Cationic Serine-Based Gemini Surfactant:Monoolein Aggregates as Viable and Efficacious Agents for DNA Complexation and Compaction: A Cytotoxicity and Physicochemical Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Cell Culture and Cytotoxicity Evaluation
2.4. Light Microscopy
2.5. Dynamic Light Scattering (DLS)
2.6. DNA Complexation: Ethidium Bromide Exclusion Assay
3. Results and Discussion
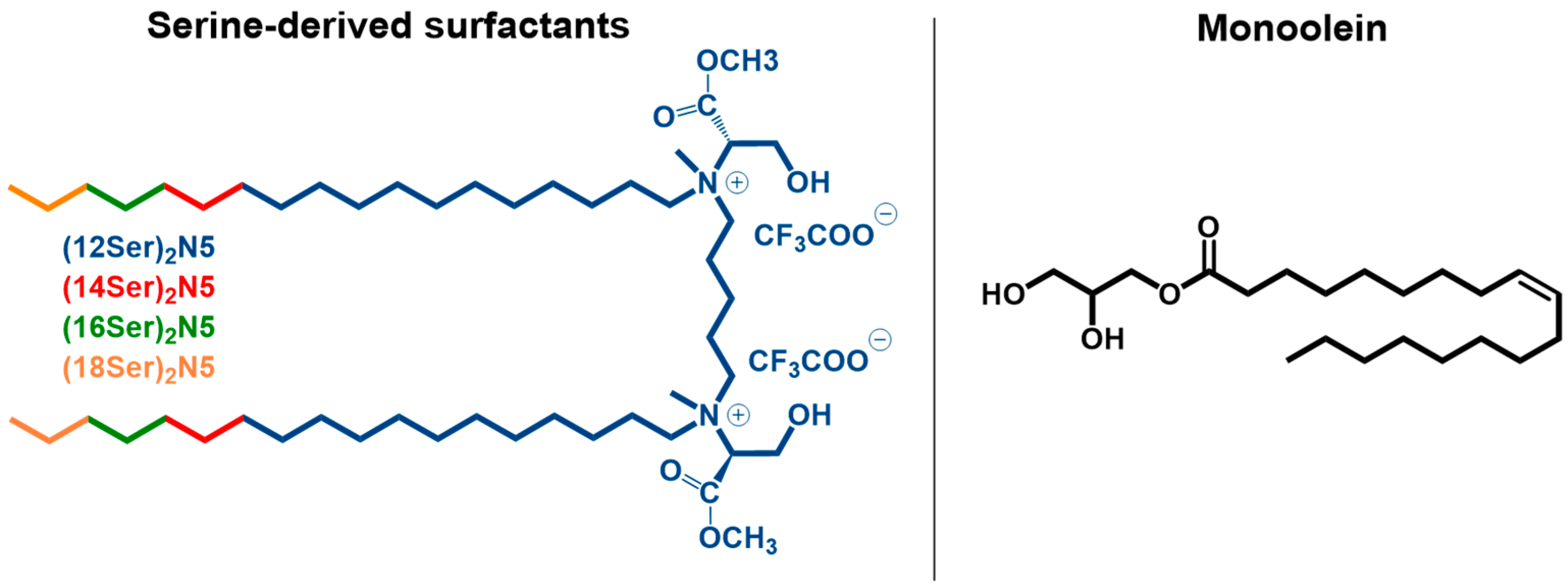
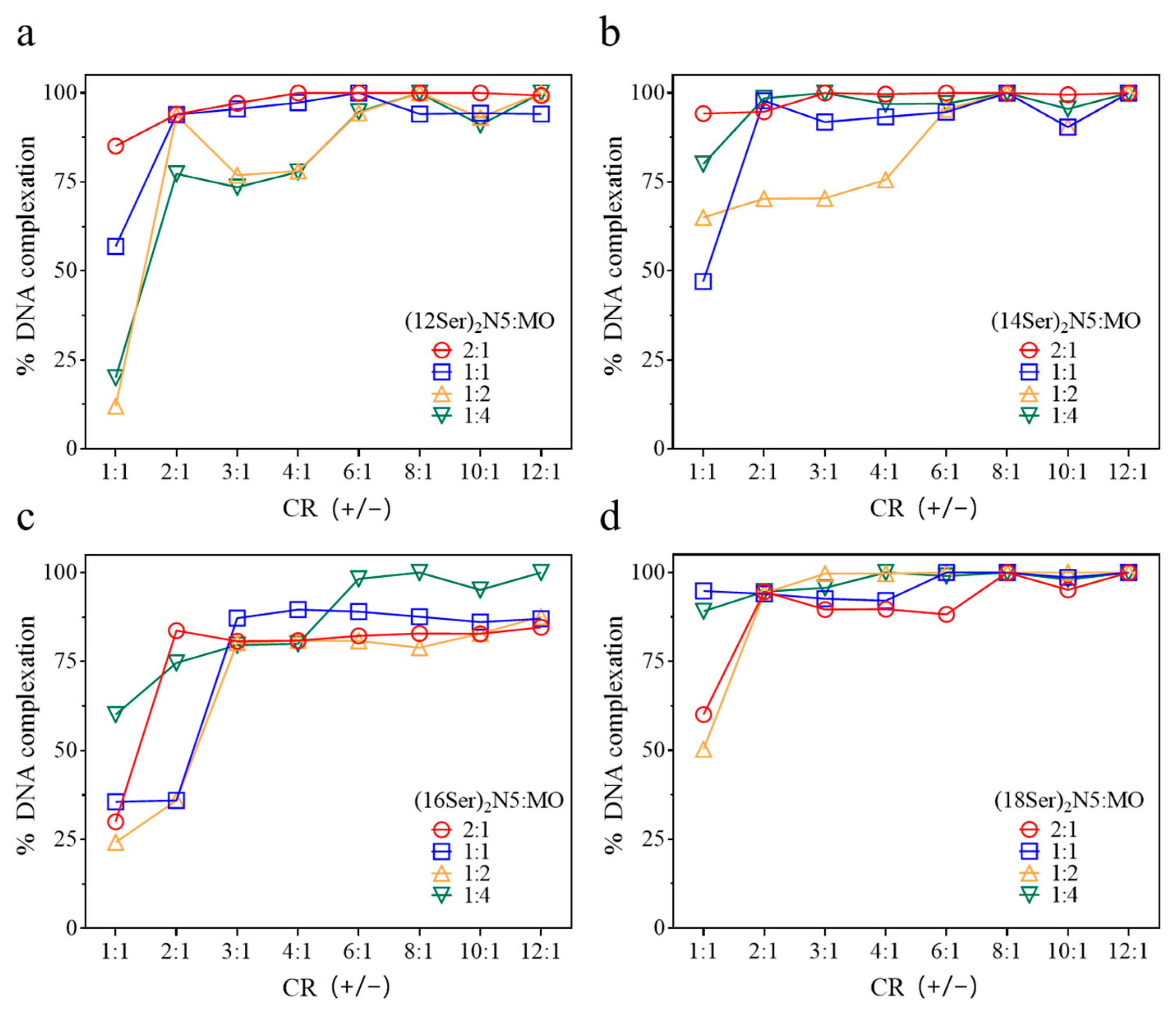
3.1. Interactions between Gemini:MO Aggregates and DNA
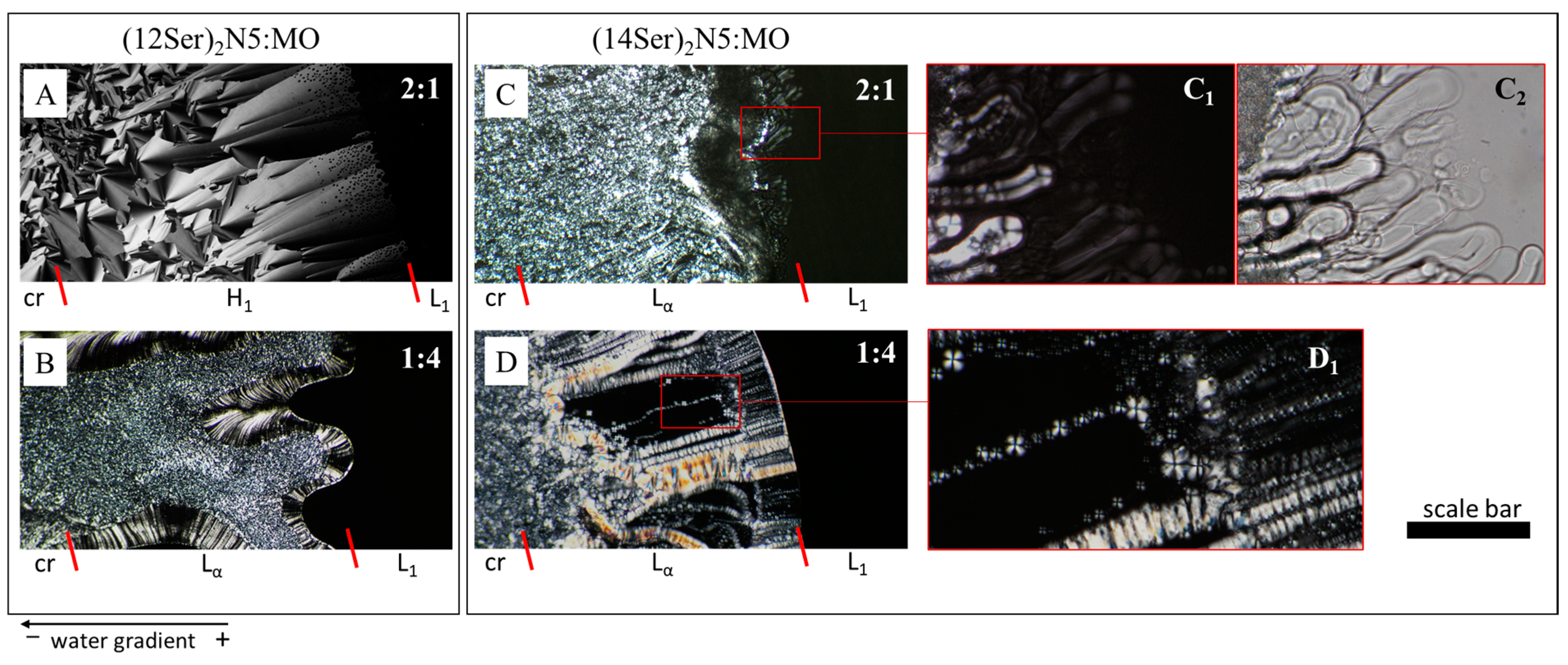
3.2. Characterization of the Gemini:MO Aggregation Behavior
3.3. Discussion of the Main Interaction/Aggregation Trends
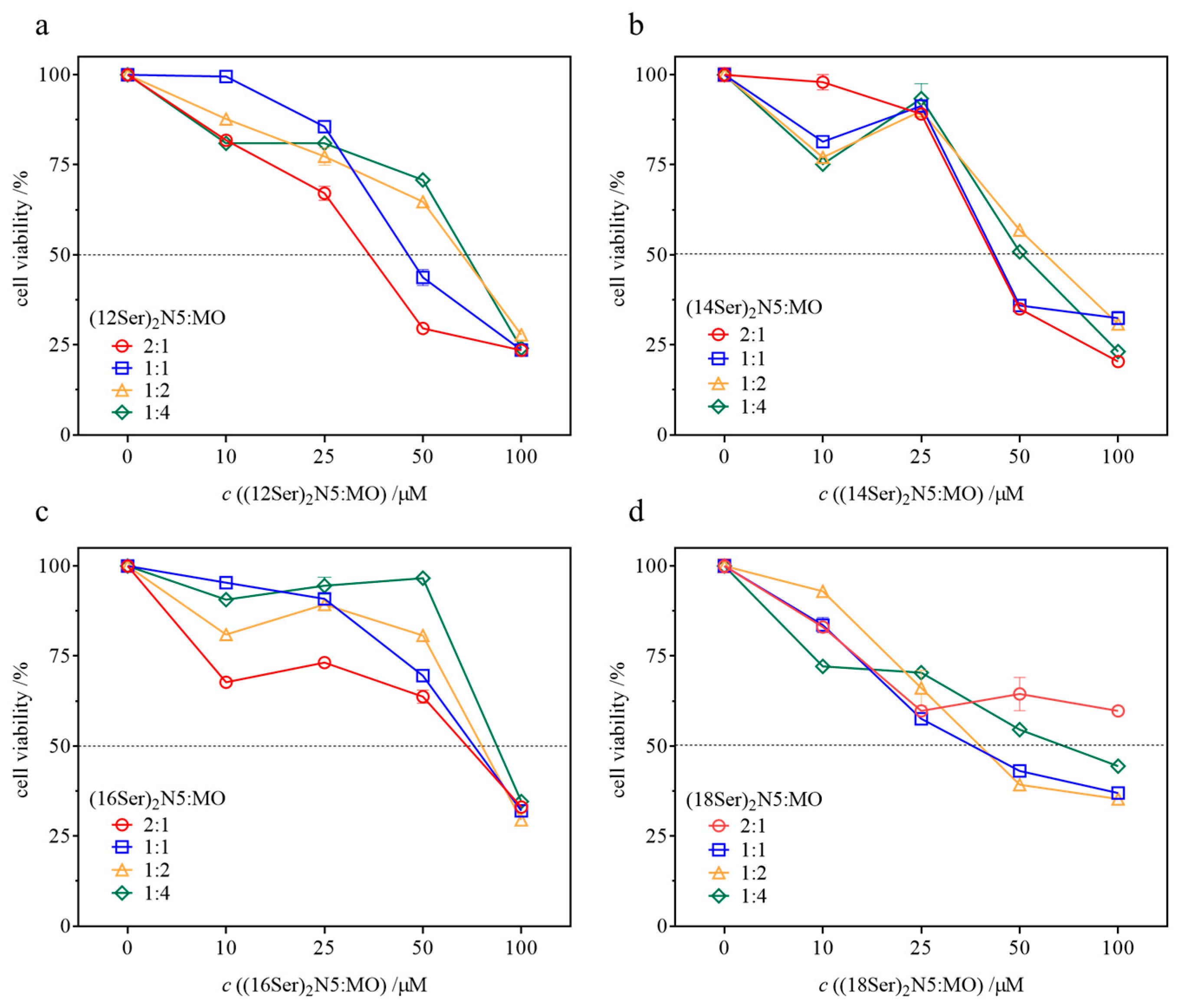
3.4. Cytotoxicity of the Gemini–MO Systems
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.B.; Conniot, J.; Avital, A.; Yao, D.; Jiang, X.; Zhou, X.; Sharf-Pauker, N.; Xiao, Y.; Adir, O.; Liang, H.; et al. Nanodelivery of nucleic acids. Nat. Rev. Methods Primers 2022, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Asami, Y.; Yoshioka, K.; Nishina, K.; Nagata, T.; Yokota, T. Drug delivery system of therapeutic oligonucleotides. Drug Discov. Today Ther. Strateg. 2016, 10, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhou, W.; Lin, L.; Chen, A.; Feng, J.; Qu, X.; Zhang, H.; Yue, J. Delivery of therapeutic oligonucleotides in nanoscale. Bioact. Mater. 2022, 7, 292–323. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Gao, Y.; He, L.; Fang, B.; Ge, W.; Yang, P.; Ju, Y.; Xie, X.; Lei, L. Biomaterial-based gene therapy. MedComm 2023, 4, e259. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhan, Y.; Zhang, Y.; Mao, C.; Xie, X.; Lin, Y. The biological applications of DNA nanomaterials: Current challenges and future directions. Signal Transduct. Target. Ther. 2021, 6, 351. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M.; Pérez-Betancourt, Y. Cationic nanostructures for vaccines design. Biomimetics 2020, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Göpfrich, K.; Platzman, I.; Spatz, J.P. DNA-based assembly of multi-compartment polymersome networks. Adv. Funct. Mater. 2020, 30, 2003480. [Google Scholar] [CrossRef]
- Koroleva, M.Y.; Nagovitsina, T.Y.; Bidanov, D.A.; Gorbachevski, O.S.; Yurtov, E.V. Nano- and microcapsules as drug-delivery systems. Resour. Effic. Technol. 2016, 2, 233–239. [Google Scholar]
- Wang, H.; Ding, S.; Zhang, Z.; Wang, L.; You, Y. Cationic micelle: A promising nanocarrier for gene delivery with high transfection efficiency. J. Gene Med. 2019, 21, e3101. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid nanoparticles─from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Oliveira, I.S.; Silva, J.P.N.; Silva, S.G.; Botelho, C.; do Vale, M.L.C.; Real Oliveira, M.; Gomes, A.C.; Marques, E.F. Effective cytocompatible nanovectors based on serine-derived gemini surfactants and monoolein for small interfering RNA delivery. J. Colloid Interface Sci. 2021, 584, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Al-Dulaymi, M.; Badea, I.; Leary, S.C.; Rehman, J.; El-Aneed, A. Cellular uptake and distribution of gemini surfactant nanoparticles used as gene delivery agents. AAPS J. 2019, 21, 98. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Badea, I.; Leary, S.C.; El-Aneed, A. The determination of gemini surfactants used as gene delivery agents in cellular matrix using validated tandem mass spectrometric method. J. Pharm. Biomed. Anal. 2019, 164, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.G.; Oliveira, I.S.; do Vale, M.L.C.; Marques, E.F. Serine-based gemini surfactants with different spacer linkages: From self-assembly to DNA compaction. Soft Matter 2014, 10, 9352–9361. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.K.; Kim, S.W. Recent advances in the development of gene delivery systems. Biomater. Res. 2019, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Arribas, N.; Martínez-Negro, M.; Villar, E.M.; Pérez, L.; Aicart, E.; Taboada, P.; Guerrero-Martínez, A.; Junquera, E. Biocompatible nanovector of siRNA consisting of arginine-based cationic lipid for gene knockdown in cancer cells. ACS Appl. Mater. Interfaces 2020, 12, 34536–34547. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Negro, M.; Sánchez-Arribas, N.; Guerrero-Martínez, A.; Moyá, M.L.; de Ilarduya, C.T.; Mendicuti, F.; Aicart, E.; Junquera, E. A non-viral plasmid DNA delivery system consisting on a lysine-derived cationic lipid mixed with a fusogenic lipid. Pharmaceutics 2019, 11, 632. [Google Scholar] [CrossRef]
- Martínez-Negro, M.; Blanco-Fernández, L.; Tentori, P.M.; Pérez, L.; Pinazo, A.; de Ilarduya, C.T.; Aicart, E.; Junquera, E. A gemini cationic lipid with histidine residues as a novel lipid-based gene nanocarrier: A biophysical and biochemical study. Nanomaterials 2018, 8, 1061. [Google Scholar] [CrossRef]
- Cheng, X.; Lee, R.J. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv. Drug Deliv. Rev. 2016, 99, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Witzigmann, D.; Leung, J.; Tam, Y.Y.C.; Cullis, P.R. On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale 2019, 11, 21733–21739. [Google Scholar] [CrossRef]
- Silva, J.P.N.; Oliveira, A.C.N.; Casal, M.; Gomes, A.C.; Coutinho, P.J.G.; Coutinho, O.P.; Oliveira, M. DODAB:monoolein-based lipoplexes as non-viral vectors for transfection of mammalian cells. Biochim. Biophys. Acta (BBA)-Biomembr. 2011, 1808, 2440–2449. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, G.; Dogan-Surmeier, S.; Paulus, M.; Albers, C.; Latarius, J.; Sternemann, C.; Schneider, E.; Tolan, M.; Nase, J. The interaction of viral fusion peptides with lipid membranes. Biophys. J. 2022, 121, 3811–3825. [Google Scholar] [CrossRef]
- Gaspar, R.; Coelho, F.; Silva, B.F.B. Lipid-Nucleic Acid Complexes: Physicochemical Aspects and Prospects for Cancer Treatment. Molecules 2020, 25, 5006. [Google Scholar] [CrossRef]
- Goreti Silva, S.; Fernandes, R.F.; Marques, E.F.; do Vale, M.L.C. Serine-based bis-quat gemini surfactants: Synthesis and micellization properties. Eur. J. Org. Chem. 2012, 2012, 345–352. [Google Scholar] [CrossRef]
- Barreleiro, P.C.A.; Lindman, B. The kinetics of DNA-cationic vesicle complex formation. J. Phys. Chem. B 2003, 107, 6208–6213. [Google Scholar] [CrossRef]
- Galindo-Murillo, R.; Cheatham, T.E., III. Ethidium bromide interactions with DNA: An exploration of a classic DNA-ligand complex with unbiased molecular dynamics simulations. Nucleic Acids Res. 2021, 49, 3735–3747. [Google Scholar] [CrossRef] [PubMed]
- Bonasera, V.; Alberti, S.; Sacchetti, A. Protocol for high-sensitivity/long linear-range spectrofluorimetric DNA quantification using ethidium bromide. BioTechniques 2007, 43, 173–176. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1976, 72, 1525–1568. [Google Scholar] [CrossRef]
- Zana, R. Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution: A review. Adv. Colloid Interface Sci. 2002, 97, 205–253. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009, I.S.I.; Biological Evaluation of Medical Devices: Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.


| System | Gemini:MO Molar Ratio | IC50/µM |
|---|---|---|
| (12Ser)2N5:MO | 2:1 | 35 ± 5 |
| 1:1 | 50 ± 5 | |
| 1:2 | 62 ± 9 | |
| 1:4 | 64 ± 5 | |
| (14Ser)2N5:MO | 2:1 | 45 ± 6 |
| 1:1 | 51 ± 8 | |
| 1:2 | 62 ± 7 | |
| 1:4 | 55 ± 8 | |
| (16Ser)2N5:MO | 2:1 | 64 ± 3 |
| 1:1 | 72 ± 3 | |
| 1:2 | 76 ± 7 | |
| 1:4 | 90 ± 7 | |
| (18Ser)2N5:MO | 2:1 | >100 |
| 1:1 | 43 ± 7 | |
| 1:2 | 46 ± 8 | |
| 1:4 | 74 ± 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, I.S.; Silva, S.G.; Gomes, A.C.; Real Oliveira, M.E.C.D.; Vale, M.L.C.d.; Marques, E.F. Cationic Serine-Based Gemini Surfactant:Monoolein Aggregates as Viable and Efficacious Agents for DNA Complexation and Compaction: A Cytotoxicity and Physicochemical Assessment. J. Funct. Biomater. 2024, 15, 224. https://doi.org/10.3390/jfb15080224
Oliveira IS, Silva SG, Gomes AC, Real Oliveira MECD, Vale MLCd, Marques EF. Cationic Serine-Based Gemini Surfactant:Monoolein Aggregates as Viable and Efficacious Agents for DNA Complexation and Compaction: A Cytotoxicity and Physicochemical Assessment. Journal of Functional Biomaterials. 2024; 15(8):224. https://doi.org/10.3390/jfb15080224
Chicago/Turabian StyleOliveira, Isabel S., Sandra G. Silva, Andreia C. Gomes, M. Elisabete C. D. Real Oliveira, M. Luísa C. do Vale, and Eduardo F. Marques. 2024. "Cationic Serine-Based Gemini Surfactant:Monoolein Aggregates as Viable and Efficacious Agents for DNA Complexation and Compaction: A Cytotoxicity and Physicochemical Assessment" Journal of Functional Biomaterials 15, no. 8: 224. https://doi.org/10.3390/jfb15080224










