internal surface area and internal space, avoiding the shadowing effect caused by a pore size that is too small. This makes it a good temporary immobilized enzyme reactor, shortening the trypsin digestion process from 16 h to 2 h. Additionally, one-step selective enrichment of phosphopeptides was also established, allowing for protein digestion and phosphopeptide enrichment to be carried out in the same system, reducing the additional loss and interference of the sample. Fe3O4@nSiO2@mSiO2@TiO2 exhibits a high sensitivity (0.5 f·mol·μL−1 β-casein), good selectivity (molar ratio of β-casein: BAS of 1:2000), and high loading capacity (120 mg/g). In skimmed milk, 13 unique phosphopeptides were extracted, while in HepG2 cells, 1904 unique phosphopeptides were extracted. Additionally, a label-free quantitative proteomic analysis was conducted to examine phosphorylated proteins. The analysis was based on one-step selective enrichment of phosphopeptides. The results were obtained from mixed samples containing BAS and different concentrations of β-casein. The study found that the uniquely phosphorylated peptides of β-casein (m/z 2061.77) showed a linear correlation with the concentration of β-casein, with R = 0.9987.
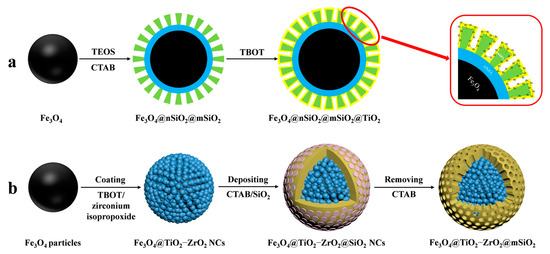
Figure 6.
Magnetic mesoporous silica materials functionalized with metal oxide, the synthesis steps of Fe3O4@nSiO2@mSiO2@TiO2 and Fe3O4@TiO2−ZrO2@mSiO2 are represented by processes (a) and (b), respectively.
Similar to IMAC, MOAC materials exhibit varying affinities of different metal oxides for phosphopeptides. TiO2 has a stronger affinity for polyphosphopeptides, while ZrO2 has a stronger affinity for monophosphopeptides. Binary bimetallic oxides of MOAC materials have attracted much attention for a more comprehensive identification of endogenous phosphopeptides. This was discussed by Li et al. [69]. Bimetallic-oxide-modified core–shell magnetic mesoporous materials (Fe3O4@TiO2-ZrO2@mSiO2) were synthesized and designed to enrich endogenous phosphopeptides in human saliva (shown in Figure 6b). The ability of these materials to enrich phosphopeptides was compared to that of the monometallic oxide composites Fe3O4@TiO2@mSiO2 and Fe3O4@ZrO2@mSiO2. Fe3O4@TiO2-ZrO2@mSiO2 detected 30 phosphopeptides in human saliva, while Fe3O4@TiO2@mSiO2 and Fe3O4@ZrO2@mSiO2 detected only 11 and 10 phosphopeptides.
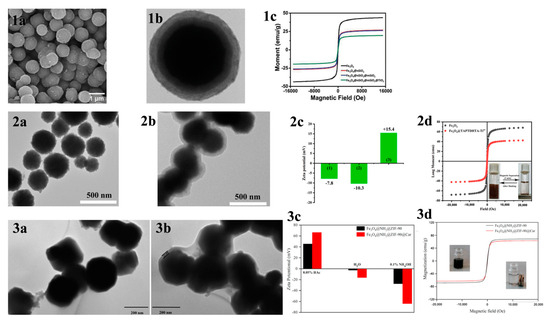
Figure 7.
(1a) SEM and (1b) TEM images of Fe3O4@nSiO2@mSiO2@TiO2; (1c) magnetic hysteresis curves of Fe3O4, Fe3O4@nSiO2, Fe3O4@nSiO2@mSiO2 and Fe3O4@nSiO2@mSiO2@TiO2. Reprinted with permission from Ref. [68]. Copyright 2024 Elsevier. (2a,2b) TEM images of the Fe3O4 NPs and Fe3O4@TAPTDHTA composites, (2c) zeta potential of the (1) Fe3O4 NPs, (2) Fe3O4@TAPTDHTA composites and (3) Fe3O4@TAPTDHTA-Ti4+ composites, and (2d) magnetic hysteresis curve of the Fe3O4 NPs (black curve) and Fe3O4@TAPTDHTA-Ti4+ composites (red curve). Reprinted with permission from Ref. [70]. Copyright 2024 Elsevier. (3a,3b) TEM micrographs of Fe3O4@NH2@ZIF-90 and Fe3O4@NH2@ZIF-90@Car, (3c) zeta potential, and (3d) magnetic hysteresis curves. Reprinted with permission from Ref. [71]. Copyright 2024 Elsevier.
Figure 7.
(1a) SEM and (1b) TEM images of Fe3O4@nSiO2@mSiO2@TiO2; (1c) magnetic hysteresis curves of Fe3O4, Fe3O4@nSiO2, Fe3O4@nSiO2@mSiO2 and Fe3O4@nSiO2@mSiO2@TiO2. Reprinted with permission from Ref. [68]. Copyright 2024 Elsevier. (2a,2b) TEM images of the Fe3O4 NPs and Fe3O4@TAPTDHTA composites, (2c) zeta potential of the (1) Fe3O4 NPs, (2) Fe3O4@TAPTDHTA composites and (3) Fe3O4@TAPTDHTA-Ti4+ composites, and (2d) magnetic hysteresis curve of the Fe3O4 NPs (black curve) and Fe3O4@TAPTDHTA-Ti4+ composites (red curve). Reprinted with permission from Ref. [70]. Copyright 2024 Elsevier. (3a,3b) TEM micrographs of Fe3O4@NH2@ZIF-90 and Fe3O4@NH2@ZIF-90@Car, (3c) zeta potential, and (3d) magnetic hysteresis curves. Reprinted with permission from Ref. [71]. Copyright 2024 Elsevier.
Immobilized metal ion affinity chromatography (IMAC) and metal oxide affinity chromatography (MOAC) are highly selective and sensitive due to their affinity interactions between metal ions and metal oxides with the phosphate groups of phosphopeptides, resulting in specific binding of phosphopeptides. A Lewis acid–base reaction was the preferred method for MOAC enrichment of monophosphopeptides, while in IMAC, the interaction between metal ions and phosphate groups was favored for polyphosphopeptide enrichment.
4.2. Magnetic COF Materials for Phosphopeptide Enrichment
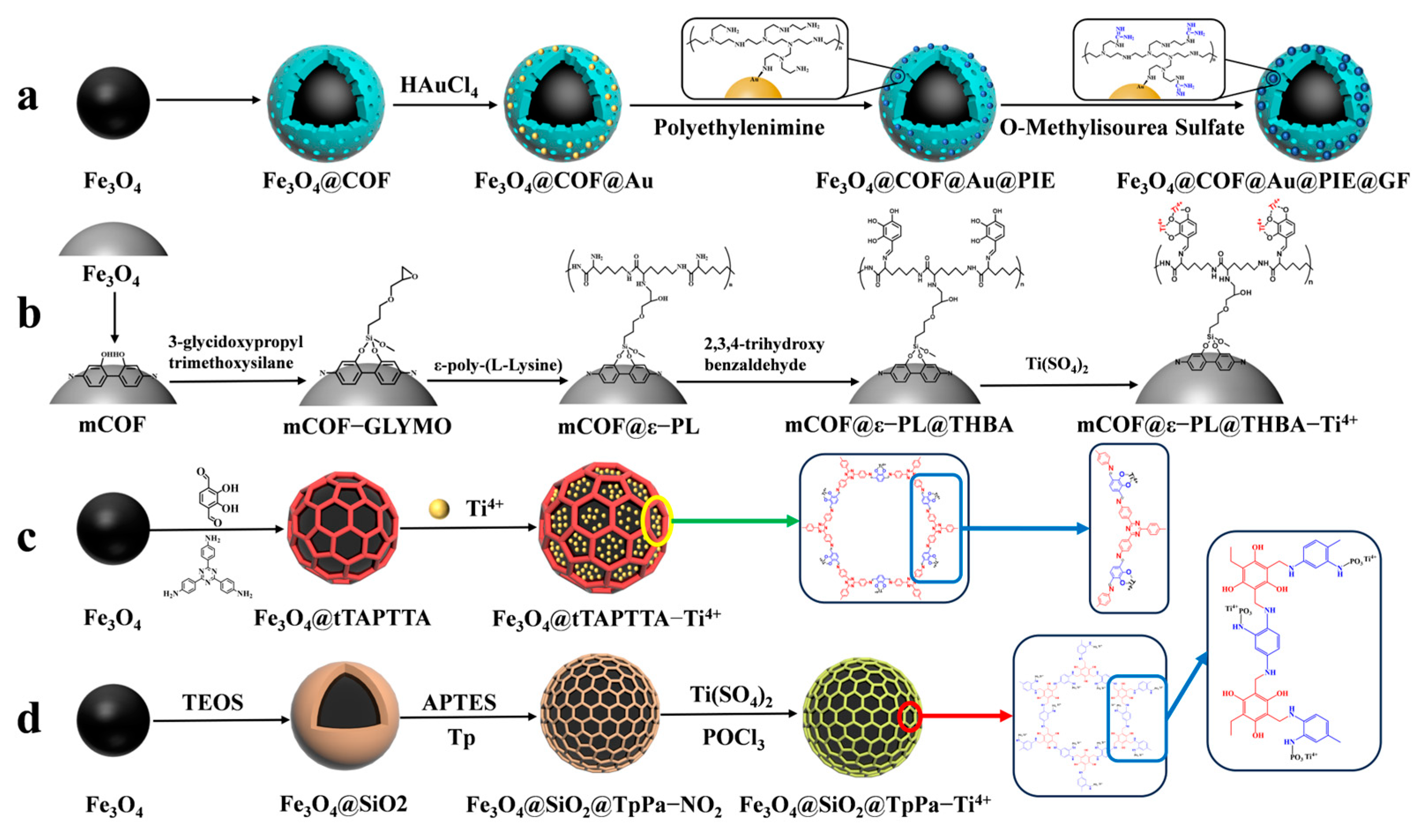
Guanidine-functionalized composites have recently been utilized to capture phosphopeptides in complex biological samples through non-covalent bonding interactions between guanidine and phosphate groups. Wang et al. [72] proposed a composite material, Fe3O4@COF@Au@PEI-GF, which is guanidino-functionalized and magnetic. This composite not only enriches phosphopeptides in human serum but also specifically traps exosomes in serum based on the force between the guanidino group and the exosomal phospholipid bilayer (as shown in Figure 8a). Immediately after, their group [73] proposed magnetic COF composites functionalized with flexible branched polymer-connected Ti4+ (mCOF@ε-PL@THBA-Ti4+). The surface of the magnetic composites was modified amino-rich ε-poly-L-lysine (ε-PL), as shown in Figure 8b, which enhances the hydrophilicity of the material and reduces the nonspecific enrichment of non-phosphopeptides. 2,3,4-trihydroxybenzaldehyde (THBA) was used to modify the material’s surface; this was achieved by reacting the amino group with the aldehyde group to provide binding sites for Ti4+. The resulting material has a low detection limit (2 f mol for β-casein digest), high selectivity (molar ratio of β-casein: BAS was 1:5000), and high loading capacity (66.7 mg/g). The material was able to detect 16 endogenous phosphopeptides in healthy human saliva.
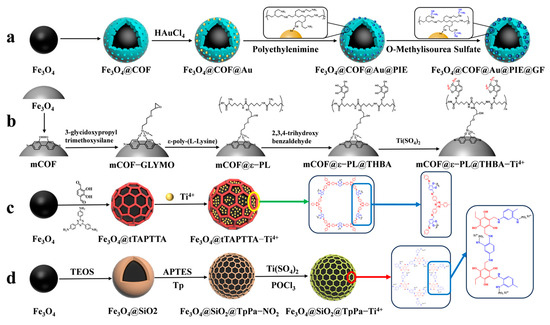
Figure 8.
Magnetic covalent organic framework materials for enrichment of phosphopeptides, the synthesis steps of Fe3O4@COF@Au@PIE@GF, mCOF@ε−PL@THBA−Ti4+, Fe3O4@tTAPTTA−Ti4+ and Fe3O4@SiO2@TpPa−Ti4+ are represented by processes (a), (b), (c) and (d), respectively.
However, the multistep modification process on the material’s surface introduces significant spatial site resistance, and the cumbersome synthesis steps have an impact on the material. Therefore, there is a need to develop a simple and more efficient magnetic composite. He et al. [70] reported a one-pot synthesis of Fe3O4@TAPTDHTA-Ti4+ magnetic composites with inherent bifunctional groups (Tz and OH) based on tpt and DHTA. The composites have a large number of binding sites for Ti4+ due to their surface-abundant o-phenol hydroxyls, and a limit of detection as low as 0.05 f mol μL−1 (β-casein digest). The synthesis is shown in Figure 7(2a–2d) and Figure 8c. Additionally, the study detected 9, 4, and 12 enriched phosphopeptides in milk, human serum, and human salivary fluid, respectively. In the HeLa cell lyses, the study detected 3333 phosphopeptides, of which 3180 (95.4%) were monophosphopeptides and 153 (4.6%) were polyphosphopeptides. Ding et al. [74] applied a layer of COFs to Fe3O4@SiO2 microspheres through the condensation reaction between mesitylene triphenol (Tp) and 2-nitro-1,4-phenylenediamine (Pa-NO2) under vacuum and acetic acid-catalyzed conditions. Nitroamidation and phosphorylation were performed in an aqueous titanium sulfate solution, resulting in Fe3O4@SiO2@TpPa-Ti4+ (as shown in Figure 8d). The COFs layer’s surface area and abundant functional groups contributed to a Ti4+ loading of up to 14% (wt%). The binding capacity for phosphopeptides was up to 200 mg/g, with a low detection limit of 0.2 f·mol·μL−1 (α-casein digest) and a good selectivity (1:5000, α-casein to BAS molar ratio). In skimmed milk, 33 phosphopeptides were enriched, while in human serum, 4 phosphopeptides were enriched. In HeLa cell lysates, 3010 phosphopeptides were identified, of which 74.8% were monophosphorylated and 25.2% were polyphosphorylated. In rat brain lysates, 1084 phosphorylated peptides were detected.
4.3. Magnetic MOF Materials for Phosphopeptids Enrichment
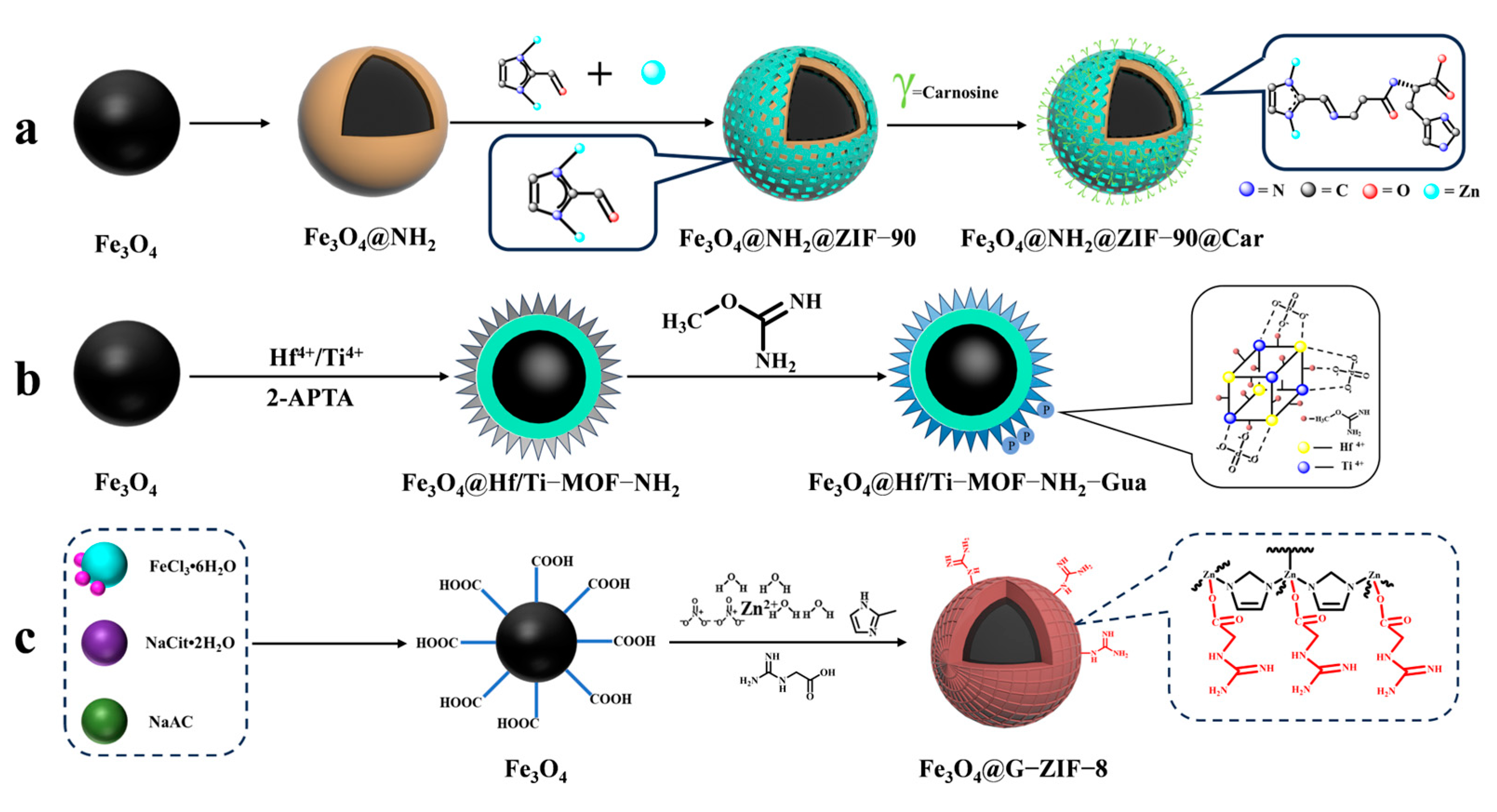
To enrich phosphopeptides, Qi et al. [71] synthesized ZIF-90 on an aminated Fe3O4 core using an in situ method. They then utilized the aldehyde group in ZIF-90 to modify the carnosine peptide (Car) on the material’s surface through imine condensation, resulting in Fe3O4@NH2@ZIF-90@Car (shown in Figure 7(3a–3d) and Figure 9a). The phosphopeptide can be adsorbed synergistically by both the Zn2+ in ZIF-90 and the imidazole in Car. Under acidic conditions, imidazole is protonated, resulting in a positive charge on Fe3O4@NH2@ZIF-90@Car. This positive charge allows it to bind to the negatively charged phosphopeptide. Upon exposure to alkaline conditions, Fe3O4@NH2@ZIF-90@Car loses its proton and separates from the phosphopeptide. The Fe3O4@NH2@ZIF-90@Car composite exhibits a low detection limit of 0.1 f mol for the β-casein digest, a high selectivity with a 1:500 mass ratio for the tryptophan-digested mixture of β-casein and bovine serum albumin, and a high size exclusion with a 1:1000 mass ratio for β-casein digest to BSA. These properties are attributed to the regularly ordered pores of the MOFs, resulting in the detection of 17 phosphopeptides in skim milk and 28 phosphopeptides in human saliva.
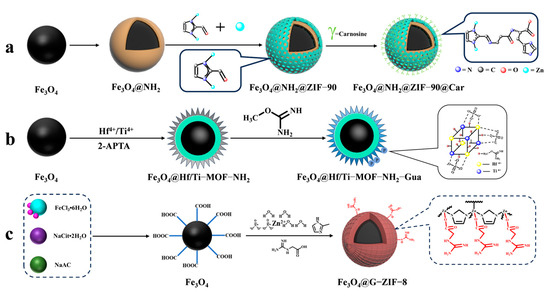
Figure 9.
Magnetic metal–organic framework materials for enrichment of phosphopeptides, the synthesis steps of Fe3O4@NH2@ZIF−90@Car, Fe3O4@Hf/Ti−MOF−NH2−Gua and Fe3O4@G−ZIF−8 are represented by processes (a), (b), and (c), respectively.
Cao et al. [75] synthesized magnetic MOF materials modified with iron and titanium using the bimetallic ion-IMAC strategy. The resulting material, Fe3O4@MIL(Fe/Ti), was used for phosphopeptide enrichment in complex samples. Li et al. [76] modified guanidinium groups on the surface of magnetic MOF composites using the bimetallic ion IMAC strategy to obtain Fe3O4@Hf/Ti-MOF-Gua (shown in Figure 9b). This modification uses multiple affinity strategies to synergistically enrich phosphopeptides, increasing the phosphopeptide selectivity in complex samples to up to 1: 2000: 2000 (α/β-casein digest–α-casein–BSA). Zhang et al. [77] developed a one-step epitaxial growth strategy to prepare magnetic guanidinium-based functionalized MOF materials with multi-affinity sites (Fe3O4@G-ZIF-8) for the enrichment of global phosphopeptides. This approach was taken due to the potential for molecules derived from MOFs to clog the MOF’s pores and the relatively cumbersome synthesis process. Figure 9c shows the results of this strategy. The MOF layer uses an IMAC-based strategy with Zn2+ and a non-covalent strategy with guanidinium groups to interact with phosphopeptides. This multi-affinity site and strategy effectively enriches phosphopeptides in biological samples, demonstrating good selectivity and an excellent sensitivity of 1 f mol for β-casein digest. Using MALDI-TOF mass spectrometry, they detected 4 and 30 phosphopeptides in human serum and human saliva, respectively. Additionally, nano-lc-MS/MS detected 25 phosphopeptides in human serum and 14 in human saliva. Table 2 presents the properties of the core-shell magnetic mesoporous materials discussed in the text, which are employed for the enrichment of phosphopeptides.

Table 2.
A representative selection of magnetic mesoporous materials that have been employed in the enrichment of phosphopeptides over the past five years.
In summary, the principle of phosphopeptide enrichment is based on the electrostatic interaction force between phosphopeptides and metal ions. The specific adsorption capacity of phosphopeptides can be enhanced by using high-valence metal ions (oxides) with stronger electrostatic interactions or bimetallic ions (oxides). The desired effect can be achieved by using mesoporous shells, such as COFs, which have a larger specific surface area or a larger number of fixed sites for the metal ions. To achieve synergistic adsorption of phosphopeptides, a variety of processes and strategies can be used, such as using imidazole or guanidinium. It is important to pay attention to their spatial site resistance to prevent clogging of the pores and to maintain a high adsorption efficiency.
5. Conclusions and Perspectives
Post-translationally modified peptides, such as glycopeptides and phosphopeptides, are closely related to human health. Enriching and identifying these peptides are of great significance. This paper summarizes the research progress for new magnetic mesoporous materials in enriching glycopeptides and phosphopeptides in the last five years. The majority of strategies for enriching glycopeptides involve modifying hydrophilic compounds. This is because the most significant distinction between glycopeptides and other endogenous peptides is that the glycosyl portion of glycopeptides is hydrophilic. In comparison to conventional mesoporous silica, COFs and MOFs are formed by covalent linkage between organic ligands or ligand linkage with metal ions, which results in a large number of sites for binding hydrophilic compounds on the surface of COFs and MOFs. When biomarkers for various diseases are explored in the future, magnetic COF and MOF materials can be targeted for self-assembly based on the characteristics of these disease markers, and faster and more accurate disease diagnosis methods can be developed.
For phosphopeptides, the majority of current enrichment strategies are based on IMAC and MOAC. However, there are various phosphopeptides with different characteristics in real samples, and a single IMAC or MOAC strategy cannot comprehensively enrich all phosphopeptides in real samples. Consequently, the majority of phosphopeptide enrichment strategies are based on multiple affinity strategies, which can compensate for the shortcomings of a single strategy. However, the use of multiple enrichment strategies simultaneously necessitates multi-step modification during the synthesis of the enriched materials. In contrast, MOFs exhibit advantages that are not comparable to mesoporous silica and COFs due to their inherent metal ions. Consequently, the simplification of the synthesis steps of magnetic mesoporous materials and the development of high-performance adsorbent materials represent dominant research focuses for the near future. Furthermore, the kinetic mechanism of the adsorption of metal oxides on phosphopeptides remains poorly understood. To gain deeper insights into the pathogenic mechanisms of aberrant post-translationally modified phosphopeptides, it is essential to enhance the investigation of the adsorption mechanism of metal oxides on phosphopeptides. The development of material science has led to the discovery of new functionalized magnetic mesoporous materials and enrichment strategies. These advancements provide new ideas for the enrichment of post-translationally modified peptides. This will play a crucial role in the discovery of disease biomarkers based on post-translationally modified peptidomics.
Author Contributions
H.F., Y.Z. and Q.Y. contributed to reviewing the background literature, writing the draft, and formatting this review article. Z.Z. contributed to validation, supervision, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NSFC, grant number 32071507.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors and funders declare no conflicts of interest.
References
- Bhandari, D.; Rafiq, S.; Gat, Y.; Gat, P.; Waghmare, R.; Kumar, V. A Review on Bioactive Peptides: Physiological Functions, Bioavailability and Safety. Int. J. Pept. Res. Ther. 2019, 26, 139–150. [Google Scholar] [CrossRef]
- Meisel, H.; FitzGerald, R.J. Biofunctional Peptides from Milk Proteins: Mineral Binding and Cytomodulatory Effects. Curr. Pharm. Des. 2003, 9, 1289–1295. [Google Scholar] [PubMed]
- Savastano, M.L.; Liu, Y.; Mels, J.; Dittrich, D.; Haus, S.; Gensberger-Reigl, S.; Pischetsrieder, M. Profiling of Multiphosphorylated Peptides in Kefir and Their Release During Simulated Gastrointestinal Digestion. ACS Omega 2019, 4, 7963–7970. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, Y.; Zhang, Z.; Wang, X.; Niu, Y.; Zhang, S.; Xu, W.; Ren, C. Advances of peptides for antibacterial applications. Colloids Surf. B Biointerfaces 2021, 202, 111682. [Google Scholar] [CrossRef]
- Messerli, F.H.; Bangalore, S.; Bavishi, C.; Rimoldi, S.F. Angiotensin-Converting Enzyme Inhibitors in Hypertension. J. Am. Coll. Cardiol. 2018, 71, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Du, A.; Jia, W. Bioaccessibility of novel antihypertensive short-chain peptides in goat milk using the INFOGEST static digestion model by effect-directed assays. Food Chem. 2023, 427, 136735. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, M.; Lu, X.; Yang, S.; Yang, M.; Fang, Y.; Lai, R.; Duan, Z. Isolation and Characterization of Poeciguamerin, a Peptide with Dual Analgesic and Anti-Thrombotic Activity from the Poecilobdella manillensis Leech. Int. J. Mol. Sci. 2023, 24, 11097. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Liceaga, A.M. Cellular antioxidant effect of bioactive peptides and molecular mechanisms underlying: Beyond chemical properties. Int. J. Food Sci. Technol. 2020, 56, 2193–2204. [Google Scholar] [CrossRef]
- Du, A.; Jia, W. Virtual screening, identification, and potential antioxidant mechanism of novel bioactive peptides during aging by a short-chain peptidomics, quantitative structure–activity relationship analysis, and molecular docking. Food Res. Int. 2023, 172, 113129. [Google Scholar] [CrossRef]
- Hart, G.W.; Copeland, R.J. Glycomics Hits the Big Time. Cell 2010, 143, 672–676. [Google Scholar] [CrossRef]
- Matsubayashi, Y. Posttranslationally Modified Small-Peptide Signals in Plants. Annu. Rev. Plant Biol. 2014, 65, 385–413. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Moutal, A.; Dorame, A.; Bellampalli, S.S.; Chefdeville, A.; Kanazawa, I.; Pham, N.Y.N.; Park, K.D.; Weimer, J.M.; Khanna, R. Phosphorylated CRMP2 Regulates Spinal Nociceptive Neurotransmission. Mol. Neurobiol. 2018, 56, 5241–5255. [Google Scholar] [CrossRef] [PubMed]
- Kelley, A.R.; Bach, S.B.H.; Perry, G. Analysis of post-translational modifications in Alzheimer’s disease by mass spectrometry. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2019, 1865, 2040–2047. [Google Scholar] [CrossRef] [PubMed]
- Kailemia, M.J.; Park, D.; Lebrilla, C.B. Glycans and glycoproteins as specific biomarkers for cancer. Anal. Bioanal. Chem. 2016, 409, 395–410. [Google Scholar] [CrossRef]
- Fert-Bober, J.; Murray, C.I.; Parker, S.J.; Van Eyk, J.E. Precision Profiling of the Cardiovascular Post-Translationally Modified Proteome. Circ. Res. 2018, 122, 1221–1237. [Google Scholar] [CrossRef]
- Neagu, A.-N.; Jayathirtha, M.; Baxter, E.; Donnelly, M.; Petre, B.A.; Darie, C.C. Applications of Tandem Mass Spectrometry (MS/MS) in Protein Analysis for Biomedical Research. Molecules 2022, 27, 2411. [Google Scholar] [CrossRef]
- Jiang, X.; Ye, M.; Zou, H. Technologies and methods for sample pretreatment in efficient proteome and peptidome analysis. Proteomics 2008, 8, 686–705. [Google Scholar] [CrossRef]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Muzio, L.L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review). Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, C.; Boll, I.; Finsen, B.; Modzel, M.; Larsen, M.R. Characterizing disease-associated changes in post-translational modifications by mass spectrometry. Expert Rev. Proteom. 2018, 15, 245–258. [Google Scholar] [CrossRef]
- Thingholm, T.E.; Jensen, O.N.; Larsen, M.R. Analytical strategies for phosphoproteomics. Proteomics 2009, 9, 1451–1468. [Google Scholar] [CrossRef]
- Wan, Y.; Cui, Z.; Ghosh, R. Fractionation of Proteins Using Ultrafiltration: Developments and Challenges. Dev. Chem. Eng. Miner. Process. 2005, 13, 121–136. [Google Scholar] [CrossRef]
- Koh, J.; Kim, B.J.; Qu, Y.; Dallas, D.C. Mass spectral profiling of caseinomacropeptide extracted from feeding material and jejunal fluid using three methods–ethanol precipitation, perchloric acid precipitation, and ultrafiltration. Food Chem. 2023, 398, 133864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Molecularly Imprinted Nanoparticles for Biomedical Applications. Adv. Mater. 2020, 32, 1806328. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Jiang, L.; Jia, Q. Application of magnetic solid phase extraction in separation and enrichment of glycoproteins and glycopeptides. Chin. Chem. Lett. 2021, 32, 2629–2636. [Google Scholar] [CrossRef]
- Aristoteli, L.P.; Molloy, P.M.; Baker, M.S. Evaluation of Endogenous Plasma Peptide Extraction Methods for Mass Spectrometric Biomarker Discovery. J. Proteome Res. 2007, 6, 571–581. [Google Scholar] [CrossRef]
- Shah, A.; Dobrovolskaia, M.A. Immunological effects of iron oxide nanoparticles and iron-based complex drug formulations: Therapeutic benefits, toxicity, mechanistic insights, and translational considerations. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 977–990. [Google Scholar]
- Ganapathe, L.S.; Mohamed, M.A.; Mohamad Yunus, R.; Berhanuddin, D.D. Magnetite (Fe3O4) Nanoparticles in Biomedical Application: From Synthesis to Surface Functionalisation. Magnetochemistry 2020, 6, 68. [Google Scholar] [CrossRef]
- Cui, H.; Li, D.; Zhang, Z. Preparation and characterization of Fe3O4 magnetic nanoparticles modified by perfluoropolyether carboxylic acid surfactant. Mater. Lett. 2015, 143, 38–40. [Google Scholar] [CrossRef]
- Yao, J.; Sun, N.; Deng, C. Recent advances in mesoporous materials for sample preparation in proteomics research. TrAC Trends Anal. Chem. 2018, 99, 88–100. [Google Scholar] [CrossRef]
- Li, Y.; Sun, N.; Hu, X.; Li, Y.; Deng, C. Recent advances in nanoporous materials as sample preparation techniques for peptidome research. TrAC Trends Anal. Chem. 2019, 120, 115658. [Google Scholar] [CrossRef]
- Cigarroa-Mayorga, O.E. Tuning the size stability of MnFe2O4 nanoparticles: Controlling the morphology and tailoring of surface properties under the hydrothermal synthesis for functionalization with myricetin. Ceram. Int. 2021, 47, 32397–32406. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Alothman, Z.A.; Ghfar, A.A. Novel Metal–Organic Framework (MOF) Based Composite Material for the Sequestration of U(VI) and Th (IV) Metal Ions from Aqueous Environment. ACS Appl. Mater. Interfaces 2017, 9, 36026–36037. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, S.Z.; Hu, Q.H.; Lu, G.Q. Magnetic Nanocomposites with Mesoporous Structures: Synthesis and Applications. Small 2011, 7, 425–443. [Google Scholar] [CrossRef]
- Yue, Q.; Sun, J.; Kang, Y.; Deng, Y. Advances in the Interfacial Assembly of Mesoporous Silica on Magnetite Particles. Angew. Chem. Int. Ed. 2020, 59, 15804–15817. [Google Scholar] [CrossRef]
- Zuo, B.; Li, W.; Wu, X.; Wang, S.; Deng, Q.; Huang, M. Recent Advances in the Synthesis, Surface Modifications and Applications of Core-Shell Magnetic Mesoporous Silica Nanospheres. Chem. Asian J. 2020, 15, 1248–1265. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Tong, L.; Huang, S.; Chen, G.; Zhu, F.; Ouyang, G. Recent advances of covalent organic frameworks and their application in sample preparation of biological analysis. TrAC Trends Anal. Chem. 2021, 136, 116182. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, Z.; Chen, X.; Wang, J. Advances in the adsorption/enrichment of proteins/peptides by metal–organic frameworks-affinity adsorbents. TrAC Trends Anal. Chem. 2022, 153, 116627. [Google Scholar] [CrossRef]
- Nisticò, R.; Cesano, F.; Garello, F. Magnetic Materials and Systems: Domain Structure Visualization and Other Characterization Techniques for the Application in the Materials Science and Biomedicine. Inorganics 2020, 8, 6. [Google Scholar] [CrossRef]
- Latham, A.H.; Williams, M.E. Controlling Transport and Chemical Functionality of Magnetic Nanoparticles. Acc. Chem. Res. 2008, 41, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Cai, W.; Feng, J.; Meng, X.; Liu, E. In situ decoration of carbon nanotubes with nearly monodisperse magnetite nanoparticles in liquid polyols. J. Mater. Chem. 2007, 17, 1188–1192. [Google Scholar] [CrossRef]
- Zhu, G.-T.; Li, X.-S.; Gao, Q.; Zhao, N.-W.; Yuan, B.-F.; Feng, Y.-Q. Pseudomorphic synthesis of monodisperse magnetic mesoporous silica microspheres for selective enrichment of endogenous peptides. J. Chromatogr. A 2012, 1224, 11–18. [Google Scholar] [CrossRef]
- Ke, F.; Qiu, L.-G.; Yuan, Y.-P.; Jiang, X.; Zhu, J.-F. Fe3O4@MOF core–shell magnetic microspheres with a designable metal–organic framework shell. J. Mater. Chem. 2012, 22, 9497. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Apweiler, R.; Hermjakob, H.; Sharon, N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim. Biophys. Acta 1999, 1473, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Q.; Huang, Y.; Lin, L.; Schlüter, H.; Wang, K.; Zhang, C.; Yang, P.; Yu, H. Boronic acid-functionalized mesoporous magnetic particles with a hydrophilic surface for the multimodal enrichment of glycopeptides for glycoproteomics. Analyst 2020, 145, 5252–5259. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Wu, H.; Sun, N.; Deng, C. Smart Hydrophilic Modification of Magnetic Mesoporous Silica with Zwitterionic L-Cysteine for Endogenous Glycopeptides Recognition. ACS Sustain. Chem. Eng. 2019, 7, 2844–2851. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Y.; Deng, Z.; Long, J.; Sun, N.; Deng, C. One-step fabrication of strongly hydrophilic mesoporous silica for comprehensive analysis of serum glycopeptidome. Talanta 2021, 234, 122713. [Google Scholar] [CrossRef]
- Yi, L.; Wang, B.; Feng, Q.; Yan, Y.; Ding, C.-F.; Mao, H. Surface functionalization modification of ultra-hydrophilic magnetic spheres with mesoporous silica for specific identification of glycopeptides in serum exosomes. Anal. Bioanal. Chem. 2023, 415, 1741–1749. [Google Scholar] [CrossRef]
- Su, P.; Li, M.; Li, X.; Yuan, X.; Gong, Z.; Wu, L.; Song, J.; Yang, Y. Glutathione functionalized magnetic covalent organic frameworks with dual-hydrophilicity for highly efficient and selective enrichment of glycopeptides. J. Chromatogr. A 2022, 1667, 462869. [Google Scholar] [CrossRef]
- Hu, X.; Wu, Y.; Deng, C. Recognition of urinary N-linked glycopeptides in kidney cancer patients by hydrophilic carbohydrate functionalized magnetic metal organic framework combined with LC-MS/MS. Microchim. Acta 2020, 187, 616. [Google Scholar] [CrossRef]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhou, X.; Cui, B. Recent advances and applications of magnetic covalent organic frameworks in food analysis. J. Chromatogr. A 2023, 1687, 463702. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, N.; Deng, C. Construction of Magnetic Covalent Organic Frameworks with Inherent Hydrophilicity for Efficiently Enriching Endogenous Glycopeptides in Human Saliva. ACS Appl. Mater. Interfaces 2020, 12, 9814–9823. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Luan, J.; Li, Y.; He, X.; Chen, L.; Zhang, Y. Hydrophilic maltose-modified magnetic metal-organic framework for highly efficient enrichment of N-linked glycopeptides. J. Chromatogr. A 2020, 1615, 460754. [Google Scholar] [CrossRef]
- Hu, X.; Liu, Q.; Wu, Y.; Deng, Z.; Long, J.; Deng, C. Magnetic metal-organic frameworks containing abundant carboxylic groups for highly effective enrichment of glycopeptides in breast cancer serum. Talanta 2019, 204, 446–454. [Google Scholar] [CrossRef]
- Qing, G.; Yan, J.; He, X.; Li, X.; Liang, X. Recent advances in hydrophilic interaction liquid interaction chromatography materials for glycopeptide enrichment and glycan separation. TrAC Trends Anal. Chem. 2020, 124, 115570. [Google Scholar] [CrossRef]
- Nasa, I.; Kettenbach, A.N. Coordination of Protein Kinase and Phosphoprotein Phosphatase Activities in Mitosis. Front. Cell Dev. Biol. 2018, 6, 30. [Google Scholar] [CrossRef]
- Day, E.K.; Sosale, N.G.; Lazzara, M.J. Cell signaling regulation by protein phosphorylation: A multivariate, heterogeneous, and context-dependent process. Curr. Opin. Biotechnol. 2016, 40, 185–192. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Z.-Z.; Chen, N.-H. Connexin 43 Phosphorylation: Implications in Multiple Diseases. Molecules 2023, 28, 4914. [Google Scholar] [CrossRef]
- Zhai, G.; Yang, L.; Luo, Q.; Wu, K.; Zhao, Y.; Wang, F. Serum phosphopeptide profiling for colorectal cancer diagnosis using liquid chromatography–mass spectrometry. Rapid Commun. Mass Spectrom. 2022, 36, e9316. [Google Scholar] [CrossRef]
- Yao, J.; Sun, N.; Wang, J.; Xie, Y.; Deng, C.; Zhang, X. Rapid synthesis of titanium (IV)-immobilized magnetic mesoporous silica nanoparticles for endogenous phosphopeptides enrichment. Proteomics 2017, 17, 1600320. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Sun, X.; Li, Y.; Deng, C.; Duan, G. Facile synthesis of Fe3O4@PDA core-shell microspheres functionalized with various metal ions: A systematic comparison of commonly-used metal ions for IMAC enrichment. Talanta 2018, 178, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.C.Y.; Tsai, C.F.; Hsu, C.C.; Sun, Y.N.; Chen, Y.J. Complementary Fe3+- and Ti4+-immobilized metal ion affinity chromatography for purification of acidic and basic phosphopeptides. Rapid Commun. Mass Spectrom. 2012, 26, 2186–2194. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Liu, X.; Sun, N.; Deng, C. Enhanced specificity of bimetallic ions via mesoporous confinement for phosphopeptides in human saliva. Talanta 2021, 233, 122587. [Google Scholar] [CrossRef]
- Liu, B.; Wang, B.; Yan, Y.; Tang, K.; Ding, C.-F. Efficient separation of phosphopeptides employing a Ti/Nb-functionalized core-shell structure solid-phase extraction nanosphere. Microchim. Acta 2021, 188, 32. [Google Scholar] [CrossRef]
- Hu, X.; Li, Y.; Miao, A.; Deng, C. Dual metal cations coated magnetic mesoporous silica probe for highly selective capture of endogenous phosphopeptides in biological samples. Microchim. Acta 2020, 187, 400. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xie, Z.; Ding, C.-F.; Deng, C.; Yan, Y. Recent advances in metal oxide affinity chromatography materials for phosphoproteomics. TrAC Trends Anal. Chem. 2023, 158, 116881. [Google Scholar] [CrossRef]
- Gao, L.; Tao, J.; Qi, L.; Jiang, X.; Shi, H.; Liu, Y.; Di, B.; Wang, Y.; Yan, F. Synthesis of a metal oxide affinity chromatography magnetic mesoporous nanomaterial and development of a one-step selective phosphopeptide enrichment strategy for analysis of phosphorylated proteins. Anal. Chim. Acta 2022, 1195, 339430. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Wu, H.; Deng, C. Magnetic mesoporous silica nanocomposites with binary metal oxides core-shell structure for the selective enrichment of endogenous phosphopeptides from human saliva. Anal. Chim. Acta 2019, 1079, 111–119. [Google Scholar] [CrossRef]
- He, Y.; Zhang, S.; Zhong, C.; Yang, Y.; Li, G.; Ji, Y.; Lin, Z. Facile synthesis of Ti4+-immobilized magnetic covalent organic frameworks for enhanced phosphopeptide enrichment. Talanta 2021, 235, 122789. [Google Scholar] [CrossRef]
- Qi, H.; Li, Z.; Zheng, H.; Jia, Q. Carnosine functionalized magnetic metal–organic framework nanocomposites for synergistic enrichment of phosphopeptides. Anal. Chim. Acta 2021, 1157, 338383. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, B.; Feng, Q.; Fang, X.; Dai, X.; Yan, Y.; Ding, C.-F. Magnetic guanidyl–functionalized covalent organic framework composite: A platform for specific capture and isolation of phosphopeptides and exosomes. Microchim. Acta 2022, 189, 330. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wang, B.; Wang, B.; Feng, Q.; Zhang, S.; Xiong, Z.; Zhang, S.; Cai, T.; Ding, C.-F.; Yan, Y. Post-synthesis of a titanium-rich magnetic COF nanocomposite with flexible branched polymers for efficient enrichment of phosphopeptides from human saliva and serum. Analyst 2023, 148, 4738–4745. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Zhao, Y.; Liu, H.; Zhang, W. Core–shell magnetic microporous covalent organic framework with functionalized Ti (IV) for selective enrichment of phosphopeptides. Analyst 2020, 145, 4341–4351. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhao, Y.; Chu, Z.; Zhang, X.; Zhang, W. Core-shell magnetic bimetallic MOF material for synergistic enrichment of phosphopeptides. Talanta 2020, 206, 120165. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-y.; Zhang, S.; Gao, W.; Hua, Y.; Lian, H.-z. Guanidyl-Functionalized Magnetic Bimetallic MOF Nanocomposites Developed for Selective Enrichment of Phosphopeptides. ACS Sustain. Chem. Eng. 2020, 8, 16422–16429. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, T.; Xie, P.; Yang, Z.; Zhang, L.; Wu, X.; Cai, Z. Epitaxial Growth of Guanidyl-Functionalized Magnetic Metal–Organic Frameworks with Multiaffinity Sites for Selective Capture of Global Phosphopeptides. ACS Appl. Mater. Interfaces 2022, 14, 39364–39374. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).