Advancements in Nanoporous Materials for Biomedical Imaging and Diagnostics
Abstract
:1. Introduction
1.1. Background and Importance
1.2. Scope and Objectives
- Summarize the types and properties of nanoporous materials utilized in biomedical applications.
- Discuss state-of-the-art synthesis and functionalization techniques that enhance the performance of nanoporous materials.
- Evaluate the use of nanoporous materials in various imaging and diagnostic applications, highlighting their mechanisms and efficacy.
- Identify the challenges and limitations associated with the use of nanoporous materials in biomedical contexts.
- Provide insights into future perspectives and potential breakthroughs in the field.
2. Types of Nanoporous Materials
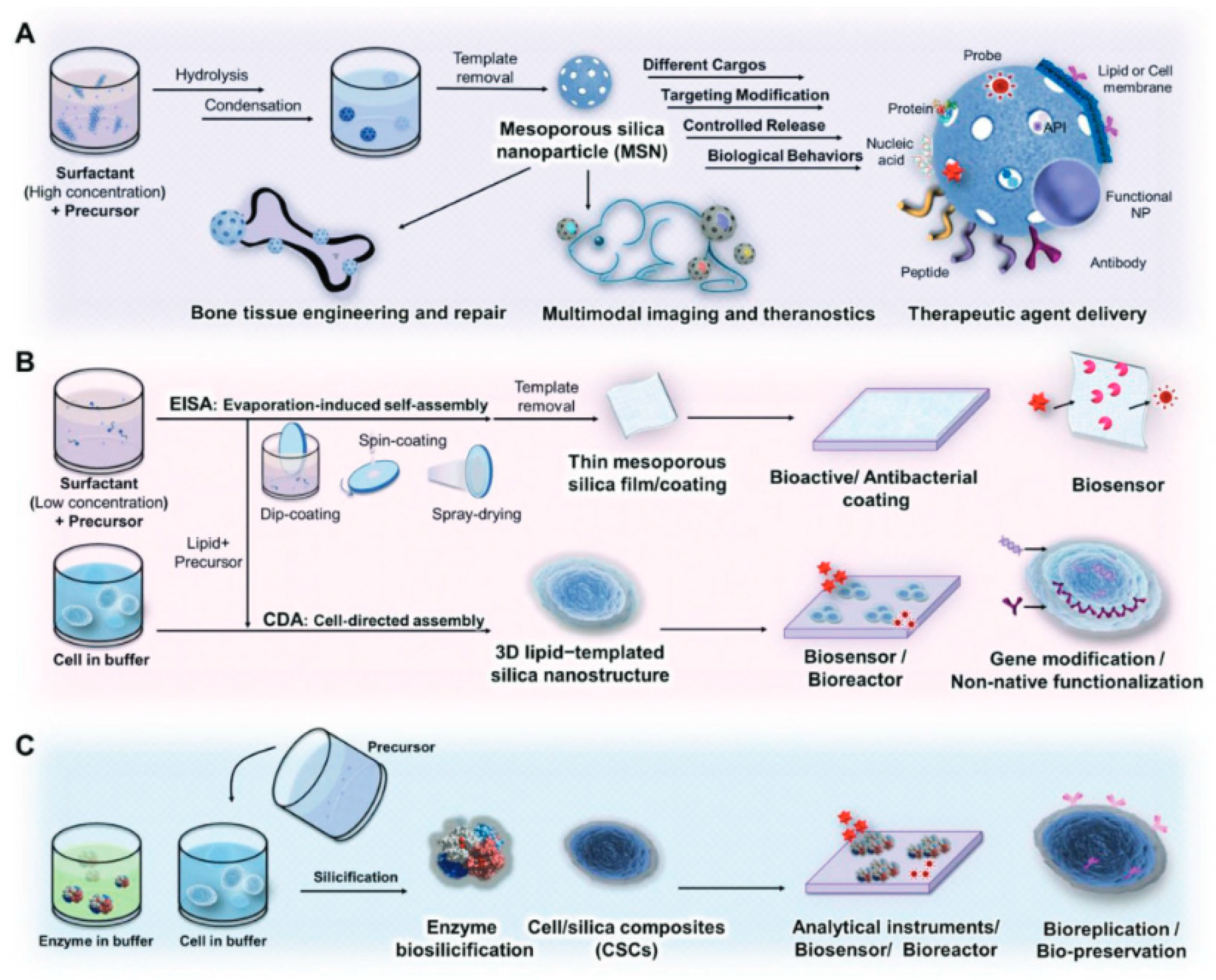
2.1. Mesoporous Silica Nanoparticles
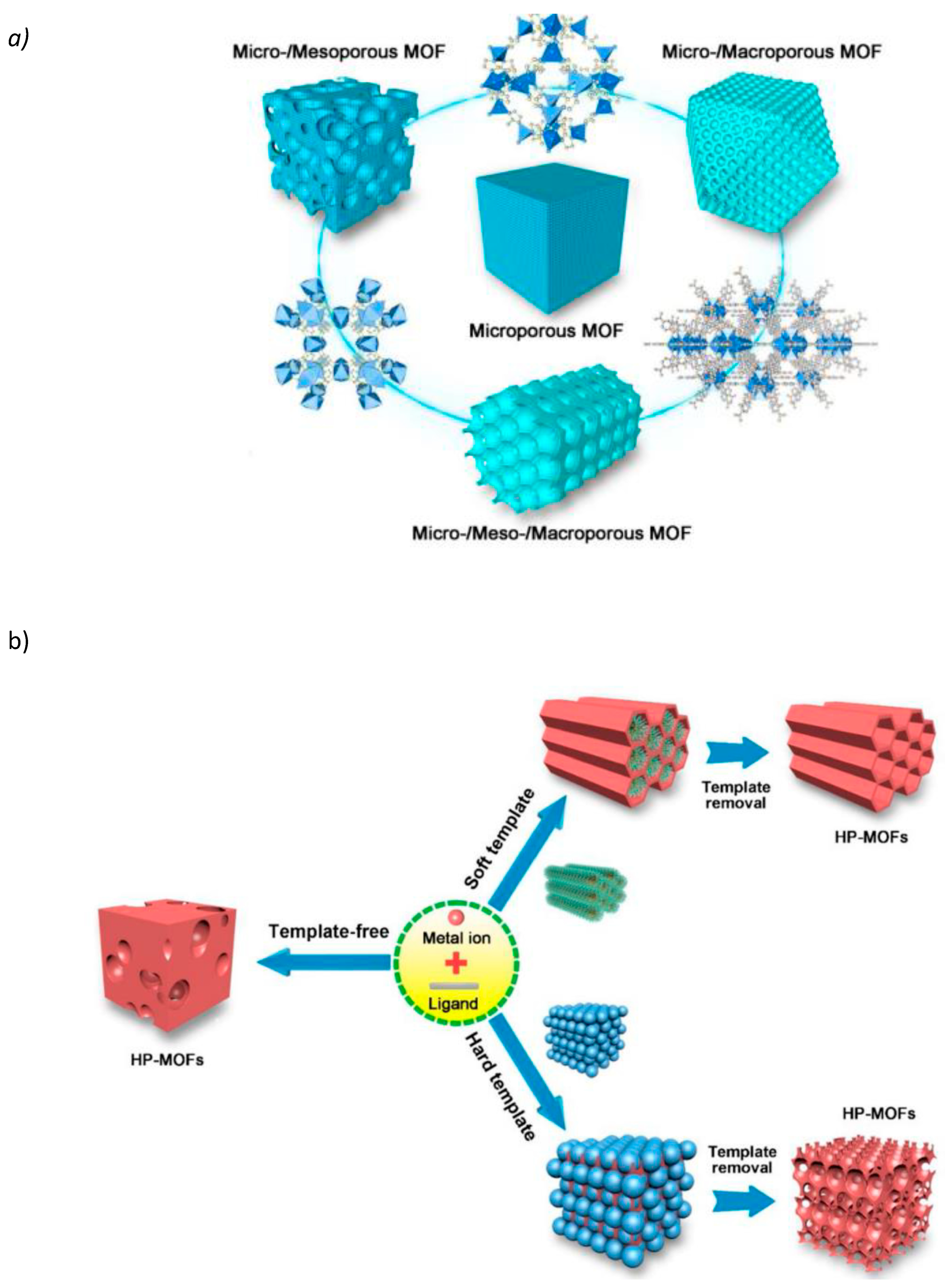
2.2. Metal–Organic Frameworks (MOFs)
2.3. Carbon-Based Nanoporous Materials
2.4. Nanoporous Gold
3. Synthesis Techniques
3.1. Sol–Gel Method
3.2. Hydrothermal Synthesis
3.3. Template-Assisted Methods
3.3.1. Approach and Mechanisms
- Soft templates: These include surfactants, block copolymers, and other molecular assemblies that form micelles or liquid crystalline phases. During the synthesis, the precursor material deposits around these micellar structures, and subsequent removal of the template (often through calcination or solvent extraction) leaves behind a porous network. This method is particularly advantageous for creating mesoporous structures with highly uniform pore sizes. However, the removal process can sometimes lead to partial collapse of the structure, affecting the uniformity and integrity of the pores.
- Hard templates: Colloidal particles (such as silica or polystyrene beads) and other solid materials are used as hard templates. The precursor material coats these hard templates, and upon removal (via etching or dissolution), a highly ordered porous structure is obtained. This method allows for excellent control over the pore size and distribution, but the process can be more complex and costly compared to soft templates.
- Sacrificial templates: These involve templates that can be selectively removed without affecting the underlying structure. Examples include polymer scaffolds that are thermally decomposable. This approach is highly beneficial for creating hierarchical structures, which are increasingly important in applications requiring multi-functional surfaces, such as biosensing and catalysis.
3.3.2. Advantages of Template-Assisted Methods
- Enhanced sensitivity: The high surface area-to-volume ratio and tunable pore sizes can significantly improve the capture and detection of biomolecules, such as proteins and nucleic acids, which are crucial in diagnostic applications. The uniform pore structure ensures consistent interactions with target molecules, leading to more reliable and reproducible results [35].
- Versatility: The ability to use a wide range of templates allows for the design of materials with diverse properties suitable for various diagnostic techniques, including optical, electrochemical, and magnetic biosensors. This versatility is particularly useful in developing multifunctional diagnostic platforms capable of detecting multiple biomarkers simultaneously [36].
- Scalability: Template-assisted methods can be scaled up for mass production, which is essential for the commercial development of diagnostic tools. The reproducibility of these methods ensures consistent quality across different batches, which is critical for clinical applications where standardization is a key requirement [37].
3.3.3. Disadvantages and Challenges
- a.
- Template Removal: The removal of templates, especially hard templates, can sometimes leave residues that contaminate the porous material, potentially affecting its performance. In biosensing applications, such impurities can lead to false positives or reduce the specificity of the assay.
- b.
- Complexity and cost: The use of hard templates often requires additional steps and specialized equipment for removal, increasing the complexity and cost of the fabrication process. This can be a barrier to the widespread adoption of these methods, particularly in resource-limited settings where low-cost diagnostic tools are needed.
- c.
- Limited scalability for certain templates: While soft templates are relatively easy to scale up, hard templates and some sacrificial templates present challenges in large-scale production. The uniformity and reproducibility of the porous structures can diminish when scaling up, potentially affecting the performance of the resulting diagnostic devices [35].
3.3.4. Applications in Disease Diagnosis
3.3.5. Future Directions
3.4. Self-Assembly Techniques
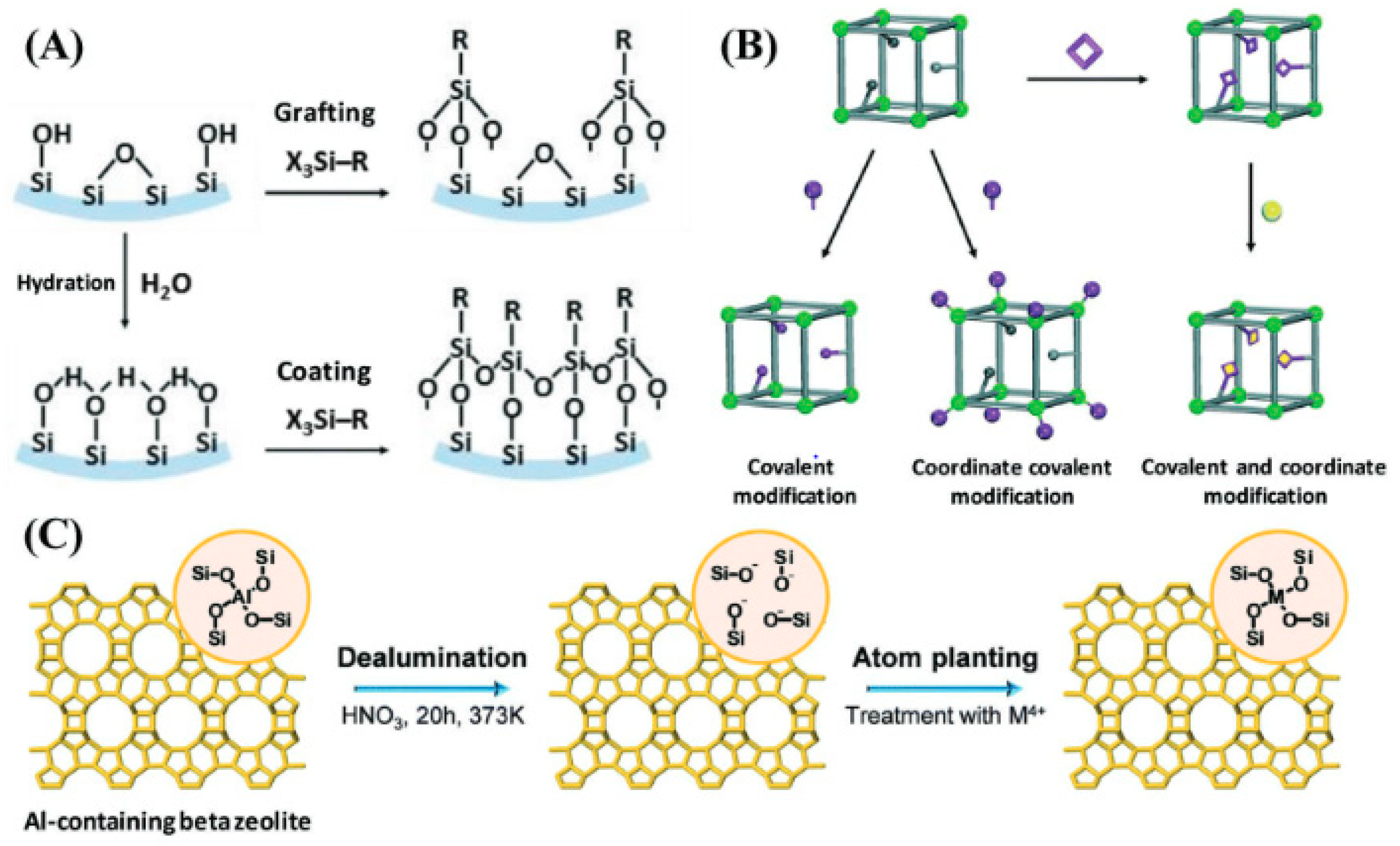
4. Functionalization and Surface Modification
4.1. Chemical Functionalization
4.2. Bioconjugation Strategies
4.3. Surface Coating and Encapsulation
5. Biomedical Imaging Applications
5.1. Fluorescence Imaging
5.2. Magnetic Resonance Imaging (MRI)
5.3. Computed Tomography (CT) Imaging
5.4. Ultrasound Imaging
5.5. Multimodal Imaging
6. Diagnostic Applications
6.1. Biosensing and Bioimaging
6.2. Disease Diagnostics
6.3. Early Detection of Cancer
6.4. In Vivo and In Vitro Applications
7. Mechanisms and Efficacy
7.1. Imaging Mechanisms
7.2. Diagnostic Mechanisms
7.3. Efficiency and Sensitivity
7.4. Targeting and Specificity
8. Challenges and Limitations
8.1. Biocompatibility and Toxicity
8.2. Stability and Degradation
8.3. Scale-Up and Manufacturing
8.4. Regulatory and Ethical Issues
8.4.1. Regulatory Pathways for Clinical Approval
- a.
- Preclinical testing
- b.
- Clinical trials
- Phase I focuses on safety and involves a small number of healthy volunteers or patients;
- Phase II assesses efficacy and side effects in a larger group:
- Phase III involves large-scale testing to confirm effectiveness, monitor side effects, and compare with commonly used treatments.
- c.
- Market approval and post-market surveillance
8.4.2. Regulatory Challenges Specific to Nanoporous Materials
- a.
- Characterization and standardization
- b.
- Risk assessment
- c.
- Manufacturing and quality control
8.4.3. Ethical Considerations
- a.
- Informed consent
- b.
- Privacy and data protection
- c.
- Equity and access
- d.
- Environmental and long-term impact
8.4.4. Regulatory and Ethical Framework Development
- Interdisciplinary collaboration: involving experts from toxicology, material science, medicine, ethics, and law to develop comprehensive guidelines;
- Public engagement: engaging the public in discussions about the benefits and risks of nanoporous materials to build trust and support informed decision-making;
- International cooperation: harmonizing regulations across countries to facilitate global access to safe and effective nanotechnology-based treatments.
9. Future Perspectives
9.1. Emerging Trends in Nanoporous Materials
9.2. Innovations in Synthesis and Functionalization
9.3. Potential Breakthroughs in Imaging and Diagnostics
9.4. Interdisciplinary Approaches
9.5. Advancements in Nanoporous Materials
- Enhanced surface area and porosity: Nanoporous materials exhibit significantly larger surface areas compared to other nanomaterials, facilitating improved interaction with biological molecules. This property enhances applications in drug delivery, catalysis, and biosensing. For instance, mesoporous silica nanoparticles can be designed with specific pore sizes for controlled drug release, an advancement over non-porous nanoparticles that lack such precise control.
- Controlled drug release: The capability of nanoporous materials to provide controlled and sustained drug release is a major advancement. This allows for the precise tuning of release rates, which is crucial for managing chronic conditions. For example, mesoporous silica nanoparticles can release drugs over extended periods of time, reducing the need for frequent dosing.
- Functionalization flexibility: The surface chemistry of nanoporous materials can be modified to enhance biocompatibility and target specificity, surpassing the functionalization capabilities of some other nanomaterials. For instance, surface modifications on mesoporous silica can improve compatibility with biological systems or target specific cells, offering a level of customization not easily achievable with other materials.
- Biocompatibility and safety: Certain nanoporous materials, such as silica, are highly biocompatible and crucial for biomedical applications. They can be engineered to degrade safely within the body, minimizing potential toxicity—a common issue with some other nanomaterials like certain metal oxides.
9.6. Disadvantages and Considerations
- Complex synthesis and scalability: The complex synthesis processes of nanoporous materials pose challenges for scalability, potentially limiting their widespread use compared to other nanomaterials that are easier to produce at scale.
- Potential for unintended bio-interactions: The high surface area and porosity of these materials, while advantageous, may lead to unintended interactions with biological molecules, potentially causing off-target effects. This includes the possible activation of the immune system or unwanted protein interactions.
- Regulatory and ethical challenges: Nanoporous materials face stringent regulatory and ethical scrutiny due to their novel properties and potential risks. Regulatory approval can be challenging, impacting their clinical application.
- Potential for accumulation and toxicity: The potential for accumulation and toxicity is a concern, particularly for materials that are not easily degraded or cleared from the body, posing long-term safety and environmental risks.
10. Conclusions
10.1. Summary of Key Findings
10.2. Implications for Biomedical Research
Nanoporous Materials in Clinical Practice: Rapid Transition from Lab to Clinic
- 1.
- Drug delivery systems
- 2.
- Imaging and diagnostics
- 3.
- Biosensing and early disease detection
- 4.
- Scaffold materials in tissue engineering
- 5.
- Translational Research and Clinical Trials
10.3. Final Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Liu, B.; Ejaz, W.; Gong, S.; Kurbanov, M.; Canakci, M.; Anson, F.; Thayumanavan, S. Engineered Interactions with Mesoporous Silica Facilitate Intracellular Delivery of Proteins and Gene Editing. Nano Lett. 2020, 20, 4014–4021. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.-C.; Xie, Y.; Deng, T.; Zink, J.I. Magnetism, Ultrasound, and Light-Stimulated Mesoporous Silica Nanocarriers for Theranostics and Beyond. J. Am. Chem. Soc. 2021, 143, 6025–6036. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.I.; Trewyn, B.G.; Lin, V.S.-Y. Mesoporous Silica Nanoparticles for Intracellular Delivery of Membrane-Impermeable Proteins. J. Am. Chem. Soc. 2007, 129, 8845–8849. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous Metal–Organic-Framework Nanoscale Carriers as a Potential Platform for Drug Delivery and Imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, I.; Patriarche, G.; Serre, C.; Boissiére, C.; Gref, R. Advanced Characterization Methodology to Unravel the Biodegradability of Metal–Organic Framework Nanoparticles in Extremely Diluted Conditions. ACS Appl. Mater. Interfaces 2024, 16, 14296–14307. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Pashow, K.M.L.; Della Rocca, J.; Huxford, R.C.; Lin, W. Hybrid Nanomaterials for Biomedical Applications. Chem. Commun. 2010, 46, 5832. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Mandal, T.K. Dually Emissive P,N-Co-Doped Carbon Dots for Fluorescent and Photoacoustic Tissue Imaging in Living Mice. Microchim. Acta 2017, 184, 1117–1125. [Google Scholar] [CrossRef]
- Johnsen, H.M.; Hossaini Nasr, S.; De Luna, R.; Filtvedt, W.; Sailor, M.J.; Klaveness, J.; Hiorth, M. Stable “Snow Lantern-like” Aggregates of Silicon Nanoparticles Suitable as a Drug Delivery Platform. Nanoscale 2024, 16, 9899–9910. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.-T.; Liu, Z. Graphene in Mice: Ultrahigh In Vivo Tumor Uptake and Efficient Photothermal Therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef]
- Lang, X.; Hirata, A.; Fujita, T.; Chen, M. Nanoporous Metal/Oxide Hybrid Electrodes for Electrochemical Supercapacitors. Nat. Nanotechnol. 2011, 6, 232–236. [Google Scholar] [CrossRef]
- Parvin, N.; Mandal, T.K. Synthesis of a Highly Fluorescence Nitrogen-Doped Carbon Quantum Dots Bioimaging Probe and Its in Vivo Clearance and Printing Applications. RSC Adv. 2016, 6, 18134–18140. [Google Scholar] [CrossRef]
- Parvin, N.; Kumar, V.; Joo, S.W.; Park, S.-S.; Mandal, T.K. Recent Advances in the Characterized Identification of Mono-to-Multi-Layer Graphene and Its Biomedical Applications: A Review. Electronics 2022, 11, 3345. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Shi, J. In Vivo Bio-Safety Evaluations and Diagnostic/Therapeutic Applications of Chemically Designed Mesoporous Silica Nanoparticles. Adv. Mater. 2013, 25, 3144–3176. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Meziani, M.J.; Sahu, S.; Sun, Y.-P. Photoluminescence Properties of Graphene versus Other Carbon Nanomaterials. Acc. Chem. Res. 2013, 46, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Nallapureddy, R.R.; Mandal, T.K.; Joo, S.W. Construction of Bimetallic Hybrid Multishell Hollow Spheres via Sequential Template Approach for Less Cytotoxic Antimicrobial Effect. IEEE Trans. Nanobiosci. 2022, 22, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhong, M.; Shi, F.; Liu, X.; Tang, Z.; Wang, Y.; Huang, Y.; Hou, H.; Xie, X.; Zhi, C. An Intrinsically Stretchable and Compressible Supercapacitor Containing a Polyacrylamide Hydrogel Electrolyte. Angew. Chem. Int. Ed. 2017, 56, 9141–9145. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Seaberg, J.; Montazerian, H.; Hossen, M.N.; Bhattacharya, R.; Khademhosseini, A.; Mukherjee, P. Hybrid Nanosystems for Biomedical Applications. ACS Nano 2021, 15, 2099–2142. [Google Scholar] [CrossRef]
- Batool, M.; Tawakkul, N.; Batool, S.; Zafar, M.N.; Nazar, M.F. Porous Metal–Organic Framework Nanoscale Carriers as a Potential Platform for Drug Delivery. In Novel Platforms for Drug Delivery Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 153–176. [Google Scholar]
- Park, W.; Shin, H.; Choi, B.; Rhim, W.-K.; Na, K.; Keun Han, D. Advanced Hybrid Nanomaterials for Biomedical Applications. Prog. Mater. Sci. 2020, 114, 100686. [Google Scholar] [CrossRef]
- Rahman, A.; Chowdhury, M.A.; Hossain, N. Green Synthesis of Hybrid Nanoparticles for Biomedical Applications: A Review. Appl. Surf. Sci. Adv. 2022, 11, 100296. [Google Scholar] [CrossRef]
- Kabtamu, D.M.; Wu, Y.; Li, F. Hierarchically Porous Metal–Organic Frameworks: Synthesis Strategies, Structure(s), and Emerging Applications in Decontamination. J. Hazard. Mater. 2020, 397, 122765. [Google Scholar] [CrossRef]
- Romero, M.P.; Buzza, H.H.; Stringasci, M.D.; Estevão, B.M.; Silva, C.C.; Pereira-da-Silva, M.A.; Inada, N.M.; Bagnato, V.S. Graphene Oxide Theranostic Effect: Conjugation of Photothermal and Photodynamic Therapies Based on an In Vivo Demonstration. Int. J. Nanomed. 2021, 16, 1601–1616. [Google Scholar] [CrossRef]
- Qi, K.; Sun, B.; Liu, S.; Zhang, M. Research Progress on Carbon Materials in Tumor Photothermal Therapy. Biomed. Pharmacother. 2023, 165, 115070. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.R.; Narayan, R.J. Recent Advances in Carbon Nanomaterials for Biomedical Applications: A Review. Curr. Opin. Biomed. Eng. 2021, 17, 100262. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, S.; Wang, X.; Xu, P. Nanoporous Gold: A Review and Potentials in Biotechnological and Biomedical Applications. Nano Sel. 2021, 2, 1437–1458. [Google Scholar] [CrossRef]
- Sondhi, P.; Lingden, D.; Bhattarai, J.K.; Demchenko, A.V.; Stine, K.J. Applications of Nanoporous Gold in Therapy, Drug Delivery, and Diagnostics. Metals 2022, 13, 78. [Google Scholar] [CrossRef]
- Ruffino, F.; Grimaldi, M.G. Nanoporous Gold-Based Sensing. Coatings 2020, 10, 899. [Google Scholar] [CrossRef]
- Qureshi, M.; Viegas, C.; Duarte, S.O.D.; Girardi, M.; Shehzad, A.; Fonte, P. Camptothecin-Loaded Mesoporous Silica Nanoparticles Functionalized with CpG Oligodeoxynucleotide as a New Approach for Skin Cancer Treatment. Int. J. Pharm. 2024, 660, 124340. [Google Scholar] [CrossRef]
- Lei, Q.; Guo, J.; Noureddine, A.; Wang, A.; Wuttke, S.; Brinker, C.J.; Zhu, W. Sol–Gel-Based Advanced Porous Silica Materials for Biomedical Applications. Adv. Funct. Mater. 2020, 30, 1909539. [Google Scholar] [CrossRef]
- Harish, V.; Ansari, M.M.; Tewari, D.; Yadav, A.B.; Sharma, N.; Bawarig, S.; García-Betancourt, M.-L.; Karatutlu, A.; Bechelany, M.; Barhoum, A. Cutting-Edge Advances in Tailoring Size, Shape, and Functionality of Nanoparticles and Nanostructures: A Review. J. Taiwan Inst. Chem. Eng. 2023, 149, 105010. [Google Scholar] [CrossRef]
- Chen, W.; Du, L.; Wu, C. Hydrothermal Synthesis of MOFs. In Metal-Organic Frameworks for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 141–157. [Google Scholar]
- Theerthagiri, J.; Chandrasekaran, S.; Salla, S.; Elakkiya, V.; Senthil, R.A.; Nithyadharseni, P.; Maiyalagan, T.; Micheal, K.; Ayeshamariam, A.; Arasu, M.V.; et al. Recent Developments of Metal Oxide Based Heterostructures for Photocatalytic Applications towards Environmental Remediation. J. Solid State Chem. 2018, 267, 35–52. [Google Scholar] [CrossRef]
- Kumar, R.; Joanni, E.; Singh, R.K.; Singh, D.P.; Moshkalev, S.A. Recent Advances in the Synthesis and Modification of Carbon-Based 2D Materials for Application in Energy Conversion and Storage. Prog. Energy Combust. Sci. 2018, 67, 115–157. [Google Scholar] [CrossRef]
- Swain, N.; Saravanakumar, B.; Kundu, M.; Schmidt-Mende, L.; Ramadoss, A. Recent Trends in Template Assisted 3D Porous Materials for Electrochemical Supercapacitors. J. Mater. Chem. A 2021, 9, 25286–25324. [Google Scholar] [CrossRef]
- Thompson, B.R.; Horozov, T.S.; Stoyanov, S.D.; Paunov, V.N. Hierarchically Structured Composites and Porous Materials from Soft Templates: Fabrication and Applications. J. Mater. Chem. A 2019, 7, 8030–8049. [Google Scholar] [CrossRef]
- Chauhan, S. Synthesis of Ordered Mesoporous Carbon by Soft Template Method. Mater. Today Proc. 2023, 81, 842–847. [Google Scholar] [CrossRef]
- Karayianni, M.; Pispas, S. Block Copolymer Solution Self-assembly: Recent Advances, Emerging Trends, and Applications. J. Polym. Sci. 2021, 59, 1874–1898. [Google Scholar] [CrossRef]
- Rao, A.; Roy, S.; Jain, V.; Pillai, P.P. Nanoparticle Self-Assembly: From Design Principles to Complex Matter to Functional Materials. ACS Appl. Mater. Interfaces 2023, 15, 25248–25274. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, W.; Yang, C.; Zhu, Z. Scaling Up DNA Self-Assembly. ACS Appl. Bio Mater. 2020, 3, 2805–2815. [Google Scholar] [CrossRef]
- Suvindran, N.; Servati, A.; Servati, P. Emerging Biomedical and Industrial Applications of Nanoporous Materials. In Advanced Functional Porous Materials Engineering Materials; Uthaman, A., Thomas, S., Li, T., Maria, H., Eds.; Springer: Cham, Switzerland, 2022; pp. 353–390. [Google Scholar]
- Tiemann, M.; Weinberger, C. Selective Modification of Hierarchical Pores and Surfaces in Nanoporous Materials. Adv. Mater. Interfaces 2021, 8, 2001153. [Google Scholar] [CrossRef]
- Joseph, S.; Saianand, G.; Benzigar, M.R.; Ramadass, K.; Singh, G.; Gopalan, A.; Yang, J.H.; Mori, T.; Al-Muhtaseb, A.H.; Yi, J.; et al. Recent Advances in Functionalized Nanoporous Carbons Derived from Waste Resources and Their Applications in Energy and Environment. Adv. Sustain. Syst. 2021, 5, 2000169. [Google Scholar] [CrossRef]
- Perovic, M.; Qin, Q.; Oschatz, M. From Molecular Precursors to Nanoparticles—Tailoring the Adsorption Properties of Porous Carbon Materials by Controlled Chemical Functionalization. Adv. Funct. Mater. 2020, 30, 1908371. [Google Scholar] [CrossRef]
- Jung, Y.; Huh, Y.; Kim, D. Recent Advances in Surface Engineering of Porous Silicon Nanomaterials for Biomedical Applications. Microporous Mesoporous Mater. 2021, 310, 110673. [Google Scholar] [CrossRef]
- Yang, S.-B.; Banik, N.; Han, B.; Lee, D.-N.; Park, J. Peptide-Based Bioconjugates and Therapeutics for Targeted Anticancer Therapy. Pharmaceutics 2022, 14, 1378. [Google Scholar] [CrossRef] [PubMed]
- Vandghanooni, S.; Barar, J.; Eskandani, M.; Omidi, Y. Aptamer-Conjugated Mesoporous Silica Nanoparticles for Simultaneous Imaging and Therapy of Cancer. TrAC Trends Anal. Chem. 2020, 123, 115759. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, X.; Liao, H.; Liang, Z.; Li, F.; Tian, J.; Ling, D. Artificially Engineered Cubic Iron Oxide Nanoparticle as a High-Performance Magnetic Particle Imaging Tracer for Stem Cell Tracking. ACS Nano 2020, 14, 2053–2062. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Yao, W.; Xiao, Y.; Dong, Z.; Huang, W.; Zhang, F.; Zhou, X.; Liang, M. Resveratrol-Modified Mesoporous Silica Nanoparticle for Tumor-Targeted Therapy of Gastric Cancer. Bioengineered 2021, 12, 6343–6353. [Google Scholar] [CrossRef]
- Kapruwan, P.; Ferré-Borrull, J.; Marsal, L.F. Nanoporous Anodic Alumina Platforms for Drug Delivery Applications: Recent Advances and Perspective. Adv. Mater. Interfaces 2020, 7, 2001133. [Google Scholar] [CrossRef]
- Peng, C.; Chen, M.; Spicer, J.B.; Jiang, X. Acoustics at the Nanoscale (Nanoacoustics): A Comprehensive Literature Review. Part II: Nanoacoustics for Biomedical Imaging and Therapy. Sens. Actuators A Phys. 2021, 332, 112925. [Google Scholar] [CrossRef] [PubMed]
- Dilnawaz, F. Multifunctional Mesoporous Silica Nanoparticles for Cancer Therapy and Imaging. Curr. Med. Chem. 2019, 26, 5745–5763. [Google Scholar] [CrossRef]
- Feng, Y.; Liao, Z.; Li, M.; Zhang, H.; Li, T.; Qin, X.; Li, S.; Wu, C.; You, F.; Liao, X.; et al. Mesoporous Silica Nanoparticles-Based Nanoplatforms: Basic Construction, Current State, and Emerging Applications in Anticancer Therapeutics. Adv. Healthc. Mater. 2023, 12, e2201884. [Google Scholar] [CrossRef]
- Barkat, A.; Beg, S.; Panda, S.K.; Alharbi, K.S.; Rahman, M.; Ahmed, F.J. Functionalized Mesoporous Silica Nanoparticles in Anticancer Therapeutics. Semin. Cancer Biol. 2021, 69, 365–375. [Google Scholar] [CrossRef]
- Bunzen, H.; Jirák, D. Recent Advances in Metal–Organic Frameworks for Applications in Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2022, 14, 50445–50462. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ge, J.; Gao, Y.; Chen, L.; Cui, J.; Zeng, J.; Gao, M. Ultrasmall Superparamagnetic Iron Oxide Nanoparticles: A next Generation Contrast Agent for Magnetic Resonance Imaging. WIREs Nanomed. Nanobiotechnol. 2022, 14, e1740. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.; Javaid, M.; Singh, R.P.; Rab, S.; Suman, R. Applications of Nanotechnology in Medical Field: A Brief Review. Glob. Heath. J. 2023, 7, 70–77. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Ridwan, S.M.; Stanishevskiy, F.Y.; Smilowitz, H.M. Iodine Nanoparticle Radiotherapy of Human Breast Cancer Growing in the Brains of Athymic Mice. Sci. Rep. 2020, 10, 15627. [Google Scholar] [CrossRef] [PubMed]
- El Ketara, S.; Ford, N.L. Time-Course Study of a Gold Nanoparticle Contrast Agent for Cardiac-Gated Micro-CT Imaging in Mice. Biomed. Phys. Eng. Express 2020, 6, 035025. [Google Scholar] [CrossRef]
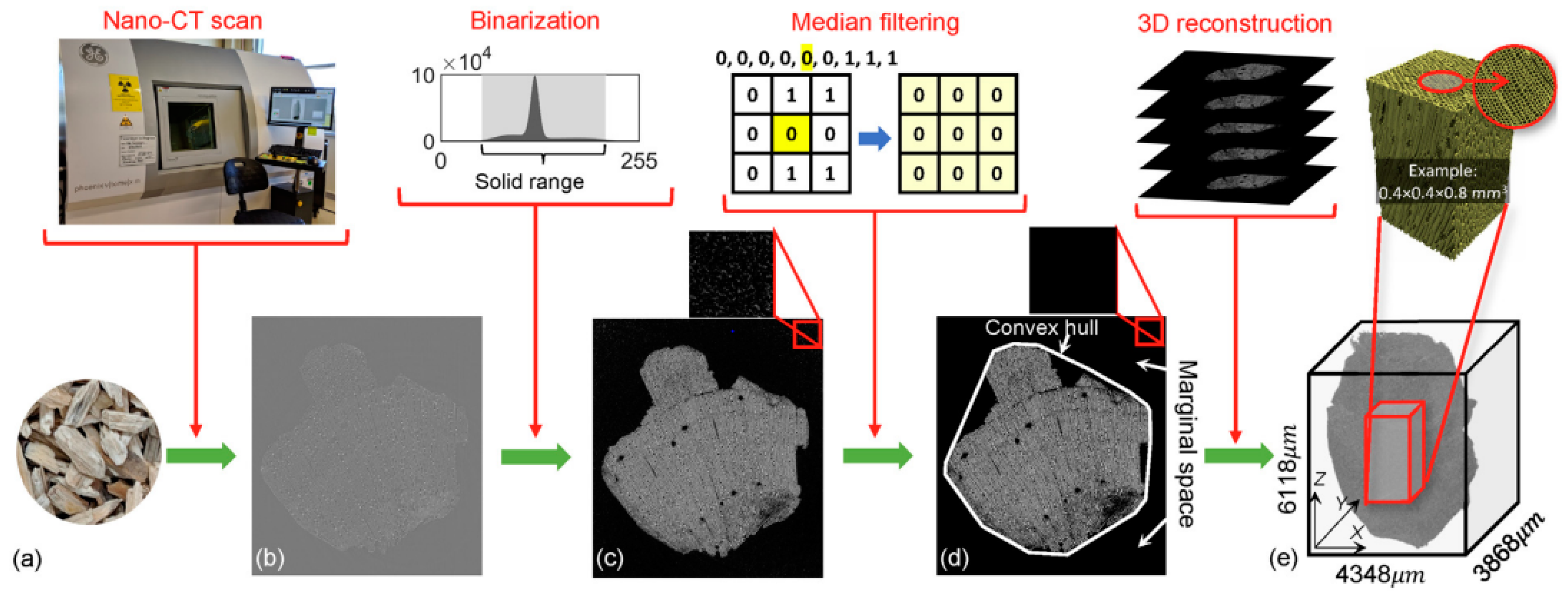
- Sun, Q.; Xia, Y.; Klinger, J.; Seifert, R.; Kane, J.; Thompson, V.; Chen, Q. X-Ray Computed Tomography-Based Porosity Analysis: Algorithms and Application for Porous Woody Biomass. Powder Technol. 2021, 388, 496–504. [Google Scholar] [CrossRef]
- Kunkalekar, R.K.; Gawas, U.B. Role of Nanoparticles in Advanced Biomedical Research. In Advances in Biological Science Research; Elsevier: Amsterdam, The Netherlands, 2019; pp. 347–361. [Google Scholar]
- Exner, A.A.; Kolios, M.C. Bursting Microbubbles: How Nanobubble Contrast Agents Can Enable the Future of Medical Ultrasound Molecular Imaging and Image-Guided Therapy. Curr. Opin. Colloid Interface Sci. 2021, 54, 101463. [Google Scholar] [CrossRef] [PubMed]
- Upputuri, P.K.; Pramanik, M. Recent Advances in Photoacoustic Contrast Agents for in Vivo Imaging. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1618. [Google Scholar] [CrossRef]
- Peng, C.; Chen, M.; Spicer, J.B.; Jiang, X. Acoustics at the Nanoscale (Nanoacoustics): A Comprehensive Literature Review. Part I: Materials, Devices and Selected Applications. Sens. Actuators A Phys. 2021, 332, 112719. [Google Scholar] [CrossRef]
- Burke, B.P.; Cawthorne, C.; Archibald, S.J. Multimodal Nanoparticle Imaging Agents: Design and Applications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20170261. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.-N.; Li, X.-D.; Chang, J.; Li, X.; Zhang, X.-N.; Li, X.-D.; Chang, J. Multimodality Imaging in Nanomedicine and Nanotheranostics. Cancer Biol. Med. 2016, 13, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Zhang, Y.; Nie, G. Recent Advances in Nanomaterials with Inherent Optical and Magnetic Properties for Bioimaging and Imaging-Guided Nucleic Acid Therapy. Bioconjug. Chem. 2020, 31, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; He, X.-W.; Li, W.-Y.; Zhang, Y.-K. Multifunctional Mesoporous Silica Nanoplatform Based on Silicon Nanoparticles for Targeted Two-Photon-Excited Fluorescence Imaging-Guided Chemo/Photodynamic Synergetic Therapy in Vitro. Talanta 2020, 209, 120552. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.K.; Pragti; Carlton Ranjith, W.A.; Shankar, U.; Kannan, R.R.; Mobin, S.M.; Bandyopadhyay, A.; Mukhopadhyay, S. Cancer-Targeted Chitosan–Biotin-Conjugated Mesoporous Silica Nanoparticles as Carriers of Zinc Complexes to Achieve Enhanced Chemotherapy In Vitro and In Vivo. ACS Appl. Bio Mater. 2022, 5, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-S. Metal–Organic Frameworks for Biosensing and Bioimaging Applications. Coord. Chem. Rev. 2017, 349, 139–155. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, H.; Zhu, X.-W.; Lu, W.; Li, D. Metal–Organic Frameworks as Photoluminescent Biosensing Platforms: Mechanisms and Applications. Chem. Soc. Rev. 2021, 50, 4484–4513. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lu, S.; Sun, S.; Hai, J.; Meng, G.; Wang, B. NIR II Light-Response Au Nanoframes: Amplification of a Pressure- and Temperature-Sensing Strategy for Portable Detection and Photothermal Therapy of Cancer Cells. Anal. Chem. 2021, 93, 14307–14316. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, B.; Sun, J.; Hu, W.; Wang, H. Recent Advances in Porous Nanostructures for Cancer Theranostics. Nano Today 2021, 38, 101146. [Google Scholar] [CrossRef]
- Malekmohammadi, S.; Mohammed, R.U.R.; Samadian, H.; Zarebkohan, A.; García-Fernández, A.; Kokil, G.R.; Sharifi, F.; Esmaeili, J.; Bhia, M.; Razavi, M.; et al. Nonordered Dendritic Mesoporous Silica Nanoparticles as Promising Platforms for Advanced Methods of Diagnosis and Therapies. Mater. Today Chem. 2022, 26, 101144. [Google Scholar] [CrossRef]
- Deng, C.; Liu, Y.; Zhou, F.; Wu, M.; Zhang, Q.; Yi, D.; Yuan, W.; Wang, Y. Engineering of Dendritic Mesoporous Silica Nanoparticles for Efficient Delivery of Water-Insoluble Paclitaxel in Cancer Therapy. J. Colloid Interface Sci. 2021, 593, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Xie, H.; Huang, H.; Li, Z.; Sun, Z.; Xu, Y.; Xiao, Q.; Yu, X.-F.; Zhao, Y.; Zhang, H.; et al. Biodegradable Black Phosphorus-Based Nanospheres for in Vivo Photothermal Cancer Therapy. Nat. Commun. 2016, 7, 12967. [Google Scholar] [CrossRef] [PubMed]
- Santhoshkumar, S.; Madhu, M.; Tseng, W.-B.; Tseng, W.-L. Gold Nanocluster-Based Fluorescent Sensors for in Vitro and in Vivo Ratiometric Imaging of Biomolecules. Phys. Chem. Chem. Phys. 2023, 25, 21787–21801. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Song, L.; Tian, Y.; Zhu, L.; Guo, K.; Zhang, H.; Wang, Z. Emodin-Conjugated PEGylation of Fe3O4 Nanoparticles for FI/MRI Dual-Modal Imaging and Therapy in Pancreatic Cancer. Int. J. Nanomed. 2021, 16, 7463–7478. [Google Scholar] [CrossRef] [PubMed]
- Welch, E.C.; Powell, J.M.; Clevinger, T.B.; Fairman, A.E.; Shukla, A. Advances in Biosensors and Diagnostic Technologies Using Nanostructures and Nanomaterials. Adv. Funct. Mater. 2021, 31, 2104126. [Google Scholar] [CrossRef]
- Bezinge, L.; Suea-Ngam, A.; DeMello, A.J.; Shih, C.-J. Nanomaterials for Molecular Signal Amplification in Electrochemical Nucleic Acid Biosensing: Recent Advances and Future Prospects for Point-of-Care Diagnostics. Mol. Syst. Des. Eng. 2020, 5, 49–66. [Google Scholar] [CrossRef]
- Noah, N.M.; Ndangili, P.M. Current Trends of Nanobiosensors for Point-of-Care Diagnostics. J. Anal. Methods Chem. 2019, 2019, 2179718. [Google Scholar] [CrossRef]
- Peng, X.; Lin, G.; Zeng, Y.; Lei, Z.; Liu, G. Mesoporous Silica Nanoparticle-Based Imaging Agents for Hepatocellular Carcinoma Detection. Front. Bioeng. Biotechnol. 2021, 9, 749381. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhen, X.; Jiang, X. Development of Mesoporous Silica-Based Nanoprobes for Optical Bioimaging Applications. Biomater. Sci. 2021, 9, 3603–3620. [Google Scholar] [CrossRef]
- Peller, M.; Böll, K.; Zimpel, A.; Wuttke, S. Metal–Organic Framework Nanoparticles for Magnetic Resonance Imaging. Inorg. Chem. Front. 2018, 5, 1760–1779. [Google Scholar] [CrossRef]
- Demir Duman, F.; Forgan, R.S. Applications of Nanoscale Metal–Organic Frameworks as Imaging Agents in Biology and Medicine. J. Mater. Chem. B 2021, 9, 3423–3449. [Google Scholar] [CrossRef] [PubMed]
- Chen-Wiegart, Y.-C.K.; Kim, S.; Vine, D.; Xiao, X.; Zhao, C.; Pfeifer, M.A.; Williams, G.J.; McNulty, I. Revealing Three-Dimensional Morphology in Nanoporous Gold Using Three-Dimensional X-Ray Fresnel Coherent Diffractive Imaging Tomography. J. Electrochem. Energy Convers. Storage 2020, 17, 041005. [Google Scholar] [CrossRef]
- Raheem, M.A.; Rahim, M.A.; Gul, I.; Zhong, X.; Xiao, C.; Zhang, H.; Wei, J.; He, Q.; Hassan, M.; Zhang, C.Y.; et al. Advances in Nanoparticles-Based Approaches in Cancer Theranostics. OpenNano 2023, 12, 100152. [Google Scholar] [CrossRef]
- Li, S.-R.; Huo, F.-Y.; Wang, H.-Q.; Wang, J.; Xu, C.; Liu, B.; Bu, L.-L. Recent Advances in Porous Nanomaterials-Based Drug Delivery Systems for Cancer Immunotherapy. J. Nanobiotechnol. 2022, 20, 277. [Google Scholar] [CrossRef] [PubMed]
- Kukkar, D.; Kukkar, P.; Kumar, V.; Hong, J.; Kim, K.-H.; Deep, A. Recent Advances in Nanoscale Materials for Antibody-Based Cancer Theranostics. Biosens. Bioelectron. 2021, 173, 112787. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zeng, J.; Shih, W.-C. Nanoporous Gold Nanocomposites as a Versatile Platform for Plasmonic Engineering and Sensing. Sensors 2017, 17, 1519. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-M.; Chiu, S.-H.; Busa, P.; Liu, C.-L.; Kankala, R.K.; Lee, C.-H. Engineered Mesoporous Silica-Based Core-Shell Nanoarchitectures for Synergistic Chemo-Photodynamic Therapies. Int. J. Mol. Sci. 2022, 23, 11604. [Google Scholar] [CrossRef] [PubMed]
- Sodagar Taleghani, A.; Ebrahimnejad, P.; Heidarinasab, A.; Akbarzadeh, A. Sugar-Conjugated Dendritic Mesoporous Silica Nanoparticles as PH-Responsive Nanocarriers for Tumor Targeting and Controlled Release of Deferasirox. Mater. Sci. Eng. C 2019, 98, 358–368. [Google Scholar] [CrossRef] [PubMed]
- van de Looij, S.M.; Hebels, E.R.; Viola, M.; Hembury, M.; Oliveira, S.; Vermonden, T. Gold Nanoclusters: Imaging, Therapy, and Theranostic Roles in Biomedical Applications. Bioconjug. Chem. 2022, 33, 4–23. [Google Scholar] [CrossRef]
- Li, D.; Zhao, J.; Ma, J.; Yang, H.; Zhang, X.; Cao, Y.; Liu, P. GMT8 Aptamer Conjugated PEGylated Ag@Au Core-Shell Nanoparticles as a Novel Radiosensitizer for Targeted Radiotherapy of Glioma. Colloids Surf. B Biointerfaces 2022, 211, 112330. [Google Scholar] [CrossRef]
- Yadegari, A.; Akbarzadeh, M.; Kargaran, F.; Mirzaee, R.; Salahshoori, I.; Nobre, M.A.L.; Khonakdar, H.A. Recent Advancements in Bio-Based Dielectric and Piezoelectric Polymers and Their Biomedical Applications. J. Mater. Chem. B 2024, 12, 5272–5298. [Google Scholar] [CrossRef] [PubMed]
- Egbuna, C.; Parmar, V.K.; Jeevanandam, J.; Ezzat, S.M.; Patrick-Iwuanyanwu, K.C.; Adetunji, C.O.; Khan, J.; Onyeike, E.N.; Uche, C.Z.; Akram, M.; et al. Toxicity of Nanoparticles in Biomedical Application: Nanotoxicology. J. Toxicol. 2021, 2021, 9954443. [Google Scholar] [CrossRef] [PubMed]
- Nienhaus, K.; Wang, H.; Nienhaus, G.U. Nanoparticles for Biomedical Applications: Exploring and Exploiting Molecular Interactions at the Nano-Bio Interface. Mater. Today Adv. 2020, 5, 100036. [Google Scholar] [CrossRef]
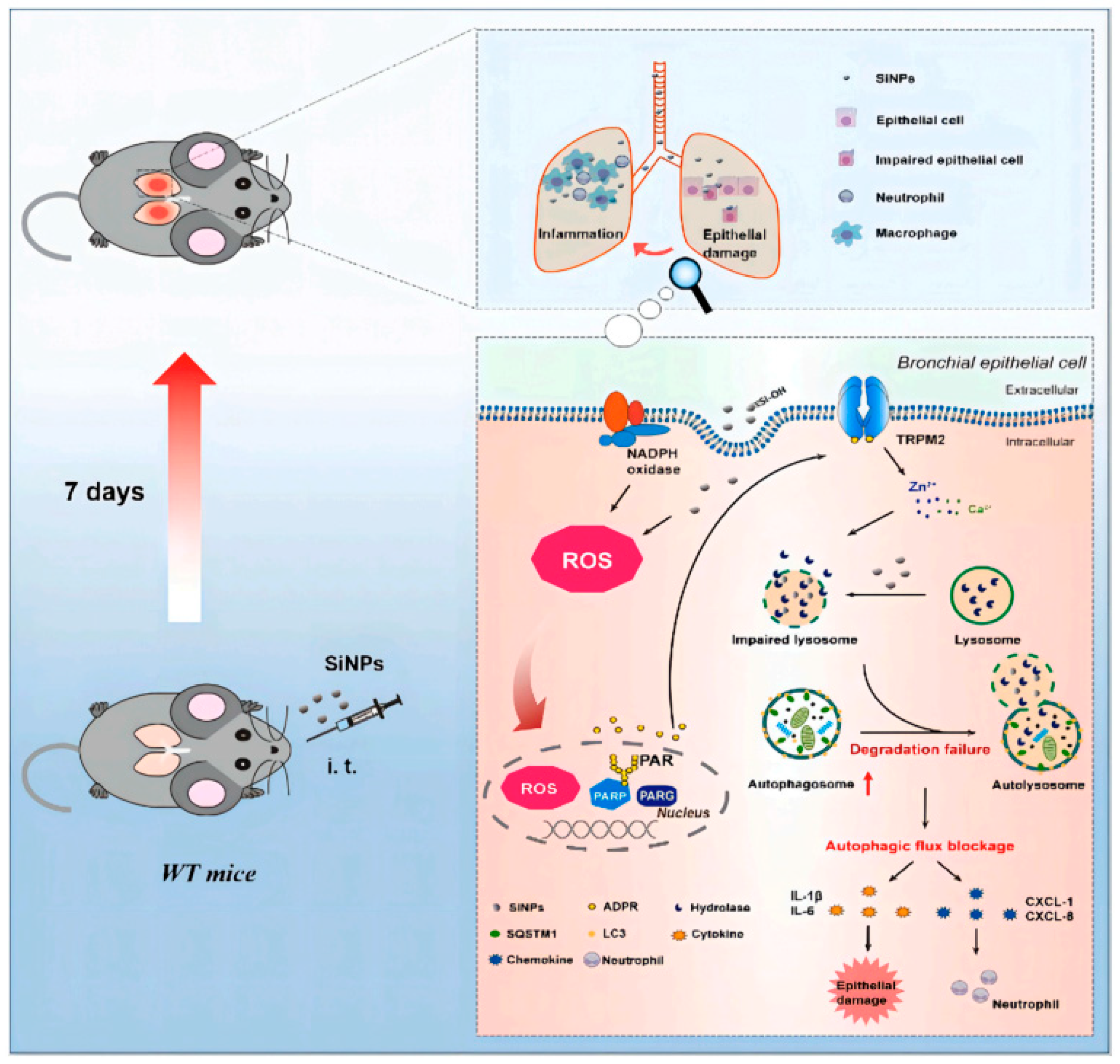
- Wang, M.; Li, J.; Dong, S.; Cai, X.; Simaiti, A.; Yang, X.; Zhu, X.; Luo, J.; Jiang, L.-H.; Du, B.; et al. Silica Nanoparticles Induce Lung Inflammation in Mice via ROS/PARP/TRPM2 Signaling-Mediated Lysosome Impairment and Autophagy Dysfunction. Part. Fibre Toxicol. 2020, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Schneid, A.d.C.; Silveira, C.P.; Galdino, F.E.; Ferreira, L.F.; Bouchmella, K.; Cardoso, M.B. Colloidal Stability and Redispersibility of Mesoporous Silica Nanoparticles in Biological Media. Langmuir 2020, 36, 11442–11449. [Google Scholar] [CrossRef] [PubMed]
- Salehipour, M.; Rezaei, S.; Rezaei, M.; Yazdani, M.; Mogharabi-Manzari, M. Opportunities and Challenges in Biomedical Applications of Metal–Organic Frameworks. J. Inorg. Organomet. Polym. Mater. 2021, 31, 4443–4462. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles. Adv. Mater. 2017, 29, 1604634. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wu, Y.; Chen, G.; Zheng, X.; Dai, M.; Peng, C. Metal-Organic Framework Membranes: Recent Development in the Synthesis Strategies and Their Application in Oil-Water Separation. Chem. Eng. J. 2021, 405, 127004. [Google Scholar] [CrossRef]
- Saldanha, P.L.; Lesnyak, V.; Manna, L. Large Scale Syntheses of Colloidal Nanomaterials. Nano Today 2017, 12, 46–63. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, Q.; Zhang, Y.; Weaver, K.P.; Phillip, W.A.; Guo, R. Facile Synthesis of a Pentiptycene-Based Highly Microporous Organic Polymer for Gas Storage and Water Treatment. ACS Appl. Mater. Interfaces 2018, 10, 15174–15182. [Google Scholar] [CrossRef]
- Shukla, S.K.; Shukla, S.K.; Govender, P.P.; Giri, N.G. Biodegradable Polymeric Nanostructures in Therapeutic Applications: Opportunities and Challenges. RSC Adv. 2016, 6, 94325–94351. [Google Scholar] [CrossRef]
- White, M.; Whittaker, R.; Gándara, C.; Stoll, E.A. A Guide to Approaching Regulatory Considerations for Lentiviral-Mediated Gene Therapies. Hum. Gene Ther. Methods 2017, 28, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Fatehi, L.; Wolf, S.M.; Ramachandran, G.; Kuzma, J. Introduction: Designing Nanobiotechnology Oversight. J. Nanopart. Res. 2011, 13, 1341–1343. [Google Scholar] [CrossRef]
- Blum, A.P.; Kammeyer, J.K.; Rush, A.M.; Callmann, C.E.; Hahn, M.E.; Gianneschi, N.C. Stimuli-Responsive Nanomaterials for Biomedical Applications. J. Am. Chem. Soc. 2015, 137, 2140–2154. [Google Scholar] [CrossRef]
- Xing, Y.; Zeng, B.; Yang, W. Light Responsive Hydrogels for Controlled Drug Delivery. Front. Bioeng. Biotechnol. 2022, 10, 1075670. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, J.; Yu, S.; Hong, S. PH- and Temperature-Responsive Radially Porous Silica Nanoparticles with High-Capacity Drug Loading for Controlled Drug Delivery. Nanotechnology 2020, 31, 335103. [Google Scholar] [CrossRef]
- de Oliveira, P.F.M.; Torresi, R.M.; Emmerling, F.; Camargo, P.H.C. Challenges and Opportunities in the Bottom-up Mechanochemical Synthesis of Noble Metal Nanoparticles. J. Mater. Chem. A 2020, 8, 16114–16141. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, F.; Lively, R.P. Manufacturing Nanoporous Materials for Energy-Efficient Separations. In Sustainable Nanoscale Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 33–81. [Google Scholar]
- Harun-Ur-Rashid, M.; Jahan, I.; Foyez, T.; Imran, A. Bin Bio-Inspired Nanomaterials for Micro/Nanodevices: A New Era in Biomedical Applications. Micromachines 2023, 14, 1786. [Google Scholar] [CrossRef]
- Refaat, A.; Yap, M.L.; Pietersz, G.; Walsh, A.P.G.; Zeller, J.; del Rosal, B.; Wang, X.; Peter, K. In Vivo Fluorescence Imaging: Success in Preclinical Imaging Paves the Way for Clinical Applications. J. Nanobiotechnol. 2022, 20, 450. [Google Scholar] [CrossRef]
- Caspani, S.; Magalhães, R.; Araújo, J.P.; Sousa, C.T. Magnetic Nanomaterials as Contrast Agents for MRI. Materials 2020, 13, 2586. [Google Scholar] [CrossRef]
- Zhu, W.; Wei, Z.; Han, C.; Weng, X. Nanomaterials as Promising Theranostic Tools in Nanomedicine and Their Applications in Clinical Disease Diagnosis and Treatment. Nanomaterials 2021, 11, 3346. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, A.; Rehman, A.; John, P. Challenges and Opportunities in Healthcare Biotechnology. In Biotechnology in Healthcare; Elsevier: Amsterdam, The Netherlands, 2022; pp. 321–342. [Google Scholar]
- Hartshorn, C.M.; Russell, L.M.; Grodzinski, P. National Cancer Institute Alliance for Nanotechnology in Cancer—Catalyzing Research and Translation toward Novel Cancer Diagnostics and Therapeutics. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1570. [Google Scholar] [CrossRef] [PubMed]
- Illert, A.L.; Stenzinger, A.; Bitzer, M.; Horak, P.; Gaidzik, V.I.; Möller, Y.; Beha, J.; Öner, Ö.; Schmitt, F.; Laßmann, S.; et al. The German Network for Personalized Medicine to Enhance Patient Care and Translational Research. Nat. Med. 2023, 29, 1298–1301. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, D.; Mohanan, S.; Choi, G.; Choy, J.-H.; Tiburcius, S.; Trinh, H.T.; Bolan, S.; Verrills, N.; Tanwar, P.; Karakoti, A.; et al. The Emergence of Nanoporous Materials in Lung Cancer Therapy. Sci. Technol. Adv. Mater. 2022, 23, 225–274. [Google Scholar] [CrossRef] [PubMed]
- Prasad Mishra, S.; Dutta, S.; Kumar Sahu, A.; Mishra, K.; Kashyap, P. Potential Application of Nanoporous Materials in Biomedical Field. In Nanopores; IntechOpen: London, UK, 2021. [Google Scholar]
- Sutrisno, L.; Ariga, K. Pore-Engineered Nanoarchitectonics for Cancer Therapy. NPG Asia Mater. 2023, 15, 21. [Google Scholar] [CrossRef]
- Fontana, F.; Liu, Z.; Hirvonen, J.; Santos, H.A. Porous Silicon Materials for Cancer and Immunotherapy. In Porous Silicon for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 571–609. [Google Scholar]
- Li, J.; Zhang, W.; Ji, W.; Wang, J.; Wang, N.; Wu, W.; Wu, Q.; Hou, X.; Hu, W.; Li, L. Near Infrared Photothermal Conversion Materials: Mechanism, Preparation, and Photothermal Cancer Therapy Applications. J. Mater. Chem. B 2021, 9, 7909–7926. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zeng, W.; Huang, P.; Zeng, X.; Mei, L. Smart Materials for Drug Delivery and Cancer Therapy. VIEW 2021, 2, 20200042. [Google Scholar] [CrossRef]
- Izhar, F.; Imran, M.; Izhar, H.; Latif, S.; Hussain, N.; Iqbal, H.M.N.; Bilal, M. Recent Advances in Metal-Based Nanoporous Materials for Sensing Environmentally-Related Biomolecules. Chemosphere 2022, 307, 135999. [Google Scholar] [CrossRef]
- Priya, A.K.; Gnanasekaran, L.; Kumar, P.S.; Jalil, A.A.; Hoang, T.K.A.; Rajendran, S.; Soto-Moscoso, M.; Balakrishnan, D. Recent Trends and Advancements in Nanoporous Membranes for Water Purification. Chemosphere 2022, 303, 135205. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvin, N.; Kumar, V.; Mandal, T.K.; Joo, S.W. Advancements in Nanoporous Materials for Biomedical Imaging and Diagnostics. J. Funct. Biomater. 2024, 15, 226. https://doi.org/10.3390/jfb15080226
Parvin N, Kumar V, Mandal TK, Joo SW. Advancements in Nanoporous Materials for Biomedical Imaging and Diagnostics. Journal of Functional Biomaterials. 2024; 15(8):226. https://doi.org/10.3390/jfb15080226
Chicago/Turabian StyleParvin, Nargish, Vineet Kumar, Tapas Kumar Mandal, and Sang Woo Joo. 2024. "Advancements in Nanoporous Materials for Biomedical Imaging and Diagnostics" Journal of Functional Biomaterials 15, no. 8: 226. https://doi.org/10.3390/jfb15080226





