Enhancing the Antimicrobial Properties of Experimental Resin-Based Dental Composites through the Addition of Quaternary Ammonium Salts
Abstract
:1. Introduction
- In dental composites: 2-methacryloxyethyl hexadecyl methyl ammonium bromide (MAE-HB) [18], cetyltrimethylammonium bromide (CTAB), dimethyldioctadecylammonium bromide (DODAB) [2,4], CHX released from a dental composite reduces bacterial adhesion to the dental material without harmful effects on the oral environment [19];
- In denture-based acrylic resins: Poly 202063A [20];
2. Materials and Methods
2.1. Chemicals and Reagents
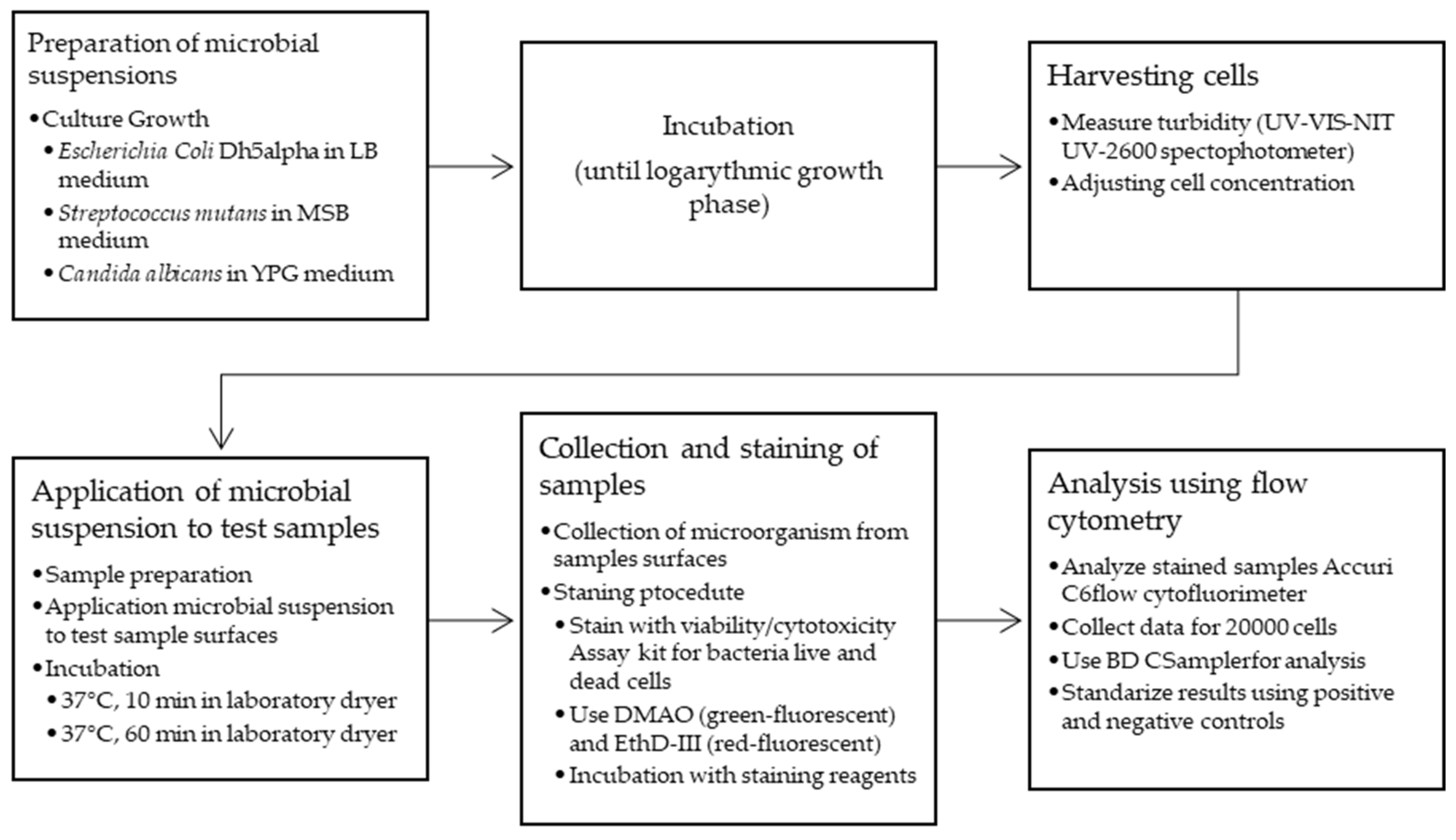
2.2. Samples Preparation
2.3. Antibacterial Properties Testing Methods
2.3.1. Procedure for Surface Bactericidal Analysis with Escherichia coli, Streptococcus mutans, and Candida albicans
2.3.2. Bacterial/Yeast Surface Colonization
3. Results
3.1. The Surface Bactericidal Analysis with Escherichia coli, Streptococcus mutans, and Candida albicans
3.2. Antibacterial and Antifungal Properties of the Surface QAS-Modified Experimental Dental Composite
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Group 1 | Group 2 | p-Value | Significance |
|---|---|---|---|
| 0.5 wt% CTAB | 1.0 wt% CTAB | 0.7488 | False |
| 0.5 wt% CTAB | 1.0 wt% DODAB | 0.9896 | False |
| 0.5 wt% CTAB | 2.0 wt% DODAB | 0.0554 | False |
| 0.5 wt% CTAB | 2.0 wt% CTAB | 0.0072 | True |
| 0.5 wt% CTAB | experimental composite | 0.0001 | True |
| 0.5 wt% CTAB | 0.5 wt% DODAB | 0.9632 | False |
| 0.5 wt% DODAB | 1.0 wt% CTAB | 0.3635 | False |
| 0.5 wt% DODAB | 1.0 wt% DODAB | 0.7081 | False |
| 0.5 wt% DODAB | 2.0 wt% DODAB | 0.0057 | True |
| 0.5 wt% DODAB | 2.0 wt% CTAB | 0.0007 | True |
| 0.5 wt% DODAB | experimental composite | 0.0001 | True |
| 1.0 wt% CTAB | 1.0 wt% DODAB | 0.9875 | False |
| 1.0 wt% CTAB | 2.0 wt% DODAB | 0.4821 | False |
| 1.0 wt% CTAB | 2.0 wt% CTAB | 0.1234 | False |
| 1.0 wt% CTAB | experimental composite | 0.0006 | True |
| 1.0 wt% DODAB | 2.0 wt% DODAB | 0.2227 | False |
| 1.0 wt% DODAB | 2.0 wt% CTAB | 0.0412 | True |
| 1.0 wt% DODAB | experimental composite | 0.0001 | True |
| 2.0 wt% DODAB | 2.0 wt% CTAB | 0.9897 | False |
| 2.0 wt% DODAB | experimental composite | 0.0596 | False |
| 2.0 wt% CTAB | Experimental composite | 0.3201 | False |
| Group 1 | Group 2 | p-Value | Significance |
|---|---|---|---|
| 0.5 wt% CTAB | 0.5 wt% DODAB | 0.0000 | True |
| 0.5 wt% CTAB | 1.0 wt% CTAB | 0.0002 | True |
| 0.5 wt% CTAB | 1.0 wt% DODAB | 0.0000 | True |
| 0.5 wt% CTAB | 2.0 wt% CTAB | 0.0000 | True |
| 0.5 wt% CTAB | 2.0 wt% DODAB | 0.0000 | True |
| 0.5 wt% CTAB | experimental composite | 0.0129 | True |
| 0.5 wt% DODAB | 1.0 wt% CTAB | 0.0002 | True |
| 0.5 wt% DODAB | 1.0 wt% DODAB | 0.0000 | True |
| 0.5 wt% DODAB | 2.0 wt% CTAB | 0.0000 | True |
| 0.5 wt% DODAB | 2.0 wt% DODAB | 0.0000 | True |
| 0.5 wt% DODAB | experimental composite | 0.0129 | True |
| 1.0 wt% CTAB | 1.0 wt% DODAB | 0.0000 | True |
| 1.0 wt% CTAB | 2.0 wt% CTAB | 0.0000 | True |
| 1.0 wt% CTAB | 2.0 wt% DODAB | 0.0000 | True |
| 1.0 wt% CTAB | experimental composite | 0.0001 | True |
| 1.0 wt% DODAB | 2.0 wt% CTAB | 0.0000 | True |
| 1.0 wt% DODAB | 2.0 wt% DODAB | 0.0000 | True |
| 1.0 wt% DODAB | experimental composite | 0.0000 | True |
| 2.0 wt% CTAB | 2.0 wt% DODAB | 0.0000 | True |
| 2.0 wt% CTAB | experimental composite | 0.0000 | True |
| 2.0 wt% DODAB | experimental composite | 0.0000 | True |
| Group 1 | Group 2 | p-Value | Significance |
|---|---|---|---|
| experimental composite | 0.5 wt% DODAB | 0.9971 | False |
| experimental composite | 1.0 wt% DODAB | 0.3490 | False |
| experimental composite | 2.0 wt% DODAB | 0.0035 | True |
| experimental composite | 0.5 wt% CTAB | 0.9994 | False |
| experimental composite | 1.0 wt% CTAB | 0.9432 | False |
| experimental composite | 2.0 wt% CTAB | 0.9970 | False |
| 0.5 wt% DODAB | 1.0 wt% DODAB | 0.7601 | False |
| 0.5 wt% DODAB | 2.0 wt% DODAB | 0.0282 | True |
| 0.5 wt% DODAB | 0.5 wt% CTAB | 0.9047 | False |
| 0.5 wt% DODAB | 1.0 wt% CTAB | 0.9999 | False |
| 0.5 wt% DODAB | 2.0 wt% CTAB | 1.0000 | False |
| 1.0 wt% DODAB | 2.0 wt% DODAB | 0.6773 | False |
| 1.0 wt% DODAB | 0.5 wt% CTAB | 0.0806 | False |
| 1.0 wt% DODAB | 1.0 wt% CTAB | 0.9047 | False |
| 1.0 wt% DODAB | 2.0 wt% CTAB | 0.7611 | False |
| 2.0 wt% DODAB | 0.5 wt% CTAB | 0.0005 | True |
| 2.0 wt% DODAB | 1.0 wt% CTAB | 0.0570 | False |
| 2.0 wt% DODAB | 2.0 wt% CTAB | 0.0285 | True |
| 0.5 wt% CTAB | 1.0 wt% CTAB | 0.7815 | False |
| 0.5 wt% CTAB | 2.0 wt% CTAB | 0.9025 | False |
| 1.0 wt% CTAB | 2.0 wt% CTAB | 1.0000 | False |
| Group 1 | Group 2 | p-Value | Significance |
|---|---|---|---|
| experimental composite | 0.5 wt% DODAB | 0.0000 | True |
| experimental composite | 1 wt% DODAB | 0.0000 | True |
| experimental composite | 2 wt% DODAB | 0.0000 | True |
| experimental composite | 0.5 wt% CTAB | 0.0000 | True |
| experimental composite | 1.0 wt% CTAB | 0.0000 | True |
| experimental composite | 2.0 wt% CTAB | 0.0000 | True |
| 0.5 wt% DODAB | 1.0 wt% DODAB | 0.0000 | True |
| 0.5 wt% DODAB | 2.0 wt% DODAB | 0.0000 | True |
| 0.5 wt% DODAB | 0.5 wt% CTAB | 0.9968 | False |
| 0.5 wt% DODAB | 1.0 wt% CTAB | 0.0205 | True |
| 0.5 wt% DODAB | 2.0 wt% CTAB | 0.6323 | False |
| 1.0 wt% DODAB | 2.0 wt% DODAB | 1.0000 | False |
| 1.0 wt% DODAB | 0.5 wt% CTAB | 0.0000 | True |
| 1.0 wt% DODAB | 1.0 wt% CTAB | 0.0000 | True |
| 1.0 wt% DODAB | 2.0 wt% CTAB | 0.0000 | True |
| 2.0 wt% DODAB | 0.5 wt% CTAB | 0.0000 | True |
| 2.0 wt% DODAB | 1.0 wt% CTAB | 0.0000 | True |
| 2.0 wt% DODAB | 2.0 wt% CTAB | 0.0000 | True |
| 0.5 wt% CTAB | 1.0 wt% CTAB | 0.9162 | False |
| 0.5 wt% CTAB | 2.0 wt% CTAB | 0.8971 | False |
| 1.0 wt% CTAB | 2.0 wt% CTAB | 1.0000 | False |
| Group 1 | Group 2 | p-Value | Significance |
|---|---|---|---|
| 0.5 wt% CTAB | 1.0 wt% CTAB | 0.001 | True |
| 0.5 wt% CTAB | 1.0 wt% DODAB | 0.001 | True |
| 0.5 wt% CTAB | 2.0 wt% DODAB | 0.001 | True |
| 0.5 wt% CTAB | 2.0 wt% CTAB | 0.001 | True |
| 0.5 wt% CTAB | experimental composite | 0.001 | True |
| 0.5 wt% CTAB | 0.5 wt% DODAB | 0.9 | False |
| 0.5 wt% DODAB | 1.0 wt% CTAB | 0.001 | True |
| 0.5 wt% DODAB | 1.0 wt% DODAB | 0.001 | True |
| 0.5 wt% DODAB | 2.0 wt% DODAB | 0.001 | True |
| 0.5 wt% DODAB | 2.0 wt% CTAB | 0.001 | True |
| 0.5 wt% DODAB | experimental composite | 0.001 | True |
| 1.0 wt% CTAB | 1.0 wt% DODAB | 0.7334 | False |
| 1.0 wt% CTAB | 2.0 wt% DODAB | 0.001 | True |
| 1.0 wt% CTAB | 2.0 wt% CTAB | 0.001 | True |
| 1.0 wt% CTAB | experimental composite | 0.001 | True |
| 1.0 wt% DODAB | 2.0 wt% DODAB | 0.001 | True |
| 1.0 wt% DODAB | 2.0 wt% CTAB | 0.001 | True |
| 1.0 wt% DODAB | experimental composite | 0.001 | True |
| 2.0 wt% DODAB | 2.0 wt% CTAB | 0.9 | False |
| 2.0 wt% DODAB | experimental composite | 0.9 | False |
| 2.0 wt% CTAB | experimental composite | 0.9 | False |
| Group 1 | Group 2 | p-Value | Significance |
|---|---|---|---|
| 0.5 wt% CTAB | 1 wt% CTAB | 0.729 | False |
| 0.5 wt% CTAB | 1 wt% DODAB | 0.9 | False |
| 0.5 wt% CTAB | 2 wt% DODAB | 0.9 | False |
| 0.5 wt% CTAB | 2 wt% CTAB | 0.0 | True |
| 0.5 wt% CTAB | experimental composite | 0.9 | False |
| 0.5 wt% CTAB | 0.5 wt% DODAB | 0.736 | False |
| 0.5 wt% DODAB | 1.0 wt% CTAB | 0.9 | False |
| 0.5 wt% DODAB | 1.0 wt% DODAB | 0.9 | False |
| 0.5 wt% DODAB | 2.0 wt% DODAB | 0.9 | False |
| 0.5 wt% DODAB | 2.0 wt% CTAB | 0.0 | True |
| 0.5 wt% DODAB | experimental composite | 0.9 | False |
| 1.0 wt% CTAB | 1.0 wt% DODAB | 0.9 | False |
| 1.0 wt% CTAB | 2.0 wt% DODAB | 0.9 | False |
| 1.0 wt% CTAB | 2.0 wt% CTAB | 0.0 | True |
| 1.0 wt% CTAB | experimental composite | 0.9 | False |
| 1.0 wt% DODAB | 2.0 wt% DODAB | 0.9 | False |
| 1.0 wt% DODAB | 2.0 wt% CTAB | 0.0 | True |
| 1.0 wt% DODAB | experimental composite | 0.9 | False |
| 2.0 wt% DODAB | 2.0 wt% CTAB | 0.0 | True |
| 2.0 wt% DODAB | experimental composite | 0.9 | False |
| 2.0 wt% CTAB | experimental composite | 0.0 | True |
| Group 1 | Group 2 | p-Value | Significance |
|---|---|---|---|
| 0.5 wt% CTAB | 1.0 wt% CTAB | 0.7488 | False |
| 0.5 wt% CTAB | 1.0 wt% DODAB | 0.9896 | False |
| 0.5 wt% CTAB | 2.0 wt% DODAB | 0.0554 | False |
| 0.5 wt% CTAB | 2.0 wt% CTAB | 0.0072 | True |
| 0.5 wt% CTAB | experimental composite | 0.0001 | True |
| 0.5 wt% CTAB | 0.5 wt% DODAB | 0.9632 | False |
| 0.5 wt% DODAB | 1.0 wt% CTAB | 0.3635 | False |
| 0.5 wt% DODAB | 1.0 wt% DODAB | 0.7081 | False |
| 0.5 wt% DODAB | 2.0 wt% DODAB | 0.0057 | True |
| 0.5 wt% DODAB | 2.0 wt% CTAB | 0.0007 | True |
| 0.5 wt% DODAB | experimental composite | 0.0001 | True |
| 1.0 wt% CTAB | 1.0 wt% DODAB | 0.9875 | False |
| 1.0 wt% CTAB | 2.0 wt% DODAB | 0.4821 | False |
| 1.0 wt% CTAB | 2.0 wt% CTAB | 0.1234 | False |
| 1.0 wt% CTAB | experimental composite | 0.0006 | True |
| 1.0 wt% DODAB | 2.0 wt% DODAB | 0.2227 | False |
| 1.0 wt% DODAB | 2.0 wt% CTAB | 0.0412 | True |
| 1.0 wt% DODAB | experimental composite | 0.0001 | True |
| 2.0 wt% DODAB | 2.0 wt% CTAB | 0.9897 | False |
| 2.0 wt% DODAB | experimental composite | 0.0596 | False |
| 2.0 wt% CTAB | experimental composite | 0.3201 | False |
References
- Seemann, R.; Flury, S.; Pfefferkorn, F.; Lussi, A.; Noack, M.J. Restorative Dentistry and Restorative Materials over the next 20 Years: A Delphi Survey. Dent. Mater. 2014, 30, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Oral Health. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 2 March 2024).
- Cheng, L.; Zhang, K.; Zhang, N.; Melo, M.A.S.; Weir, M.D.; Zhou, X.D.; Bai, Y.X.; Reynolds, M.A.; Xu, H.H.K. Developing a New Generation of Antimicrobial and Bioactive Dental Resins. J. Dent. Res. 2017, 96, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Zalega, M.; Nowak, J.; Bociong, K. The Influence of Quaternary Ammonium Salts on Mechanical Properties of Light-Cured Resin Dental Composites. Polimery/Polymers 2023, 68, 195–205. [Google Scholar] [CrossRef]
- Phillips, R.W.; Shen, C.; Rawls, H.R.; Anusavice, K.J. Phillip’s Science of Dental Materials; Elsevier Health Sciences: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Qiu, W.; Zhou, Y.; Li, Z.; Huang, T.; Xiao, Y.; Cheng, L.; Peng, X.; Zhang, L.; Ren, B. Application of Antibiotics/Antimicrobial Agents on Dental Caries. Biomed Res. Int. 2020, 2020, 5658212. [Google Scholar] [CrossRef] [PubMed]
- Favaro, J.C.; Detomini, T.R.; Maia, L.P.; Poli, R.C.; Guiraldo, R.D.; Lopes, M.B.; Berger, S.B. Anticaries Agent Based on Silver Nanoparticles and Fluoride: Characterization and Biological and Remineralizing Effects—An In Vitro Study. Int. J. Dent. 2022, 2022, 9483589. [Google Scholar] [CrossRef] [PubMed]
- Ahlstrom, B.; Chelminska-Bertilsson, M.; Thompson, R.A.; Edebo, L. Long-Chain Alkanoylcholines, a New Category of Soft Antimicrobial Agents That Are Enzymatically Degradable. Antimicrob. Agents Chemother. 1995, 39, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial Quaternary Ammonium Compounds in Dental Materials: A Systematic Review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Niu, L.N.; Ma, S.; Li, J.; Tay, F.R.; Chen, J. hua Quaternary Ammonium-Based Biomedical Materials: State-of-the-Art, Toxicological Aspects and Antimicrobial Resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef] [PubMed]
- Alkattan, R.; Rojo, L.; Deb, S. Antimicrobials in Dentistry. Appl. Sci. 2021, 11, 3279. [Google Scholar] [CrossRef]
- Montoya, C.; Roldan, L.; Yu, M.; Valliani, S.; Ta, C.; Yang, M.; Orrego, S. Smart Dental Materials for Antimicrobial Applications. Bioact. Mater. 2023, 24, 1–19. [Google Scholar] [CrossRef]
- Weng, Y.; Guo, X.; Chong, V.J.; Howard, L.; Gregory, R.L.; Xie, D.; Weng, Y.; Guo, X.; Chong, V.J.; Howard, L.; et al. Synthesis and Evaluation of a Novel Antibacterial Dental Resin Composite with Quaternary Ammonium Salts. J. Biomed. Sci. Eng. 2011, 4, 147–157. [Google Scholar] [CrossRef]
- Chrószcz, M.; Barszczewska-Rybarek, I. Synthesis of Novel Urethane-Dimethacrylate Monomer Containing Two Quaternary Ammonium Groups for Applications in Dentistry. Proceedings 2020, 67, 3. [Google Scholar] [CrossRef]
- He, J.; Söderling, E.; Vallittu, P.K.; Lassila, L.V.J. Investigation of Double Bond Conversion, Mechanical Properties, and Antibacterial Activity of Dental Resins with Different Alkyl Chain Length Quaternary Ammonium Methacrylate Monomers (QAM). J. Biomater. Sci. Polym. Ed. 2013, 24, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, S.; Zhou, X.; Xu, H.H.K.; Weir, M.D.; Ge, Y.; Li, M.; Wang, S.; Li, Y.; Xu, X.; et al. Effect of Antibacterial Dental Adhesive on Multispecies Biofilms Formation. J. Dent. Res. 2015, 94, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.H.; Ma, S.; Chen, J.H.; Chai, Z.G.; Li, F.; Wang, Y.J. Antibacterial Activity and Bonding Ability of an Adhesive Incorporating an Antibacterial Monomer DMAE-CB. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90B, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yu, F.; Sun, X.; Dong, Y.; Lin, P.T.; Yu, H.H.; Xiao, Y.H.; Chai, Z.G.; Xing, X.D.; Chen, J.H. Antibacterial Activity of a Modified Unfilled Resin Containing a Novel Polymerizable Quaternary Ammonium Salt MAE-HB. Sci. Rep. 2016, 6, 33858. [Google Scholar] [CrossRef] [PubMed]
- Larissa, P.; Gambrill, B.; de Carvalho, R.D.P.; Picolo, M.Z.D.; Cavalli, V.; Boaro, L.C.C.; Prokopovich, P.; Cogo-Müller, K. Development, Characterization and Antimicrobial Activity of Multilayer Silica Nanoparticles with Chlorhexidine Incorporated into Dental Composites. Dent. Mater. 2023, 39, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Pesci-Bardon, C.; Fosse, T.; Serre, D.; Madinier, I. In Vitro Antiseptic Properties of an Ammonium Compound Combined with Denture Base Acrylic Resin. Gerodontology 2006, 23, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, J.M.; Zeiger, D.N.; Tang, K.; Lin-Gibson, S.; Fowler, B.O.; Lin, N.J. Synthesis and Characterization of Dimethacrylates Containing Quaternary Ammonium Functionalities for Dental Applications. Dent. Mater. 2012, 28, 219–228. [Google Scholar] [CrossRef]
- Chladek, G.; Barszczewska-Rybarek, I.; Chrószcz-Porębska, M.; Mertas, A. The Effect of Quaternary Ammonium Polyethylenimine Nanoparticles on Bacterial Adherence, Cytotoxicity, and Physical and Mechanical Properties of Experimental Dental Composites. Sci. Rep. 2023, 13, 17497. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.M.; Chrószcz, M.W.; Chladek, G. Physicochemical and Mechanical Properties of Bis-Gma/Tegdma Dental Composite Resins Enriched with Quaternary Ammonium Polyethylenimine Nanoparticles. Materials 2021, 14, 2037. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.; Moore, L.E. Cationic Antiseptics: Diversity of Action under a Common Epithet. J. Appl. Microbiol. 2005, 99, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.B.; Carmona-Ribeiro, A.M. Cationic Lipids and Surfactants as Antifungal Agents: Mode of Action. J. Antimicrob. Chemother. 2006, 58, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Lindstedt, M.; Allenmark, S.; Thompson, R.A.; Edebo, L. Antimicrobial Activity of Betaine Esters, Quaternary Ammonium Amphiphiles Which Spontaneously Hydrolyze into Nontoxic Components. Antimicrob. Agents Chemother. 1990, 34, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Gottenbos, B.; Van Der Mei, H.C.; Klatter, F.; Nieuwenhuis, P.; Busscher, H.J. In Vitro and in Vivo Antimicrobial Activity of Covalently Coupled Quaternary Ammonium Silane Coatings on Silicone Rubber. Biomaterials 2002, 23, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Abduraimova, A.; Molkenova, A.; Duisembekova, A.; Mulikova, T.; Kanayeva, D.; Atabaev, T.S. Cetyltrimethylammonium Bromide (CTAB)-Loaded SiO2–Ag Mesoporous Nanocomposite as an Efficient Antibacterial Agent. Nanomaterials 2021, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Tsuchido, T.; Matsumura, Y. Antimicrobial Cationic Surfactant, Cetyltrimethylammonium Bromide, Induces Superoxide Stress in Escherichia Coli Cells. J. Appl. Microbiol. 2011, 110, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Mad Salim, M.; Nik Malek, N.A.N.; Ramli, N.I.; Mohd Hanim, S.A.; Hamdan, S. Antibacterial Activity of CTAB-Modified Zeolite NaY with Different CTAB Loading. Malays. J. Fundam. Appl. Sci. 2014, 10, 129–133. [Google Scholar] [CrossRef]
- Martins, L.S.; Nomura, D.A.; Duarte, E.L.; Riske, K.A.; Lamy, M.T.; Rozenfeld, J.H.K. Structural Characterization of Cationic DODAB Bilayers Containing C24:1 β-Glucosylceramide. Biochim. Biophys. Acta Biomembr. 2019, 1861, 643–650. [Google Scholar] [CrossRef]
- Sharma, V.K.; Srinivasan, H.; Garc Ia Sakai, V.; Mitra, S. Dioctadecyldimethylammonium Bromide, a Surfactant Model for the Cell Membrane: Importance of Microscopic Dynamics. Struct. Dyn. 2020, 7, 51301. [Google Scholar] [CrossRef]
- Kleczewska, J.; Bieliński, D.M.; Nowak, J.; Sokołowski, J.; Łukomska-Szymańska, M. Dental Composites Based on Dimethacrylate Resins Reinforced by Nanoparticulate Silica. Polym. Polym. Compos. 2016, 24, 411–418. [Google Scholar] [CrossRef]
- Olczak, K.; Jakubowski, W.; Szymański, W. Bactericidal Activity of Graphene Oxide Tests for Selected Microorganisms. Materials 2023, 16, 4199. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, W.; Walkowiak, B. Resistance of Oxidative Stress in Biofilm and Planktonic Cells. Braz. Arch. Biol. Technol. 2015, 58, 300–308. [Google Scholar] [CrossRef]
- Mamizuka, E.M.; Carmonaribeiro, A.M. Cationic Liposomes as Antimicrobial Agents. Commun. Curr. Res. Educ. Top. Trends Appl. Microbiol. 2007, 2, 636–647. [Google Scholar]
- Ishikawa, S.; Matsumura, Y.; Katoh-Kubo, K.; Tsuchido, T. Antibacterial Activity of Surfactants against Escherichia Coli Cells Is Influenced by Carbon Source and Anaerobiosis. J. Appl. Microbiol. 2002, 93, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.T.; Galvão, C.N.; Betancourt, Y.P.; Mathiazzi, B.I.; Carmona-Ribeiro, A.M. Microbicidal Dispersions and Coatings from Hybrid Nanoparticles of Poly (Methyl Methacrylate), Poly (Diallyl Dimethyl Ammonium) Chloride, Lipids, and Surfactants. Int. J. Mol. Sci. 2019, 20, 6150. [Google Scholar] [CrossRef] [PubMed]
- Nadagouda, M.N.; Vijayasarathy, P.; Sin, A.; Nam, H.; Khan, S.; Parambath, J.B.M.; Mohamed, A.A.; Han, C. Antimicrobial Activity of Quaternary Ammonium Salts: Structure-Activity Relationship. Med. Chem. Res. 2022, 31, 1663–1678. [Google Scholar] [CrossRef]
- Melo, L.D.; Palombo, R.R.; Petri, D.F.S.; Bruns, M.; Pereira, E.M.A.; Carmona-Ribeiro, A.M. StructureÀActivity Relationship for Quaternary Ammonium Compounds Hybridized with Poly(Methyl Methacrylate). ACS Appl. Mater. Interfaces 2011, 3, 1933–1939. [Google Scholar] [CrossRef]



| Ingredient Name | Molecular Weight [g/mol] | Purity [%] | Ratio [wt%] | LOT/ Batch | Manufacturer |
|---|---|---|---|---|---|
| bisphenol A glycerolate dimethacrylate (bis-GMA) | 512 | >97 | 40 | MIKCR9254 | Sigma-Aldrich, St. Louis, MO, USA |
| diurethane dimethacrylate (UDMA) | 470 | >97 | 40 | #MKCG8230 | Sigma-Aldrich, Steinheim, Germany |
| 2-hydroxyethyl methacrylate (HEMA) | 130 | >97 | 10 | #GTBC3071V | Sigma-Aldrich, Steinheim, Germany |
| triethylene glycol dimethacrylate (TEGDMA) | 470 | >95 | 10 | #STBH8825 | Sigma-Aldrich, Steinheim, Germany |
| camphorquinone (CQ) | 166 | >97 | 0.4 | 09003AQV | Sigma-Aldrich, St. Louis, MO, USA |
| 2-(dimethylamine)ethyl methacrylate (DMAEMA) | 157 | >98 | 0.9 | #BCBZ6476 | Sigma-Aldrich, Steinheim, Germany |
| butylated hydroxytoluene (BHT) | 220 | >99 | 0.1 | #116K0036 | Sigma-Aldrich, St. Louis, MO, USA |
| silica Arsil | <150 g/dm3 | >95 | 45 | 260321 | Zakłady Chemiczne Rudniki S.A., Rudniki, Poland |
| 3-methacrylooxypropyltri-methoxysilane (γ-MPTS) | 196 | >95 | - | 20.10.2020 | Unisil Sp. z o.o, Tarnów, Poland |
| dimethyldioctadecylammonium bromide (DODAB) | 631 | >98 | 0.5–2.0 | BCBR19922V | Sigma-Aldrich, Steinheim, Germany |
| cetyltrimethylammonium bromide (CTAB) | 364 | >98 | 0.5–2.0 | SLCH0757 | Sigma-Aldrich, Product of China |
| QAS [wt%] | Material | Time [min] | Escherichia coli | Streptococcus mutans | Candida albicans |
|---|---|---|---|---|---|
| Dead Cells [%] | Dead Cells [%] | Dead Cells [%] | |||
| 0.0 | experimental composite | 10 | 21.0 | 33.8 | 13.9 |
| 0.5 | CTAB | 10 | 19.4 | 35.0 | 10.2 |
| 1.0 | CTAB | 10 | 20.1 | 37.6 | 12.2 |
| 2.0 | CTAB | 10 | 37.2 | 40.6 | 15.4 |
| 0.5 | DODAB | 10 | 21.1 | 32.9 | 10.0 |
| 1.0 | DODAB | 10 | 22.7 | 35.6 | 10.5 |
| 2.0 | DODAB | 10 | 26.1 | 35.0 | 15.6 |
| 0.0 | experimental composite | 60 | 28.9 | 44.6 | 15.0 |
| 0.5 | CTAB | 60 | 18.8 | 42.9 | 13.0 |
| 1.0 | CTAB | 60 | 24.0 | 57.3 | 15.6 |
| 2.0 | CTAB | 60 | 65.0 | 73.9 | 23.9 |
| 0.5 | DODAB | 60 | 25.1 | 45.9 | 12.2 |
| 1.0 | DODAB | 60 | 35.8 | 54.7 | 15.6 |
| 2.0 | DODAB | 60 | 54.5 | 68.9 | 21.7 |
| QAS [wt%] | Material | Number of Living Cells | Number of Dead Cells | Sum | Average Percent of Living Cells [%] |
|---|---|---|---|---|---|
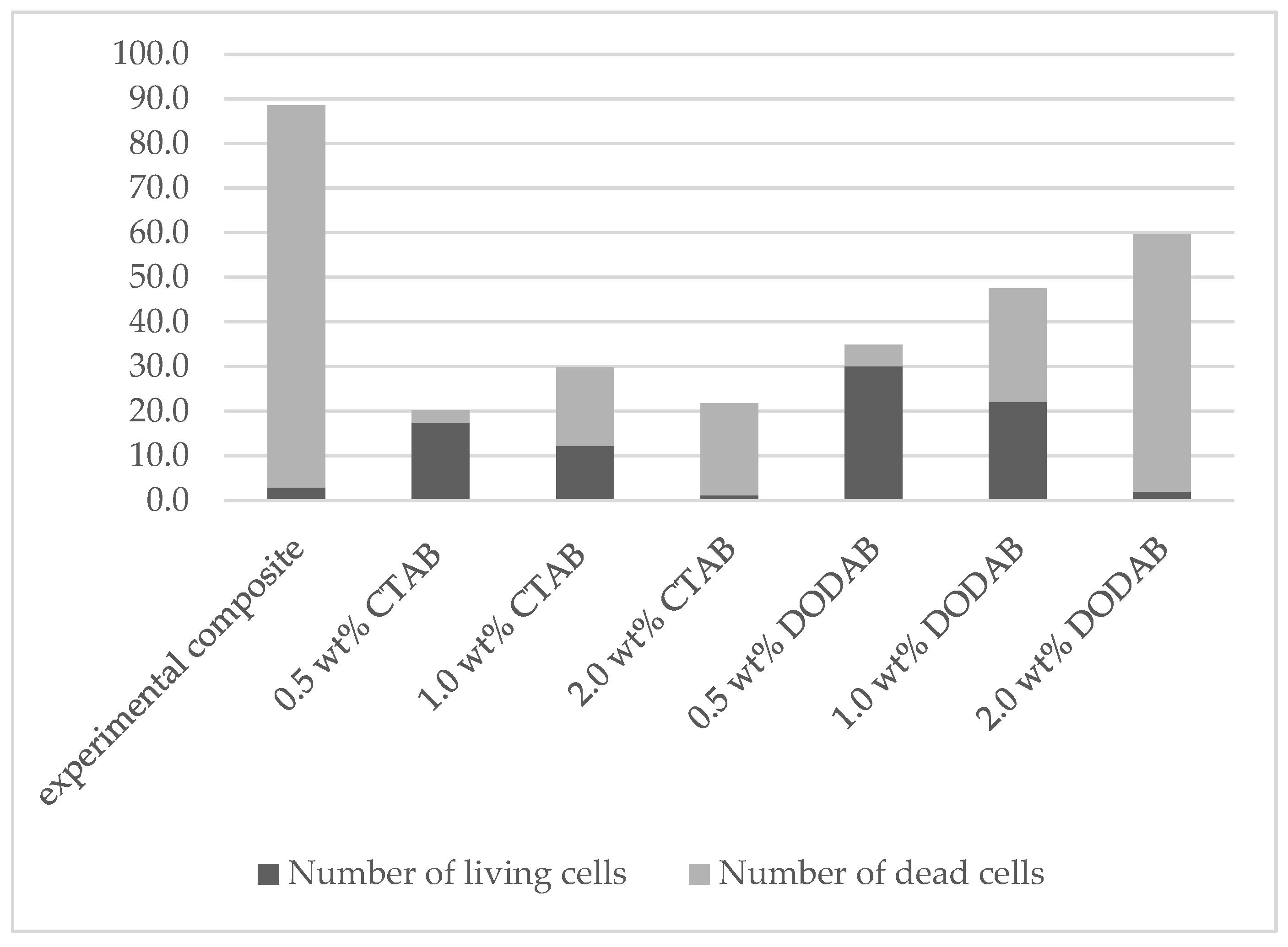
| 0.0 | experimental composite | 2.9 ± 1.0 | 85.6 ± 7.9 | 88.5 ± 8.2 | 3.3 ± 0.8 |
| 0.5 | CTAB | 17.5 ± 3.5 | 2.7 ± 1.2 | 20.2 ± 3.5 | 86.4 ± 5.9 |
| 1.0 | CTAB | 12.2 ± 3.2 | 17.7 ± 2.6 | 29.9 ± 3.3 | 40.6 ± 8.1 |
| 2.0 | CTAB | 1.2 ± 0.6 | 20.6 ± 3.9 | 21.8 ± 3.8 | 5.7 ± 2.9 |
| 0.5 | DODAB | 30.1 ± 4.3 | 4.8 ± 1.2 | 34.9 ± 4.8 | 86.2 ± 3.0 |
| 1.0 | DODAB | 22.1 ± 4.3 | 25.4 ± 3.9 | 47.5 ± 5.2 | 46.4 ± 6.9 |
| 2.0 | DODAB | 2.0 ± 1.1 | 57.6 ± 8.3 | 59.6 ± 8.6 | 3.3 ± 1.6 |
| QAS [wt%] | Material | Number of Living Cells | Number of Dead Cells | Sum | Average Percent of Living Cells [%] |
|---|---|---|---|---|---|
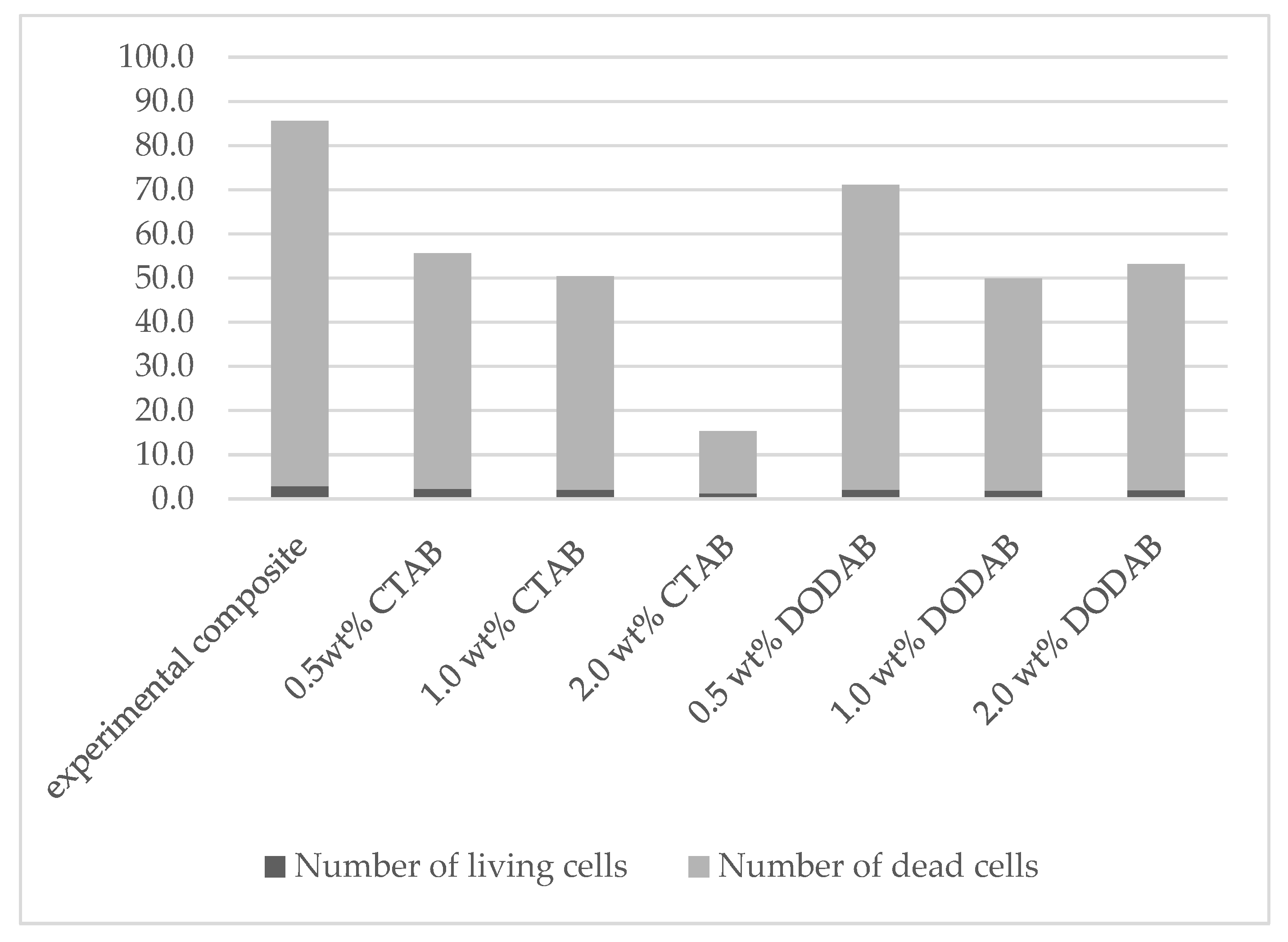
| 0.0 | experimental composite | 2.9 ± 1.0 | 82.7 ± 5.5 | 85.6 ± 6.0 | 3.4 ± 0.9 |
| 0.5 | CTAB | 2.3 ± 1.1 | 53.4 ± 5.9 | 55.7 ± 6.4 | 4.1 ± 1.8 |
| 1.0 | CTAB | 2.1 ± 0.7 | 48.4 ± 4.0 | 50.5 ± 4.2 | 4.1 ± 1.3 |
| 2.0 | CTAB | 1.2 ± 0.6 | 14.1 ± 4.4 | 15.3 ± 4.3 | 8.5 ± 4.7 |
| 0.5 | DODAB | 2.1 ± 0.7 | 69.0 ± 5.3 | 71.1 ± 5.3 | 3.0 ± 1.1 |
| 1.0 | DODAB | 1.9 ± 1.0 | 48.0 ± 6.8 | 49.9 ± 7.2 | 3.7 ± 1.8 |
| 2.0 | DODAB | 2.0 ± 1.1 | 51.2 ± 8.7 | 53.2 ± 8.8 | 3.8 ± 1.9 |
| QAS [wt%] | Material | Number of Living Cells | Number of Dead Cells | Sum | Average Percent of Living Cells [%] |
|---|---|---|---|---|---|
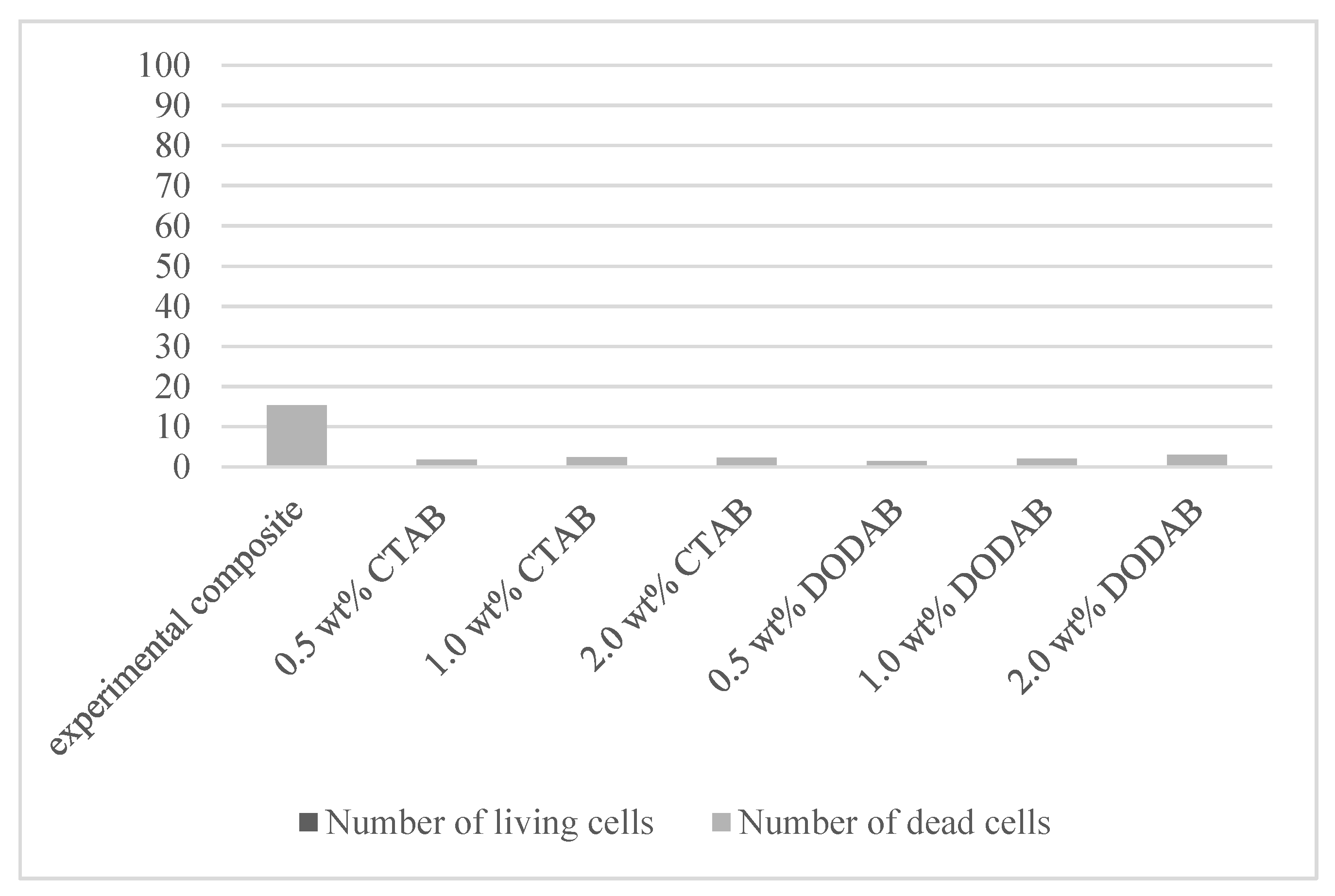
| 0.0 | experimental composite | 50.9 ± 7.9 | 15.4 ± 2.8 | 66.3 ± 10.1 | 76.8 ± 2.2 |
| 0.5 | CTAB | 25.2 ± 2.8 | 1.8 ± 0.9 | 27.0 ± 2.4 | 93.2 ± 3.3 |
| 1.0 | CTAB | 21.4 ± 2.3 | 2.4 ± 0.8 | 23.8 ± 2.6 | 90.0 ± 3.2 |
| 2.0 | CTAB | 12.2 ± 4.8 | 2.3 ± 1.2 | 14.5 ± 4.8 | 82.7 ± 8.2 |
| 0.5 | DODAB | 31.3 ± 4.6 | 1.4 ± 0.5 | 32.7 ± 4.6 | 95.6 ± 1.8 |
| 1.0 | DODAB | 23.6 ± 3.2 | 2.1 ± 1.0 | 25.7 ± 2.8 | 91.6 ± 4.2 |
| 2.0 | DODAB | 17.1 ± 4.7 | 3.0 ± 1.3 | 20.1 ± 5.0 | 84.9 ± 7.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, J.; Zalega, M.; Jakubowski, W.; Domarecka, M.; Sokołowski, J.; Bociong, K. Enhancing the Antimicrobial Properties of Experimental Resin-Based Dental Composites through the Addition of Quaternary Ammonium Salts. J. Funct. Biomater. 2024, 15, 213. https://doi.org/10.3390/jfb15080213
Nowak J, Zalega M, Jakubowski W, Domarecka M, Sokołowski J, Bociong K. Enhancing the Antimicrobial Properties of Experimental Resin-Based Dental Composites through the Addition of Quaternary Ammonium Salts. Journal of Functional Biomaterials. 2024; 15(8):213. https://doi.org/10.3390/jfb15080213
Chicago/Turabian StyleNowak, Joanna, Maja Zalega, Witold Jakubowski, Monika Domarecka, Jerzy Sokołowski, and Kinga Bociong. 2024. "Enhancing the Antimicrobial Properties of Experimental Resin-Based Dental Composites through the Addition of Quaternary Ammonium Salts" Journal of Functional Biomaterials 15, no. 8: 213. https://doi.org/10.3390/jfb15080213






