Enhanced Antibacterial Properties of Titanium Surfaces through Diversified Ion Plating with Silver Atom Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.1.1. Substrates
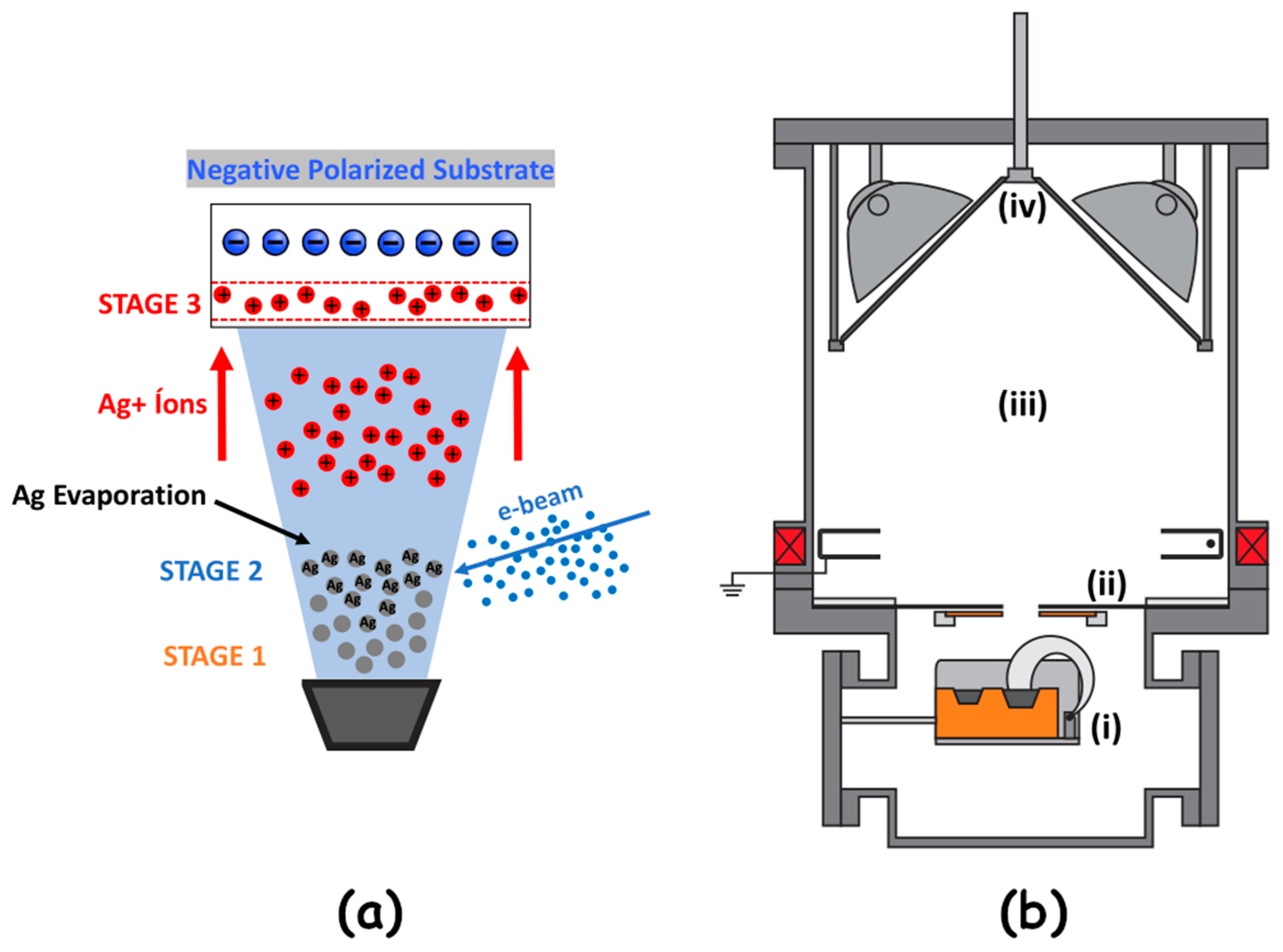
2.1.2. Diversified Ion Plating Process
2.1.3. Stoichiometry
2.1.4. Surface Morphology and Elemental Composition
2.1.5. Wettability
2.1.6. Surface Roughness
2.1.7. Bacterial Suspension Immersion Tests
2.1.8. Statistical Analysis
3. Results and Discussion
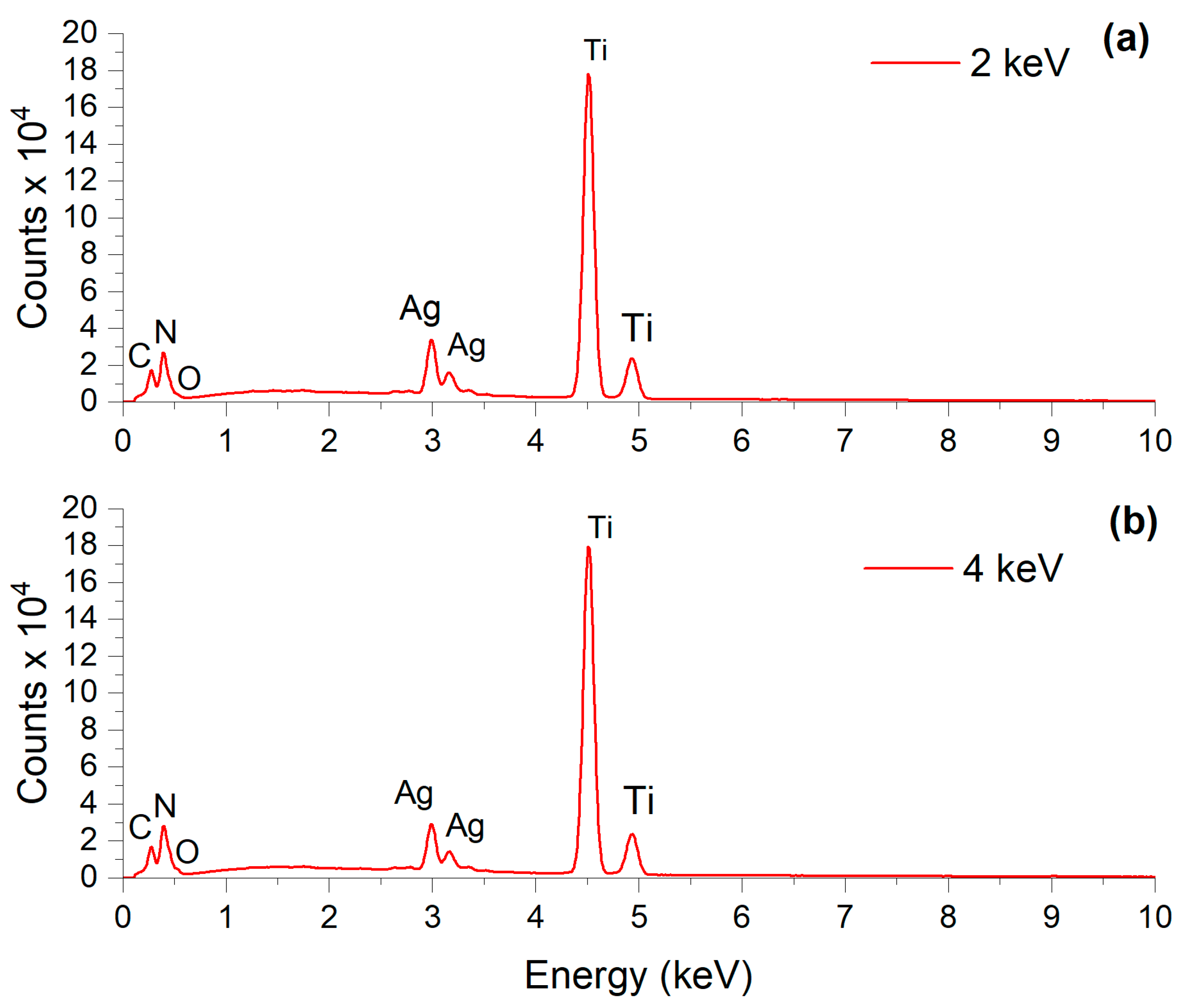
3.1. Surface and Chemical Analysis
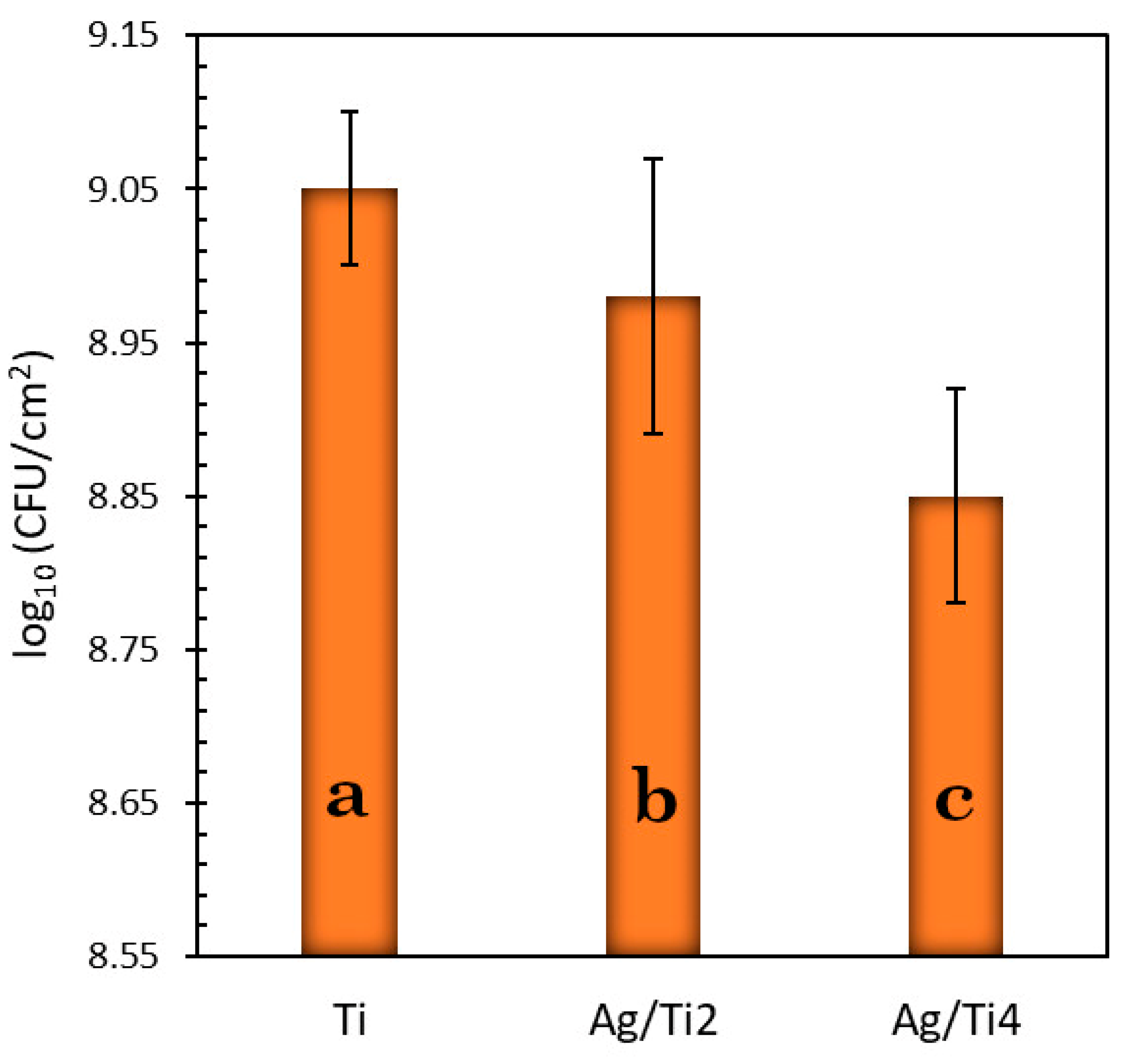
3.2. Statistical Results of the Microbiological Essay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [PubMed]
- Tuson, H.; Weibel, D.B. Bacteria–surface interactions. Soft Matter 2013, 9, 4368–4380. [Google Scholar] [CrossRef]
- Blunk, B.; Perkins, M.; Walsh, D.; Chauhan, V.; Camara, M.; Williams, P.; Aylott, J.; Hardie, K. Use of nanosensor technology to investigate biofilm formation and resulting malodour in washing machines. Access Microbiol. 2019, 1. [Google Scholar] [CrossRef]
- Gahlot, R.; Nigam, C.; Kumar, V.; Yadav, G.; Anupurba, S. Catheter-related bloodstream infections. Int. J. Crit. Illn. Inj. Sci. 2014, 4, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Busscher, H.J.; Rinastiti, M.; Siswomihardjo, W.; van der Mei, H.C. Biofilm Formation on Dental Restorative and Implant Materials. J. Dent. Res. 2010, 89, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Koo, H.; Ren, D. Effects of Material Properties on Bacterial Adhesion and Biofilm Formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef]
- van der Vaart, T.W.; Prins, J.M.; Soetekouw, R.; van Twillert, G.; Veenstra, J.; Herpers, B.L.; Rozemeijer, W.; Jansen, R.R.; Bonten, M.J.; van der Meer, J.T. All-Cause and Infection-Related Mortality in Staphylococcus aureus Bacteremia, a Multicenter Prospective Cohort Study. Open Forum Infect. Dis. 2022, 9, ofac653. [Google Scholar] [CrossRef]
- Baena-Monroy, T.; Moreno-Maldonado, V.; Franco-Martínez, F.; Aldape-Barrios, B.; Quindós, G.; Sánchez-Vargas, L.O. Staphylococcus aureus and Streptococcus mutans Colonization in Patients Wearing Dental Prosthesis. Med. Oral Patol. Oral Cir. Bucal 2005, 10, E27–E39. [Google Scholar]
- Singh, R.; Ray, P.; Das, A.; Sharma, M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Antimicrob. Chemother. 2010, 65, 1955–1958. [Google Scholar] [CrossRef]
- Lewis, N.; Parmar, N.; Hussain, Z.; Baker, G.; Green, I.; Howlett, J.; Kearns, A.; Cookson, B.; McDonald, A.; Wilson, M.; et al. Colonization of dentures by Staphylococcus aureus and MRSA in out-patient and in-patient populations. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1823–1826. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ren, D. Controlling Streptococcus mutans and Staphylococcus aureus biofilms with direct current and chlorhexidine. AMB Express 2017, 7, 204. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.; Webber, M.A. Novel approaches to the treatment of bacterial biofilm infections. Br. J. Pharmacol. 2017, 174, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Jolivet-Gougeon, A.; Bonnaure-Mallet, M. Biofilms as a mechanism of bacterial resistance. Drug Discov. Today Technol. 2014, 11, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Orapiriyakul, W.; Young, P.S.; Damiati, L.; Tsimbouri, P.M. Antibacterial surface modification of titanium implants in orthopaedics. J. Tissue Eng. 2018, 9, 2041731418789838. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. 43. Gram-Positive Pathogens, 3rd ed. In Staphylococcal Biofilms; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 699–711. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1128/9781683670131.ch43 (accessed on 12 June 2024).
- Yoshinari, M.; Kato, T.; Matsuzaka, K.; Hayakawa, T.; Shiba, K. Prevention of biofilm formation on titanium surfaces modified with conjugated molecules comprised of antimicrobial and titanium-binding peptides. Biofouling 2010, 26, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Prasher, P.; Singh, M.; Mudila, H. Oligodynamic Effect of Silver Nanoparticles: A Review. BioNanoScience 2018, 8, 951–962. [Google Scholar] [CrossRef]
- Lampé, I.; Beke, D.; Biri, S.; Csarnovics, I.; Csik, A.; Dombrádi, Z.; Hajdu, P.; Hegedűs, V.; Rácz, R.; Varga, I.; et al. Investigation of silver nanoparticles on titanium surface created by ion implantation technology. J. Nanomed. 2019, 208, 4709–4721. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Gould, M.; Ali, M.A. A review of current advancements for wound healing: Biomaterial applications and medical devices. J. Biomed. Mater. Res. 2022, 110, 2542–2573. [Google Scholar] [CrossRef]
- Haugen, H.J.; Makhtari, S.; Ahmadi, S.; Hussain, B. The Antibacterial and Cytotoxic Effects of Silver Nanoparticles Coated Titanium Implants: A Narrative Review. Materials 2022, 15, 5025. [Google Scholar] [CrossRef]
- Coman, A.N.; Mare, A.; Tanase, C.; Bud, E.; Rusu, A. Silver-Deposited Nanoparticles on the Titanium Nanotubes Surface as a Promising Antibacterial Material into Implants. Metals 2021, 11, 92. [Google Scholar] [CrossRef]
- Scheuerman, T.R.; Camper, A.K.; Hamilton, M.A. Effects of Substratum Topography on Bacterial Adhesion. J. Colloid Interface Sci. 1998, 208, 23–33. [Google Scholar] [CrossRef]
- Carvalho, B.M.; Pellizer, E.P.; Moraes, S.L.D.; Falcón-Antenucci, R.M.; Ferreira Júnior, J.S. Tratamentos de superfície nos implantes dentários. Rev. Cir. Traumatol. Buco-Maxilo-Fac. 2009, 9, 123. [Google Scholar] [CrossRef]
- Giurlani, W.; Zangari, G.; Gambinossi, F.; Passaponti, M.; Salvietti, E.; Di Benedetto, F.; Caporali, S.; Innocenti, M. Electroplating for Decorative Applications: Recent Trends in Research and Development. Coatings 2018, 8, 260. [Google Scholar] [CrossRef]
- Stepanov, A.L. Applications of ion implantation for modification of TiO2: A review. Rev. Adv. Mater. Sci. 2012, 30, 150–465. [Google Scholar]
- Jain, I.P.; Agarwal, G. Ion beam induced surface and interface engineering. Surf. Sci. Rep. 2011, 66, 77–172. [Google Scholar] [CrossRef]
- Nascimento, C.D.; Souza, E.G.; Aguzzoli, C.; Cruz, R.L. Effects of oxygen on the resistivity in Au thin films with Ti-Al adhesion layer. J. Vac. Sci. Technol. B 2019, 37, 052202. [Google Scholar] [CrossRef]
- Rautray, T.R.; Narayanan, R.; Kim, K. Ion implantation of titanium-based biomaterials. Prog. Mater. Sci. 2011, 56, 1137–1177. [Google Scholar] [CrossRef]
- Strafford, K.N. Surface Engineering: Processes and Applications; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Soares, T.P.; Garcia, C.S.C.; Roesch-Ely, M.; Maia da Costa, M.E.H.; Giovanela, M.; Aguzzoli, C. Cytotoxicity and antibacterial efficacy of silver deposited onto titanium plates by low-energy ion implantation. J. Mater. Res. 2018, 33, 2545–2553. [Google Scholar] [CrossRef]
- Flandrois, J.P.; Fardel, G.; Carret, G. Early Stages of In Vitro Killing Curve of LY146032 and Vancomycin for Staphylococcus aureus. Antimicrob. Agents Chemother. 1988, 32, 454–457. [Google Scholar] [CrossRef]
- ASTM F67; Standard Specification for Unalloyed Titanium for Surgical Implant Applications. ASTM International: West Conshohocken, PA, USA, 2006.
- Echeverrigaray, F.G.; Echeverrigaray, S.; Delamare, A.P.L.; Wanke, C.H.; Figueroa, C.A.; Baumvol, I.J.R.; Aguzzoli, C. Antibacterial properties obtained by low-energy silver implantation in stainless steel surfaces. Surf. Coat. Technol. 2016, 307, 345–351. [Google Scholar] [CrossRef]
- de Fraga Malfatti, C.; de Castro, V.V.; Bullmann, M.; Aguzzoli, C. Antibacterial Surfaces Treated with Metal Ions Incorporation by Low-Energy Ionic Ion Implantation: An Opinion. J. Biomed. Res. Environ. Sci. 2024, 5, 271–278. [Google Scholar] [CrossRef]
- Cruz, R.L.; Nascimento, C.D.; Souza, E.G.; Aguzzoli, C.; Moraes, A.C.B.; Lund, R.G. On the Adhesion of Protein in Nitrided Metallic Coatings for Electrosurgical Electrodes of Stainless Steel. Mater. Res. 2022, 25, 1. [Google Scholar] [CrossRef]
- Peralta, M.A.; da Silva, M.A.; Ortega, M.G.; Cabrera, J.L.; Paraje, M.G. Antifungal activity of a prenylated flavonoid from Dalea elegans against Candida albicans biofilms. Phytomedicine 2015, 22, 975–980. [Google Scholar] [CrossRef]
- Berne, C.; Ellison, C.K.; Ducret, A.; Brun, Y.V. Bacterial adhesion at the single-cell level. Nat. Rev. Microbiol. 2018, 16, 616–627. [Google Scholar] [CrossRef]
- Kreve, S.; Reis, A.C.D. Bacterial adhesion to biomaterials: What regulates this attachment? A review. Jpn. Dent. Sci. Rev. 2021, 57, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.D.; Granemann Souza, E.; Aguzzoli, C. Correlation between electrical and compositional properties in cadmium sulfide deposited by laser ablation. Thin Solid Films 2018, 651, 39–41. [Google Scholar] [CrossRef]
- Mayer, M. SIMNRA User’s Guide; Report IPP—Max-Planck-Institut fur Plasmaphysik; 1997; Volume 9. Available online: https://mam.home.ipp.mpg.de/Report%20IPP%209-113.pdf (accessed on 12 June 2024).
- Ziegler, J.P.; Ziegler, M.D.; Biersack, J.P. The Program Stopping and Range of Ion in Matter (SRIM) 2013 Pro. Available online: http://www.srim.org (accessed on 12 June 2024).
- Zeraik, A.N.; Nitschke, M. Biosurfactants as agents to reduce adhesion of pathogenic bacteria to polystyrene surfaces: Effect of temperature and hydrophobicity. Curr. Microbiol. 2010, 61, 554. [Google Scholar] [CrossRef]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell Infect. Microbiol. 2014, 4, 112. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 12, 643722. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial Silver in Medicinal and Consumer Applications: A Patent Review of the Past Decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Tlili, A.; Jabiol, J.; Behra, R.; Gil-Allu´e, C.; Gessner, M.O. Chronic Exposure Effects of Silver Nanoparticles on Stream Microbial Decomposer Communities and Ecosystem Functions. Environ. Sci. Technol. 2017, 51, 2447–2455. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, D.S.; El-Baky, R.M.A.; Sandle, T.; Mandour, S.A.; Ahmed, E.F. Antimicrobial Activity of Silver-Treated Bacteria against other Multi-Drug Resistant Pathogens in Their Environment. Antibiotics 2020, 9, 181. [Google Scholar] [CrossRef] [PubMed]


| Sample | Contact Angle (°) | Contact Angle Image | Ra (µm) |
|---|---|---|---|
| Ti | 107.6 (1.01) a |  | 0.19 (0.01) a |
| Ag/Ti2 | 97.3 (0.48) c |  | 0.10 (0.03) b |
| Ag/Ti4 | 102.7 (0.55) b |  | 0.20 (0.01) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, E.G.; Nascimento, C.d.D.d.; Aguzzoli, C.; Santillán, E.S.B.; Cuevas-Suárez, C.E.; Nascente, P.d.S.; Piva, E.; Lund, R.G. Enhanced Antibacterial Properties of Titanium Surfaces through Diversified Ion Plating with Silver Atom Deposition. J. Funct. Biomater. 2024, 15, 164. https://doi.org/10.3390/jfb15060164
Souza EG, Nascimento CdDd, Aguzzoli C, Santillán ESB, Cuevas-Suárez CE, Nascente PdS, Piva E, Lund RG. Enhanced Antibacterial Properties of Titanium Surfaces through Diversified Ion Plating with Silver Atom Deposition. Journal of Functional Biomaterials. 2024; 15(6):164. https://doi.org/10.3390/jfb15060164
Chicago/Turabian StyleSouza, Everton Granemann, Chiara das Dores do Nascimento, Cesar Aguzzoli, Elena Sarai Baena Santillán, Carlos Enrique Cuevas-Suárez, Patricia da Silva Nascente, Evandro Piva, and Rafael Guerra Lund. 2024. "Enhanced Antibacterial Properties of Titanium Surfaces through Diversified Ion Plating with Silver Atom Deposition" Journal of Functional Biomaterials 15, no. 6: 164. https://doi.org/10.3390/jfb15060164
APA StyleSouza, E. G., Nascimento, C. d. D. d., Aguzzoli, C., Santillán, E. S. B., Cuevas-Suárez, C. E., Nascente, P. d. S., Piva, E., & Lund, R. G. (2024). Enhanced Antibacterial Properties of Titanium Surfaces through Diversified Ion Plating with Silver Atom Deposition. Journal of Functional Biomaterials, 15(6), 164. https://doi.org/10.3390/jfb15060164










