Nanomaterials in Immunology: Bridging Innovative Approaches in Immune Modulation, Diagnostics, and Therapy
Abstract
:1. Introduction
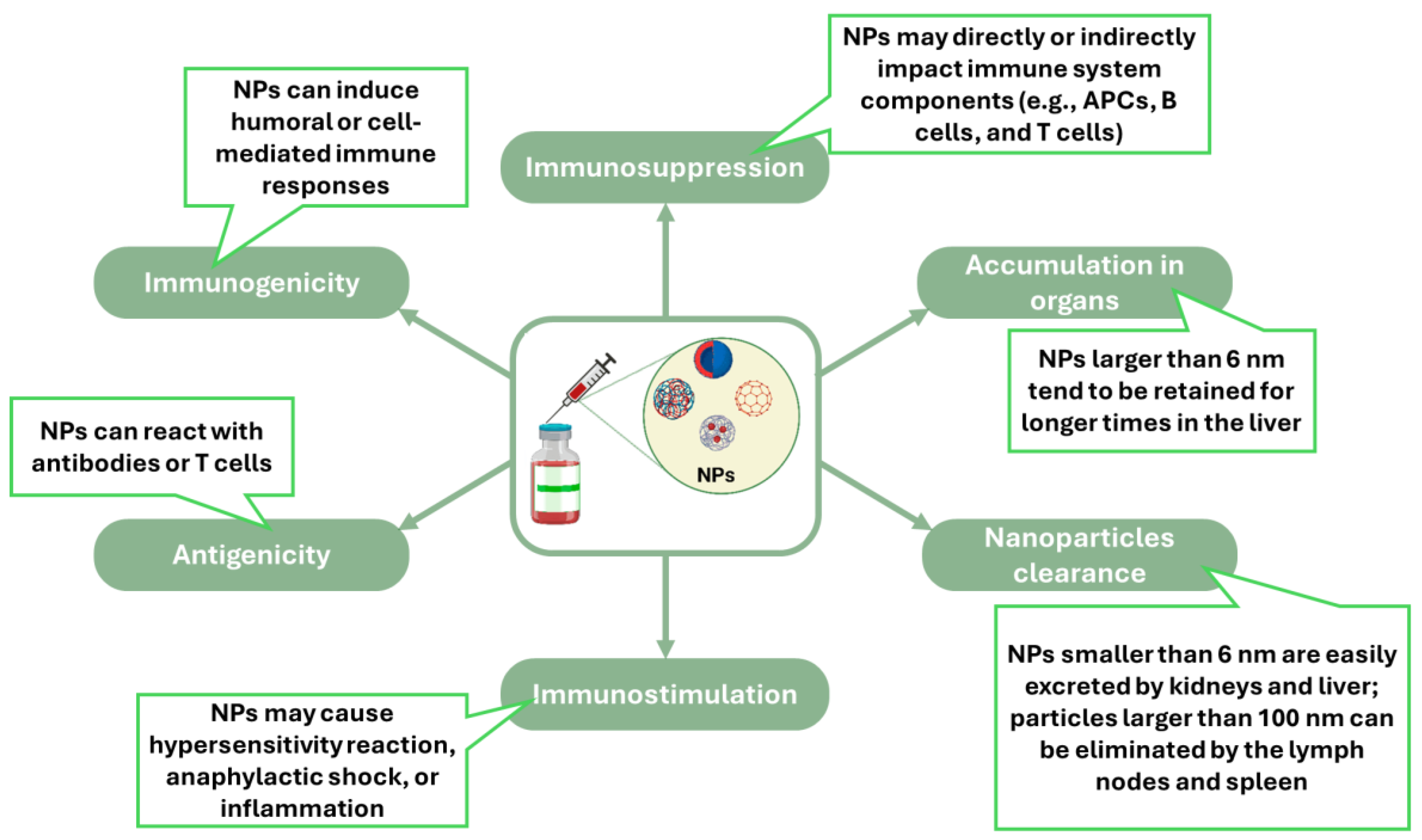
2. Fundamental Interactions between Nanomaterials and the Immune System
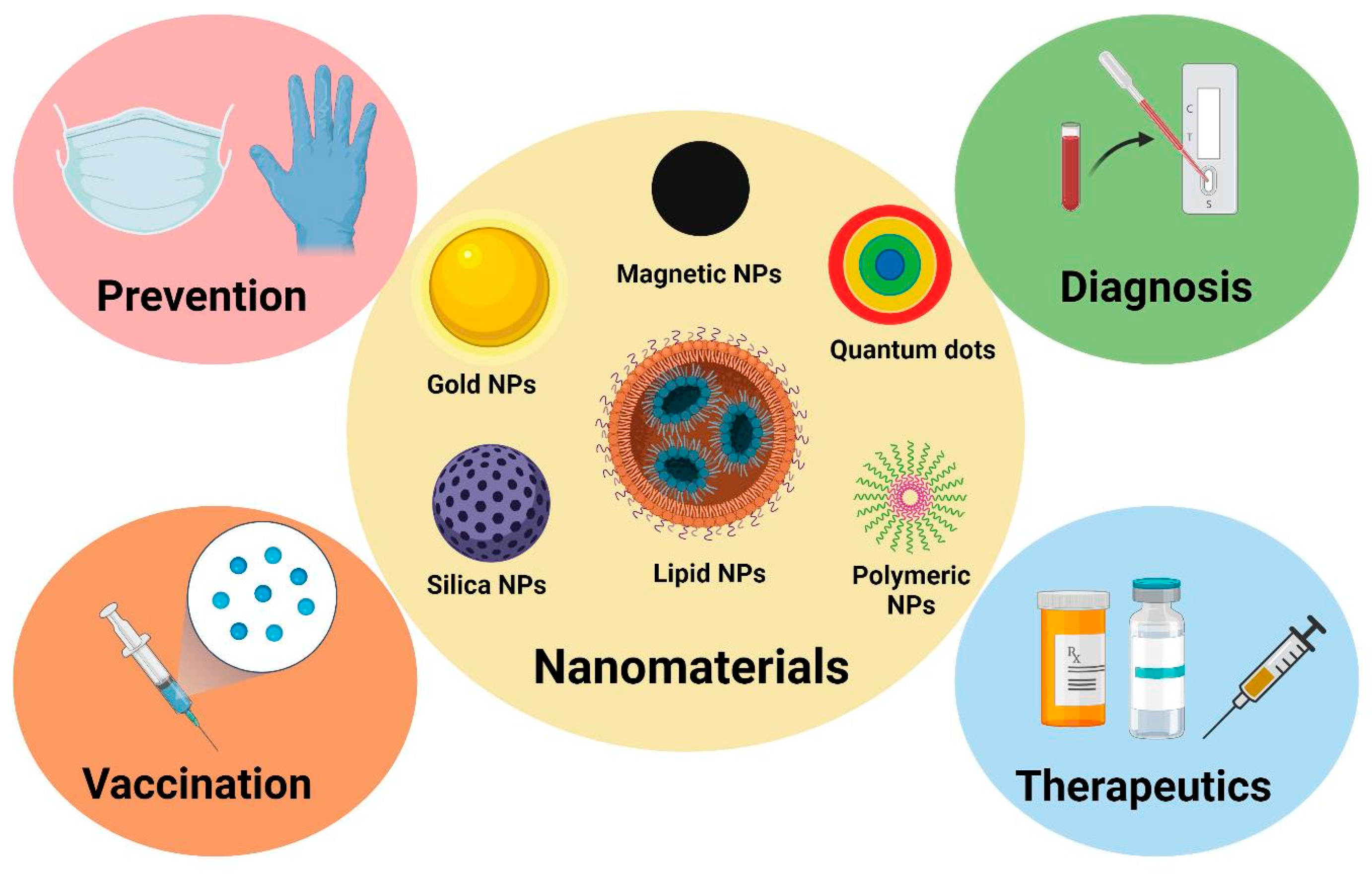
3. Nanomaterials in Immunodiagnosis
4. Nanomaterials in Vaccine Development
5. Nanomaterials in Immunotherapy
5.1. Targeted Drug Delivery Systems for Immunomodulation
5.2. Nanoparticles in Cancer Immunotherapy
6. Safety and Biocompatibility
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.; Leo, O. Key concepts in immunology. Vaccine 2010, 28, C2–C13. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Davis, M.M. The science and medicine of human immunology. Science 2020, 369, eaay4014. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Pepper, M.; Thomas, P.G. Principles and therapeutic applications of adaptive immunity. Cell 2024, 187, 2052–2078. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, R.; Safaei, M.; Mozaffari, H.R.; Moradpoor, H.; Karami, S.; Golshah, A.; Salimi, B.; Karami, H. The Role of Nanomaterials in the Treatment of Diseases and Their Effects on the Immune System. Open Access Maced. J. Med. Sci. 2019, 7, 1884–1890. [Google Scholar] [CrossRef]
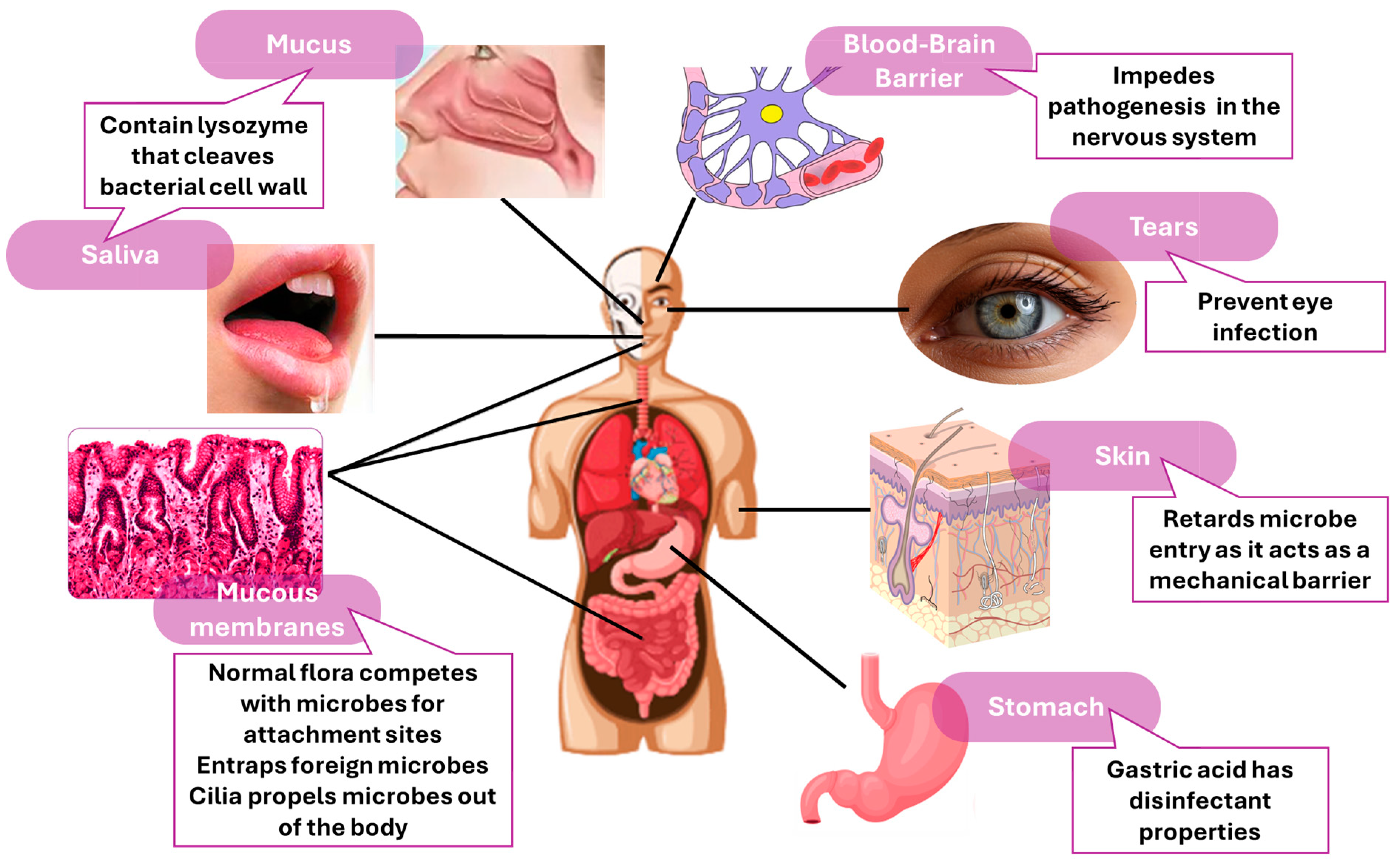
- Günther, J.; Seyfert, H.M. The first line of defence: Insights into mechanisms and relevance of phagocytosis in epithelial cells. Semin. Immunopathol. 2018, 40, 555–565. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.R.; Levy, O. Innate immunity. In Clinical Immunology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 39–53. [Google Scholar]
- Pal, A.; Chakravarty, A.K. (Eds.) Chapter 4-Basic concepts of immunogenetics. In Genetics and Breeding for Disease Resistance of Livestock; Academic Press: Cambridge, MA, USA, 2020; pp. 95–100. [Google Scholar]
- Smith, D.M.; Simon, J.K.; Baker Jr, J.R. Applications of nanotechnology for immunology. Nat. Rev. Immunol. 2013, 13, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Mobeen, H.; Safdar, M.; Fatima, A.; Afzal, S.; Zaman, H.; Mehdi, Z. Emerging applications of nanotechnology in context to immunology: A comprehensive review. Front. Bioeng. Biotechnol. 2022, 10, 1024871. [Google Scholar] [CrossRef] [PubMed]
- Lenders, V.; Koutsoumpou, X.; Sargsian, A.; Manshian, B.B. Biomedical nanomaterials for immunological applications: Ongoing research and clinical trials. Nanoscale Adv. 2020, 2, 5046–5089. [Google Scholar] [CrossRef]
- Mabrouk, M.; Das, D.B.; Salem, Z.A.; Beherei, H.H. Nanomaterials for Biomedical Applications: Production, Characterisations, Recent Trends and Difficulties. Molecules 2021, 26, 1077. [Google Scholar] [CrossRef]
- Pandey, S. Advance Nanomaterials for Biosensors. Biosensors 2022, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Zhang, A.; Su, M. Nanomaterials for Biosensing Applications. Nanomaterials 2016, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Mitarotonda, R.; Giorgi, E.; Eufrasio-da-Silva, T.; Dolatshahi-Pirouz, A.; Mishra, Y.K.; Khademhosseini, A.; Desimone, M.F.; De Marzi, M.; Orive, G. Immunotherapeutic nanoparticles: From autoimmune disease control to the development of vaccines. Biomater. Adv. 2022, 135, 212726. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Luzuriaga, M.A.; Shahrivarkevishahi, A.; Herbert, F.C.; Wijesundara, Y.H.; Gassensmith, J.J. Biomaterials and nanomaterials for sustained release vaccine delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2021, 13, e1735. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.A.; Obeid, M.A.; Bashatwah, R.M.; Serrano-Aroca, Á.; Mishra, V.; Mishra, Y.; El-Tanani, M.; Hromić-Jahjefendić, A.; Kapoor, D.N.; Goyal, R.; et al. Nanomaterials and Their Impact on the Immune System. Int. J. Mol. Sci. 2023, 24, 2008. [Google Scholar] [CrossRef] [PubMed]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Qi, Y.; Liu, G.; Song, Y.; Jiang, X.; Du, B. Size-dependent in vivo transport of nanoparticles: Implications for delivery, targeting, and clearance. ACS Nano 2023, 17, 20825–20849. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B. Hide and Seek: Nanomaterial Interactions With the Immune System. Front. Immunol. 2019, 10, 133. [Google Scholar] [CrossRef]
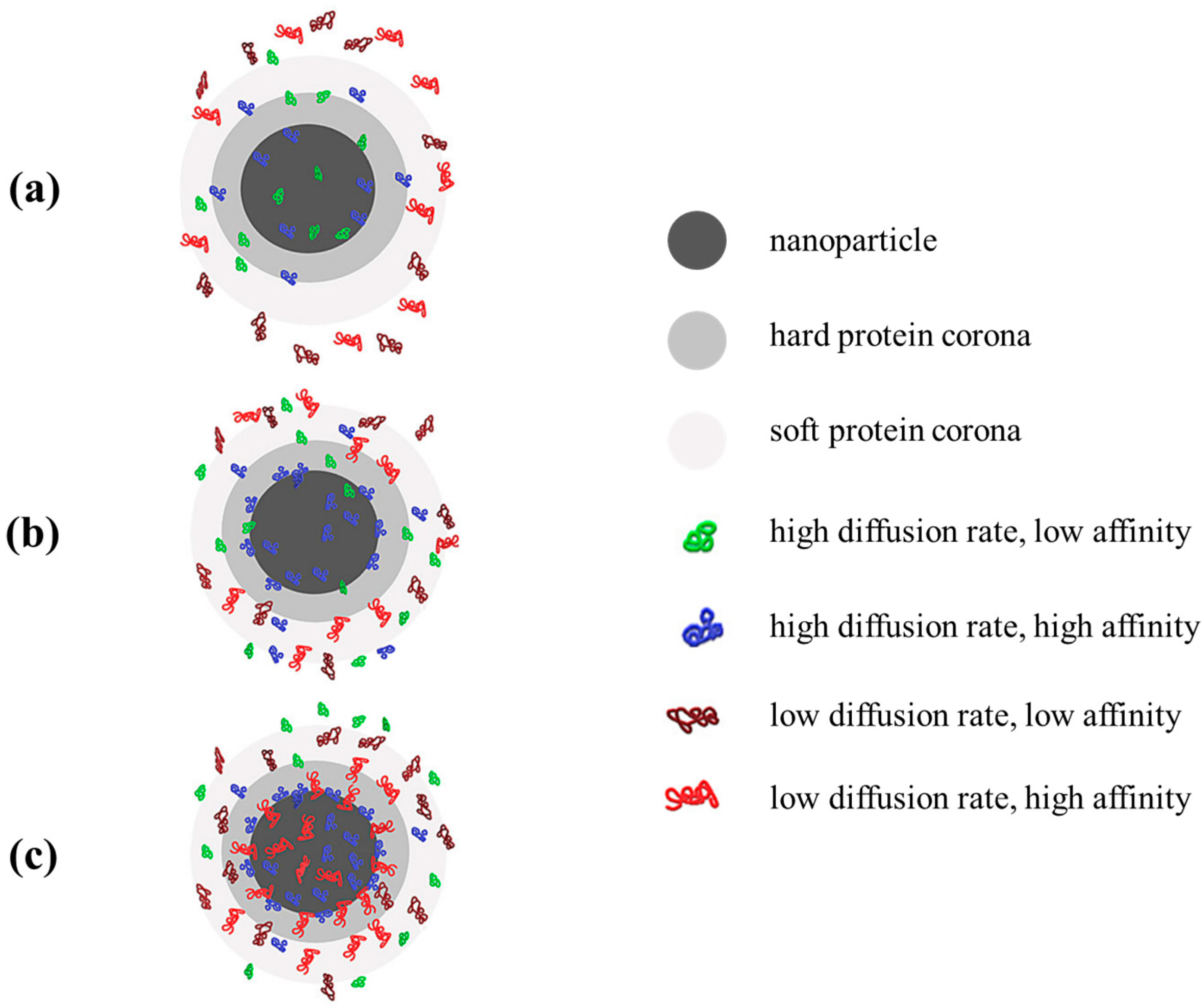
- Bashiri, G.; Padilla, M.S.; Swingle, K.L.; Shepherd, S.J.; Mitchell, M.J.; Wang, K. Nanoparticle protein corona: From structure and function to therapeutic targeting. Lab Chip 2023, 23, 1432–1466. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Bîrcă, A.C.; Grumezescu, A.M. New Applications of Lipid and Polymer-Based Nanoparticles for Nucleic Acids Delivery. Pharmaceutics 2021, 13, 2053. [Google Scholar] [CrossRef] [PubMed]
- Moraru, C.; Mincea, M.; Menghiu, G.; Ostafe, V. Understanding the Factors Influencing Chitosan-Based Nanoparticles-Protein Corona Interaction and Drug Delivery Applications. Molecules 2020, 25, 4758. [Google Scholar] [CrossRef] [PubMed]
- La-Beck, N.M.; Gabizon, A.A. Nanoparticle Interactions with the Immune System: Clinical Implications for Liposome-Based Cancer Chemotherapy. Front. Immunol. 2017, 8, 416. [Google Scholar] [CrossRef] [PubMed]
- Gref, R.; Lück, M.; Quellec, P.; Marchand, M.; Dellacherie, E.; Harnisch, S.; Blunk, T.; Müller, R. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): Influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf. B Biointerfaces 2000, 18, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Nasrullah, M.; Meenakshi Sundaram, D.N.; Claerhout, J.; Ha, K.; Demirkaya, E.; Uludag, H. Nanoparticles and cytokine response. Front. Bioeng. Biotechnol. 2023, 11, 1243651. [Google Scholar] [CrossRef] [PubMed]
- Metzloff, A.E.; Padilla, M.S.; Gong, N.; Billingsley, M.M.; Han, X.; Merolle, M.; Mai, D.; Figueroa-Espada, C.G.; Thatte, A.S.; Haley, R.M. Antigen Presenting Cell Mimetic Lipid Nanoparticles for Rapid mRNA CAR T Cell Cancer Immunotherapy. Adv. Mater. 2024, 36, 2313226. [Google Scholar] [CrossRef] [PubMed]
- Benne, N.; Ter Braake, D.; Stoppelenburg, A.J.; Broere, F. Nanoparticles for Inducing Antigen-Specific T Cell Tolerance in Autoimmune Diseases. Front. Immunol. 2022, 13, 864403. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T.K.; Maldonado, R.A. Nanoparticles for the Induction of Antigen-Specific Immunological Tolerance. Front. Immunol. 2018, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Siefert, A.L.; Caplan, M.J.; Fahmy, T.M. Artificial bacterial biomimetic nanoparticles synergize pathogen-associated molecular patterns for vaccine efficacy. Biomaterials 2016, 97, 85–96. [Google Scholar] [CrossRef]
- Liu, Q.; Jia, J.; Yang, T.; Fan, Q.; Wang, L.; Ma, G. Pathogen-mimicking polymeric nanoparticles based on dopamine polymerization as vaccines adjuvants induce robust humoral and cellular immune responses. Small 2016, 12, 1744–1757. [Google Scholar] [CrossRef]
- Petersen, L.K.; Ramer-Tait, A.E.; Broderick, S.R.; Kong, C.-S.; Ulery, B.D.; Rajan, K.; Wannemuehler, M.J.; Narasimhan, B. Activation of innate immune responses in a pathogen-mimicking manner by amphiphilic polyanhydride nanoparticle adjuvants. Biomaterials 2011, 32, 6815–6822. [Google Scholar] [CrossRef]
- Barberio, A.E.; Smith, S.G.; Correa, S.; Nguyen, C.; Nhan, B.; Melo, M.; Tokatlian, T.; Suh, H.; Irvine, D.J.; Hammond, P.T. Cancer Cell Coating Nanoparticles for Optimal Tumor-Specific Cytokine Delivery. ACS Nano 2020, 14, 11238–11253. [Google Scholar] [CrossRef]
- Ilinskaya, A.N.; Dobrovolskaia, M.A. Immunosuppressive and anti-inflammatory properties of engineered nanomaterials. Br. J. Pharmacol. 2014, 171, 3988–4000. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Dai, H.; Fei, Z.; Chai, Y.; Hao, Y.; Fan, Q.; Dong, Z.; Zhu, Y.; Xu, J.; Ma, Q.; et al. Immunosuppressive Nanoparticles for Management of Immune-Related Adverse Events in Liver. ACS Nano 2021, 15, 9111–9125. [Google Scholar] [CrossRef]
- Zhuang, J.; Holay, M.; Park, J.H.; Fang, R.H.; Zhang, J.; Zhang, L. Nanoparticle Delivery of Immunostimulatory Agents for Cancer Immunotherapy. Theranostics 2019, 9, 7826–7848. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood-brain barrier with nanoparticles. J. Control. Release 2018, 270, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Long, H.; Zhou, T.; Liu, Y.; Zhao, J.; Han, J.; Yang, X.; Yu, Y.; Chen, F.; Shi, S. Blood-brain barrier Permeable nanoparticles for Alzheimer’s disease treatment by selective mitophagy of microglia. Biomaterials 2022, 288, 121690. [Google Scholar] [CrossRef]
- Lu, L.; Qi, S.; Chen, Y.; Luo, H.; Huang, S.; Yu, X.; Luo, Q.; Zhang, Z. Targeted immunomodulation of inflammatory monocytes across the blood-brain barrier by curcumin-loaded nanoparticles delays the progression of experimental autoimmune encephalomyelitis. Biomaterials 2020, 245, 119987. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.K.; Prajapati, V.K. Molecular and immunological toxic effects of nanoparticles. Int. J. Biol. Macromol. 2018, 107, 1278–1293. [Google Scholar] [CrossRef]
- Patel, U.; Rathnayake, K.; Hunt, E.C.; Singh, N. Role of Nanomaterials in COVID-19 Prevention, Diagnostics, Therapeutics, and Vaccine Development. J. Nanotheranostics 2022, 3, 151–176. [Google Scholar] [CrossRef]
- Xia, F.; Zuo, X.; Yang, R.; Xiao, Y.; Kang, D.; Vallée-Bélisle, A.; Gong, X.; Yuen, J.D.; Hsu, B.B.; Heeger, A.J.; et al. Colorimetric detection of DNA, small molecules, proteins, and ions using unmodified gold nanoparticles and conjugated polyelectrolytes. Proc. Natl. Acad. Sci. USA 2010, 107, 10837–10841. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Pan, Y.; Sun, Z.; Li, J.; Yi, Y.; Yang, J.; Li, G. An electrochemical biosensor for sensitive analysis of the SARS-CoV-2 RNA. Biosens. Bioelectron. 2021, 186, 113309. [Google Scholar] [CrossRef] [PubMed]
- Choosang, J.; Khumngern, S.; Thavarungkul, P.; Kanatharana, P.; Numnuam, A. An ultrasensitive label-free electrochemical immunosensor based on 3D porous chitosan–graphene–ionic liquid–ferrocene nanocomposite cryogel decorated with gold nanoparticles for prostate-specific antigen. Talanta 2021, 224, 121787. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.-K.; Kim, K.; Park, J.; Im, H.; Maher, S.; Kim, M.-G. Plasmon color-preserved gold nanoparticle clusters for high sensitivity detection of SARS-CoV-2 based on lateral flow immunoassay. Biosens. Bioelectron. 2022, 205, 114094. [Google Scholar] [CrossRef] [PubMed]
- Hryniewicz, B.M.; Volpe, J.; Bach-Toledo, L.; Kurpel, K.C.; Deller, A.E.; Soares, A.L.; Nardin, J.M.; Marchesi, L.F.; Simas, F.F.; Oliveira, C.C.; et al. Development of polypyrrole (nano)structures decorated with gold nanoparticles toward immunosensing for COVID-19 serological diagnosis. Mater. Today Chem. 2022, 24, 100817. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Jia, H.; Duan, X.; Lu, L.; Tian, Q.; Chen, S.; Xu, J.; Jiang, F. Label-free electrochemical immunosensor for the detection of prostate specific antigen based three-dimensional Au nanoparticles/MoS2-graphene aerogels composite. Inorg. Chem. Commun. 2020, 119, 108122. [Google Scholar] [CrossRef]
- Ghanavati, M.; Tadayon, F.; Bagheri, H. A novel label-free impedimetric immunosensor for sensitive detection of prostate specific antigen using Au nanoparticles/MWCNTs- graphene quantum dots nanocomposite. Microchem. J. 2020, 159, 105301. [Google Scholar] [CrossRef]
- Xie, J.; Tang, M.-Q.; Chen, J.; Zhu, Y.-H.; Lei, C.-B.; He, H.-W.; Xu, X.-H. A sandwich ELISA-like detection of C-reactive protein in blood by citicoline-bovine serum albumin conjugate and aptamer-functionalized gold nanoparticles nanozyme. Talanta 2020, 217, 121070. [Google Scholar] [CrossRef] [PubMed]
- Hepel, M. Magnetic Nanoparticles for Nanomedicine. Magnetochemistry 2020, 6, 3. [Google Scholar] [CrossRef]
- Bhandari, D.; Chen, F.-C.; Bridgman, R.C. Magnetic Nanoparticles Enhanced Surface Plasmon Resonance Biosensor for Rapid Detection of Salmonella Typhimurium in Romaine Lettuce. Sensors 2022, 22, 475. [Google Scholar] [CrossRef]
- Qiu, Y.; Jiang, K.; Wu, J.; Mi, H.; Peng, Y.-K.; Go, Y.Y.; Park, H.J.; Lee, J.-H. Fast and sensitive immuno-PCR assisted by plasmonic magnetic nanoparticles. Appl. Mater. Today 2021, 23, 101054. [Google Scholar] [CrossRef]
- Sun, J.; Liu, F.; Yu, W.; Fu, D.; Jiang, Q.; Mo, F.; Wang, X.; Shi, T.; Wang, F.; Pang, D.W.; et al. Visualization of vaccine dynamics with quantum dots for immunotherapy. Angew. Chem. 2021, 133, 24477–24485. [Google Scholar] [CrossRef]
- Liu, F.; Sun, J.; Yu, W.; Jiang, Q.; Pan, M.; Xu, Z.; Mo, F.; Liu, X. Quantum dot-pulsed dendritic cell vaccines plus macrophage polarization for amplified cancer immunotherapy. Biomaterials 2020, 242, 119928. [Google Scholar] [CrossRef] [PubMed]
- Kheirollahpour, M.; Mehrabi, M.; Dounighi, N.M.; Mohammadi, M.; Masoudi, A. Nanoparticles and Vaccine Development. Pharm. Nanotechnol. 2020, 8, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Lozano, D.; Larraga, V.; Vallet-Regí, M.; Manzano, M. An Overview of the Use of Nanoparticles in Vaccine Development. Nanomedicine 2023, 13, 1828. [Google Scholar] [CrossRef] [PubMed]
- Curley, S.M.; Putnam, D. Biological Nanoparticles in Vaccine Development. Front. Bioeng. Biotechnol. 2022, 10, 867119. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jeong, M.; Park, J.; Jung, H.; Lee, H. Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics. Exp. Mol. Med. 2023, 55, 2085–2096. [Google Scholar] [CrossRef]
- Wilson, B.; Geetha, K.M. Lipid nanoparticles in the development of mRNA vaccines for COVID-19. J. Drug Deliv. Sci. Technol. 2022, 74, 103553. [Google Scholar] [CrossRef] [PubMed]
- Shimosakai, R.; Khalil, I.A.; Kimura, S.; Harashima, H. mRNA-Loaded Lipid Nanoparticles Targeting Immune Cells in the Spleen for Use as Cancer Vaccines. Pharmaceuticals 2022, 15, 1017. [Google Scholar] [CrossRef]
- Sasaki, K.; Sato, Y.; Okuda, K.; Iwakawa, K.; Harashima, H. mRNA-Loaded Lipid Nanoparticles Targeting Dendritic Cells for Cancer Immunotherapy. Pharmaceutics 2022, 14, 1572. [Google Scholar] [CrossRef]
- Chen, J.; Ye, Z.; Huang, C.; Qiu, M.; Song, D.; Li, Y.; Xu, Q. Lipid nanoparticle-mediated lymph node–targeting delivery of mRNA cancer vaccine elicits robust CD8+ T cell response. Proc. Natl. Acad. Sci. USA 2022, 119, e2207841119. [Google Scholar] [CrossRef] [PubMed]
- Henson, T.R.; Richards, K.A.; Gandhapudi, S.K.; Woodward, J.G.; Sant, A.J. R-DOTAP Cationic Lipid Nanoparticles Outperform Squalene-Based Adjuvant Systems in Elicitation of CD4 T Cells after Recombinant Influenza Hemagglutinin Vaccination. Viruses 2023, 15, 538. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Kumari, S.; Sillman, S.; Chaudhari, J.; Lai, D.C.; Vu, H.L.X. A Single-Dose Intramuscular Immunization of Pigs with Lipid Nanoparticle DNA Vaccines Based on the Hemagglutinin Antigen Confers Complete Protection against Challenge Infection with the Homologous Influenza Virus Strain. Vaccines 2023, 11, 1596. [Google Scholar] [CrossRef] [PubMed]
- Pine, M.; Arora, G.; Hart, T.M.; Bettini, E.; Gaudette, B.T.; Muramatsu, H.; Tombácz, I.; Kambayashi, T.; Tam, Y.K.; Brisson, D. Development of an mRNA-lipid nanoparticle vaccine against Lyme disease. Mol. Ther. 2023, 31, 2702–2714. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Fan, N.; Huang, H.; Jiang, X.; Qin, S.; Xiao, W.; Zheng, Q.; Zhang, Y.; Duan, X.; Qin, Z. mRNA vaccines against SARS-CoV-2 variants delivered by lipid nanoparticles based on novel ionizable lipids. Adv. Funct. Mater. 2022, 32, 2204692. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Zhang, Z.; Liu, F.; Lu, H.; Yu, C.; Sun, H.; Long, J.; Cao, Y.; Mai, J.; Miao, Y.; et al. Monkeypox virus quadrivalent mRNA vaccine induces immune response and protects against vaccinia virus. Signal Transduct. Target. Ther. 2023, 8, 172. [Google Scholar] [CrossRef]
- Grego, E.A.; Siddoway, A.C.; Uz, M.; Liu, L.; Christiansen, J.C.; Ross, K.A.; Kelly, S.M.; Mallapragada, S.K.; Wannemuehler, M.J.; Narasimhan, B. Polymeric Nanoparticle-Based Vaccine Adjuvants and Delivery Vehicles. In Nanoparticles for Rational Vaccine Design; Gill, H.S., Compans, R.W., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 29–76. [Google Scholar]
- Wibowo, D.; Jorritsma, S.H.T.; Gonzaga, Z.J.; Evert, B.; Chen, S.; Rehm, B.H.A. Polymeric nanoparticle vaccines to combat emerging and pandemic threats. Biomaterials 2021, 268, 120597. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Grumezescu, A.M. Polymer-Based Nanosystems—A Versatile Delivery Approach. Materials 2021, 14, 6812. [Google Scholar] [CrossRef]
- Amin, M.K.; Boateng, J. Surface functionalization of PLGA nanoparticles for potential oral vaccine delivery targeting intestinal immune cells. Colloids Surf. B Biointerfaces 2023, 222, 113121. [Google Scholar] [CrossRef]
- Du, X.; Xue, J.; Jiang, M.; Lin, S.; Huang, Y.; Deng, K.; Shu, L.; Xu, H.; Li, Z.; Yao, J.; et al. A Multiepitope Peptide, rOmp22, Encapsulated in Chitosan-PLGA Nanoparticles as a Candidate Vaccine Against Acinetobacter baumannii Infection. Int. J. Nanomed. 2021, 16, 1819–1836. [Google Scholar] [CrossRef]
- Yang, Y.; Teng, Z.; Lu, Y.; Luo, X.; Mu, S.; Ru, J.; Zhao, X.; Guo, H.; Ran, X.; Wen, X.; et al. Enhanced immunogenicity of foot and mouth disease DNA vaccine delivered by PLGA nanoparticles combined with cytokine adjuvants. Res. Vet. Sci. 2021, 136, 89–96. [Google Scholar] [CrossRef]
- AbdelAllah, N.H.; Gaber, Y.; Rashed, M.E.; Azmy, A.F.; Abou-Taleb, H.A.; AbdelGhani, S. Alginate-coated chitosan nanoparticles act as effective adjuvant for hepatitis A vaccine in mice. Int. J. Biol. Macromol. 2020, 152, 904–912. [Google Scholar] [CrossRef]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in nanomaterial-based targeted drug delivery systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef]
- Egwu, C.O.; Aloke, C.; Onwe, K.T.; Umoke, C.I.; Nwafor, J.; Eyo, R.A.; Chukwu, J.A.; Ufebe, G.O.; Ladokun, J.; Audu, D.T.; et al. Nanomaterials in Drug Delivery: Strengths and Opportunities in Medicine. Molecules 2024, 29, 2584. [Google Scholar] [CrossRef] [PubMed]
- Yildirimer, L.; Thanh, N.T.; Loizidou, M.; Seifalian, A.M. Toxicology and clinical potential of nanoparticles. Nano Today 2011, 6, 585–607. [Google Scholar] [CrossRef]
- Mendez-Fernandez, A.; Cabrera-Fuentes, H.A.; Velmurugan, B.; Irei, J.; Boisvert, W.A.; Lu, S.; Hausenloy, D. Nanoparticle delivery of cardioprotective therapies. Cond. Med. 2020, 3, 18–30. [Google Scholar]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yang, J.; Lu, A.; Gong, J.; Yang, Y.; Lin, X.; Li, M.; Xu, H. Nanoparticles in ocular applications and their potential toxicity. Front. Mol. Biosci. 2022, 9, 931759. [Google Scholar] [CrossRef] [PubMed]
- Rai, G.; Gauba, P.; Dang, S. Recent advances in nanotechnology for Intra-nasal drug delivery and clinical applications. J. Drug Deliv. Sci. Technol. 2023, 86, 104726. [Google Scholar] [CrossRef]
- Khatun, S.; Putta, C.L.; Hak, A.; Rengan, A.K. Immunomodulatory nanosystems: An emerging strategy to combat viral infections. Biomater. Biosyst. 2023, 9, 100073. [Google Scholar] [CrossRef]
- El-Deeb, N.M.; Abo-Eleneen, M.A.; Al-Madboly, L.A.; Sharaf, M.M.; Othman, S.S.; Ibrahim, O.M.; Mubarak, M.S. Biogenically Synthesized Polysaccharides-Capped Silver Nanoparticles: Immunomodulatory and Antibacterial Potentialities Against Resistant Pseudomonas aeruginosa. Front. Bioeng. Biotechnol. 2020, 8, 643. [Google Scholar] [CrossRef] [PubMed]
- Diez-Echave, P.; Ruiz-Malagón, A.J.; Molina-Tijeras, J.A.; Hidalgo-García, L.; Vezza, T.; Cenis-Cifuentes, L.; Rodríguez-Sojo, M.J.; Cenis, J.L.; Rodríguez-Cabezas, M.E.; Rodríguez-Nogales, A.; et al. Silk fibroin nanoparticles enhance quercetin immunomodulatory properties in DSS-induced mouse colitis. Int. J. Pharm. 2021, 606, 120935. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Fu, H.; Yu, J.; Sun, Y.; Huang, H.; Tang, Y.; Shen, N.; Duan, Y. MicroRNA-125a-Loaded Polymeric Nanoparticles Alleviate Systemic Lupus Erythematosus by Restoring Effector/Regulatory T Cells Balance. ACS Nano 2020, 14, 4414–4429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, D.; He, M.; Lin, M.; Tu, C.; Zhang, B. Polymeric nanoparticles containing rapamycin and autoantigen induce antigen-specific immunological tolerance for preventing vitiligo in mice. Hum. Vaccines Immunother. 2021, 17, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
- Aslan, B.; Ozpolat, B.; Sood, A.K.; Lopez-Berestein, G. Nanotechnology in cancer therapy. J. Drug Target. 2013, 21, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, J.; Gu, P.; Fan, X. The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment. Bioact. Mater. 2021, 6, 1973–1987. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Gowd, V.; Ahmad, A.; Tarique, M.; Suhail, M.; Zughaibi, T.A.; Tabrez, S.; Khan, R. Advancement of cancer immunotherapy using nanoparticles-based nanomedicine. Semin. Cancer Biol. 2022, 86, 624–644. [Google Scholar] [CrossRef]
- Shams, F.; Golchin, A.; Azari, A.; Mohammadi Amirabad, L.; Zarein, F.; Khosravi, A.; Ardeshirylajimi, A. Nanotechnology-based products for cancer immunotherapy. Mol. Biol. Rep. 2022, 49, 1389–1412. [Google Scholar] [CrossRef]
- Goldberg, M.S. Improving cancer immunotherapy through nanotechnology. Nat. Rev. Cancer 2019, 19, 587–602. [Google Scholar] [CrossRef]
- Cavalcanti, I.D.L.; Soares, J.C.S. (Eds.) Conventional Chemotherapy Versus Targeted Therapy. In Advances in Cancer Treatment: From Systemic Chemotherapy to Targeted Therapy; Springer International Publishing: Cham, Switzerland, 2021; pp. 79–89. [Google Scholar]
- Joo, W.D.; Visintin, I.; Mor, G. Targeted cancer therapy--are the days of systemic chemotherapy numbered? Maturitas 2013, 76, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, M.; Manavalan, R.; Kathiresan, K. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J. Pharm. Pharm. Sci. 2011, 14, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Fang, C.; Zhang, K.; Su, C. Recent Advances in Nanoparticles-Based Platforms Targeting the PD-1/PD-L1 Pathway for Cancer Treatment. Pharmaceutics 2022, 14, 1581. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-h.; Huang, L.-j.; Zhou, H.-l.; Shan, Y.-m.; Chen, F.-m.; Lehto, V.-P.; Xu, W.-j.; Luo, L.-q.; Yu, H.-j. Engineered nanomedicines block the PD-1/PD-L1 axis for potentiated cancer immunotherapy. Acta Pharmacol. Sin. 2022, 43, 2749–2758. [Google Scholar] [CrossRef] [PubMed]
- Kiaie, S.H.; Salehi-Shadkami, H.; Sanaei, M.J.; Azizi, M.; Shokrollahi Barough, M.; Nasr, M.S.; Sheibani, M. Nano-immunotherapy: Overcoming delivery challenge of immune checkpoint therapy. J. Nanobiotechnology 2023, 21, 339. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Zhang, C.; Yan, J.; Hou, X.; Du, S.; Zeng, C.; Zhao, W.; Deng, B.; McComb, D.W.; et al. Biomimetic nanoparticles deliver mRNAs encoding costimulatory receptors and enhance T cell mediated cancer immunotherapy. Nat. Commun. 2021, 12, 7264. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Beiss, V.; Wang, C.; Wang, L.; Steinmetz, N.F. Plant Viral Nanoparticle Conjugated with Anti-PD-1 Peptide for Ovarian Cancer Immunotherapy. Int. J. Mol. Sci. 2021, 22, 9733. [Google Scholar] [CrossRef]
- Neek, M.; Tucker, J.A.; Butkovich, N.; Nelson, E.L.; Wang, S.W. An Antigen-Delivery Protein Nanoparticle Combined with Anti-PD-1 Checkpoint Inhibitor Has Curative Efficacy in an Aggressive Melanoma Model. Adv. Ther. 2020, 3, 2000122. [Google Scholar] [CrossRef]
- He, Y.; de Araújo Júnior, R.F.; Cruz, L.J.; Eich, C. Functionalized Nanoparticles Targeting Tumor-Associated Macrophages as Cancer Therapy. Pharmaceutics 2021, 13, 1670. [Google Scholar] [CrossRef]
- Zhao, C.; Pang, X.; Yang, Z.; Wang, S.; Deng, H.; Chen, X. Nanomaterials targeting tumor associated macrophages for cancer immunotherapy. J. Control. Release 2022, 341, 272–284. [Google Scholar] [CrossRef]
- Chen, C.; Song, M.; Du, Y.; Yu, Y.; Li, C.; Han, Y.; Yan, F.; Shi, Z.; Feng, S. Tumor-Associated-Macrophage-Membrane-Coated Nanoparticles for Improved Photodynamic Immunotherapy. Nano Lett. 2021, 21, 5522–5531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, J.; Li, C.; Lu, Y.; Cheng, L.; Liu, J. A targeting black phosphorus nanoparticle based immune cells nano-regulator for photodynamic/photothermal and photo-immunotherapy. Bioact. Mater. 2021, 6, 472–489. [Google Scholar] [CrossRef]
- Bahmani, B.; Gong, H.; Luk, B.T.; Haushalter, K.J.; DeTeresa, E.; Previti, M.; Zhou, J.; Gao, W.; Bui, J.D.; Zhang, L.; et al. Intratumoral immunotherapy using platelet-cloaked nanoparticles enhances antitumor immunity in solid tumors. Nat. Commun. 2021, 12, 1999. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chu, Z.; Yang, J.; Qian, H.; Xu, J.; Chen, B.; Tian, T.; Chen, H.; Xu, Y.; Wang, F. Immunogenic Cell Death Augmented by Manganese Zinc Sulfide Nanoparticles for Metastatic Melanoma Immunotherapy. ACS Nano 2022, 16, 15471–15483. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Luan, X.; Paholak, H.J.; Burnett, J.P.; Stevers, N.O.; Sansanaphongpricha, K.; He, M.; Chang, A.E.; Li, Q.; Sun, D. Depleting Tumor-Associated Tregs Via Nanoparticle-Mediated Hyperthermia to Enhance Anti-CTLA-4 Immunotherapy. Nanomedicine 2020, 15, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Sun, Q.; Zhang, X.; Zheng, L.; Yang, X.; He, N.; Pang, Y.; Wang, X.; Lai, Z.; Zheng, W.; et al. A novel doxorubicin/CTLA-4 blocker co-loaded drug delivery system improves efficacy and safety in antitumor therapy. Cell Death Dis. 2024, 15, 386. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Francis, D.M.; Sestito, L.F.; Archer, P.A.; Manspeaker, M.P.; O’Melia, M.J.; Thomas, S.N. Thermosensitive hydrogel releasing nitric oxide donor and anti-CTLA-4 micelles for antitumor immunotherapy. Nat. Commun. 2022, 13, 1479. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Li, T.; Guo, Y.; Sun, C.; Xianyu, B.; Xu, H. Selenium-containing nanoparticles combine the NK cells mediated immunotherapy with radiotherapy and chemotherapy. Adv. Mater. 2020, 32, 1907568. [Google Scholar] [CrossRef] [PubMed]
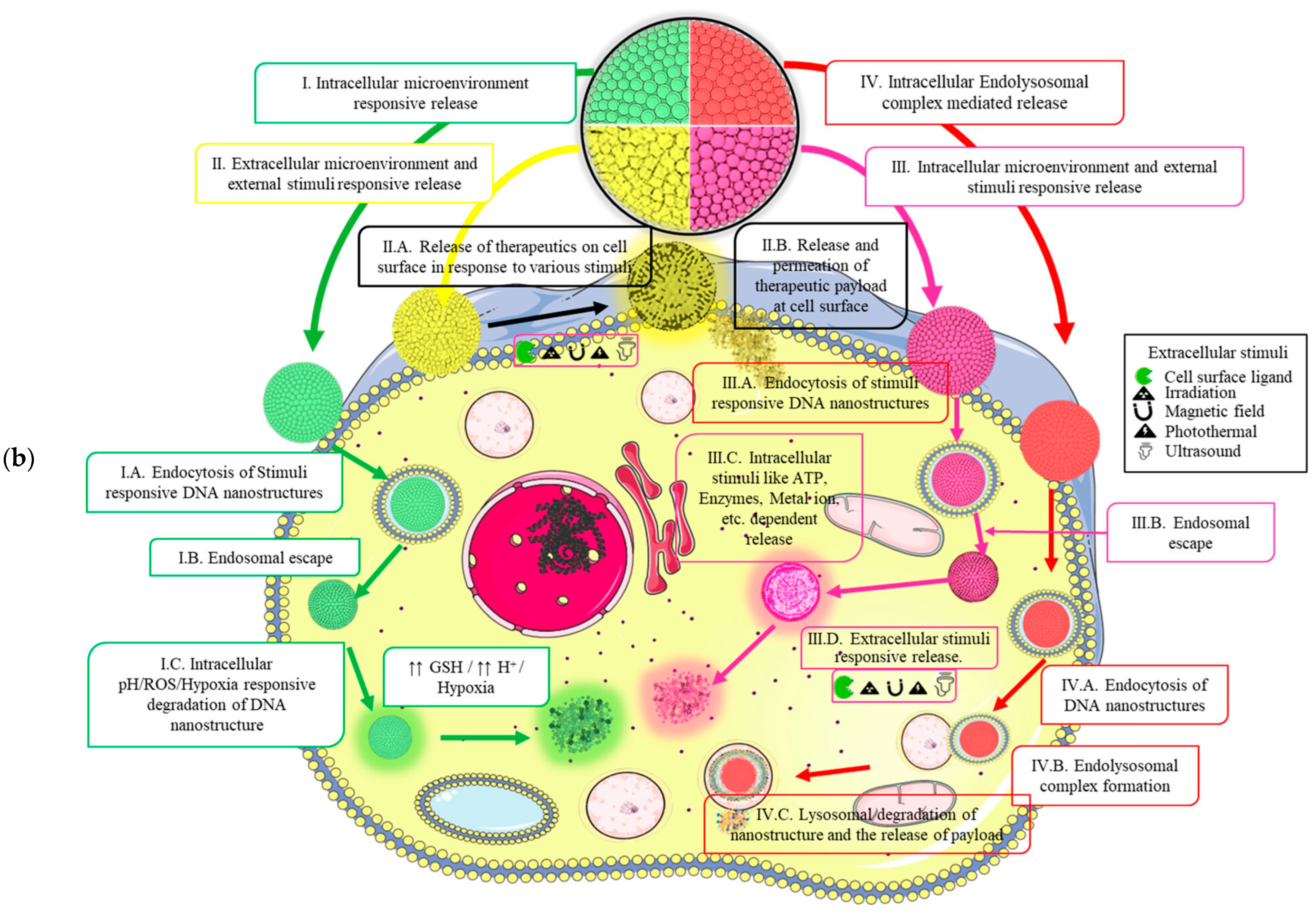
- Jabbari, A.; Sameiyan, E.; Yaghoobi, E.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. Aptamer-based targeted delivery systems for cancer treatment using DNA origami and DNA nanostructures. Int. J. Pharm. 2023, 646, 123448. [Google Scholar] [CrossRef] [PubMed]
- Weiden, J.; Bastings, M.M.C. DNA origami nanostructures for controlled therapeutic drug delivery. Curr. Opin. Colloid Interface Sci. 2021, 52, 101411. [Google Scholar] [CrossRef]
- Kumar, M.; Jha, A.; Mishra, B. DNA-Based Nanostructured Platforms as Drug Delivery Systems. Chem Bio Eng. 2024, 1, 179–198. [Google Scholar] [CrossRef]
- Xu, F.; Xia, Q.; Wang, P. Rationally Designed DNA Nanostructures for Drug Delivery. Front. Chem. 2020, 8, 751. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, Y.; Wu, Q.; Lou, B.; Liu, Z. DNA nanostructures for stimuli-responsive drug delivery. Smart Mater. Med. 2022, 3, 66–84. [Google Scholar] [CrossRef]
- Li, C.; Wu, B.; Chen, S.; Hao, K.; Yang, J.; Cao, H.; Yang, S.; Wu, Z.-S.; Shen, Z. Structural requirement of G-quadruplex/aptamer-combined DNA macromolecule serving as efficient drug carrier for cancer-targeted drug delivery. Eur. J. Pharm. Biopharm. 2021, 159, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wang, D.; Zhang, Q.; Zhang, Y.; Peng, R.; Tan, W. Leveraging Aptamer-Based DNA Nanotechnology for Bioanalysis and Cancer Therapeutics. Acc. Mater. Res. 2024, 5, 438–452. [Google Scholar] [CrossRef]
- Fang, Y.; Lin, S.; Yang, F.; Situ, J.; Lin, S.; Luo, Y. Aptamer-Conjugated Multifunctional Polymeric Nanoparticles as Cancer-Targeted, MRI-Ultrasensitive Drug Delivery Systems for Treatment of Castration-Resistant Prostate Cancer. BioMed Res. Int. 2020, 2020, 9186583. [Google Scholar] [CrossRef]
- Saleh, T.; Soudi, T.; Shojaosadati, S.A. Aptamer functionalized curcumin-loaded human serum albumin (HSA) nanoparticles for targeted delivery to HER-2 positive breast cancer cells. Int. J. Biol. Macromol. 2019, 130, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, X.; Duan, J.; Yang, X.-D. Targeted Treatment of Colon Cancer with Aptamer-Guided Albumin Nanoparticles Loaded with Docetaxel. Int. J. Nanomed. 2020, 15, 6737–6748. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Di, Z.; Li, L.; Zhao, J.; Zheng, L. An aptamer-driven DNA nanodevice for improved delivery of synthetic immunostimulants. Nano Res. 2024. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Higashisaka, K.; Tsutsumi, Y. Biocompatibility of Nanomaterials. In Nanomaterials in Pharmacology, Lu, Z.-R., Sakuma, S., Eds.; Springer: New York, NY, USA, 2016; pp. 185–199. [Google Scholar]
- Kyriakides, T.R.; Raj, A.; Tseng, T.H.; Xiao, H.; Nguyen, R.; Mohammed, F.S.; Halder, S.; Xu, M.; Wu, M.J.; Bao, S.; et al. Biocompatibility of nanomaterials and their immunological properties. Biomed. Mater. 2021, 16, 042005. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B.; Hayes, A.W. The impact of immunotoxicity in evaluation of the nanomaterials safety. Toxicol. Res. Appl. 2018, 2, 2397847318755579. [Google Scholar] [CrossRef]
- Dusinska, M.; Tulinska, J.; El Yamani, N.; Kuricova, M.; Liskova, A.; Rollerova, E.; Rundén-Pran, E.; Smolkova, B. Immunotoxicity, genotoxicity and epigenetic toxicity of nanomaterials: New strategies for toxicity testing? Food Chem. Toxicol. 2017, 109, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Hassanen, E.I.; Khalaf, A.A.; Tohamy, A.F.; Mohammed, E.R.; Farroh, K.Y. Toxicopathological and immunological studies on different concentrations of chitosan-coated silver nanoparticles in rats. Int. J. Nanomed. 2019, 14, 4723–4739. [Google Scholar] [CrossRef] [PubMed]
- Aksakal, F.I.; Ciltas, A. Impact of copper oxide nanoparticles (CuO NPs) exposure on embryo development and expression of genes related to the innate immune system of zebrafish (Danio rerio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 223, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wen, X.; Hu, Y.; Zhang, X.; Wang, D.; Yin, S. Copper nanoparticles induced oxidation stress, cell apoptosis and immune response in the liver of juvenile Takifugu fasciatus. Fish Shellfish. Immunol. 2019, 84, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, H.; He, M.; Chen, B.; Yang, B.; Hu, B. Size-dependent cytotoxicity study of ZnO nanoparticles in HepG2 cells. Ecotoxicol. Environ. Saf. 2019, 171, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Zha, S.; Rong, J.; Guan, X.; Tang, Y.; Han, Y.; Liu, G. Immunotoxicity of four nanoparticles to a marine bivalve species, Tegillarca granosa. J. Hazard. Mater. 2019, 377, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef]
- Liu, X.Y.; Nothias, J.M.; Scavone, A.; Garfinkel, M.; Millis, J.M. Biocompatibility investigation of polyethylene glycol and alginate-poly-L-lysine for islet encapsulation. Asaio. J 2010, 56, 241–245. [Google Scholar] [CrossRef]
- Zheng, H.; Wen, S.; Zhang, Y.; Sun, Z. Organosilane and polyethylene glycol functionalized magnetic mesoporous silica nanoparticles as carriers for CpG immunotherapy in vitro and in vivo. PLoS ONE 2015, 10, e0140265. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Atibalentja, D.F.; Yao, L.E.; Park, J.; Kuruvilla, S.; Felsher, D.W. Anti-PD-L1 F(ab) Conjugated PEG-PLGA Nanoparticle Enhances Immune Checkpoint Therapy. Nanotheranostics 2022, 6, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fan, Y.; Hao, X.; Yang, C.; Peng, Y.; Guo, R.; Shi, X.; Cao, X. Adoptive cellular immunotherapy of tumors via effective CpG delivery to dendritic cells using dendrimer-entrapped gold nanoparticles as a gene vector. J. Mater. Chem. B 2020, 8, 5052–5063. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Shou, X.; Zhang, Y.; Li, Z.; Wu, G.; Wu, D.; Wu, J.; Shi, S.; Wang, S. Cell membrane-encapsulated magnetic nanoparticles for enhancing natural killer cell-mediated cancer immunotherapy. Nanomed. Nanotechnol. Biol. Med. 2021, 32, 102333. [Google Scholar] [CrossRef] [PubMed]
- Kasinathan, K.; Marimuthu, K.; Murugesan, B.; Samayanan, S.; Panchu, S.J.; Swart, H.C.; Savariroyan, S.R.I. Synthesis of biocompatible chitosan functionalized Ag decorated biocomposite for effective antibacterial and anticancer activity. Int. J. Biol. Macromol. 2021, 178, 270–282. [Google Scholar] [CrossRef]
- Wagner, J.; Gößl, D.; Ustyanovska, N.; Xiong, M.; Hauser, D.; Zhuzhgova, O.; Hočevar, S.; Taskoparan, B.; Poller, L.; Datz, S.; et al. Mesoporous Silica Nanoparticles as pH-Responsive Carrier for the Immune-Activating Drug Resiquimod Enhance the Local Immune Response in Mice. ACS Nano 2021, 15, 4450–4466. [Google Scholar] [CrossRef]
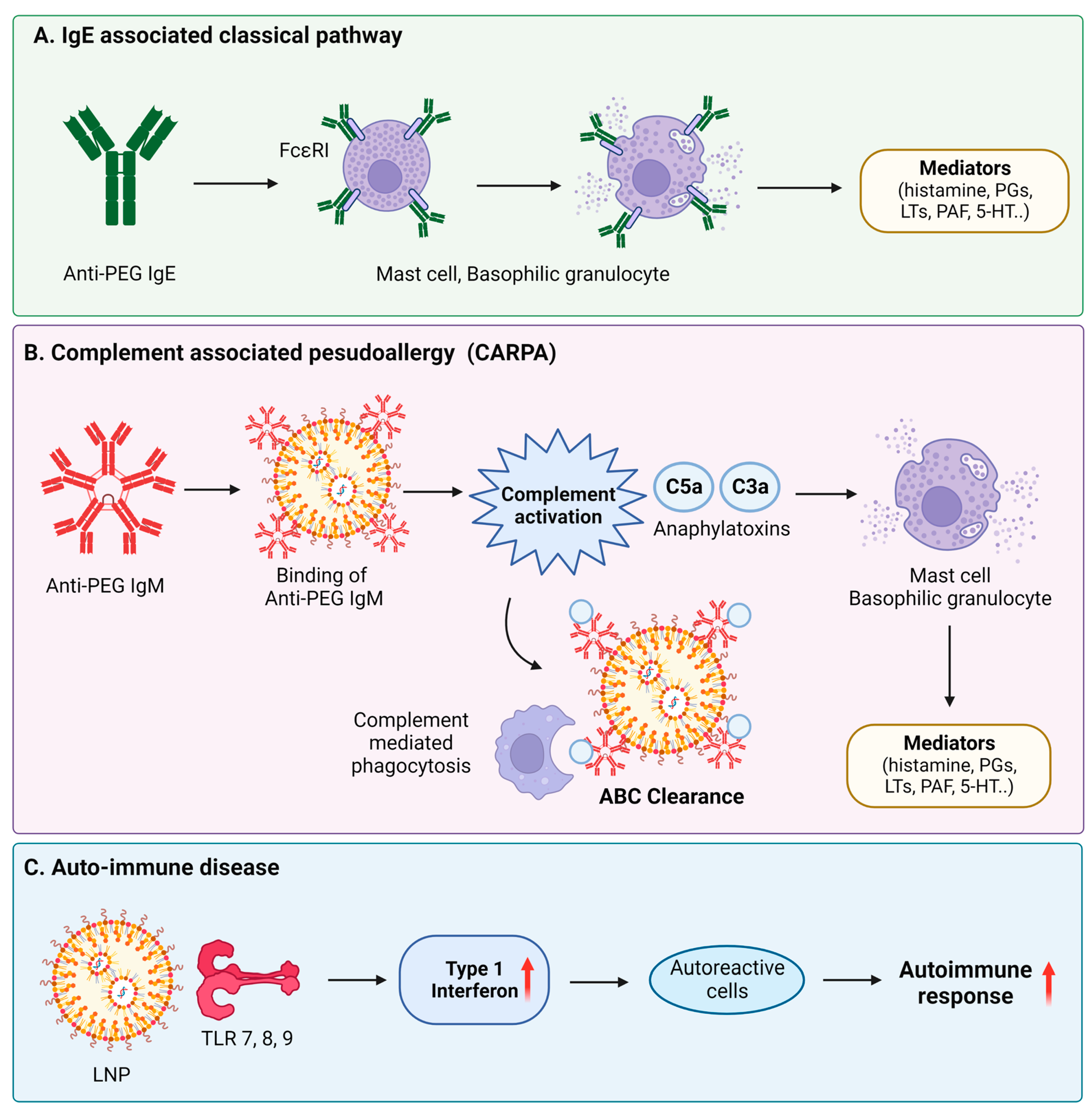
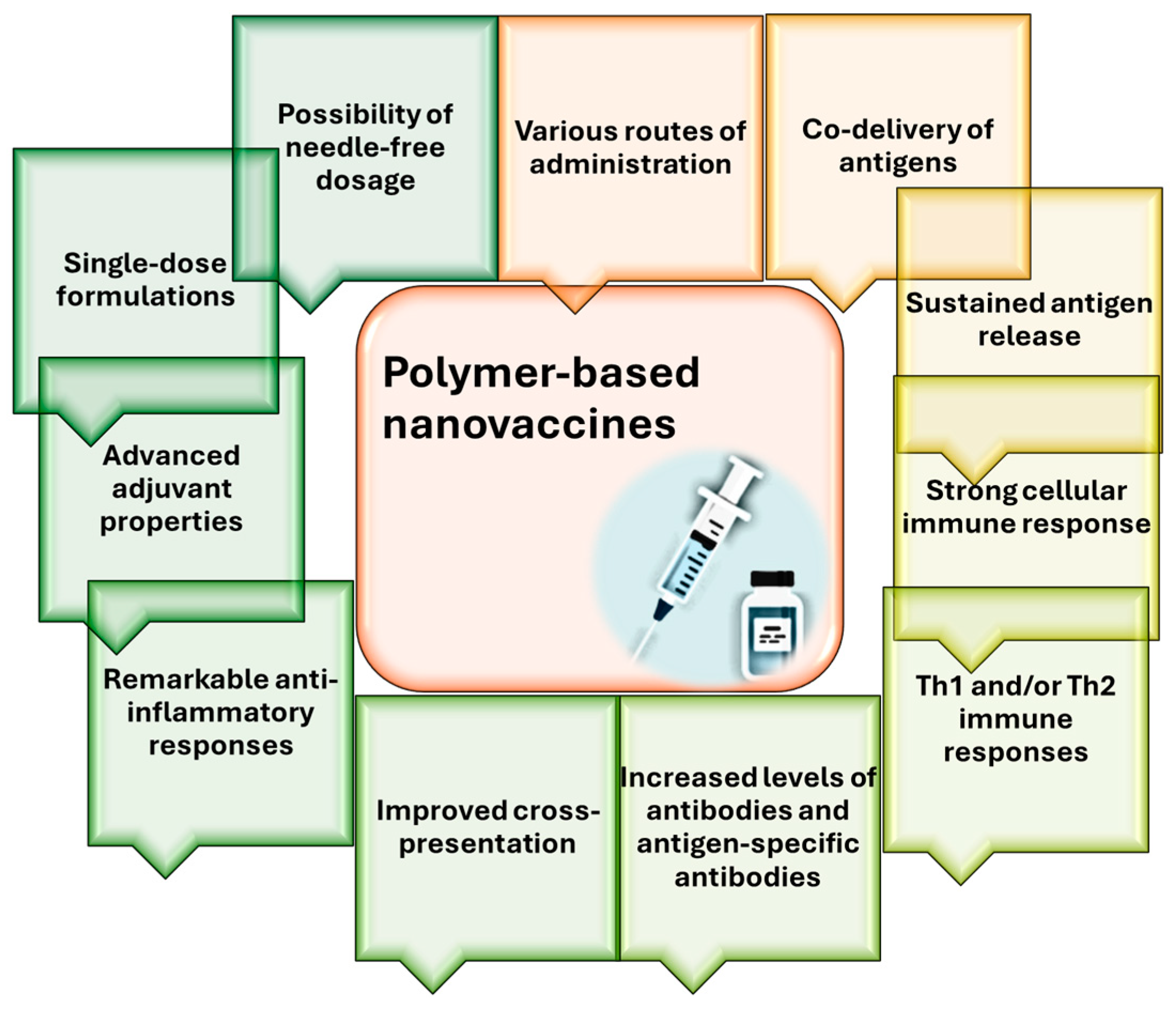
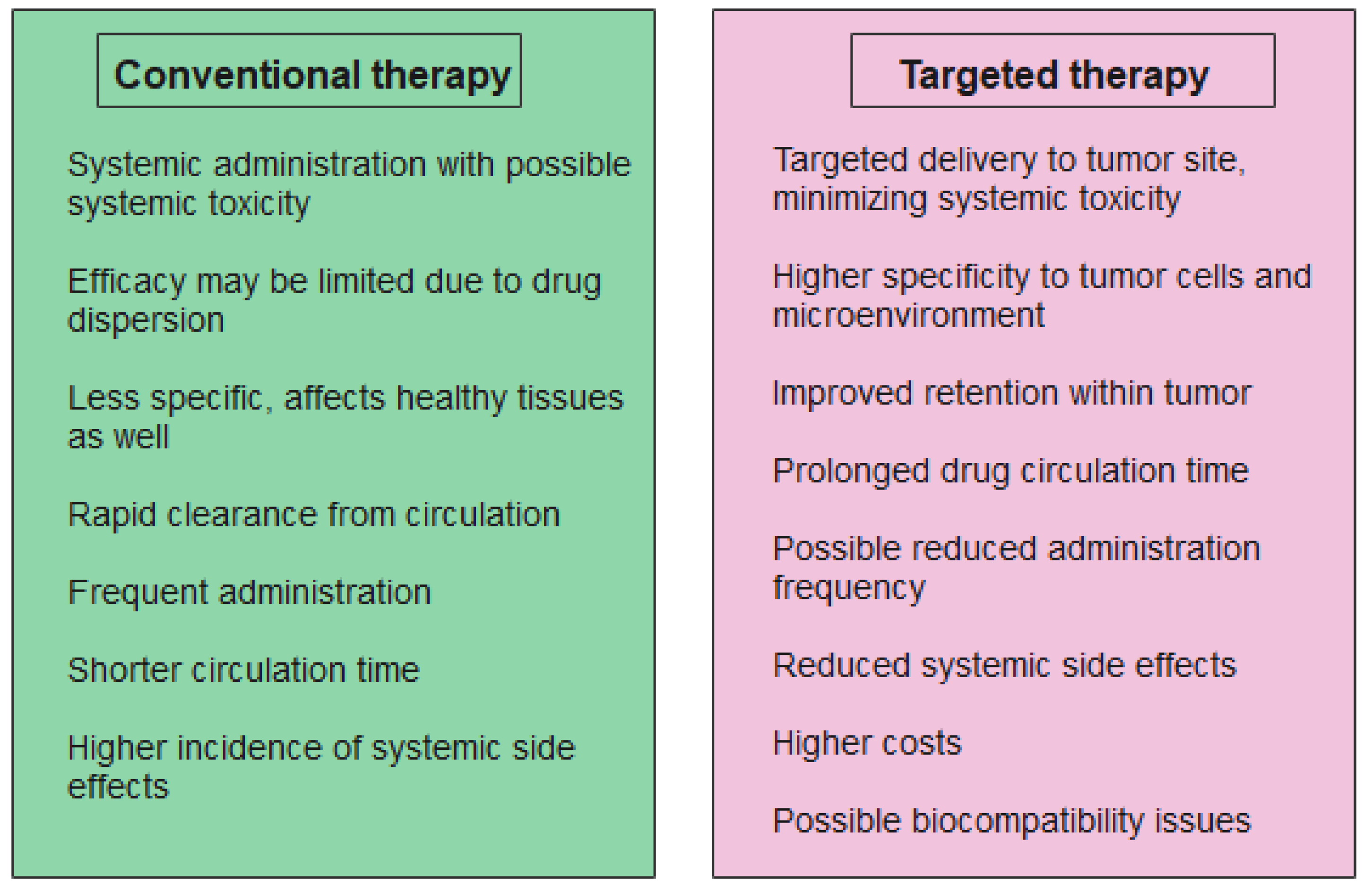
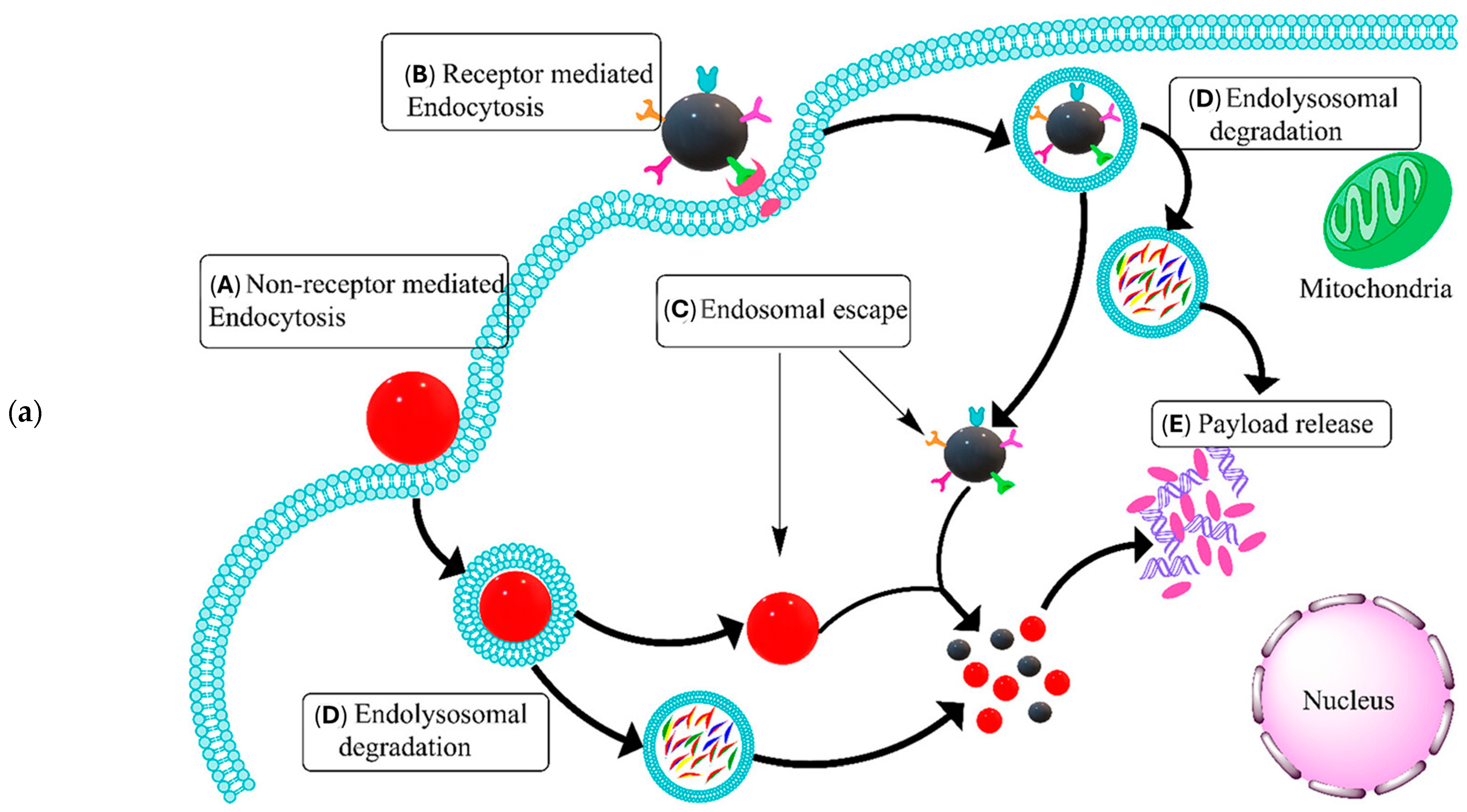
| Nanomaterial | Target | Findings | Ref. |
|---|---|---|---|
| Plasmon color-preserved AuNP clusters | Detection of SARS-CoV-2 | Selective detection of the SARS-CoV-2 nucleocapsid protein Detection at low concentrations Improved detection compared with available COVID-19 test kits | [46] |
| Polypyrrole (nano)structures decorated with AuNPs | COVID-19 serological diagnosis | Provided optimal surfaces for protein attachment Sensitively detected specific antibodies Rapid detection (less than one hour) Low-cost alternative | [47] |
| Au NPs/MoS2-graphene aerogels composite | Detection of prostate-specific antigen | Excellent conductivity, which accelerates the electron transport of the electrode interface Amplified the electrochemical signal Detection at low concentrations Stable and selective | [48] |
| Au NPs/MWCNTs-graphene quantum dots nanocomposite | Sensitive detection of prostate-specific antigen | Excellent linear relationship between the impedance change and different concentrations of PSA Detection at low concentrations Stable, selective, and reproductible | [49] |
| Citicoline-bovine serum albumin conjugate and aptamer-functionalized AuNPs nanozyme | Detection of C-reactive protein | High accuracy and sensitivity for detection in blood Low detection limit Low-cost Stability and selectivity | [50] |
| Targeted Disease | LNPs-Based Vaccine | Findings | Ref. |
|---|---|---|---|
| Influenza | R-DOTAP (1,2-dioleoyl-3-trimethylammonium-propane) cationic LPNs | Outperformed conventional adjuvants in promoting peptide-specific CD4 T cell responses Enhanced immune response through different mechanisms R-DOTAP alone activated and differentiated CD4 T cells | [64] |
| Influenza A virus of swine (IAV-S) | Hemagglutinin Antigen LPNs | Provided robust systemic and mucosal responses in pigs Induced antibody and T-cell responses led to complete protective immunity in vaccinated pigs | [65] |
| Lyme disease | mRNA-LNPs | Induced stronger antibody and T-cell responses More robust CD8+ and CD4+ T cell responses Led to higher levels of antigen-specific memory B cells | [66] |
| SARS-CoV-2 variants | 4N4T-LNPs | Showed higher mRNA translation efficiency compared to existing delivery systems Induced stronger immune responses Higher levels of RBD-specific IgG and neutralizing antibodies | [67] |
| Monkeypox virus (MPXV) | mRNA-LNPs | Induced MPXV-specific antibodies, potent neutralizing antibodies, and efficient No side effects | [68] |
| Route of Administration | Advantages | Disadvantages |
|---|---|---|
| Oral | Non-invasive High patient compliance | Degradation in the gastrointestinal tract First-pass metabolism Poor absorption |
| Transdermal | Non-invasive Large surface area for administration Good patient compliance | Possible issues with drug absorption Irritation |
| Intravenous | Systemic NP delivery Prompt response | Risk of systemic toxicity Pain in the administration area Invasive |
| Inhalation | Non-invasive Large surface area for administration Avoidance of first-pass metabolism | Possible pulmonary clearance Risk of local toxicity The potential of passing into the systemic circulation |
| Ophthalmic | Direct delivery to ocular tissues Minimizes systemic side effects | Toxicity in certain conditions Potential irritation Limited to ocular treatments |
| Intranasal | Non-invasive Direct delivery to the brain | Mucociliary clearance Possible irritation |
| NP System | Targeted Cancer Type | Findings | Ref. |
|---|---|---|---|
| Resiquimod-loaded platelet membrane-coated nanoparticles | Solid tumors (colorectal and breast cancer models) | Enhanced intratumoral delivery of resiquimod Increase in effector and central memory T cells Significant reduction in lung metastasis | [107] |
| Biomimetic NPs loaded with mRNAs encoding costimulatory receptors (OX40) | Various tumors | Delivery of OX40 mRNA to T cells enhanced antitumor activity Tumor regression Increased response to checkpoint blockade | [100] |
| Manganese zinc sulfide NPs | Metastatic melanoma | Induction of ICD Activation of cytotoxic T cells Reprogramming of tumor microenvironment Metastasis inhibition | [108] |
| Iron oxide nanoparticles (IONPs) | Breast tumors | Local hyperthermia via PTT depletes tumor-associated Tregs Increased efficacy of anti-CTLA-4 immunotherapy Inhibited tumor growth | [109] |
| Doxorubicin/CTLA-4 blocker-co-loaded liposomes | Various tumors | Promoted CD8+ T cell activation Stimulated cytokine production Effective tumor cell eradication | [110] |
| Thermosensitive hydrogel (F127-g-gelatin) releasing S-nitroso glutathione (GSNO) and anti-CTLA-4 micelles | Melanoma | Sustained release of GSNO and anti-CTLA-4 within the tumor microenvironment Potentiation of abscopal effects Improved antitumor immune response | [111] |
| Selenium-containing NPs with doxorubicin | Lung metastasis and subcutaneous tumors | Radiation-responsive SeNPs release selenic acid Increased NK cell-mediated immunotherapy Targeted doxorubicin delivery | [112] |
| NP System | Toxic Effect | Ref. |
|---|---|---|
| Chitosan-coated AgNPs | Dose-dependent toxicity in rats, severe at 50 mg/kg with hepatic and renal congestion Oxidative stress is evidenced by increased malondialdehyde (MDA) and decreased glutathione (GSH) Elevated liver enzymes (ALT, AST) and altered protein levels Renal damage is indicated by increased urea nitrogen and creatinine levels | [129] |
| Copper oxide NPs (CuO NPs) | Toxic effects on zebrafish embryos Decreased heartbeat Gene expression changes related to antioxidant and immune systems Increased mortality | [130] |
| Copper nanoparticles (Cu NPs) | Inhibition of growth in Takifugu fasciatus Increased oxidative stress Altered mitochondrial function Immune response activation | [131] |
| Zinc oxide (ZnO) NPs | Size- and dose-dependent cytotoxicity in HepG2 cells Release of Zn2+ Increased oxidative stress Inflammatory response | [132] |
| Different NPs (nZnO, nFe2O3, nCuO, and MWCNT) | ROS induction DNA damage Alteration of neurotransmitter levels and gene expressions related to immune and neurotransmitter systems | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croitoru, G.-A.; Pîrvulescu, D.-C.; Niculescu, A.-G.; Epistatu, D.; Rădulescu, M.; Grumezescu, A.M.; Nicolae, C.-L. Nanomaterials in Immunology: Bridging Innovative Approaches in Immune Modulation, Diagnostics, and Therapy. J. Funct. Biomater. 2024, 15, 225. https://doi.org/10.3390/jfb15080225
Croitoru G-A, Pîrvulescu D-C, Niculescu A-G, Epistatu D, Rădulescu M, Grumezescu AM, Nicolae C-L. Nanomaterials in Immunology: Bridging Innovative Approaches in Immune Modulation, Diagnostics, and Therapy. Journal of Functional Biomaterials. 2024; 15(8):225. https://doi.org/10.3390/jfb15080225
Chicago/Turabian StyleCroitoru, George-Alexandru, Diana-Cristina Pîrvulescu, Adelina-Gabriela Niculescu, Dragoș Epistatu, Marius Rădulescu, Alexandru Mihai Grumezescu, and Carmen-Larisa Nicolae. 2024. "Nanomaterials in Immunology: Bridging Innovative Approaches in Immune Modulation, Diagnostics, and Therapy" Journal of Functional Biomaterials 15, no. 8: 225. https://doi.org/10.3390/jfb15080225








