Abstract
Regeneration of articular cartilage remains a challenge for patients who have undergone cartilage injury, osteochondritis dissecans and osteoarthritis. Here, we describe a new recombinant silk fibroin with basic fibroblast growth factor (bFGF) binding peptide, which has a genetically introduced sequence PLLQATLGGGS, named P7. In this study, we cultured a human mesenchymal cell line derived from bone marrow, UE6E7-16, in wild-type fibroin sponge (FS) and recombinant silk fibroin sponge with P7 peptide (P7 FS). We compared cell proliferation, chondrogenic differentiation and cartilaginous tissue formation between the two types of sponge. After stimulation with bFGF at 3 ng/mL, P7 FS showed significantly higher cell growth (1.2-fold) and higher cellular DNA content (5.6-fold) than did wild-type FS. To promote chondrogenic differentiation, cells were cultured in the presence of TGF-β at 10 ng/mL for 28 days. Immunostaining of P7 FS showed SOX9-positive cells comparable to wild-type FS. Alcian-Blue staining of P7 FS also showed cartilaginous tissue formation equivalent to wild-type FS. A significant increase in cell proliferation in P7 FS implies future clinical application of this transgenic fibroin for regeneration of articular cartilage. To produce cartilaginous tissue efficiently, transgenic fibroin sponges and culture conditions must be improved. Such changes should include the selection of growth factors involved in chondrogenic differentiation and cartilage formation.
1. Introduction
Regeneration of articular cartilage remains a challenge for patients with cartilage injury, osteochondritis dissecans and osteoarthritis. To date, many approaches to solving this problem have been investigated. Among them, the local transplantation of cells with biomaterials has been tested in experimental animal models of articular cartilage defects [1,2,3,4,5,6]. At present, autologous chondrocyte implantation is generally applied to limited cartilage defects of the knee [7,8]. However, treatment of large defects of articular cartilage has not been established.
In animal models, several types of scaffold have been combined with mesenchymal stem cells to test their efficacy in repairing articular cartilage defects. Thus far, inclusion of a scaffold has had limited benefits in the regeneration of articular cartilage [9,10,11,12,13,14,15]. The most serious issue has been inadequate strength to support physiological loads applied to articular cartilage. Fibroin sponge has been reported as a scaffold material with suitable mechanical properties matching those of articular cartilage. In addition, fibroin has low inflammatory properties [16] and provides good attachment, benefiting cell viability, growth and function [17]. Previously, we collected chondrocytes from Japanese white rabbits and cultured cells with silk fibroin sponge (FS). We found that well-defined cartilage tissue was produced in FS [18]. Furthermore, in osteochondral defects of the rabbit patella, wrapping with FS containing chondrocytes successfully produced hyaline-like cartilage [19]. These findings suggest that FS covered with cultured cells, such as chondrocytes, has the potential to support the repair of large osteochondral defects.
To use such tissue engineering technology for the treatment of large osteochondral defects, increasing the number of cells cultured in FS is quite important because adult human cells generally have limited ability to proliferate. Among cell growth factors, basic fibroblast growth factor (bFGF) has potent mitogenic effects [20]. To increase the proliferative activity of cells seeded in FS, we previously generated transgenic silkworm fibroin fused to basic fibroblast growth factor (bFGF). Chondrocytes seeded in bFGF-fused FS showed higher cell growth activity than did wild-type. However, those in bFGF-fused FS showed lower expression levels of type II collagen than did wild-type [21]. A possible reason for the limited usefulness of bFGF-fused FS was that the conformation of genetically produced bFGF was unstable and denatured during processing. To overcome this issue, we recently developed recombinant silk fibroin combined with basic fibroblast growth factor (bFGF) binding peptide [22]. This approach has considerable potential for therapeutic application because a silk fibroin matrix can be combined with bFGF in amounts appropriate for timed release. This newly developed matrix could be applied to tissue defects or wound areas, thereby utilizing the patient’s own bFGF, leading to rapid tissue construction or wound healing.
In this study, we cultured a human mesenchymal cell line derived from bone marrow, UE6E7-16, with wild-type or recombinant silk fibroin with bFGF-binding peptide. We compared cell proliferation, chondrogenic differentiation, and cartilaginous tissue formation between sponges.
2. Materials and Methods
2.1. Preparation of Silk Fibroin Sponge
Silk fibroin sponge (FS) was processed as described previously [18]. In brief, an aqueous solution of silk fibroin was prepared by dissolving silkworm (Bombyx mori) cocoons in 9M LiBr, followed by dialysis against pure water. FS was formed as a result. The aqueous solution of silk fibroin (4 w/v%) was mixed with dimethyl sulfoxide (DMSO) at 1% v/v and was frozen at −20 °C followed by thawing. After removal of DMSO, FS was sterilized by autoclaving. This sponge was designated as wild-type FS.
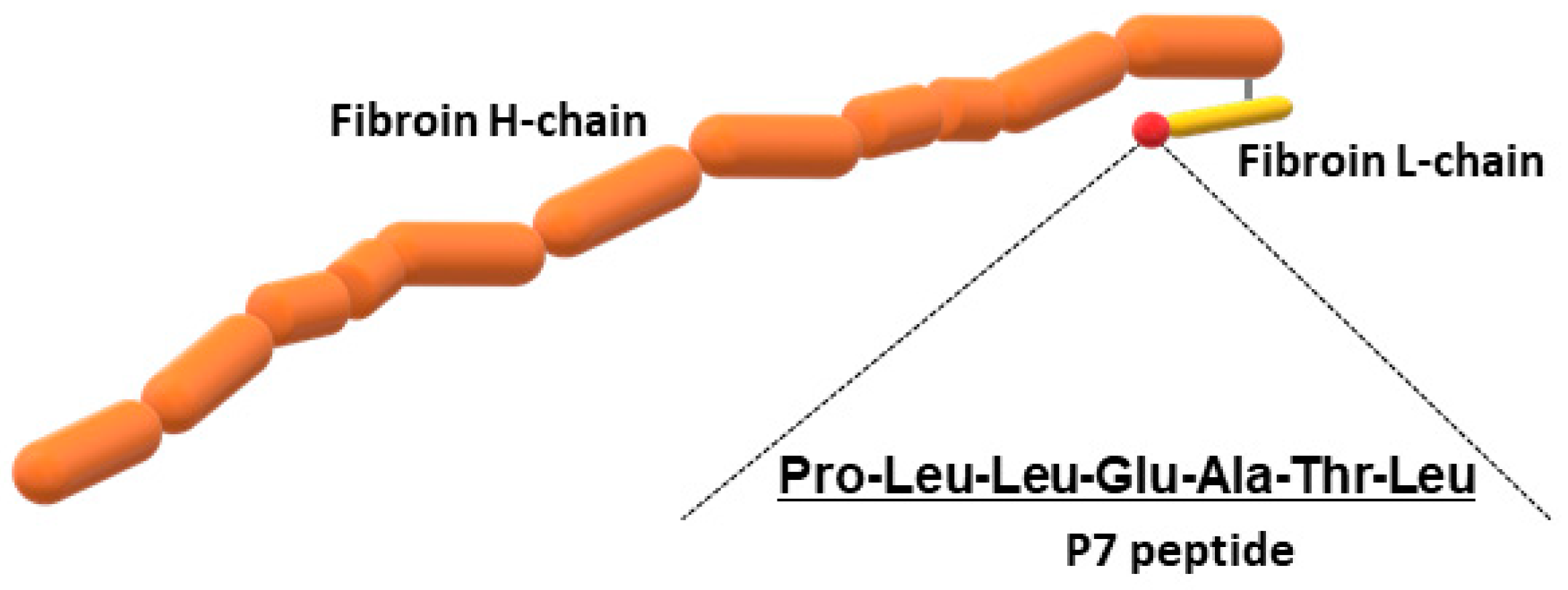
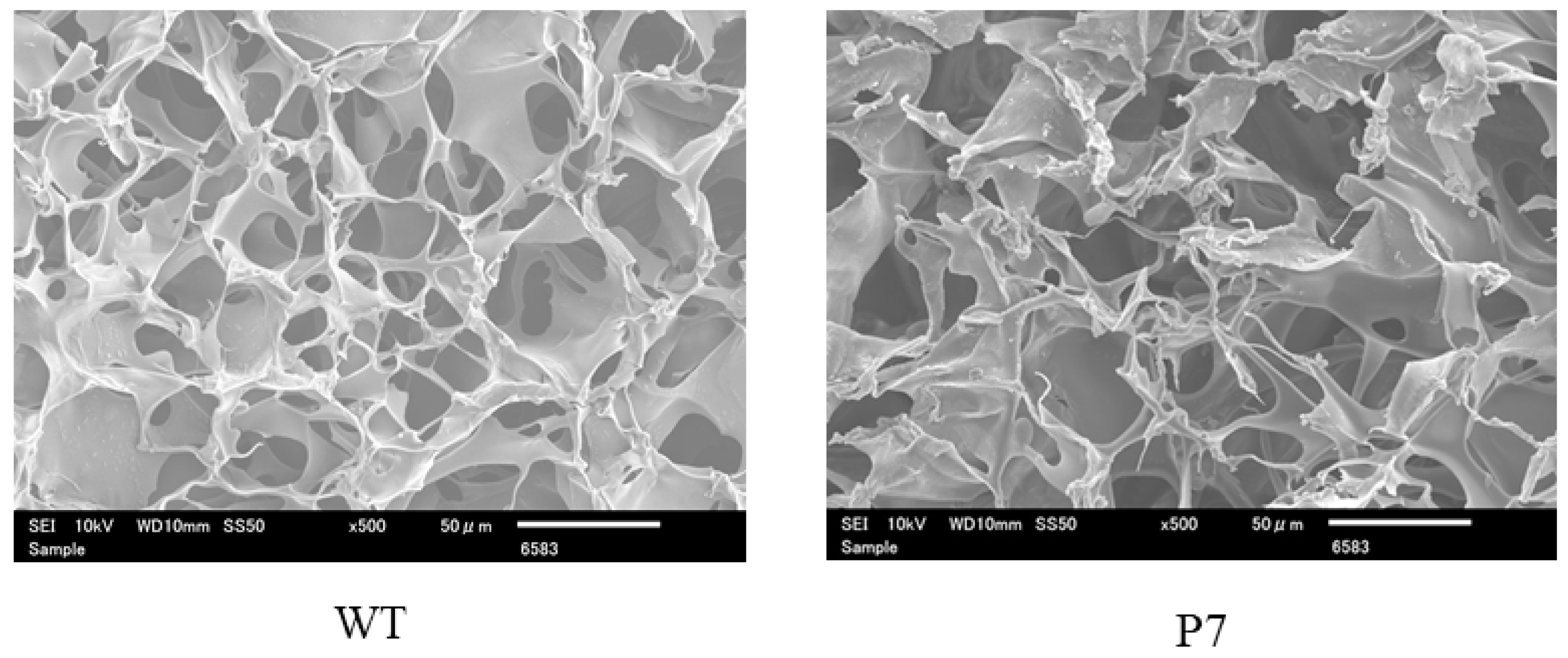
In addition to wild-type FS, we prepared recombinant silk fibroin with bFGF-binding peptide, which has a genetically introduced sequence PLLQATLGGGS, named P7. The oligonucleotide sequence encoding P7 was inserted at the C-terminus of the fibroin L-coding sequence (Figure 1). The presence of P7 peptide in the recombinant fibroin molecules was confirmed by ELISA [22]. Since P7 peptide has high homology to the immunoglobulin-like domain III of bFGF receptors [22], P7-fused FS (P7 FS) was expected to increase cultured cell growth and promote tissue regeneration. Images of wild-type and P7 FS were obtained by scanning electron microscopy and are shown in Figure 2. For cell culture experiments, FS was shaped into disk form in 6 mm Φ and 1.5 mm thicknesses.
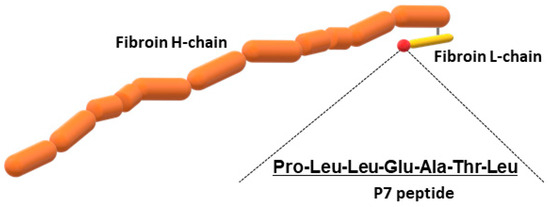
Figure 1.
Schematic drawing of recombinant silk fibroin fused to P7 peptide.
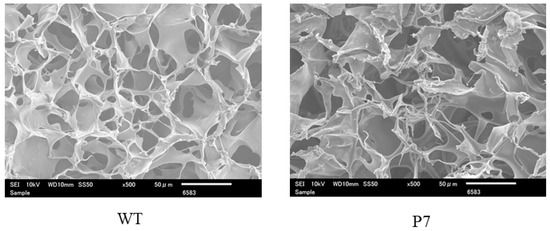
Figure 2.
Scanning electron microscopic images of wild-type (WT) and P7 fibroin sponge. P7 fibroin sponge (P7) is a recombinant silk fibroin with bFGF-binding peptide, named P7. Scale bars = 50 µm.
2.2. Cell Culture and Chondrogenic Differentiation
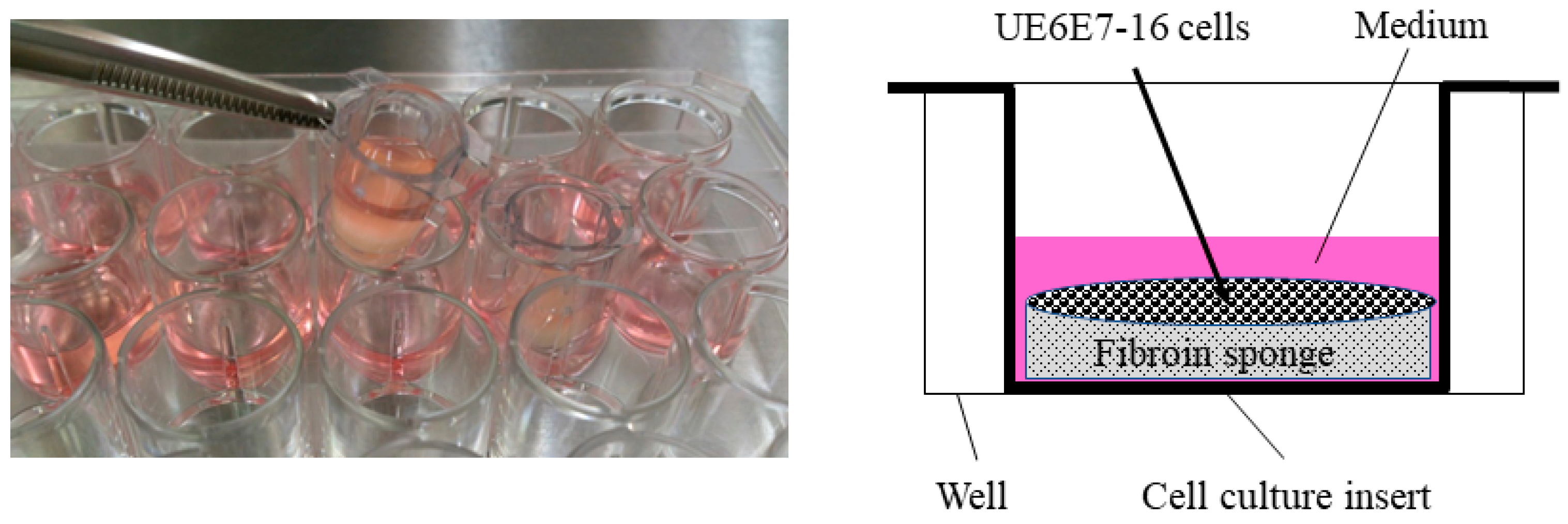
A human mesenchymal cell line derived from bone marrow, UE6E7-16 (RCB2163), was purchased from RIKEN BioResource Research Center (Tsukuba, Japan) and used in this study. For cell proliferation assays, cells were cultured in POWEREDBY10 (GlycoTechnica, Yokohama, Japan) for 1 day, then seeded in FS (1.0 × 106 cells/sponge) in DMEM containing 0.5% FBS. To stabilize FS in the medium, a cell culture insert (CORNING, Corning, NY, USA) was placed in a 24-well plate (Figure 3), where cells were cultured for an additional 3 days in the presence or absence of bFGF (KAKEN Pharmaceutical Co., Ltd., Tokyo, Japan).
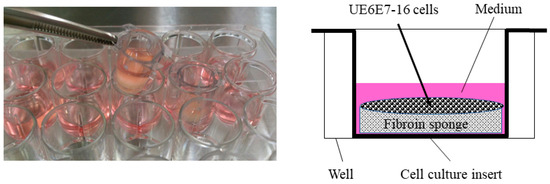
Figure 3.
Cell culture in fibroin sponge using a cell culture insert. Cells were cultured in the presence of bFGF for 3 days and subjected to a cell proliferation assay. Following stimulation with bFGF, cells were also cultured in the presence of TGF-β3 for 28 days to evaluate chondrogenic differentiation and cartilaginous tissue formation in the sponge. bFGF, basic fibroblast growth factor; TGF, transforming growth factor.
For chondrogenic differentiation, cells were cultured in POWEREDBY10 for 1 day, then seeded in FS (1.0 × 106 cells/sponge) in DMEM containing 0.5% FBS, using a cell culture insert. The cells were further cultured with a human mesenchymal stem cell chondrogenic differentiation medium bullet kit (Lonza, Walkersville, MD, USA) in the presence of TGF-β3 (Lonza) at 10 ng/mL for up to 28 days. Basic FGF at 3 ng/mL was added only for the first 3 days. The medium containing the growth factors was changed every day throughout the culture period.
2.3. Cell Proliferation Assay
On day 3, cells were subjected to proliferation assays using a cell counting kit-8 (CCK-8, Dojindo, Kumamoto, Japan). Briefly, cells were incubated for an appropriate length of time in the incubator at 37 °C, after which the supernatant was transferred to a 96-well plate. After adding CCK-8 solution to each well of the plate, the absorbance at 450 nm was measured using a microplate reader.
Cells were also subject to quantification of genomic DNA using a DNA extraction kit according to the user manual (NucleoSpin Tissue, MACHEREY-NAGEL, Duren, Germany). Before using the kit, the FS was minced with scissors and stirred in a solution using a vortex, followed by cell collection. Three to four sponges were tested in each experimental condition.
2.4. Chondrogenic Differentiation and Cartilaginous Tissue Formation
On day 28, FS was subjected to histological analyses. FS was fixed with 10% formalin and embedded in paraffin. Midsagittal sections 6 μm thick were mounted on silane-coated slides. Sections were reacted with a rabbit monoclonal antibody against Sox9 (EPR14335, Abcam, Cambridge, UK, 1:2000). Immunostaining was performed as previously described [23]. Signals were detected using diaminobenzidine (DAB). For the negative control sections, the same procedures were used except that the primary antibody was replaced by nonimmune rabbit immunoglobulin G. Four regions (0.3 mm × 2.4 mm in size) containing FS and seeded cells were analyzed in each section. The number of Sox9-positive cells and total cells was counted, and the percentage of Sox-9 positive cells was calculated. Three to four sponges for wild-type and P7 FS were evaluated.
To evaluate cartilaginous tissue formation, sections were subject to Alcian-Blue staining. The blue-stained areas in fibroin sponges were defined as cartilaginous tissue.
2.5. Statistical Analyses
The significance of differences between groups was assessed by t-test, Mann–Whitney’s U-test, or one-way ANOVA. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. Proliferation of UE6E7-16 Cells Cultured in FS
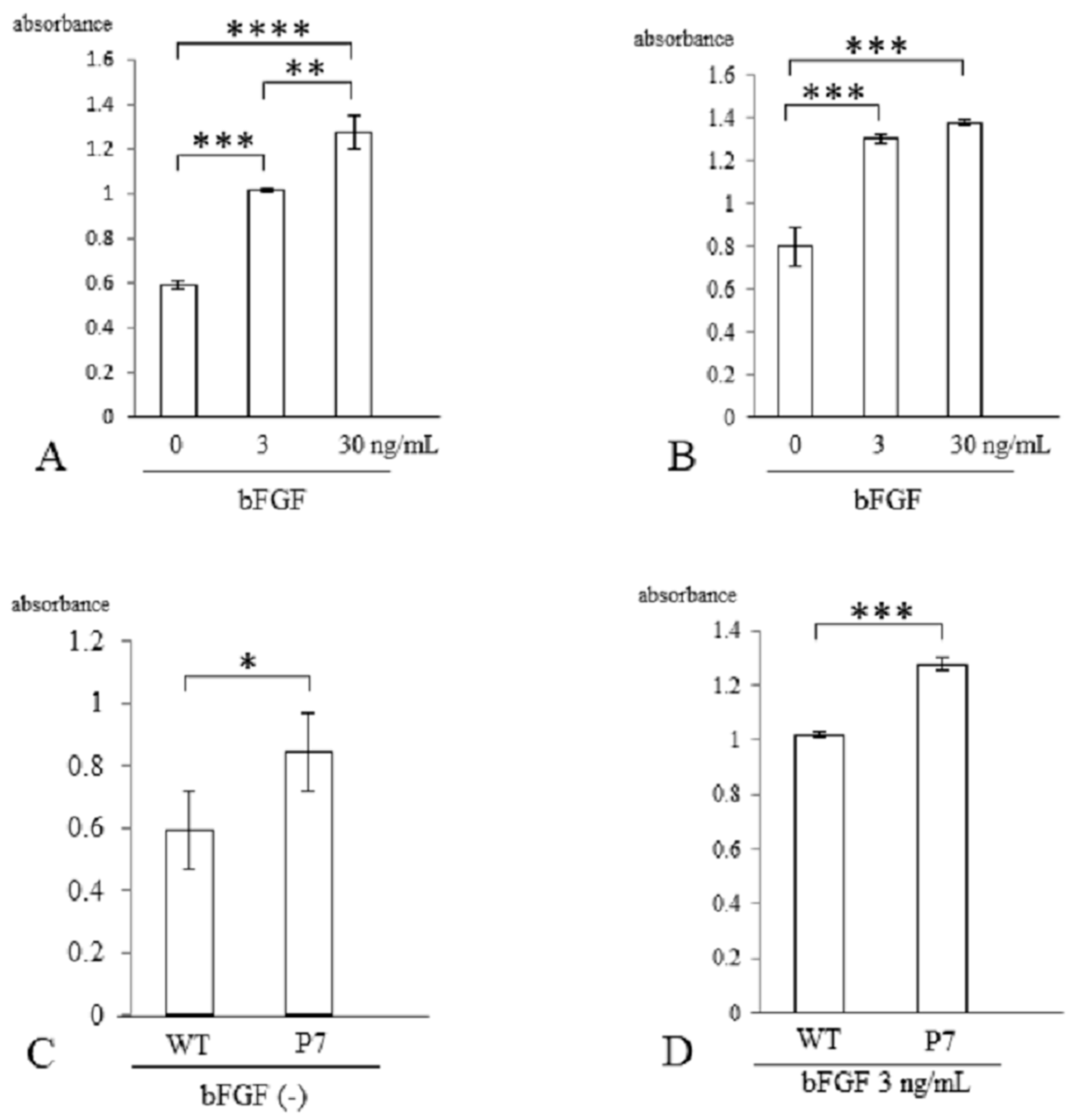
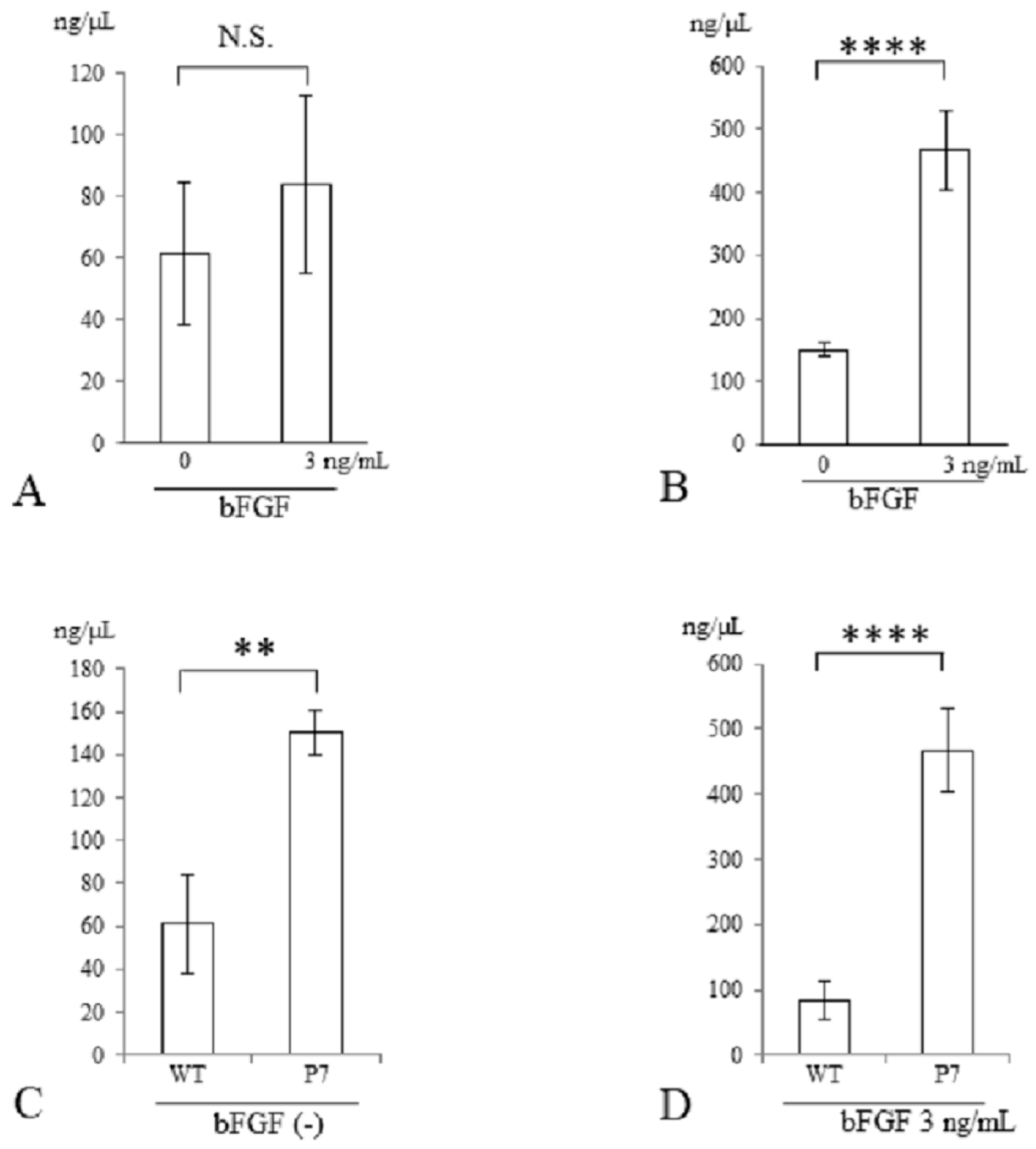
Three days after stimulation with bFGF, cell proliferation was assessed with a CCK-8 kit. In wild-type FS, cell growth was significantly elevated with bFGF at 3 ng/mL (1.7-fold, p < 0.005) and 30 ng/mL (2.2-fold, p < 0.001) compared to cells without stimulation. There was also a significant difference between 3 and 30 ng/mL (p < 0.01) (Figure 4A). In P7 FS, cell growth was significantly elevated with bFGF at 3 ng/mL (1.6-fold) and 30 ng/mL (1.8-fold) compared to cultures lacking stimulation (p < 0.005). However, no significant difference was detected between 3 and 30 ng/mL (Figure 4B).
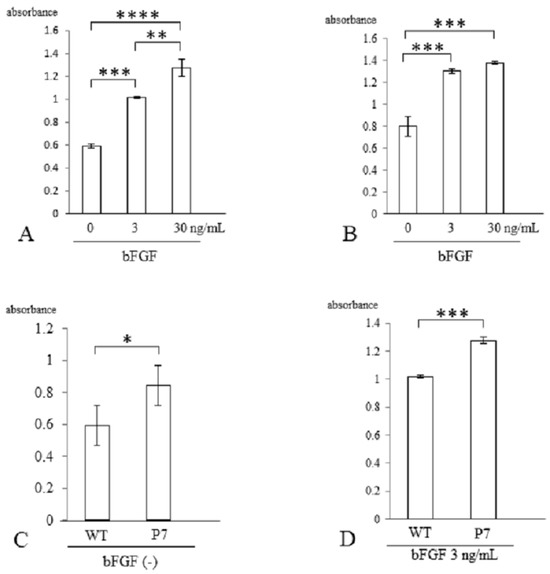
Figure 4.
Cell proliferation of UE6E7-16 cells cultured in FS for 3 days. (A) Wild-type FS. Stimulation with bFGF increased cell growth in a dose-dependent manner. (B) P7 FS. Stimulation with bFGF increased cell growth but not in a dose-dependent manner. (C) Comparison between wild-type and P7 FS without stimulation of bFGF. (D) Comparison between wild-type and P7 FS after stimulation with bFGF. Error bars show standard deviation. Significant difference, * p < 0.05, ** p < 0.01, *** p < 0.005, **** p < 0.001. WT, wild-type; FS, fibroin sponge; bFGF, basic fibroblast growth factor.
When comparing wild-type and P7 FS after 3 days of cultivation, P7 FS showed significantly higher cell numbers (1.4-fold) than wild-type FS (p < 0.05) (Figure 4C). After stimulation with bFGF at 3 ng/mL, P7 FS showed significantly higher cell growth (1.3-fold) than wild-type FS (p < 0.005) (Figure 4D).
3.2. Measurement of DNA Content of UE6E7-16 Cells Cultured in FS
Three days after stimulation with bFGF at 3 ng/mL, the genomic DNA content of cells seeded in FS was measured. In wild-type FS, the total DNA contents were similar in cultures with and without bFGF stimulation (Figure 5A). In contrast, in P7 FS, they were significantly elevated with bFGF stimulation compared to those without stimulation (3.1-fold, p < 0.001) (Figure 5B).
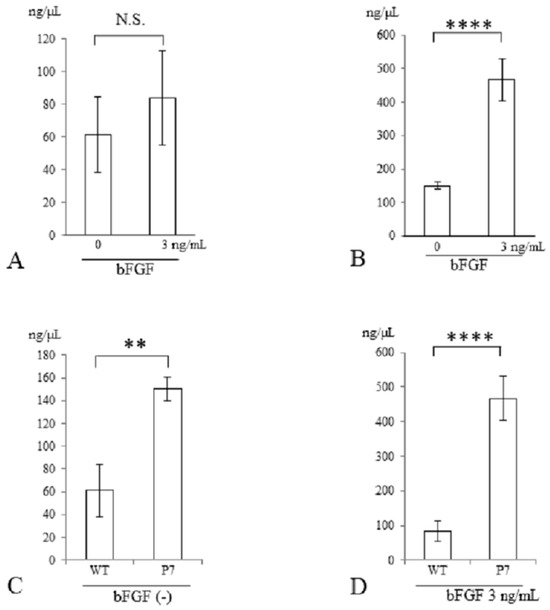
Figure 5.
DNA quantification of UE6E7-16 cells cultured in FS for 3 days. (A) Wild-type FS. (B) P7 FS. (C) Comparison between wild-type and P7 FS without stimulation of bFGF. (D) Comparison between wild-type and P7 FS after stimulation with bFGF. Error bars show standard deviation. Significant difference, ** p < 0.01, **** p < 0.001. N.S., not significant; WT, wild-type; FS, fibroin sponge; bFGF, basic fibroblast growth factor.
In a comparison between wild-type and P7 FS after 3 days of cultivation, P7 FS showed significantly higher DNA content than did wild-type FS (2.5-fold, p < 0.01) (Figure 5C). After stimulation with bFGF at 3 ng/mL, P7 FS showed significantly higher DNA content than did wild-type FS (5.6-fold, p < 0.001) (Figure 5D).
3.3. Chondrocyte Differentiation of UE6E7-16 Cells in FS
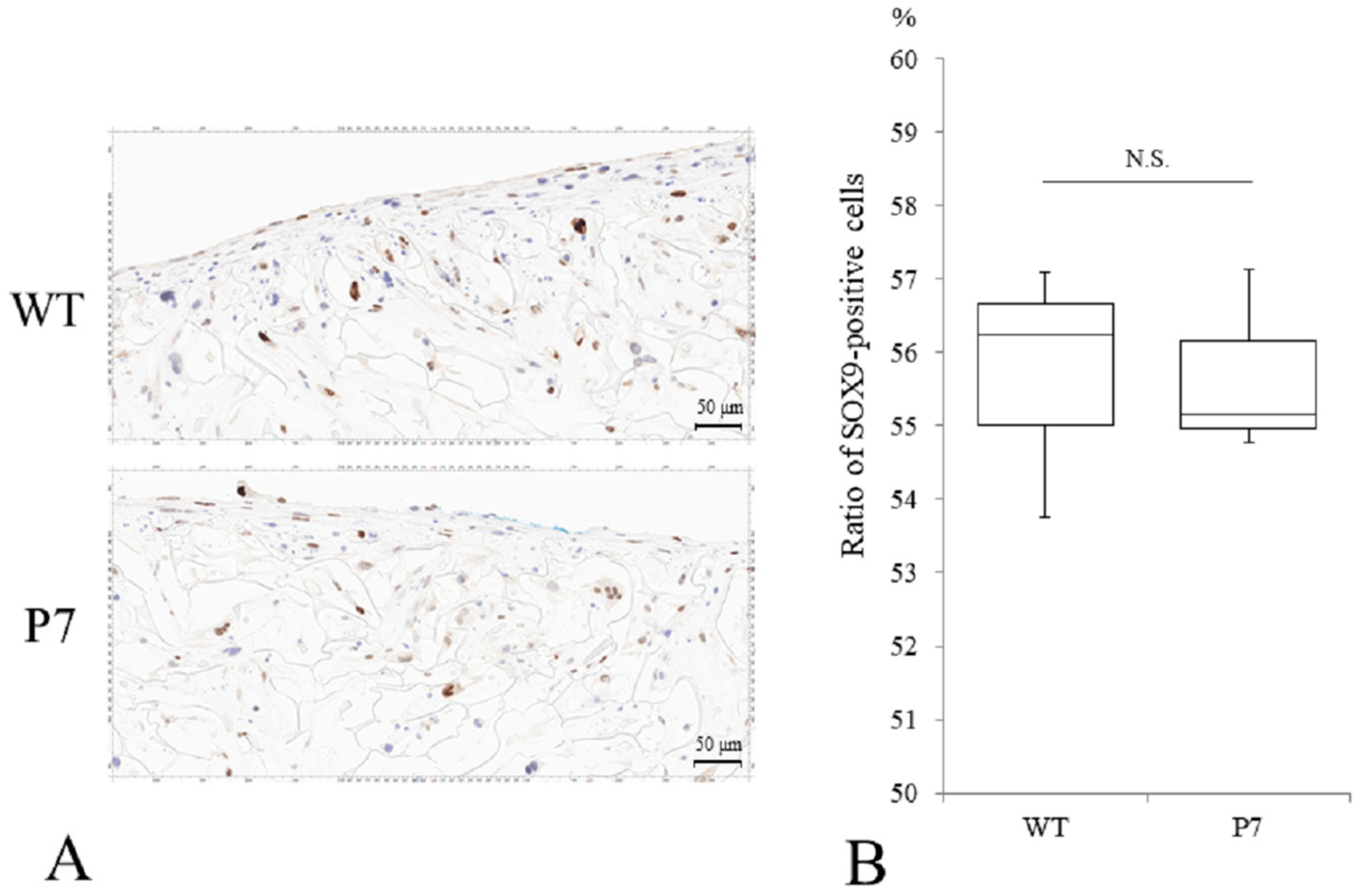
Immunostaining of P7 FS showed SOX9-positive cells comparable to that seen in wild-type FS (Figure 6A). The median ratio of Sox9-positive cells to total cells was 56.3% for wild-type and 55.2% for P7, respectively, with no significant difference (Figure 6B).
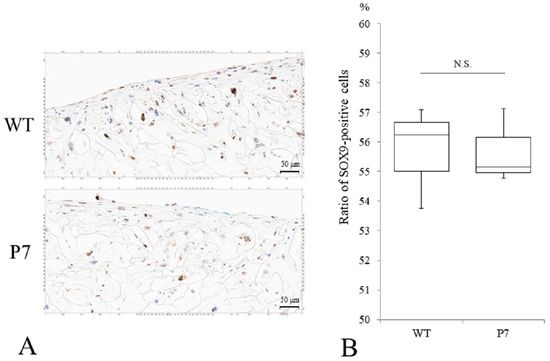
Figure 6.
Chondrogenic differentiation of UE6E7-16 cells cultured in FS for 28 days. (A) Immunostaining of SOX9 for WT and P7 FS. Scale bars = 50 µm. (B) A median ratio (%) of SOX9-positive cells to total cells. Thick horizontal lines, boxes, and whiskers show median, interquartile range (IQR), and most extreme points from the limits of the box, respectively. N.S., not significant; WT, wild-type; FS, fibroin sponge.
3.4. Cartilaginous Tissue Formation in FS
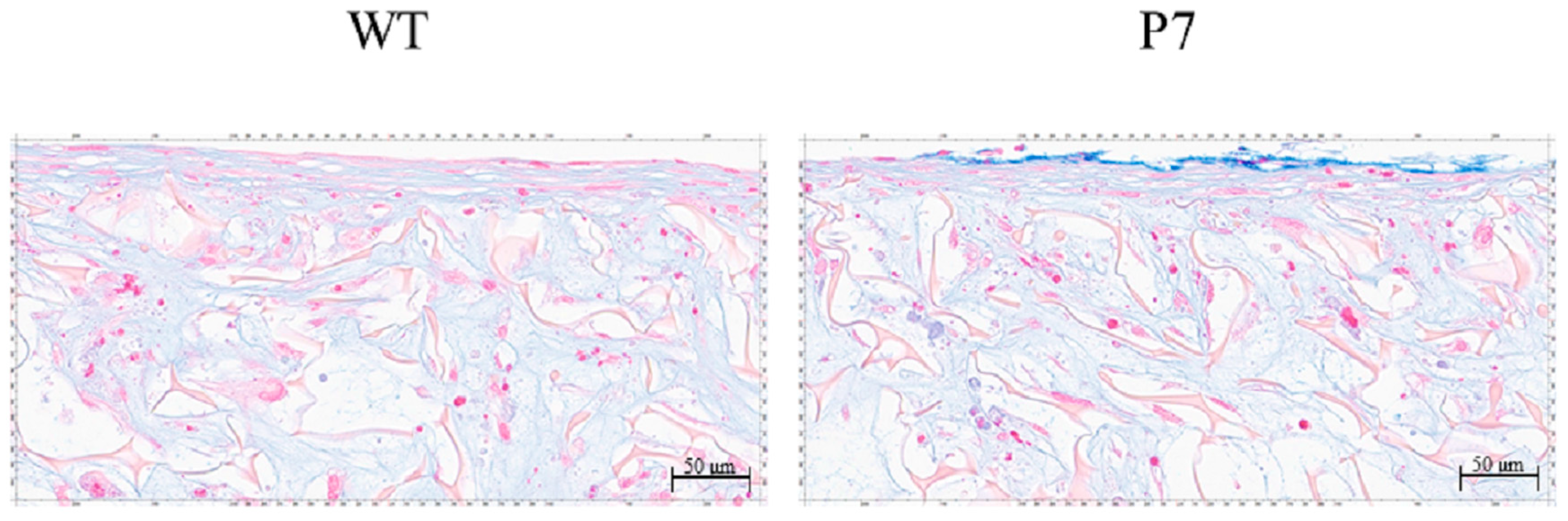
In both wild-type and P7 FS, cartilaginous tissue, which was rendered light blue by Alcian-Blue staining, was formed among fibroin fibers in the superficial layer of the sponges. P7 FS showed an equivalent amount of cartilaginous tissue to that seen in wild-type FS (Figure 7).
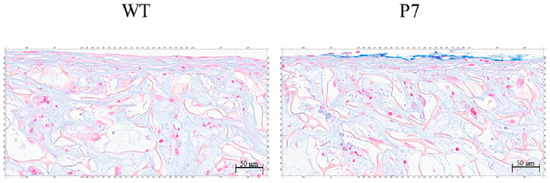
Figure 7.
Cartilaginous tissue formation by UE6E7-16 cells cultured in FS for 28 days. Cartilaginous tissue is rendered light blue with Alcian-Blue staining. P7 FS shows an equivalent amount of cartilaginous tissue to that seen in wild-type FS. Scale bars = 50 µm. WT, wild-type; FS, fibroin sponge.
4. Discussion
In the present study, we analyzed cell proliferation and cartilaginous tissue formation by a human mesenchymal cell line, UE6E7-16, cultured in P7 FS, with comparisons to wild-type FS. The results show that P7 FS significantly increased cell growth compared to wild-type; however, chondrogenic differentiation and cartilaginous tissue formation were comparable.
We previously showed that proliferation of primary articular chondrocytes in fibroin sponges was significantly increased on bFGF-fused fibroin sponges compared with wild-type. In contrast, the expression levels of a gene encoding collagen type II α 1 chain were lower in bFGF-fused FS than wild-type [21]. This observation supports our present results showing increased cell growth but not enhanced cartilaginous tissue formation in P7 FS, although the cells and fibroin sponges used were different from those previously investigated [21].
Silk fibroin sponges have a mechanically stable and porous structure [24,25,26]. Consequently, it is expected that they could be used as a scaffold source material for musculoskeletal tissue regeneration, including bone, tendon, ligament and articular cartilage [27]. Since treatment of large defects of articular cartilage is clinically challenging, we investigated cell proliferation, chondrogenic differentiation, and cartilaginous tissue formation in FS. When using bio-synthetic materials for the treatment of large tissue defects, it is important to use materials loaded with a large number of cells. We used a human bone marrow-derived mesenchymal cell line, UE6E7-16, and chose bFGF as a growth factor, as it possesses mitogenic activity for many cell types [28,29,30]. Because bFGF-fused FS had limited effects on cell proliferation and cartilage matrix production [21], we developed P7 FS and characterized cell proliferation, chondrogenic differentiation and cartilaginous tissue formation. In the cell proliferation assay, P7 FS showed a slight increase in the presence of bFGF compared with wild-type FS (Figure 4D). On the other hand, quantification of genomic DNA extracted from cells was markedly increased with bFGF stimulation in P7 FS compared to wild-type FS (Figure 5D). This could be due to differences in measurement methods between WST-8 formazan and DNA content. The former method quantified WST-8 formazan in supernatants whereas the latter quantified DNA content from total cells. Notably, P7 FS showed significantly higher cell growth and greater cellular DNA content than wild-type FS even when lacking bFGF stimulation (Figure 4C and Figure 5C). This may suggest that P7 FS allows efficient binding to low levels of bFGF in the medium.
Previous studies have shown that bFGF inhibited chondrocyte differentiation and cartilaginous matrix production [31,32,33,34]. In terms of cartilage regeneration using scaffolds, Deng et al. used gelatin microspheres loaded with bFGF for controlled and sustained release and stimulated repair of knee cartilage defects in rabbits. Their histological evidence showed that previous cartilaginous defects were filled with hyaline-like cartilage, illustrating the potential of a bFGF scaffold to promote chondrogenesis [35]. Others have reported a positive effect of bFGF on cell differentiation and viability despite bFGF-mediated downregulation of collagen type II mRNA [36].
Human mesenchymal stem cells (hMSCs) undergo multi-lineage differentiation and are capable of rapid proliferation. Given their ready availability, they represent a promising candidate for cartilage tissue engineering [37,38]. To induce chondrogenic differentiation, hMSCs require the appropriate signals. Several previous studies have demonstrated that a variety of growth factors, such as bFGF, bone morphogenic proteins (BMP), insulin-like growth factor-I (IGF-I) and TGF-β, can induce the differentiation of mesenchymal cells to chondrocytes under certain culture conditions [39,40,41,42,43,44]. In the present study, to effectively differentiate UE6E7-16 cells from chondrocytes, we cultured the cells in chondrogenic differentiation medium with 10 ng/mL of TGF-β. However, chondrogenic differentiation and cartilaginous tissue formation in P7 FS were comparable to those observed with wild-type FS. A previous study analyzed the use of extracellular matrix-based hydrogel scaffolds with TGF-β and collagen binding domain-bFGF. They reported that hMSCs differentiated from chondrocytes and the differentiation process could be regulated by the controlled release of growth factors [45]. Taken together with these observations, it is evident that both bFGF and TGF-β play important roles in chondrogenic differentiation of hMSCs, but stimulation with such growth factors needs to be regulated.
In the present study, bFGF was added for the first 3 days, whereas TGF-β was added to the chondrogenic differentiation medium throughout the 28 days culture period. Basic FGF could bind to the P7 peptide and exert its mitogenic ability on the cells. In contrast, TGF-β could not act on the cells efficiently since it was simply added to the medium. Therefore, insufficient TGF-β signaling to the cells seeded in FS may be a possible reason for the lack of enhanced chondrogenic differentiation and cartilaginous tissue formation observed in P7 FS. Given P7 FS’s high binding capacity for bFGF, it is not surprising that it generated more cells than the wild-type FS. However, the cells did not differentiate to chondrocytes or produce cartilage matrices. Presumably, this was due to insufficient TGF-β signaling. To enhance the chondrogenic differentiation and formation of cartilaginous tissue in P7 FS, improvements, such as providing a TGF-β-binding domain or peptide in the fibroin, will be necessary.
In this study, we could not investigate expression levels of genes involved in chondrocyte differentiation due to difficulties in extraction of mRNAs from cells seeded in FS. In addition, production of cartilage matrix was not measured. To better understand differences in chondrocyte differentiation and production of cartilage matrix between wild-type and P7 FS, cartilage-related gene expression analyses or measurements of cartilage matrix such as glycosaminoglycan (GAG) or type II collagen will be needed.
There are some limitations in this study. First, the content of the P7-fused L-chain to total L-chain in transgenic fibroin was approximately 25% [22]. In addition, we did not analyze the in vitro binding ability of bFGF to the P7 peptide. Improvement of transgenic fibroins with a higher ratio of P7-fused L-chain is probably necessary. Moreover, in vitro studies of the binding ability of bFGF to the P7 peptide are crucial for application of this material to future clinical use. Second, we used a human mesenchymal cell line, UE6E7-16, but not MSCs from bone marrow. Considering clinical application of the fibroin sponges, experiments using human MSCs from bone marrow will be indispensable. Third, cells were not seeded in deep layers of fibroin sponges, i.e., most of the cells were observed in the superficial layers of FS. For the treatment of large defects of articular cartilage, technology to allow seeded cells to expand to interior regions of FS is desirable. Nevertheless, P7 FS successfully increased a human mesenchymal cell line, UE6E7-16, compared to wild-type FS, which implies future clinical application of this transgenic fibroin for the treatment of large articular cartilage defects.
5. Conclusions
P7 FS increased growth of a human mesenchymal cell line, UE6E7-16, compared with wild-type FS, but it did not promote chondrogenic differentiation or cartilaginous tissue formation. To produce cartilaginous tissue efficiently, improvements of the transgenic fibroin sponges and culture conditions including regulation of growth factors involved in chondrogenic differentiation and cartilage formation are required.
Author Contributions
Conceptualization, A.N. and K.N.; methodology, M.Y., K.S., A.N. and K.N.; software, M.Y.; validation, M.Y.; formal analysis, M.Y.; investigation, M.Y. and K.S.; resources, Y.T.; data curation, M.Y.; writing—original draft preparation, M.Y.; writing—review and editing, A.N.; visualization, M.Y.; supervision, A.N., Y.T. and K.N.; project administration, M.Y., A.N. and K.N.; funding acquisition, A.N. and K.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Grants-in-Aid for Scientific Research of the Japan Society for the Promotion of Science, Grant Number JP 20K09442 to K.N. and JP 17K11033 to K.N.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
We thank Dennis Burger and Shiomi’s lab. for providing P7 silkworm cocoons, and the experimental farm of Shinshu university for providing wild-type silkworm cocoons. We also thank Haruna Mabuchi, for providing images of scanning electron microscope of fibroin sponges.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Peng, L.; Zhou, Y.; Lu, W.; Zhu, W.; Li, Y.; Chen, K.; Zhang, G.; Xu, J.; Deng, Z.; Wang, D. Characterization of a novel polyvinyl alcohol/chitosan porous hydrogel combined with bone marrow mesenchymal stem cells and its application in articular cartilage repair. BMC Musculoskelet. Disord. 2019, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zhu, F.; Li, X.; Liang, Q.; Zhuo, Z.; Huang, J.; Duan, L.; Xiong, J.; Wang, D. Repair of osteochondral defects using injectable chitosan-based hydrogel encapsulated synovial fluid-derived mesenchymal stem cells in a rabbit model. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 541–551. [Google Scholar] [CrossRef]
- Bothe, F.; Deubel, A.K.; Hesse, E.; Lotz, B.; Groll, J.; Werner, C.; Richter, W.; Hagmann, S. Treatment of Focal Cartilage Defects in Minipigs with Zonal Chondrocyte/Mesenchymal Progenitor Cell Constructs. Int. J. Mol. Sci. 2019, 20, 653. [Google Scholar] [CrossRef]
- Fernandes, T.L.; Shimomura, K.; Asperti, A.; Pinheiro, C.C.G.; Caetano, H.V.A.; Oliveira, C.R.G.C.M.; Nakamura, N.; Hernandez, A.J.; Bueno, D.F. Development of a Novel Large Animal Model to Evaluate Human Dental Pulp Stem Cells for Articular Cartilage Treatment. Stem Cell Rev. Rep. 2018, 14, 734–743. [Google Scholar] [CrossRef]
- He, A.; Liu, L.; Luo, X.; Liu, Y.; Liu, Y.; Liu, F.; Wang, X.; Zhang, Z.; Zhang, W.; Liu, W.; et al. Repair of osteochondral defects with in vitro engineered cartilage based on autologous bone marrow stromal cells in a swine model. Sci. Rep. 2017, 7, 40489. [Google Scholar] [CrossRef]
- Itokazu, M.; Wakitani, S.; Mera, H.; Tamamura, Y.; Sato, Y.; Takagi, M.; Nakamura, H. Transplantation of Scaffold-Free Cartilage-Like Cell-Sheets Made from Human Bone Marrow Mesenchymal Stem Cells for Cartilage Repair: A Preclinical Study. Cartilage 2016, 7, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Jakubiec, A.; Biant, L.; Tawy, G. The biomechanical and functional outcomes of autologous chondrocyte implantation for articular cartilage defects of the knee: A systematic review. Knee 2023, 44, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Krill, M.; Early, N.; Everhart, J.S.; Flanigan, D.C. Autologous Chondrocyte Implantation (ACI) for Knee Cartilage Defects: A Review of Indications, Technique, and Outcomes. JBJS Rev. 2018, 6, e5. [Google Scholar] [CrossRef]
- Wakitani, S.; Goto, T.; Pineda, S.J.; Young, R.G.; Mansour, J.M.; Caplan, A.I.; Goldberg, V.M. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J. Bone Jt. Surg. Am. 1994, 76, 579–592. [Google Scholar] [CrossRef]
- Løken, S.; Jakobsen, R.B.; Arøen, A.; Heir, S.; Shahdadfar, A.; Brinchmann, J.E.; Engebretsen, L.; Reinholt, F.P. Bone marrow mesenchymal stem cells in a hyaluronan scaffold for treatment of an osteochondral defect in a rabbit model. Knee Surg. Sport. Traumatol. Arthrosc. 2008, 16, 896–903. [Google Scholar] [CrossRef]
- Kayakabe, M.; Tsutsumi, S.; Watanabe, H.; Kato, Y.; Takagishi, K. Transplantation of autologous rabbit BM-derived mesenchymal stromal cells embedded in hyaluronic acid gel sponge into osteochondral defects of the knee. Cytotherapy 2006, 8, 343–353. [Google Scholar] [CrossRef]
- Fan, H.; Hu, Y.; Zhang, C.; Li, X.; Lv, R.; Qin, L.; Zhu, R. Cartilage regeneration using mesenchymal stem cells and a PLGA gelatin/chondroitin/hyaluronate hybrid scaffold. Biomaterials 2006, 27, 4573–4580. [Google Scholar] [CrossRef]
- Xie, J.; Han, Z.; Naito, M.; Maeyama, A.; Kim, S.H.; Kim, Y.H.; Matsuda, T. Articular cartilage tissue engineering based on a mechano-active scaffold made of poly(L-lactide-co-epsilon-caprolactone): In vivo performance in adult rabbits. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, L.R.; Chen, A.C.; Werpy, N.M.; Williams, A.A.; Kisiday, J.D.; Su, A.W.; Cory, E.; Morley, P.S.; McIlwraith, C.W.; Sah, R.L.; et al. Addition of Mesenchymal Stem Cells to Autologous Platelet-Enhanced Fibrin Scaffolds in Chondral Defects: Does It Enhance Repair? J. Bone Jt. Surg. 2016, 98, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, Z.; Denslin, V.; Ren, X.; Lee, C.S.; Yap, F.L.; Lee, E.H. Repair of Osteochondral Defects With Predifferentiated Mesenchymal Stem Cells of Distinct Phenotypic Character Derived From a Nanotopographic Platform. Am. J. Sport. Med. 2020, 48, 1735–1747. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.; Motta, A.; Freddi, G.; Cannas, M. In vitro evaluation of the inflammatory potential of the silk fibroin. J. Biomed. Mater. Res. 1999, 46, 382–389. [Google Scholar] [CrossRef]
- Minoura, N.; Aiba, S.; Gotoh, Y.; Tsukada, M.; Imai, Y. Attachment and growth of cultured fibroblast cells on silk protein matrices. J. Biomed. Mater. Res. 1995, 29, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Tomita, Y.; Morita, Y.; Hattori, K.; Harada, Y.; Sonobe, M.; Wakitani, S.; Tamada, Y. Culture of chondrocytes in fibroin-hydrogel sponge. Bio-Med. Mater. Eng. 2003, 13, 309–316. [Google Scholar]
- Hirakata, E.; Tomita, N.; Tamada, Y.; Suguro, T.; Nakajima, M.; Kambe, Y.; Yamada, K.; Yamamoto, K.; Kawakami, M.; Otaka, A.; et al. Early tissue formation on whole-area osteochondral defect of rabbit patella by covering with fibroin sponge. J. Biomed. Mater. Res. Part. B 2016, 104B, 1474–1482. [Google Scholar] [CrossRef]
- Schweigerer, L.; Neufeld, G.; Friedman, J.; Abraham, J.A.; Fiddes, J.C.; Gospodarowicz, D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature 1987, 325, 257–259. [Google Scholar] [CrossRef]
- Kambe, Y.; Kojima, K.; Tamada, Y.; Tomita, N.; Kameda, T. Silk fibroin sponges with cell growth-promoting activity induced by genetically fused basic fibroblast growth factor. J. Biomed. Mater. Res. Part. A 2015, 104A, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Burger, D.; Yamada, N.; Uchino, K.; Shiomi, K.; Tamada, Y. Production of recombinant silk fibroin with basic fibroblast growth factor binding affinity. J. Silk Sci. Tech. Jpn. 2021, 29, 67–77. [Google Scholar]
- Nakajima, A.; Shimizu, S.; Moriya, H.; Yamazaki, M. Expression of FGFR3, STAT1 and cyclin-dependent kinase inhibitor p21 during endochondral ossification: Differential role of FGFR3 in skeletal development and fracture repair. Endocrinology 2003, 144, 4659–4668. [Google Scholar] [CrossRef]
- Tamada, Y. New process to form a silk fibroin porous 3-D structure. Biomacromolecules 2005, 6, 3100–3106. [Google Scholar] [CrossRef]
- Burger, D.; Beaumont, M.; Rosenau, T.; Tamada, Y. Porous silk fibroin/cellulose hydrogels for bone tissue engineering via a novel combined process based on sequential regeneration and porogen leaching. Molecules 2020, 25, 5097. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Masuda, Y.; Ishikawa, K.; Tamada, Y. Fractionation of regenerated silk fibroin and characterization of the fractions. Molecules 2021, 26, 6317. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Turner, N.; Grose, R. Fibroblast growth factor signalling: From development to cancer. Nat. Rev. Cancer 2010, 10, 116–129. [Google Scholar] [CrossRef]
- Hull, M.A.; Brough, J.L.; Powe, D.G.; Carter, G.I.; Jenkins, D.; Hawkey, C.J. Expression of basic fibroblast growth factor in intact and ulcerated human gastric mucosa. Gut 1998, 43, 525–536. [Google Scholar] [CrossRef]
- Smith, G.M.; Hale, J.H. Macrophage/Microglia regulation of astrocytic tenascin: Synergistic action of transforming growth factor-beta and basic fibroblast growth factor. J. Neurosci. 1997, 17, 9624–9633. [Google Scholar] [CrossRef]
- Weiss, S.; Hennig, T.; Bock, R.; Steck, E.; Richter, W. Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells. J. Cell Physiol. 2010, 223, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.H.; Jo, C.H.; Seong, S.C.; Lee, J.C.; Lee, M.C. Effect of serum and growth factors on chondrogenic differentiation of synovium-derived stromal cells. Tissue Eng. Part. A 2009, 15, 3401–3415. [Google Scholar] [CrossRef] [PubMed]
- Ellman, M.B.; An, H.S.; Muddasani, P.; Im, H.J. Biological impact of the fibroblast growth factor family on articular cartilage and intervertebral disc homeostasis. Gene 2008, 420, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Shimokawa, H.; Fukada, K.; Suzuki, S.; Shibata, S.; Ohya, K.; Kuroda, T. Localization and inhibitory effect of basic fibroblast growth factor on chondrogenesis in cultured mouse mandibular condyle. J. Bone Min. Metab. 2003, 21, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Huang, S.; Zhou, S.; He, L.; Jin, Y. Cartilage regeneration using a novel gelatin-chondroitin-hyaluronan hybrid scaffold containing bFGF-impregnated microspheres. J. Microencapsul. 2007, 24, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Schmal, H.; Zwingmann, J.; Fehrenbach, M.; Finkenzeller, G.; Stark, G.B.; Südkamp, N.P.; Hartl, D.; Mehlhorn, A.T. bFGF influences human articular chondrocyte differentiation. Cytotherapy 2007, 9, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Sulcanese, L.; Prencipe, G.; Canciello, A.; Cerveró-Varona, A.; Perugini, M.; Mauro, A.; Russo, V.; Barboni, B. Stem-Cell-Driven Chondrogenesis: Perspectives on Amnion-Derived Cells. Cells 2024, 13, 744. [Google Scholar] [CrossRef] [PubMed]
- Owaidah, A.Y. Induced pluripotent stem cells in cartilage tissue engineering: A literature review. Biosci. Rep. 2024, 44, BSR20232102. [Google Scholar] [CrossRef]
- Chimal-Monroy, J.; de Leon, L.D. Differential effects of transforming growth factors beta 1, beta 2, beta 3 and beta 5 on chondrogenesis in mouse limb bud mesenchymal cells. Int. J. Dev. Biol. 2003, 41, 91–102. [Google Scholar]
- Sekiya, I.; Larson, B.L.; Vuoristo, J.T.; Reger, R.L.; Prockop, D.J. Comparison of effect of BMP2,-4, and-6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell Tissue Res. 2005, 320, 269–276. [Google Scholar] [CrossRef]
- Wang, L.; Detamore, M.S. Insulin like growth factor I improves chondrogenesis of predifferentiated human umbilical cord mesenchymal stromal cells. J. Orthop. Res. 2009, 27, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.E.; Bendele, A.M.; Thompson, D.L.; Littau, A.; Waggie, K.S.; Reardon, B.; Ellsworth, J.L. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthr. Cartil. 2005, 13, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Handorf, A.M.; Li, W.J. Fibroblast growth factor-2 primes human mesenchymal stem cells for enhanced chondrogenesis. PLoS ONE 2011, 6, e22887. [Google Scholar] [CrossRef] [PubMed]
- Nasrabadi, D.; Rezaeiani, S.; Eslaminejad, M.B.; Shabani, A. Improved Protocol for Chondrogenic Differentiation of Bone Marrow Derived Mesenchymal Stem Cells -Effect of PTHrP and FGF-2 on TGFbeta1/BMP2-Induced Chondrocytes Hypertrophy. Stem Cell Rev. Rep. 2018, 14, 755–766. [Google Scholar] [CrossRef]
- Du, M.; Liang, H.; Mou, C.; Li, X.; Sun, J.; Zhuang, Y.; Xiao, Z.; Chen, B.; Dai, J. Regulation of human mesenchymal stem cells differentiation into chondrocytes in extracellular matrix-based hydrogel scaffolds. Colloids Surf. B Biointerfaces 2014, 114, 316–323. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).