An Evaluation of the Cytocompatibility of Endodontic Bioceramics in Human Periodontal-Ligament-Derived Cells
Abstract
1. Introduction
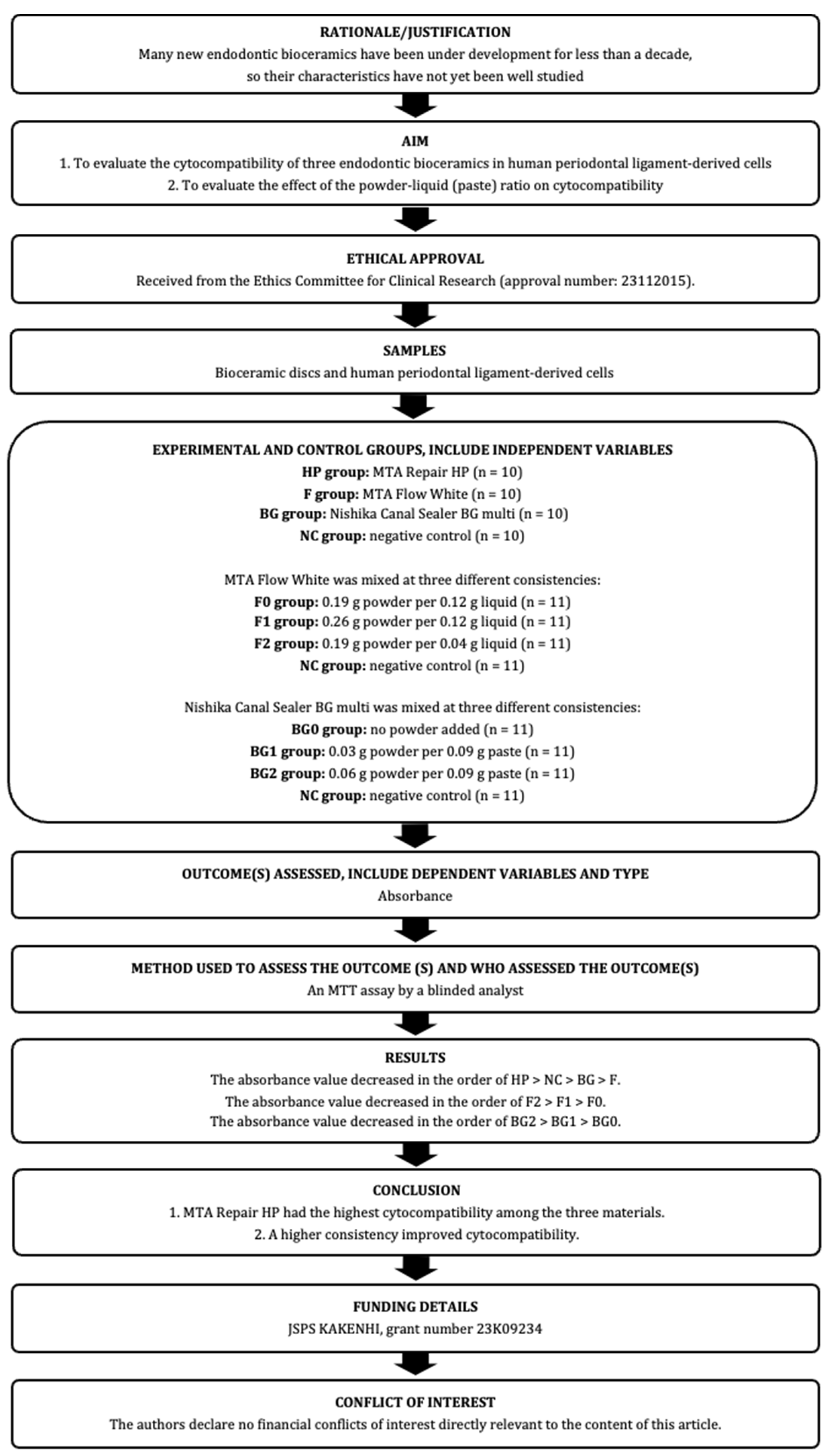
2. Materials and Methods
2.1. Human Periodontal-Ligament-Derived Cells (hPDLCs)
2.2. Preparation of Bioceramic Discs
2.3. Cytocompatibility
2.4. Effects of the Powder–Liquid (Paste) Ratio of F or BG on Cytocompatibility
2.5. Statistical Analyses
2.6. Sample Size
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siqueira, J.F., Jr.; Rôças, I.N. Clinical implications and microbiology of bacterial persistence after treatment procedures. J. Endod. 2008, 34, 1291–1301.e1293. [Google Scholar] [CrossRef]
- Hoen, M.M.; Pink, F.E. Contemporary endodontic retreatments: An analysis based on clinical treatment findings. J. Endod. 2002, 28, 834–836. [Google Scholar] [CrossRef]
- Buckley, M.; Spångberg, L.S. The prevalence and technical quality of endodontic treatment in an American subpopulation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1995, 79, 92–100. [Google Scholar] [CrossRef]
- da Silva, G.F.; Guerreiro-Tanomaru, J.M.; Sasso-Cerri, E.; Tanomaru-Filho, M.; Cerri, P.S. Histological and histomorphometrical evaluation of furcation perforations filled with MTA, CPM and ZOE. Int. Endod. J. 2011, 44, 100–110. [Google Scholar] [CrossRef]
- Ruparel, N.B.; Ruparel, S.B.; Chen, P.B.; Ishikawa, B.; Diogenes, A. Direct effect of endodontic sealers on trigeminal neuronal activity. J. Endod. 2014, 40, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Rastegar, A.F.; Kettering, J.D.; Pitt Ford, T.R. Bacterial leakage of mineral trioxide aggregate as a root-end filling material. J. Endod. 1995, 21, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.F.; Carnes, D.L.; del Rio, C.E. Longitudinal sealing ability of mineral trioxide aggregate as a root-end filling material. J. Endod. 1996, 22, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Pitt Ford, T.R.; McKendry, D.J.; Abedi, H.R.; Miller, D.A.; Kariyawasam, S.P. Histologic assessment of mineral trioxide aggregate as a root-end filling in monkeys. J. Endod. 1997, 23, 225–228. [Google Scholar] [CrossRef]
- Torabinejad, M.; Ford, T.R.; Abedi, H.R.; Kariyawasam, S.P.; Tang, H.M. Tissue reaction to implanted root-end filling materials in the tibia and mandible of guinea pigs. J. Endod. 1998, 24, 468–471. [Google Scholar] [CrossRef]
- Tanomaru-Filho, M.; de Oliveira, B.V.; Tavares, K.; Rodrigues, E.M.; Torres, F.F.E.; Guerreiro-Tanomaru, J.M. Effect of radiopacifier and liquid in the physicochemical and biological properties of calcium silicate clinker Angelus: A laboratory investigation. Aust. Endod. J. 2023, 50, 52–59. [Google Scholar] [CrossRef]
- Silva, E.J.; Carvalho, N.K.; Zanon, M.; Senna, P.M.; De-Deus, G.; Zuolo, M.L.; Zaia, A.A. Push-out bond strength of MTA HP, a new high-plasticity calcium silicate-based cement. Braz. Oral Res. 2016, 30, e84. [Google Scholar] [CrossRef]
- Ferreira, C.M.A.; Sassone, L.M.; Gonçalves, A.S.; de Carvalho, J.J.; Tomás-Catalá, C.J.; García-Bernal, D.; Oñate-Sánchez, R.E.; Rodríguez-Lozano, F.J.; Silva, E. Physicochemical, cytotoxicity and in vivo biocompatibility of a high-plasticity calcium-silicate based material. Sci. Rep. 2019, 9, 3933. [Google Scholar] [CrossRef]
- Pelepenko, L.E.; Saavedra, F.; Antunes, T.B.M.; Bombarda, G.F.; Gomes, B.; Zaia, A.A.; Camilleri, J.; Marciano, M.A. Physicochemical, antimicrobial, and biological properties of White-MTAFlow. Clin. Oral Investig. 2021, 25, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.V.; da Silva, G.R.; Souza, G.L.; Magalhães, T.E.A.; Barbosa, G.L.R.; Turrioni, A.P.; Moura, C.C.G. A laboratory evaluation of cell viability, radiopacity and tooth discoloration induced by regenerative endodontic materials. Int. Endod. J. 2020, 53, 1140–1152. [Google Scholar] [CrossRef]
- Washio, A.; Nakagawa, A.; Nishihara, T.; Maeda, H.; Kitamura, C. Physicochemical properties of newly developed bioactive glass cement and its effects on various cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 373–380. [Google Scholar] [CrossRef]
- Jo, S.B.; Kim, H.K.; Lee, H.N.; Kim, Y.J.; Dev Patel, K.; Campbell Knowles, J.; Lee, J.H.; Song, M. Physical Properties and Biofunctionalities of Bioactive Root Canal Sealers In Vitro. Nanomaterials 2020, 10, 1750. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Morotomi, T.; Washio, A.; Yada, N.; Matsuo, K.; Teshima, H.; Yokota, K.; Kitamura, C. In vitro and in vivo effects of a novel bioactive glass-based cement used as a direct pulp capping agent. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 161–168. [Google Scholar] [CrossRef]
- Nagendrababu, V.; Murray, P.E.; Ordinola-Zapata, R.; Peters, O.A.; Rôças, I.N.; Siqueira, J.F., Jr.; Priya, E.; Jayaraman, J.; Shaju, J.P.; Camilleri, J.; et al. PRILE 2021 guidelines for reporting laboratory studies in Endodontology: A consensus-based development. Int. Endod. J. 2021, 54, 1482–1490. [Google Scholar] [CrossRef]
- Nagendrababu, V.; Murray, P.E.; Ordinola-Zapata, R.; Peters, O.A.; Rôças, I.N.; Siqueira, J.F., Jr.; Priya, E.; Jayaraman, J.; Pulikkotil, S.J.; Suresh, N.; et al. PRILE 2021 guidelines for reporting laboratory studies in Endodontology: Explanation and elaboration. Int. Endod. J. 2021, 54, 1491–1515. [Google Scholar] [CrossRef]
- Hong, H.H.; Chou, T.A.; Hong, A.; Huang, Y.F.; Yen, T.H.; Liang, C.H.; Hong, A.; Hsiao, H.Y.; Nien, C.Y. Calcitriol and enamel matrix derivative differentially regulated cemento-induction and mineralization in human periodontal ligament-derived cells. J. Periodontol. 2022, 93, 1553–1565. [Google Scholar] [CrossRef] [PubMed]
- Arima, M.; Hasegawa, D.; Yoshida, S.; Mitarai, H.; Tomokiyo, A.; Hamano, S.; Sugii, H.; Wada, N.; Maeda, H. R-spondin 2 promotes osteoblastic differentiation of immature human periodontal ligament cells through the Wnt/β-catenin signaling pathway. J. Periodontal Res. 2019, 54, 143–153. [Google Scholar] [CrossRef]
- Tang, X.; Han, J.; Meng, H.; Zhao, Y.; Wang, H.; Liu, J.; Lin, L.; Zhang, D.; Li, C.; Ma, C. Downregulation of RANKL and RANKL/osteoprotegerin ratio in human periodontal ligament cells during their osteogenic differentiation. J. Periodontal Res. 2016, 51, 125–132. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Ling, J.; Gao, Y.; Xiao, Y. Effects of varied ionic calcium and phosphate on the proliferation, osteogenic differentiation and mineralization of human periodontal ligament cells in vitro. J. Periodontal Res. 2012, 47, 374–382. [Google Scholar] [CrossRef]
- Jäger, A.; Götz, W.; Lossdörfer, S.; Rath-Deschner, B. Localization of SOST/sclerostin in cementocytes in vivo and in mineralizing periodontal ligament cells in vitro. J. Periodontal Res. 2010, 45, 246–254. [Google Scholar] [CrossRef]
- Inada, R.N.H.; Queiroz, M.B.; Lopes, C.S.; Silva, E.C.A.; Torres, F.F.E.; da Silva, G.F.; Guerreiro-Tanomaru, J.M.; Cerri, P.S.; Tanomaru-Filho, M. Biocompatibility, bioactive potential, porosity, and interface analysis calcium silicate repair cements in a dentin tube model. Clin. Oral Investig. 2023, 27, 3839–3853. [Google Scholar] [CrossRef]
- Tomás-Catalá, C.J.; Collado-González, M.; García-Bernal, D.; Oñate-Sánchez, R.E.; Forner, L.; Llena, C.; Lozano, A.; Moraleda, J.M.; Rodríguez-Lozano, F.J. Biocompatibility of New Pulp-capping Materials NeoMTA Plus, MTA Repair HP, and Biodentine on Human Dental Pulp Stem Cells. J. Endod. 2018, 44, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Kim, E.; Lee, S.; Park, S.; Chen, D.; Shin, S.J.; Kim, E.; Kim, S. Comparison of Biocompatibility of Calcium Silicate-Based Sealers and Epoxy Resin-Based Sealer on Human Periodontal Ligament Stem Cells. Materials 2020, 13, 5242. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.N.; Hong, J.U.; Kim, S.M.; Jang, J.H.; Chang, H.S.; Hwang, Y.C.; Hwang, I.N.; Oh, W.M. Anti-inflammatory and Osteogenic Effects of Calcium Silicate-based Root Canal Sealers. J. Endod. 2019, 45, 73–78. [Google Scholar] [CrossRef]
- López-García, S.; Pecci-Lloret, M.R.; Guerrero-Gironés, J.; Pecci-Lloret, M.P.; Lozano, A.; Llena, C.; Rodríguez-Lozano, F.J.; Forner, L. Comparative Cytocompatibility and Mineralization Potential of Bio-C Sealer and TotalFill BC Sealer. Materials 2019, 12, 3087. [Google Scholar] [CrossRef]
- Benetti, F.; de Azevedo Queiroz, Í.O.; Oliveira, P.H.C.; Conti, L.C.; Azuma, M.M.; Oliveira, S.H.P.; Cintra, L.T.A. Cytotoxicity and biocompatibility of a new bioceramic endodontic sealer containing calcium hydroxide. Braz. Oral Res. 2019, 33, e042. [Google Scholar] [CrossRef]
- Cintra, L.T.A.; Benetti, F.; de Azevedo Queiroz, Í.O.; Ferreira, L.L.; Massunari, L.; Bueno, C.R.E.; de Oliveira, S.H.P.; Gomes-Filho, J.E. Evaluation of the Cytotoxicity and Biocompatibility of New Resin Epoxy-based Endodontic Sealer Containing Calcium Hydroxide. J. Endod. 2017, 43, 2088–2092. [Google Scholar] [CrossRef] [PubMed]
- Cintra, L.T.A.; Benetti, F.; de Azevedo Queiroz, Í.O.; de Araújo Lopes, J.M.; Penha de Oliveira, S.H.; Sivieri Araújo, G.; Gomes-Filho, J.E. Cytotoxicity, Biocompatibility, and Biomineralization of the New High-plasticity MTA Material. J. Endod. 2017, 43, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shen, Y.; Stojicic, S.; Haapasalo, M. Biocompatibility of two novel root repair materials. J. Endod. 2011, 37, 793–798. [Google Scholar] [CrossRef] [PubMed]



| Materials | Manufactures | Composition |
|---|---|---|
| MTA Repair HP | Angelus (Brazil) | Powder: calcium silicate, calcium aluminate, calcium oxide, calcium tungstate Liquid: purified water, plasticizer |
| MTA Flow White | Ultradent Products (USA) | Powder: tricalcium silicate, dicalcium silicate, calcium sulfate, etc. Gel: purified water, thickening agent |
| Nishika Canal Sealer BG Multi | Nippon Shika Yakuhin (Japan) | Paste A: fatty acids, bismuth bicarbonate, silicon dioxide Paste B: magnesium oxide, purified water, calcium silicate glass, silicon dioxide, etc. Powder: calcium silicate glass, calcium hydroxide |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Mean (SD) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HP | 0.91 | 1.06 | 1.57 | 1.81 | 1.67 | 1.59 | 1.17 | 0.83 | 1.32 | 1.44 | 1.34 (0.33) a |
| BG | 0.15 | 0.41 | 0.43 | 0.63 | 0.76 | 0.15 | 0.27 | 0.33 | 0.27 | 0.43 | 0.38 (0.20) b |
| F | −0.01 | 0.01 | 0.06 | 0.02 | 0.01 | 0.03 | 0.02 | 0.01 | 0.03 | 0.06 | 0.03 (0.02) b |
| NC | 1.28 | 1.13 | 1.27 | 1.90 | 1.54 | 1.67 | 1.05 | 0.96 | 1.92 | 1.22 | 1.39 (0.34) a |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Mean (SD) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HP | 3.12 | 3.16 | 2.93 | 2.81 | 2.85 | 2.66 | 2.15 | 2.52 | 3.18 | 3.28 | 2.87 (0.35) a |
| BG | −0.08 | 2.22 | 1.38 | 2.42 | 1.90 | 1.21 | 1.34 | 0.85 | 1.94 | 2.62 | 1.58 (0.81) b |
| F | 0.01 | 0.02 | −0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.04 | 2.13 | 0.23 (0.67) c |
| NC | 2.47 | 2.99 | 2.62 | 2.79 | 2.45 | 2.45 | 2.45 | 2.39 | 3.07 | 3.08 | 2.64 (0.32) a |
| 7 Days | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Mean (SD) | p-Value | |
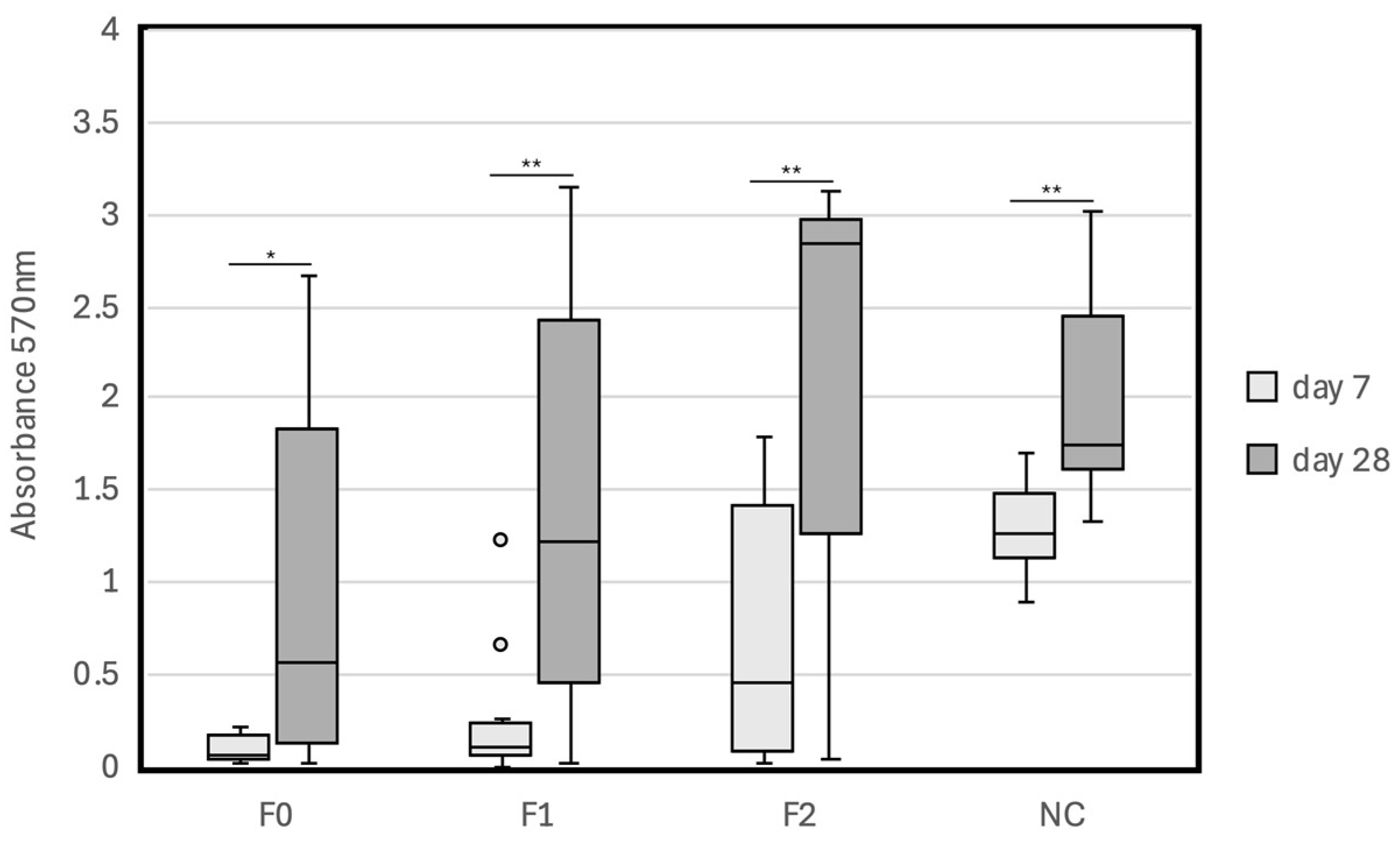
| F0 | 0.03 | 0.02 | 0.01 | 0.05 | 0.07 | 0.22 | 0.01 | 0.18 | 0.22 | 0.06 | 0.14 | 0.09 (0.08) | reference |
| F1 | 0.00 | 0.23 | 1.22 | 0.67 | 0.04 | 0.05 | 0.06 | 0.10 | 0.04 | 0.22 | 0.25 | 0.26 (0.37) | 0.165 |
| F2 | 0.01 | 1.49 | 1.72 | 0.45 | 1.35 | 0.08 | 0.75 | 0.26 | 0.07 | 1.80 | 0.06 | 0.73 (0.72) | 0.015 |
| NC | 1.37 | 1.20 | 1.70 | 1.54 | 1.58 | 0.90 | 1.26 | 1.05 | 0.90 | 1.21 | 1.41 | 1.28 (0.27) | <0.001 |
| 28 Days | |||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Mean (SD) | p-Value | |
| F0 | 2.34 | 0.03 | 0.16 | 1.32 | 2.67 | 0.55 | 0.39 | 0.01 | 2.61 | 0.07 | 1.03 | 1.02 (1.06) | reference |
| F1 | 0.02 | 1.21 | 2.71 | 1.01 | 3.15 | 2.13 | 3.11 | 0.01 | 0.75 | 1.64 | 0.13 | 1.44 (1.20) | 0.388 |
| F2 | 0.04 | 3.04 | 2.92 | 1.43 | 2.55 | 1.09 | 3.14 | 3.09 | 2.88 | 0.97 | 2.85 | 2.18 (1.09) | 0.02 |
| NC | 1.75 | 1.60 | 2.08 | 1.32 | 1.69 | 2.08 | 1.41 | 2.86 | 3.02 | 1.64 | 2.80 | 2.02 (0.61) | 0.015 |
| 7 Days | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Mean (SD) | p-Value | |
| BG0 | 0.62 | 0.39 | 0.62 | 0.48 | 0.83 | 0.58 | 0.62 | 0.22 | 0.16 | 0.28 | 0.18 | 0.45 (0.22) | reference |
| BG1 | 0.69 | 0.57 | 0.56 | 1.06 | 0.94 | 0.62 | 0.52 | 0.28 | 0.28 | 0.23 | 0.25 | 0.55 (0.28) | 0.398 |
| BG2 | 1.01 | 1.07 | 1.17 | 1.15 | 1.14 | 0.96 | 1.29 | 0.38 | 0.49 | 0.39 | 0.57 | 0.87 (0.34) | <0.01 |
| NC | 1.24 | 1.14 | 1.74 | 1.65 | 1.58 | 0.94 | 1.20 | 0.97 | 1.04 | 1.20 | 1.37 | 1.28 (0.28) | <0.001 |
| 28 Days | |||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Mean (SD) | p-Value | |
| BG0 | 2.62 | 2.09 | 1.89 | 0.78 | 2.31 | 1.35 | 2.80 | 1.11 | 2.17 | 0.40 | 1.10 | 1.69 (0.79) | reference |
| BG1 | 2.78 | 1.87 | 2.35 | 1.03 | 2.97 | 1.69 | 3.06 | 1.88 | 1.75 | 0.68 | 0.80 | 1.89 (0.84) | 0.563 |
| BG2 | 2.93 | 2.95 | 2.81 | 0.83 | 2.40 | 2.75 | 2.96 | 2.16 | 2.18 | 1.01 | 1.18 | 2.20 (0.82) | 0.157 |
| NC | 2.70 | 1.36 | 1.76 | 0.97 | 2.85 | 1.78 | 2.04 | 2.89 | 2.41 | 1.49 | 2.21 | 2.04 (0.63) | 0.267 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aka, A.; Matsuura, T.; Yoshimura, A. An Evaluation of the Cytocompatibility of Endodontic Bioceramics in Human Periodontal-Ligament-Derived Cells. J. Funct. Biomater. 2024, 15, 231. https://doi.org/10.3390/jfb15080231
Aka A, Matsuura T, Yoshimura A. An Evaluation of the Cytocompatibility of Endodontic Bioceramics in Human Periodontal-Ligament-Derived Cells. Journal of Functional Biomaterials. 2024; 15(8):231. https://doi.org/10.3390/jfb15080231
Chicago/Turabian StyleAka, Asuka, Takashi Matsuura, and Atsutoshi Yoshimura. 2024. "An Evaluation of the Cytocompatibility of Endodontic Bioceramics in Human Periodontal-Ligament-Derived Cells" Journal of Functional Biomaterials 15, no. 8: 231. https://doi.org/10.3390/jfb15080231
APA StyleAka, A., Matsuura, T., & Yoshimura, A. (2024). An Evaluation of the Cytocompatibility of Endodontic Bioceramics in Human Periodontal-Ligament-Derived Cells. Journal of Functional Biomaterials, 15(8), 231. https://doi.org/10.3390/jfb15080231







