Recent Advancements in Chitosan-Based Biomaterials for Wound Healing
Abstract
:1. Introduction
2. Chitosan Properties
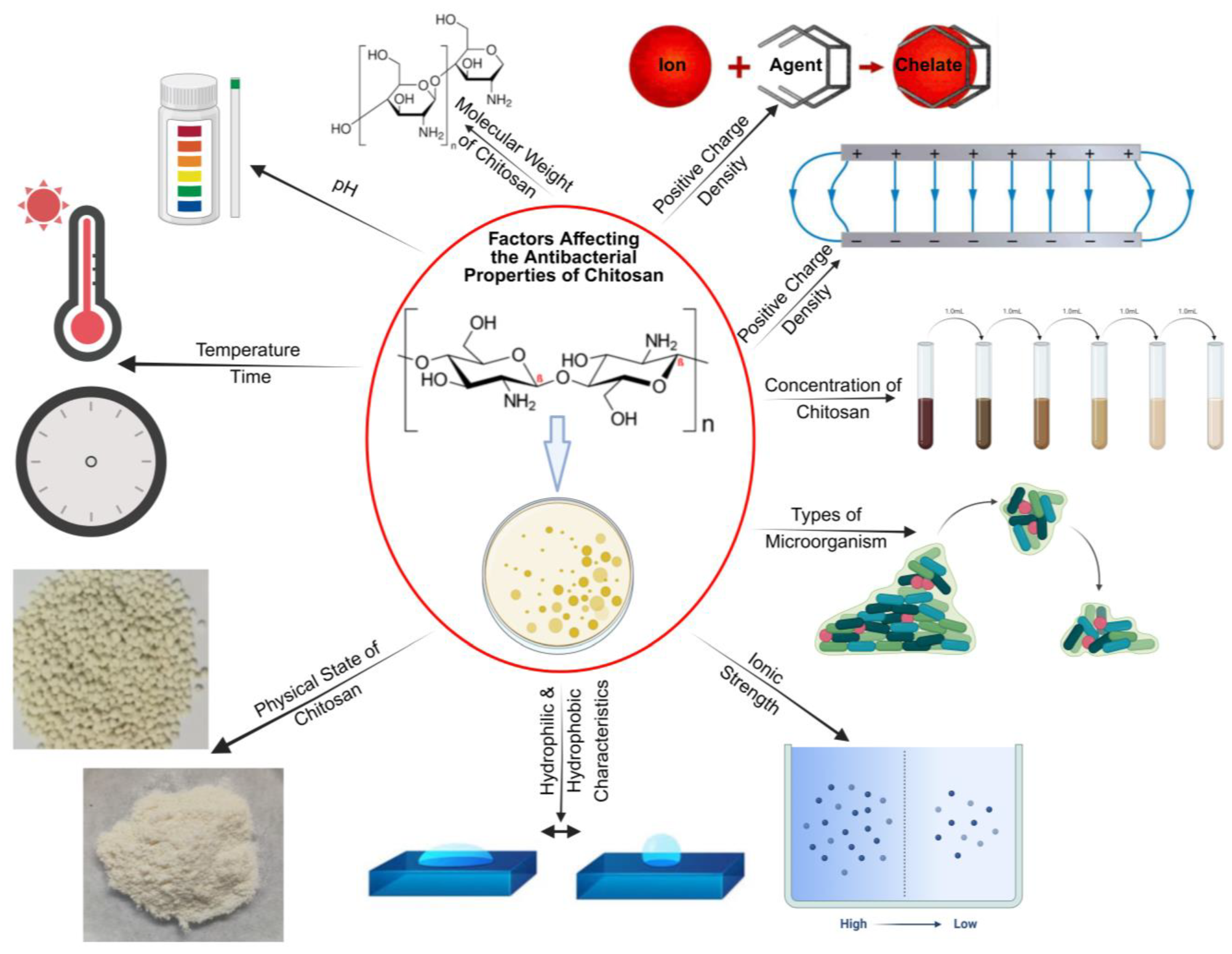
2.1. Antibacterial Property
2.2. Mucoadhesive Property
2.3. High Fluid Absorption Capacity
2.4. Biocompatibility
2.5. Biodegradability
2.6. Anti-Inflammatory Response
2.7. Antioxidant Property
3. Types of Chitosan and PEC Films
3.1. Chitosan–Heparin Sodium (CS-HS) Hydrogels
3.2. Chitosan–Sodium Alginate (CS-SA) Hydrogels
3.3. Chitosan–Gum Arabic (CS-GA) Films
3.4. Chitosan–Pectin (CS-P) Films
3.5. Chitosan–Poly (AAm-co-IA)-AgNO3 IPC Films
4. Chitosan-Based Innovations in Drug Delivery Systems
5. Wound Healing Products
6. Research Gaps
6.1. Standardization Challenges and Manufacturing Variability
6.2. Limited Clinical Translation
6.3. Long-Term Biocompatibility and Biodegradability
6.4. Integration with Existing and Emerging Therapies
6.5. Environmental and Economic Considerations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.; Hao, L.T.; Park, J.; Oh, D.X.; Hwang, D.S. Nanochitin and Nanochitosan: Chitin Nanostructure Engineering with Multiscale Properties for Biomedical and Environmental Applications. Adv. Mater. Deerfield Beach Fla 2023, 35, e2203325. [Google Scholar] [CrossRef] [PubMed]
- Abourehab, M.A.S.; Pramanik, S.; Abdelgawad, M.A.; Abualsoud, B.M.; Kadi, A.; Ansari, M.J.; Deepak, A. Recent Advances of Chitosan Formulations in Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 10975. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Alemu, D.; Getachew, E.; Mondal, A.K. Study on the Physicochemical Properties of Chitosan and Their Applications in the Biomedical Sector. Int. J. Polym. Sci. 2023, 2023, 5025341. [Google Scholar] [CrossRef]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A Review on Properties, Biological Activities and Recent Progress in Biomedical Applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Elamri, A.; Zdiri, K.; Bouzir, D.; Hamdaoui, M. Use of chitosan as antimicrobial, antiviral and antipollution agent in textile finishing. Fibres Text. 2022, 29, 51–70. [Google Scholar] [CrossRef]
- Prete, S.; Dattilo, M.; Patitucci, F.; Pezzi, G.; Parisi, O.I.; Puoci, F. Natural and Synthetic Polymeric Biomaterials for Application in Wound Management. J. Funct. Biomater. 2023, 14, 455. [Google Scholar] [CrossRef]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef]
- Ojeda-Hernández, D.D.; Canales-Aguirre, A.A.; Matias-Guiu, J.; Gomez-Pinedo, U.; Mateos-Díaz, J.C. Potential of Chitosan and Its Derivatives for Biomedical Applications in the Central Nervous System. Front. Bioeng. Biotechnol. 2020, 8, 389. [Google Scholar] [CrossRef]
- de Sousa Victor, R.; Marcelo da Cunha Santos, A.; Viana de Sousa, B.; de Araújo Neves, G.; Navarro de Lima Santana, L.; Rodrigues Menezes, R. A Review on Chitosan’s Uses as Biomaterial: Tissue Engineering, Drug Delivery Systems and Cancer Treatment. Materials 2020, 13, 4995. [Google Scholar] [CrossRef]
- Shu, M.; Long, S.; Huang, Y.; Li, D.; Li, H.; Li, X. High Strength and Antibacterial Polyelectrolyte Complex CS/HS Hydrogel Films for Wound Healing. Soft Matter. 2019, 15, 7686–7694. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, N.; Abarca, R.L.; Linares-Flores, C. Use of Chitosan-Based Polyelectrolyte Complexes for Its Potential Application in Active Food Packaging: A Review of Recent Literature. Int. J. Mol. Sci. 2023, 24, 11535. [Google Scholar] [CrossRef] [PubMed]
- Potaś, J.; Szymańska, E.; Basa, A.; Hafner, A.; Winnicka, K. Tragacanth Gum/Chitosan Polyelectrolyte Complexes-Based Hydrogels Enriched with Xanthan Gum as Promising Materials for Buccal Application. Materials 2020, 14, 86. [Google Scholar] [CrossRef]
- Baskan, H.; Esentürk, I.; Dösler, S.; Sarac, A.S.; Karakas, H. Electrospun Nanofibers of Poly (Acrylonitrile-Co-Itaconic Acid)/Silver and Polyacrylonitrile/Silver: In Situ Preparation, Characterization, and Antimicrobial Activity. J. Ind. Text. 2021, 50, 1594–1624. [Google Scholar] [CrossRef]
- Li, J.; van Ewijk, G.; van Dijken, D.J.; van der Gucht, J.; de Vos, W.M. Single-Step Application of Polyelectrolyte Complex Films as Oxygen Barrier Coatings. ACS Appl. Mater. Interfaces 2021, 13, 21844–21853. [Google Scholar] [CrossRef]
- Emonds, S.; Kamp, J.; Borowec, J.; Roth, H.; Wessling, M. Polyelectrolyte Complex Tubular Membranes via a Salt Dilution Induced Phase Inversion Process. Adv. Eng. Mater. 2021, 23, 2001401. [Google Scholar] [CrossRef]
- Volod’ko, A.V.; Davydova, V.N.; Petrova, V.A.; Romanov, D.P.; Pimenova, E.A.; Yermak, I.M. Comparative Analysis of the Functional Properties of Films Based on Carrageenans, Chitosan, and Their Polyelectrolyte Complexes. Mar. Drugs 2021, 19, 704. [Google Scholar] [CrossRef]
- Mndlovu, H.; du Toit, L.C.; Kumar, P.; Choonara, Y.E.; Marimuthu, T.; Kondiah, P.P.D.; Pillay, V. Bioplatform Fabrication Approaches Affecting Chitosan-Based Interpolymer Complex Properties and Performance as Wound Dressings. Molecules 2020, 25, 222. [Google Scholar] [CrossRef]
- Sanchez, M.F.; Guzman, M.L.; Apas, A.L.; Alovero, F.D.L.; Olivera, M.E. Sustained Dual Release of Ciprofloxacin and Lidocaine from Ionic Exchange Responding Film Based on Alginate and Hyaluronate for Wound Healing. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2021, 161, 105789. [Google Scholar] [CrossRef]
- Haririan, Y.; Asefnejad, A. Biopolymer Hydrogels and Synergistic Blends for Tailored Wound Healing. Int. J. Biol. Macromol. 2024, 279, 135519. [Google Scholar] [CrossRef]
- Soubhagya, A.S.; Balagangadharan, K.; Selvamurugan, N.; Sathya Seeli, D.; Prabaharan, M. Preparation and Characterization of Chitosan/Carboxymethyl Pullulan/Bioglass Composite Films for Wound Healing. J. Biomater. Appl. 2022, 36, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P.; Kumar, S.; Schloss, R.S.; Berthiaume, F.; Langrana, N.A. Chitosan-Polygalacturonic Acid Complex Dressing Improves Diabetic Wound Healing and Hair Growth in Diabetic Mice. Biochem. Biophys. Res. Commun. 2024, 696, 149502. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, L.; Zhang, J.; Zhao, Z.; Yu, W.; Tan, Z.; Gao, P.; Chen, X. Combination of Natural Polyanions and Polycations Based on Interfacial Complexation for Multi-Functionalization of Wound Dressings. Front. Bioeng. Biotechnol. 2022, 10, 1006584. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan: Drug Delivery and Biomedical Applications; Jana, S., Jana, S., Eds.; Springer: Singapore, 2019; pp. 457–489. ISBN 9789811502637. [Google Scholar]
- Gheorghiță, D.; Moldovan, H.; Robu, A.; Bița, A.-I.; Grosu, E.; Antoniac, A.; Corneschi, I.; Antoniac, I.; Bodog, A.D.; Băcilă, C.I. Chitosan-Based Biomaterials for Hemostatic Applications: A Review of Recent Advances. Int. J. Mol. Sci. 2023, 24, 10540. [Google Scholar] [CrossRef]
- Elbialy, N.S.; Mohamed, N. Alginate-Coated Caseinate Nanoparticles for Doxorubicin Delivery: Preparation, Characterisation, and in Vivo Assessment. Int. J. Biol. Macromol. 2020, 154, 114–122. [Google Scholar] [CrossRef]
- Petrescu, N.; Crisan, B.; Aghiorghiesei, O.; Sarosi, C.; Mirica, I.C.; Lucaciu, O.; Iușan, S.A.L.; Dirzu, N.; Apostu, D. Gradual Drug Release Membranes and Films Used for the Treatment of Periodontal Disease. Membranes 2022, 12, 895. [Google Scholar] [CrossRef]
- Mistry, P. Application of Polyelectrolyte Complex (PEC) Dressing on Diabetic Wound. Master’s Thesis, Rutgers University-School of Graduate Studies, Piscataway, NJ, USA, 2023. [Google Scholar]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicrobial Properties of Chitosan from Different Developmental Stages of the Bioconverter Insect Hermetia Illucens. Sci. Rep. 2022, 12, 8084. [Google Scholar] [CrossRef]
- Safari, S.; Barani, M.; Sadrmohammadi, R. Antimicrobial Properties of Tissue Conditioner Modified with Chitosan and Green-Synthesized Silver Nanoparticles: A Promising Approach for Preventing Denture Stomatitis. BMC Oral Health 2024, 24, 146. [Google Scholar] [CrossRef]
- Nasaj, M.; Chehelgerdi, M.; Asghari, B.; Ahmadieh-Yazdi, A.; Asgari, M.; Kabiri-Samani, S.; Sharifi, E.; Arabestani, M. Factors Influencing the Antimicrobial Mechanism of Chitosan Action and Its Derivatives: A Review. Int. J. Biol. Macromol. 2024, 277, 134321. [Google Scholar] [CrossRef]
- Li, A.; Ma, B.; Hua, S.; Ping, R.; Ding, L.; Tian, B.; Zhang, X. Chitosan-Based Injectable Hydrogel with Multifunction for Wound Healing: A Critical Review. Carbohydr. Polym. 2024, 333, 121952. [Google Scholar] [CrossRef]
- Mura, P.; Maestrelli, F.; Cirri, M.; Mennini, N. Multiple Roles of Chitosan in Mucosal Drug Delivery: An Updated Review. Mar. Drugs 2022, 20, 335. [Google Scholar] [CrossRef]
- Edo, G.I.; Yousif, E.; Al-Mashhadani, M.H. Chitosan: An Overview of Biological Activities, Derivatives, Properties, and Current Advancements in Biomedical Applications. Carbohydr. Res. 2024, 542, 109199. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, P.; Ramalingam, B.; Das, S.K. Fabrication of Chitosan-Reinforced Multifunctional Graphene Nanocomposite as Antibacterial Scaffolds for Hemorrhage Control and Wound-Healing Application. ACS Biomater. Sci. Eng. 2020, 6, 5911–5929. [Google Scholar] [CrossRef]
- Hafezi, F.; Scoutaris, N.; Douroumis, D.; Boateng, J. 3D Printed Chitosan Dressing Crosslinked with Genipin for Potential Healing of Chronic Wounds. Int. J. Pharm. 2019, 560, 406–415. [Google Scholar] [CrossRef]
- Li, X.; Ma, M.; Ahn, D.U.; Huang, X. Preparation and Characterization of Novel Eggshell Membrane-Chitosan Blend Films for Potential Wound-Care Dressing: From Waste to Medicinal Products. Int. J. Biol. Macromol. 2019, 123, 477–484. [Google Scholar] [CrossRef]
- Kalinova, R.; Dimitrov, I. Functional Polyion Complex Micelles for Potential Targeted Hydrophobic Drug Delivery. Molecules 2022, 27, 2178. [Google Scholar] [CrossRef]
- Liu, M.; Jin, J.; Zhong, X.; Liu, L.; Tang, C.; Cai, L. Polysaccharide Hydrogels for Skin Wound Healing. Heliyon 2024, 10, e35014. [Google Scholar] [CrossRef]
- Jayanudin; Lestari, R.S.D.; Barleany, D.R.; Pitaloka, A.B.; Yulvianti, M.; Prasetyo, D.; Anggoro, D.V.; Ruhiatna, A. Chitosan-Graft-Poly(Acrylic Acid) Superabsorbent’s Water Holding in Sandy Soils and Its Application in Agriculture. Polymers 2022, 14, 5175. [Google Scholar] [CrossRef]
- Neblea, I.E.; Chiriac, A.-L.; Zaharia, A.; Sarbu, A.; Teodorescu, M.; Miron, A.; Paruch, L.; Paruch, A.M.; Olaru, A.G.; Iordache, T.-V. Introducing Semi-Interpenetrating Networks of Chitosan and Ammonium-Quaternary Polymers for the Effective Removal of Waterborne Pathogens from Wastewaters. Polymers 2023, 15, 1091. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, L.; Li, Y.; Zhang, X.; Xu, S.; Yang, G.; Delair, T. Chitosan-Based Colloidal Polyelectrolyte Complexes for Drug Delivery: A Review. Carbohydr. Polym. 2020, 238, 116126. [Google Scholar] [CrossRef]
- Wu, K.; Yan, Z.; Wu, Z.; Li, J.; Zhong, W.; Ding, L.; Zhong, T.; Jiang, T. Recent Advances in the Preparation, Antibacterial Mechanisms, and Applications of Chitosan. J. Funct. Biomater. 2024, 15, 318. [Google Scholar] [CrossRef]
- Rajinikanth B, S.; Rajkumar, D.S.R.; K, K.; Vijayaragavan, V. Chitosan-Based Biomaterial in Wound Healing: A Review. Cureus 2024, 16, e55193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liang, Y.; Huang, S.; Guo, B. Chitosan-Based Self-Healing Hydrogel Dressing for Wound Healing. Adv. Colloid Interface Sci. 2024, 332, 103267. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, D.G.; Yaneva, Z.L. Antioxidant Properties and Redox-Modulating Activity of Chitosan and Its Derivatives: Biomaterials with Application in Cancer Therapy. BioResearch Open Access 2020, 9, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, A.; Gómez-Gil, V.; Ortega, M.A.; Asúnsolo, Á.; Coca, S.; Román, J.S.; Álvarez-Mon, M.; Buján, J.; García-Honduvilla, N. Chitosan Hydrogels Functionalized with Either Unfractionated Heparin or Bemiparin Improve Diabetic Wound Healing. Biomed. Pharmacother. 2020, 129, 110498. [Google Scholar] [CrossRef]
- Garshasbi, H.; Salehi, S.; Naghib, S.M.; Ghorbanzadeh, S.; Zhang, W. Stimuli-Responsive Injectable Chitosan-Based Hydrogels for Controlled Drug Delivery Systems. Front. Bioeng. Biotechnol. 2022, 10, 1126774. [Google Scholar] [CrossRef]
- Bagher, Z.; Ehterami, A.; Safdel, M.H.; Khastar, H.; Semiari, H.; Asefnejad, A.; Davachi, S.M.; Mirzaii, M.; Salehi, M. Wound Healing with Alginate/Chitosan Hydrogel Containing Hesperidin in Rat Model. J. Drug Deliv. Sci. Technol. 2020, 55, 101379. [Google Scholar] [CrossRef]
- Farasati Far, B.; Omrani, M.; Naimi Jamal, M.R.; Javanshir, S. Multi-Responsive Chitosan-Based Hydrogels for Controlled Release of Vincristine. Commun. Chem. 2023, 6, 28. [Google Scholar] [CrossRef]
- Jagtap, P.; Patil, K.; Dhatrak, P. Polyelectrolyte Complex for Drug Delivery in Biomedical Applications: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1183, 012007. [Google Scholar] [CrossRef]
- Petrila, L.-M.; Bucatariu, F.; Mihai, M.; Teodosiu, C. Polyelectrolyte Multilayers: An Overview on Fabrication, Properties, and Biomedical and Environmental Applications. Materials 2021, 14, 4152. [Google Scholar] [CrossRef]
- Mirabile, B. Optimization and Characterization of a Polyelectrolyte Complex for In Vivo Abdominal Adhesion Prevention. Master’s Thesis, Rutgers University-School of Graduate Studies, Piscataway, NJ, USA, 2021. [Google Scholar]
- Xu, T.; Gao, C.; Feng, X.; Huang, M.; Yang, Y.; Shen, X.; Tang, X. Cinnamon and Clove Essential Oils to Improve Physical, Thermal and Antimicrobial Properties of Chitosan-Gum Arabic Polyelectrolyte Complexed Films. Carbohydr. Polym. 2019, 217, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Gao, C.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Structure, Physical and Antioxidant Properties of Chitosan-Gum Arabic Edible Films Incorporated with Cinnamon Essential Oil. Int. J. Biol. Macromol. 2019, 134, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Phonrachom, O.; Charoensuk, P.; Kiti, K.; Saichana, N.; Kakumyan, P.; Suwantong, O. Potential Use of Propolis-Loaded Quaternized Chitosan/Pectin Hydrogel Films as Wound Dressings: Preparation, Characterization, Antibacterial Evaluation, and in Vitro Healing Assay. Int. J. Biol. Macromol. 2023, 241, 124633. [Google Scholar] [CrossRef]
- Soubhagya, A.S.; Moorthi, A.; Prabaharan, M. Preparation and Characterization of Chitosan/Pectin/ZnO Porous Films for Wound Healing. Int. J. Biol. Macromol. 2020, 157, 135–145. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Y.; Zhou, C.; Zhang, C.; Liu, B. Engineering pH Responsive Carboxyethyl Chitosan and Oxidized Pectin -Based Hydrogels with Self-Healing, Biodegradable and Antibacterial Properties for Wound Healing. Int. J. Biol. Macromol. 2023, 253, 127364. [Google Scholar] [CrossRef]
- Guo, H.; Ran, W.; Jin, X.; Huang, Y.; Long, F.; Xiao, Y.; Gan, R.-Y.; Wu, Y.; Gao, H. Development of Pectin/Chitosan-Based Electrospun Biomimetic Nanofiber Membranes Loaded with Dihydromyricetin Inclusion Complexes for Wound Healing Application. Int. J. Biol. Macromol. 2024, 278, 134526. [Google Scholar] [CrossRef]
- Szulc, M.; Lewandowska, K. Biomaterials Based on Chitosan and Its Derivatives and Their Potential in Tissue Engineering and Other Biomedical Applications—A Review. Molecules 2022, 28, 247. [Google Scholar] [CrossRef]
- Fatehi Hassanabad, A.; Zarzycki, A.N.; Jeon, K.; Dundas, J.A.; Vasanthan, V.; Deniset, J.F.; Fedak, P.W.M. Prevention of Post-Operative Adhesions: A Comprehensive Review of Present and Emerging Strategies. Biomolecules 2021, 11, 1027. [Google Scholar] [CrossRef]
- Potaś, J.; Wilczewska, A.Z.; Misiak, P.; Basa, A.; Winnicka, K. Optimization of Multilayer Films Composed of Chitosan and Low-Methoxy Amidated Pectin as Multifunctional Biomaterials for Drug Delivery. Int. J. Mol. Sci. 2022, 23, 8092. [Google Scholar] [CrossRef]
- Wasupalli, G.K.; Verma, D. Thermosensitive Injectable Hydrogel Based on Chitosan-Polygalacturonic Acid Polyelectrolyte Complexes for Bone Tissue Engineering. Carbohydr. Polym. 2022, 294, 119769. [Google Scholar] [CrossRef]
- Wasupalli, G.K.; Verma, D. Development of Chitosan-Polygalacturonic Acid Polyelectrolyte Complex Fibrous Scaffolds Using the Hydrothermal Treatment for Bone Tissue Engineering. J. Biomed. Mater. Res. A 2023, 111, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Wasupalli, G.K.; Verma, D. Injectable and Thermosensitive Nanofibrous Hydrogel for Bone Tissue Engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110343. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, J.; Raad, I. Antimicrobial Compositions and Uses Thereof. U.S. Patent EP3419676A4, 28 November 2023. Available online: https://patentimages.storage.googleapis.com/a2/ff/ab/722606e03a0d28/US11826332.pdf (accessed on 10 November 2024).
- Lin, Y.; Wang, X.; Liu, Q.; Fang, Y. Preparation and Application of Chitosan-Based Polyelectrolyte Complex Materials: An Overview. Pap. Biomater. 2022, 7, 1–19. [Google Scholar]
- Ghahremani-Nasab, M.; Akbari-Gharalari, N.; Rahmani Del Bakhshayesh, A.; Ghotaslou, A.; Ebrahimi-Kalan, A.; Mahdipour, M.; Mehdipour, A. Synergistic Effect of Chitosan-Alginate Composite Hydrogel Enriched with Ascorbic Acid and Alpha-Tocopherol under Hypoxic Conditions on the Behavior of Mesenchymal Stem Cells for Wound Healing. Stem Cell Res. Ther. 2023, 14, 326. [Google Scholar] [CrossRef]
- Kraskouski, A.; Mashkin, M.; Kulikouskaya, V.; Savich, V.; Sidarenka, A.; Pinchuk, S.; Li, R. Design of Highly Porous Materials Based on Chitosan/Pectin Interpolyelectrolyte Complex for Wound Healing Application. Adv. Polym. Technol. 2024, 2024, 8747902. [Google Scholar] [CrossRef]
- Jafari, H.; Ghaffari-Bohlouli, P.; Podstawczyk, D.; Nie, L.; Shavandi, A. Tannic Acid Post-Treatment of Enzymatically Crosslinked Chitosan-Alginate Hydrogels for Biomedical Applications. Carbohydr. Polym. 2022, 295, 119844. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V.; Ghiorghita, C.A. Chitosan-Based Polyelectrolyte Complex Cryogels with Elasticity, Toughness and Delivery of Curcumin Engineered by Polyions Pair and Cryostructuration Steps. Gels 2022, 8, 240. [Google Scholar] [CrossRef]
- Dingalwar, A. Polyelectrolyte Complex-Based Multiparticulate Drug Delivery System: A Special Emphasis on Chitosan and Alginate. Asian J. Pharm. 2024, 18, 276. [Google Scholar] [CrossRef]
- Khatibi, N.; Naimi-Jamal, M.R.; Balalaie, S.; Shokoohmand, A. Development and Evaluation of a pH-Sensitive, Naturally Crosslinked Alginate-Chitosan Hydrogel for Drug Delivery Applications. Front. Biomater. Sci. 2024, 3, 1457540. [Google Scholar] [CrossRef]
- Hoang, H.T.; Vu, T.T.; Karthika, V.; Jo, S.-H.; Jo, Y.-J.; Seo, J.-W.; Oh, C.-W.; Park, S.-H.; Lim, K.T. Dual Cross-Linked Chitosan/Alginate Hydrogels Prepared by Nb-Tz ‘Click’ Reaction for pH Responsive Drug Delivery. Carbohydr. Polym. 2022, 288, 119389. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Chen, K.; Jin, M.; Vu, S.H.; Jung, S.; He, N.; Zheng, Z.; Lee, M.-S. Application of Chitosan/Alginate Nanoparticle in Oral Drug Delivery Systems: Prospects and Challenges. Drug Deliv. 2022, 29, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Scolari, I.R.; Páez, P.L.; Sánchez-Borzone, M.E.; Granero, G.E. Promising Chitosan-Coated Alginate-Tween 80 Nanoparticles as Rifampicin Coadministered Ascorbic Acid Delivery Carrier Against Mycobacterium Tuberculosis. AAPS PharmSciTech 2019, 20, 67. [Google Scholar] [CrossRef]
- Ahmady, A.R.; Razmjooee, K.; Saber-Samandari, S.; Toghraie, D. Fabrication of Chitosan-Gelatin Films Incorporated with Thymol-Loaded Alginate Microparticles for Controlled Drug Delivery, Antibacterial Activity and Wound Healing: In-Vitro and in-Vivo Studies. Int. J. Biol. Macromol. 2022, 223, 567–582. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Twarowski, B.; Fecka, I.; Tuberoso, C.I.G.; Jerković, I. Thymol as a Component of Chitosan Systems-Several New Applications in Medicine: A Comprehensive Review. Plants 2024, 13, 362. [Google Scholar] [CrossRef]
- Chitosan Market Size to Worth USD 91.99 Billion by 2033. Available online: https://finance.yahoo.com/news/chitosan-market-size-worth-usd-151000576.html?guccounter=1&guce_referrer=aHR0cHM6Ly93d3cuZ29vZ2xlLmNvbS8&guce_referrer_sig=AQAAALboqm0hARusiwymxOqTX7OJJX6-b7vBksTnwuPZ3m6m06zdkCDepkUcFkYkZ3oxttq_ETrMmQwH3r_P_w9eIN45ThR5K8zdpRRyPdS5hDALwK5kxtN6F_fr1mt21GiLCK2Ruy1I3sfDVbQGTOAGDmmoTs5z_CYT0Qfz0i7Yoc1_ (accessed on 12 July 2024).
- HemCon® Bandage PRO. Available online: https://tricolbiomedical.com/product/hemcon-bandage-pro/ (accessed on 12 July 2024).
- Alnufaiy, B.M.; Lambarte, R.N.A.; Al-Hamdan, K.S. The Osteogenetic Potential of Chitosan Coated Implant: An In Vitro Study. J. Stem Cells Regen. Med. 2020, 16, 44–49. [Google Scholar] [CrossRef]
- Weißpflog, J.; Vehlow, D.; Müller, M.; Kohn, B.; Scheler, U.; Boye, S.; Schwarz, S. Characterization of Chitosan with Different Degree of Deacetylation and Equal Viscosity in Dissolved and Solid State–Insights by Various Complimentary Methods. Int. J. Biol. Macromol. 2021, 171, 242–261. [Google Scholar] [CrossRef]
- Sundaram, M.N.; Pradeep, A.; Varma, P.K.; Jayakumar, R. Different Forms of Chitosan and Its Derivatives as Hemostatic Agent and Tissue Sealants. In Chitosan for Biomaterials IV: Biomedical Applications; Jayakumar, R., Prabaharan, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–28. ISBN 978-3-030-83021-2. [Google Scholar]
- Celox FAQ Na-Celox Medical 2018.
- Goh, S.S.C.; Nand, P.; Alison, P. Successful Use of Celox (Chitosan) as a Haemostatic Agent in Cardiothoracic Surgery. Heart Lung Circ. 2019, 28, S113. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Chitosan-Based Scaffolds Incorporated with Silver Nanoparticles for the Treatment of Infected Wounds. Pharmaceutics 2024, 16, 327. [Google Scholar] [CrossRef]
- 47-Skin-USA. Available online: https://us.47skin.com/pages/what-is-silver-chitoderm (accessed on 12 July 2024).
- Zhang, H.; Wu, X.; Quan, L.; Ao, Q. Characteristics of Marine Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2022, 20, 372. [Google Scholar] [CrossRef]

| Product Types | Types of Chitosan Used | Manufacturing Variability in the Casting/Drying Process | Thickness | Advantages | Area of Improvement for Clinical Use | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Degrees of Deacetylation | Molecular Weights | Source(s) | ||||||
| 75–96% | 15–700 kDa (low and medium MW) |
| Layer by layer: room temperature to 150°C drying. Self-assembly: hot plate/molds from 60 to 100 °C. Casting: drying temperature from 37 to 50 °C. Fabrication: freeze-dried to 60 °C using different techniques. Lyophilization: hot air and supercritical CO2 drying at constant 33 °C. Freeze drying: different techniques such as hot air, fused zirconia/alumina/silica/crucible, supercritical CO2 drying, NH3 evaporation methods, supercritical CO2 impregnation/deposition (SSI/D) method, and multilayering in a polyion solution. | 300 nm to 220 µM |
|
| [11,12,18,21,22,23,24,25,26,27,28,29,30,31,32,33,34] |
| Product Name | Company/ Founded | Product Type | Chitosan(%) | Deacetylation(D)/Acetylation (A) (MW) | Application | Country of Origin | FDA Approval (Year) | References |
|---|---|---|---|---|---|---|---|---|
| HemCon Bandages | Tricol Biomedical 2001 | Dressing | 90–95% | D—70–95% A-85–95% (100–500 kDa) | Prevent Bleeding Antibacterial | Oregon, USA | Yes (2003) | [80,81] |
| ChitoFlex Pro Hemostatic Dressing | Tricol Biomedical 2001 | Dressing | ~95% | D—70–95% A—85–95% (100–500 kDa) | Control Bleeding Antibacterial | Oregon, USA | Yes (2003) | [80,83] |
| Celox Gauze | Celox 2010 | Gauze Dressing | 10–30% by weight | D: 70–95% A: 5–30% (10–1000 kDa) | Emergency Bleeding Control | Crewe, UK | Yes (unclassified medical device) | [84,85] |
| Silver Chitoderm | 47Skin 2018 | Cosmetic Product | 70–90% | D—70–95% A—5–30% (50–1000 kDa) | Scar Reduction | USA | Not subjected to premarket approval as it is cosmetic product | [86,87] |
| Serial # | Patent Number | Applications and Significant Features | Brief Product Description |
|---|---|---|---|
| 1. | US-11160901-B2 | Bioadhesive chitosan gel to control bleeding and promote healing with scar reduction without obscuring or interfering with access to a surgical field. | This is an aqueous chitosan gel system of novel non-scarring, non-interfering, transparent, stable, and solubilized chitosan, which is aimed at controlling bleeding. The gel system comprises water, chitosan, acid, a plasticizer, a rheology modifying agent, antioxidant stabilizing, alcohol, and multivalent salt. Furthermore, specific components within the chitosan gel system can comprise multiple types of organic acids, such as bifunctional/unfunctional/multifunctional acids, phosphoric acids, and salt. |
| 2. | US-11718828-B2 | Cartilage gel for cartilage repair, comprising chitosan and chondrocytes. | This invention concerns methods for obtaining an implantable cartilage gel for the tissue repair of hyaline cartilage, comprising chitosan hydrogel and cells that are capable of forming hyaline cartilage; said method comprises a step for the amplification of primary cells in a 3-D structure with a physical hydrogel of chitosan. Furthermore, an additional step for re-differentiation and induction for synthesizing the ECM through cells such as the articular chondrocytes and mesenchymal stem cells will be integrated for better repair properties. |
| 3. | US-20230270914-A1 | Designed for easy and effective application to control severe bleeding in various wound conditions. Composed of chitosan-based Celox particles and woven gauze substrate. | The invention relates to a hemostatic dressing that combines Celox, a chitosan-based material known for its blood-clotting properties, with a gauze substrate. This combination enhances the effectiveness of the dressing in controlling severe bleeding. The gauze provides a supportive matrix for the Celox particles, ensuring they remain in place and making the application easier and more efficient in emergencies. |
| 4. | US4572906-A | Chitosan-based wound dressing materials. | This patent mentions an invention of a surgical dressing to help protect wounds during healing. The dressing proposes a proprietary blend of gelatin and chitosan in a weight ratio of about 3:1 to 1:3. Furthermore, it also suggests incorporating a plasticizer ranging from 0 to 40% w/v as per the combined weight of gelatin and chitosan. |
| 5. | US9192574-B2 | Chitosan paste wound dressing. | This patent proposes a method of treating a wound with a ready-to-use composition. The composition contains a high concentration of water-soluble chitosan in a phosphate-containing solution. It is a paste at typical room temperature, has a pH of at least 4, adheres to the body tissue/surgical site, and has a total residence time of 1 day. |
| 6. | CN101816802-B | A hemostatic dressing mostly composed of chitosan and alginic acid. | This patent describes a medical dressing made from chitosan, a biocompatible and biodegradable polysaccharide derived from chitin, which is found in the exoskeleton of crustaceans. The dressing is designed for wound care, particularly to control bleeding and promote healing. |
| 7. | CN107530470-B | Chitosan wound dressing comprises chitosan chitin, at least one triprotic acid, and at least one solubilizing acid. | This invention focuses on the development of a wound dressing that leverages the beneficial properties of chitosan, a biopolymer known for its biocompatibility, biodegradability, and antimicrobial properties. The dressing is designed to enhance wound healing by creating a moist environment, which is conducive to tissue repair and regeneration. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, J.; Patel, D.; Rananavare, D.; Hudson, D.; Tran, M.; Schloss, R.; Langrana, N.; Berthiaume, F.; Kumar, S. Recent Advancements in Chitosan-Based Biomaterials for Wound Healing. J. Funct. Biomater. 2025, 16, 45. https://doi.org/10.3390/jfb16020045
Shah J, Patel D, Rananavare D, Hudson D, Tran M, Schloss R, Langrana N, Berthiaume F, Kumar S. Recent Advancements in Chitosan-Based Biomaterials for Wound Healing. Journal of Functional Biomaterials. 2025; 16(2):45. https://doi.org/10.3390/jfb16020045
Chicago/Turabian StyleShah, Jahnavi, Dhruv Patel, Dnyaneshwari Rananavare, Dev Hudson, Maxwell Tran, Rene Schloss, Noshir Langrana, Francois Berthiaume, and Suneel Kumar. 2025. "Recent Advancements in Chitosan-Based Biomaterials for Wound Healing" Journal of Functional Biomaterials 16, no. 2: 45. https://doi.org/10.3390/jfb16020045
APA StyleShah, J., Patel, D., Rananavare, D., Hudson, D., Tran, M., Schloss, R., Langrana, N., Berthiaume, F., & Kumar, S. (2025). Recent Advancements in Chitosan-Based Biomaterials for Wound Healing. Journal of Functional Biomaterials, 16(2), 45. https://doi.org/10.3390/jfb16020045







