Recombinant Humanized Collagen Enhances Secreted Protein Levels of Fibroblasts and Facilitates Rats’ Skin Basement Membrane Reinforcement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture
2.3. Recombinant Humanized Collagen Complexes Synthesis
2.4. UVC Injury
2.5. CCK-8 Assay
2.6. Rats’ Skin Sample Preparation
2.7. Hematoxylin-Eosin Staining (H&E) Observation of BM
2.8. ELISA
2.9. Immunofluorescence (IF)
2.10. Statistical Analysis
3. Results
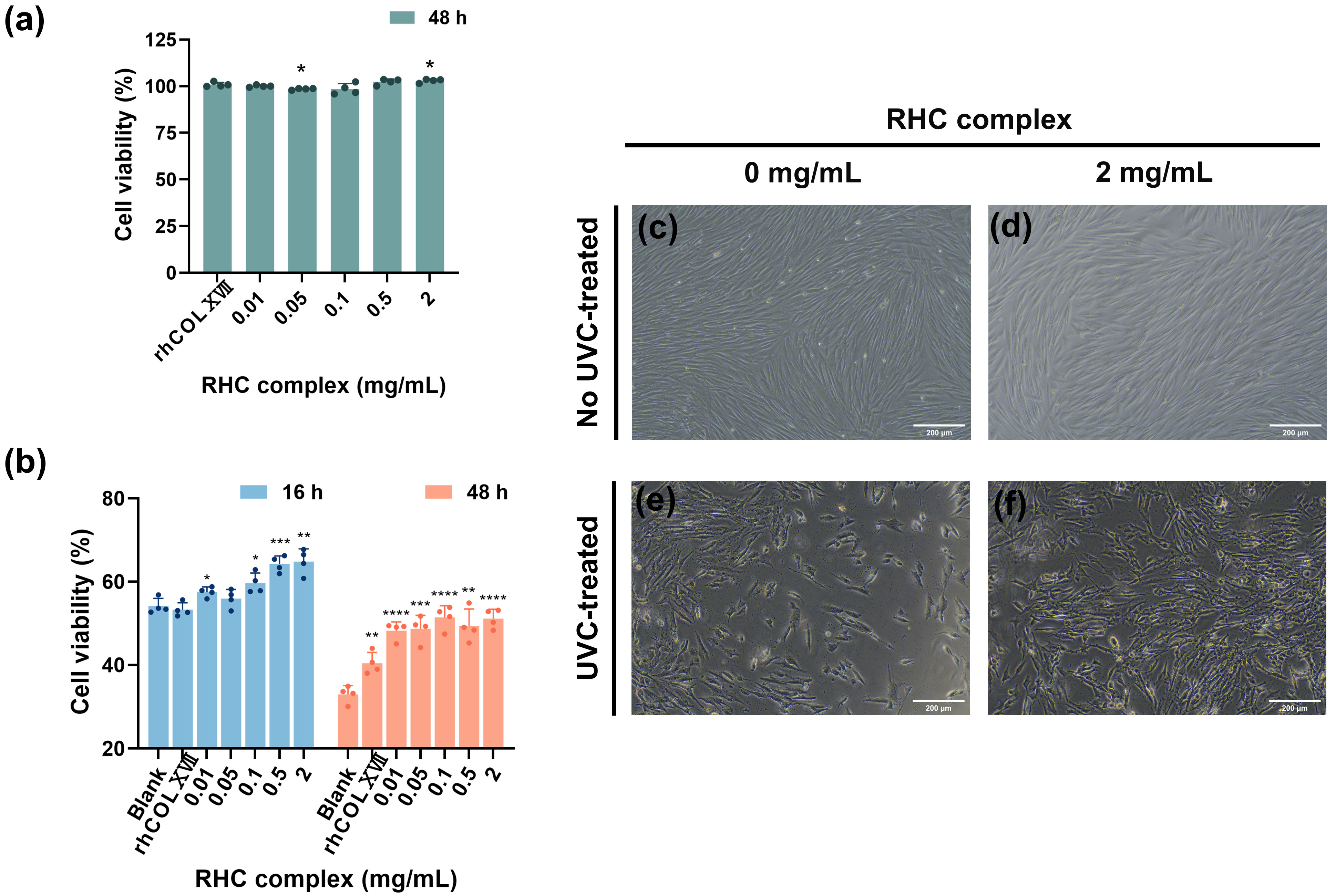
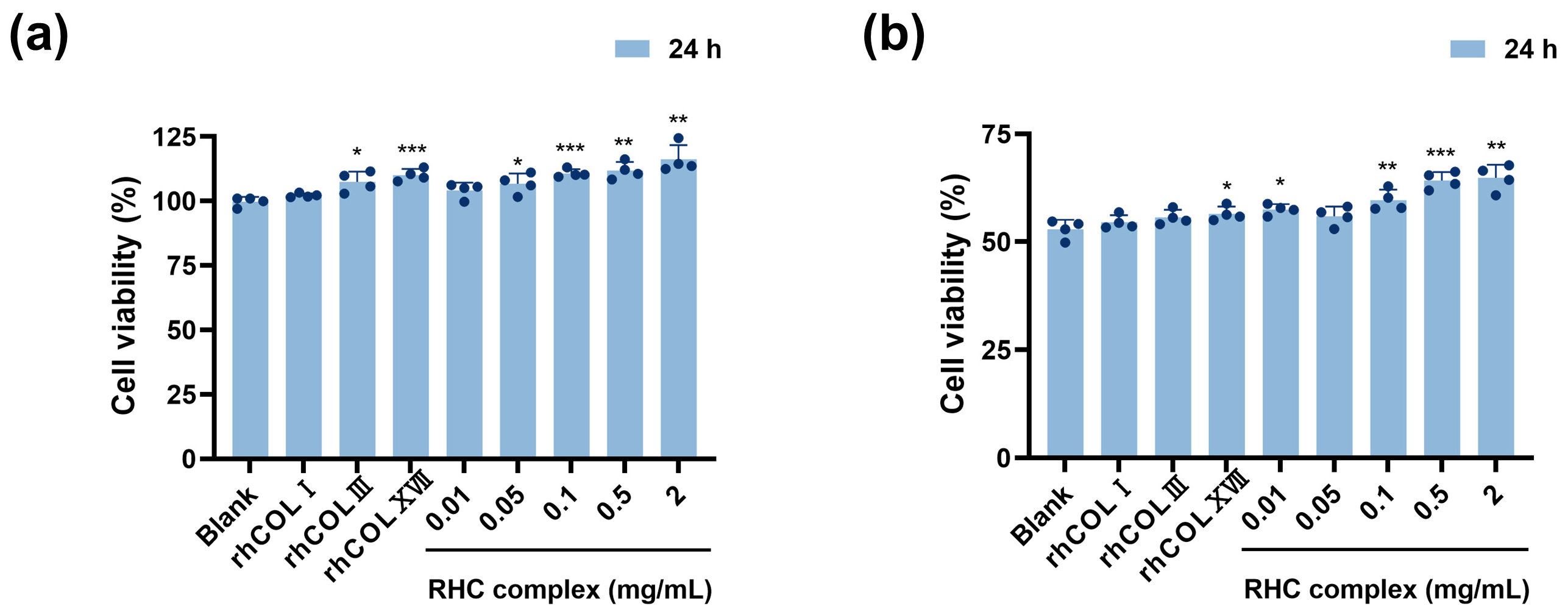
3.1. The RHC Complex Alleviated the UVC Injury in HFF-1 Cell Viability
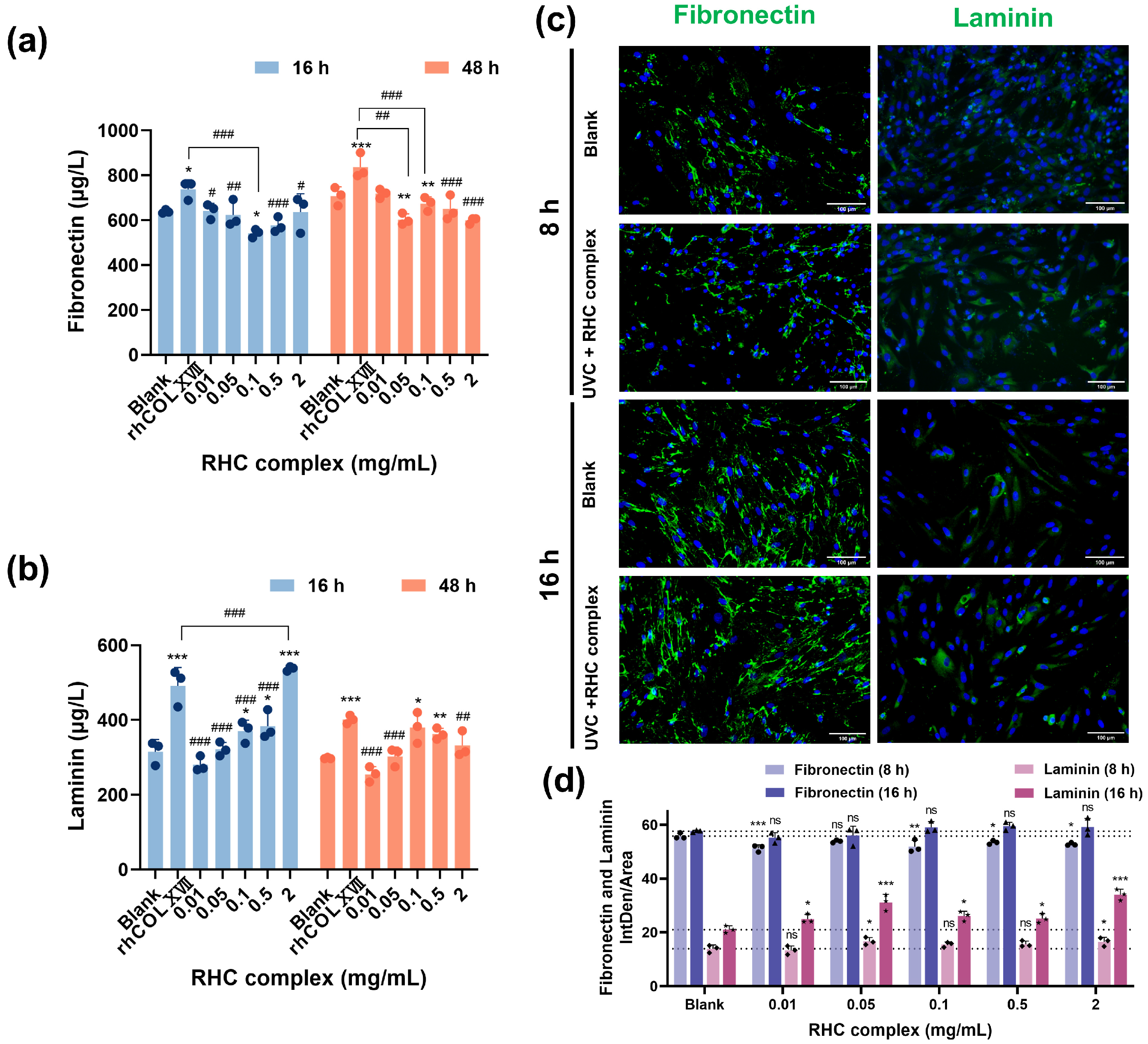
3.2. The RHC Complex Inhibited the Down-Expression of BM Protein in UVC-Damaged HFF-1 Cells
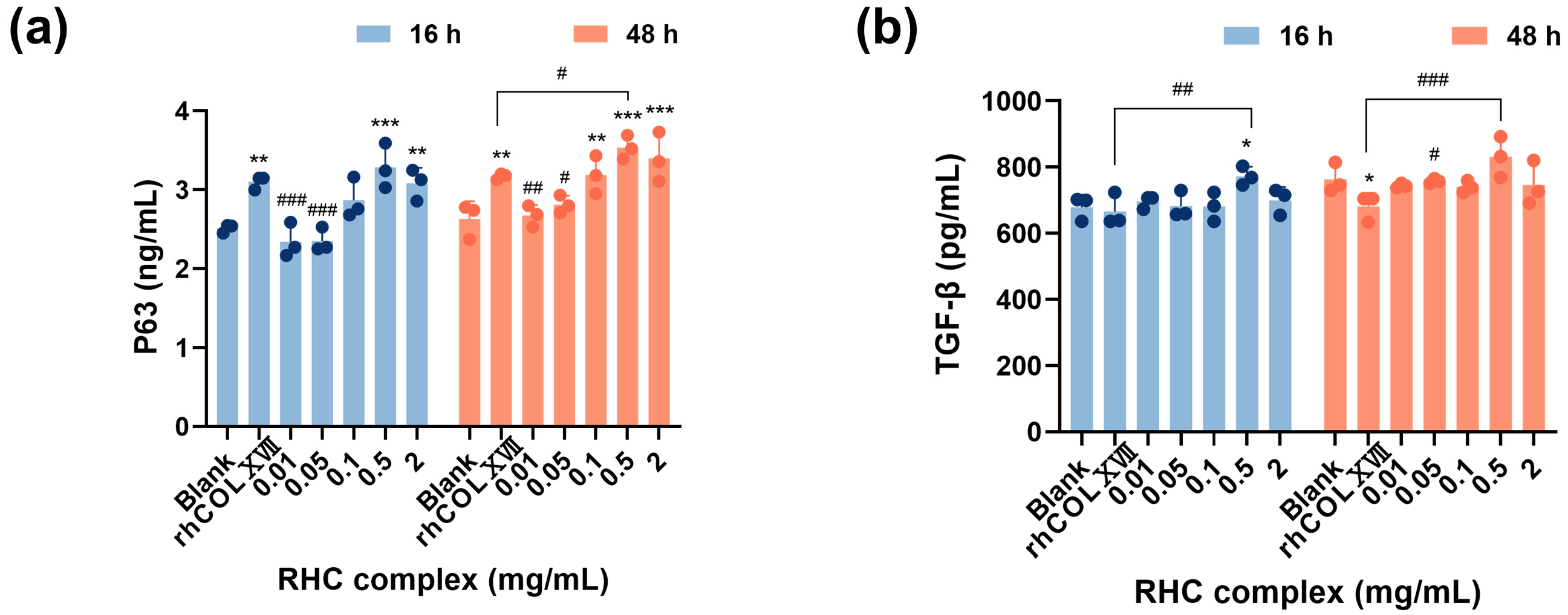
3.3. The RHC Complex Inhibited the Down-Expression of Cell Growth Regulators by UVC Injury in HFF-1 Cells
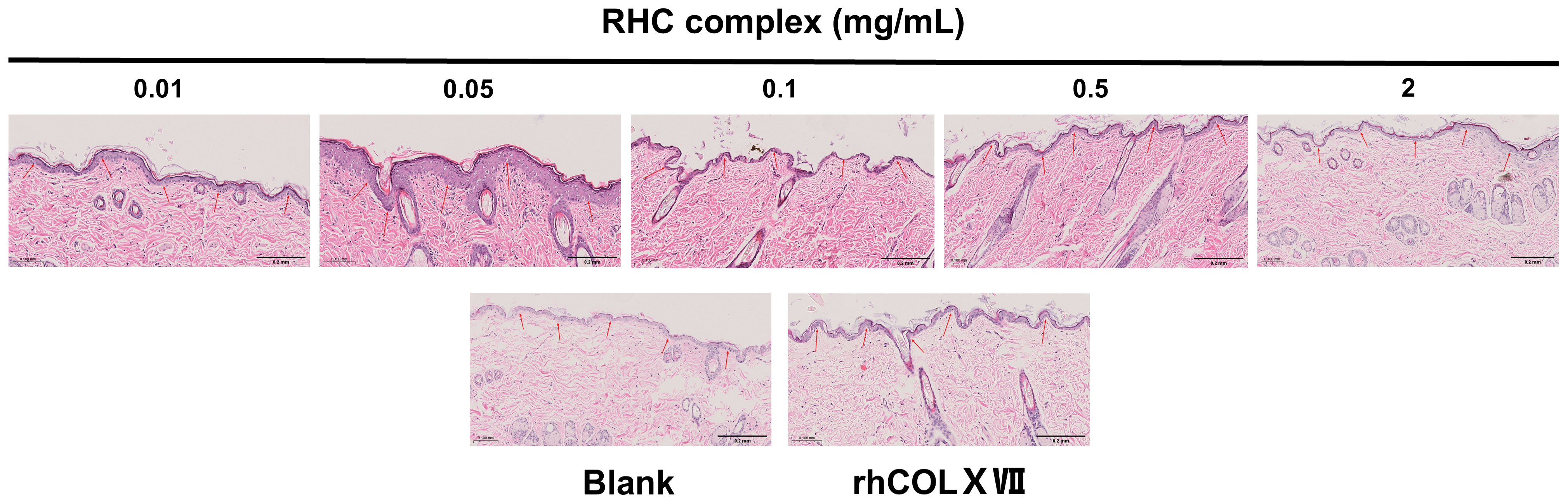
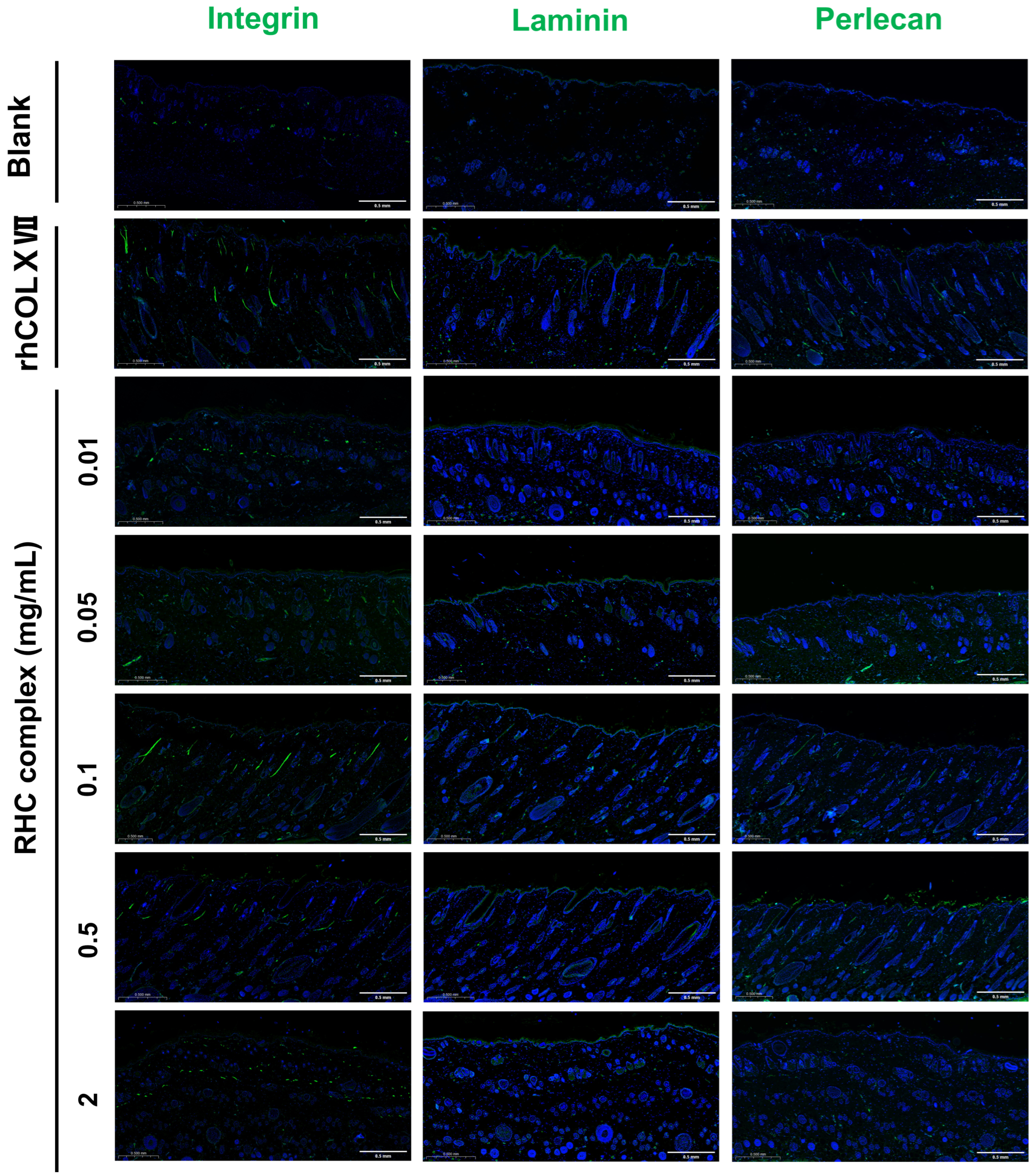
3.4. The RHC Complex Reinforcing Effect of the BM in a Dorsal Rat Skin Model
The RHC Complex Promoted the Expression of Laminin, Perlecan, and Integrin Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECM | extracellular matrix |
| RHC | recombinant humanized collagen |
| BM | basement membrane |
| rhCOLI | recombinant humanized type I collagen |
| rhCOLIII | recombinant humanized type III collagen |
| rhCOLXVII | recombinant humanized type XVII collagen |
| COLI | type I collagen |
| COLIII | type III collagen |
| COLIV | type IV collagen |
| FN | fibronectin |
| LN | laminin |
References
- Bai, S.; Xia, Z.; Ao, J.; Yang, R. Collagen and skin wound repair. J. Pract. Dermatol. 2022, 15, 296–298. (In Chinese) [Google Scholar]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef]
- Chen, J. Collagen Market Growth and Disorganization. Knowl. Econ. (Direct Mark. China) 2022, 624, 49–51. (In Chinese) [Google Scholar]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens--structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.M.; Nishiyama, A. The NG2 chondroitin sulfate proteoglycan: A multifunctional proteoglycan associated with immature cells. Perspect. Dev. Neurobiol. 1996, 3, 245–259. [Google Scholar]
- Cadar, E.; Pesterau, A.M.; Prasacu, I.; Ionescu, A.M.; Pascale, C.; Dragan, A.L.; Sirbu, R.; Tomescu, C.L. Marine Antioxidants from Marine Collagen and Collagen Peptides with Nutraceuticals Applications: A Review. Antioxidants 2024, 13, 919. [Google Scholar] [CrossRef]
- Rahman, A.; Rehmani, R.; Pirvu, D.G.; Huang, S.M.; Puri, S.; Arcos, M. Unlocking the Therapeutic Potential of Marine Collagen: A Scientific Exploration for Delaying Skin Aging. Mar. Drugs 2024, 22, 159. [Google Scholar] [CrossRef] [PubMed]
- Breitkreutz, D.; Koxholt, I.; Thiemann, K.; Nischt, R. Skin basement membrane: The foundation of epidermal integrity--BM functions and diverse roles of bridging molecules nidogen and perlecan. BioMed Res. Int. 2013, 2013, 179784. [Google Scholar] [CrossRef] [PubMed]
- Krieg, T.; Aumailley, M. The extracellular matrix of the dermis: Flexible structures with dynamic functions. Exp. Dermatol. 2011, 20, 689–695. [Google Scholar] [CrossRef]
- Roger, M.; Fullard, N.; Costello, L.; Bradbury, S.; Markiewicz, E.; O’Reilly, S.; Darling, N.; Ritchie, P.; Maatta, A.; Karakesisoglou, I.; et al. Bioengineering the microanatomy of human skin. J. Anat. 2019, 234, 438–455. [Google Scholar] [CrossRef]
- Roig-Rosello, E.; Rousselle, P. The Human Epidermal Basement Membrane: A Shaped and Cell Instructive Platform That Aging Slowly Alters. Biomolecules 2020, 10, 1607. [Google Scholar] [CrossRef]
- Raja, E.; Clarin, M.; Yanagisawa, H. Matricellular Proteins in the Homeostasis, Regeneration, and Aging of Skin. Int. J. Mol. Sci. 2023, 24, 14274. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.R.; Woodley, D.T.; Katz, S.I.; Martin, G.R. Structure and function of basement membrane. J. Investig. Dermatol. 1982, 79 (Suppl. S1), 69s–72s. [Google Scholar] [CrossRef] [PubMed]
- Fleischmajer, R.; Timpl, R. Ultrastructural localization of fibronectin to different anatomic structures of human skin. J. Histochem. Cytochem. 1984, 32, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Xie, Z.; Tu, C.L. Calcium regulation of keratinocyte differentiation. Expert Rev. Endocrinol. Metab. 2012, 7, 461–472. [Google Scholar] [CrossRef]
- Watt, F.M.; Fujiwara, H. Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb. Perspect. Biol. 2011, 3, a005124. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Segger, D.; Degwert, J.; Schunck, M.; Zague, V.; Oesser, S. Oral Supplementation of Specific Collagen Peptides Has Beneficial Effects on Human Skin Physiology: A Double-Blind, Placebo-Controlled Study. Ski. Pharmacol. Phys. 2014, 27, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Skrzynski, S.; Smiechowski, K.; Kolodziejczak, A. The review of versatile application of collagen. Polym. Adv. Technol. 2017, 28, 4–9. [Google Scholar] [CrossRef]
- Bai, M.X.; Kang, N.; Xu, Y.; Wang, J.; Shuai, X.X.; Liu, C.J.; Jiang, Y.X.; Du, Y.; Gong, P.; Lin, H.; et al. The influence of tag sequence on recombinant humanized collagen (rhCol) and the evaluation of rhCol on Schwann cell behaviors. Regen. Biomater. 2023, 10, rbad089. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.N. Bovine spongiform encephalopathy—Some surprises for biochemists. IUBMB Life 2005, 57, 273–276. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Zhu, Y.; Li, H.; He, F.; Liu, S.B.; Yang, X.; Wang, L.; Zeng, H.; Dai, J.C.; Hu, L.A. Recombinant humanized collagen remodels endometrial immune microenvironment of chronic endometritis through macrophage immunomodulation. Regen. Biomater. 2023, 10, rbad033. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.S.; Hao, J.Y.; Lei, H.; Chen, Y.R.; Liu, L.; Jia, L.P.; Gu, J.; Kang, H.P.; Shi, J.J.; He, J.; et al. Recombinant collagen for the repair of skin wounds and photo-aging damage. Regen. Biomater. 2024, 11, rbae108. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; Zhang, Y.; Zhu, Z.L.; Gao, R.C. Recombinant human collagen digestates exhibit higher protective effect on UVA-damaged skin fibroblasts than animal-derived collagens. J. Funct. Foods 2024, 113, 106035. [Google Scholar] [CrossRef]
- Fertala, A. Three Decades of Research on Recombinant Collagens: Reinventing the Wheel or Developing New Biomedical Products? Bioengineering 2020, 7, 155. [Google Scholar] [CrossRef] [PubMed]
- Izawa, M.; Otaka, Y.; Sakagami, H.; Tanuma, S.I.; Amano, S.; Uota, S.; Inomata, M.; Kato, Y.; Kadokura, H.; Yokose, S.; et al. Comprehensive Study of Anti-UVC Activity and Cytotoxicity of Hot-water Soluble Herb Extracts. In Vivo 2023, 37, 1540–1551. [Google Scholar] [CrossRef] [PubMed]
- Keyes, B.E.; Fuchs, E. Stem cells: Aging and transcriptional fingerprints. J. Cell Biol. 2018, 217, 79–92. [Google Scholar] [CrossRef]
- Zhou, B.Y.; Tu, T.; Gao, Z.; Wu, X.L.; Wang, W.B.; Liu, W. Impaired collagen fibril assembly in keloids with enhanced expression of lumican and collagen V. Arch. Biochem. Biophys. 2021, 697, 108676. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.L. Mechanism of Action of Collagen and Epidermal Growth Factor: A Review on Theory and Research Methods. Mini-Rev. Med. Chem. 2024, 24, 453–477. [Google Scholar] [CrossRef]
- Li, W.; Chi, N.W.; Rathnayake, R.A.C.; Wang, R. Distinctive roles of fibrillar collagen I and collagen III in mediating fibroblast-matrix interaction: A nanoscopic study. Biochem. Biophys. Res. Commun. 2021, 560, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Byun, K.A.; Oh, S.; Yang, J.Y.; Park, H.J.; Chung, M.S.; Son, K.H.; Byun, K. A Mixture of Topical Forms of Polydeoxyribonucleotide, Vitamin C, and Niacinamide Attenuated Skin Pigmentation and Increased Skin Elasticity by Modulating Nuclear Factor Erythroid 2-like 2. Molecules 2022, 27, 1276. [Google Scholar] [CrossRef]
- Oldak, L.; Chludzinska-Kasperuk, S.; Milewska, P.; Grubczak, K.; Reszec, J.; Gorodkiewicz, E. Laminin-5, Fibronectin, and Type IV Collagen as Potential Biomarkers of Brain Glioma Malignancy. Biomedicines 2022, 10, 2290. [Google Scholar] [CrossRef]
- Jeong, S.; Yoon, S.; Kim, S.; Jung, J.; Kor, M.; Shin, K.; Lim, C.; Han, H.S.; Lee, H.; Park, K.Y.; et al. Anti-Wrinkle Benefits of Peptides Complex Stimulating Skin Basement Membrane Proteins Expression. Int. J. Mol. Sci. 2020, 21, 73. [Google Scholar] [CrossRef]
- Aumailley, M. Laminins and interaction partners in the architecture of the basement membrane at the dermal-epidermal junction. Exp. Dermatol. 2021, 30, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Lavorgna, T.R.; Gressett, T.E.; Chastain, W.H.; Bix, G.J. Perlecan: A review of its role in neurologic and musculoskeletal disease. Front. Physiol. 2023, 14, 1189731. [Google Scholar] [CrossRef]
- Pozzi, A.; Yurchenco, P.D.; Iozzo, R.V. The nature and biology of basement membranes. Matrix Biol. 2017, 57–58, 1–11. [Google Scholar] [CrossRef]
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q.; Cui, Y. Targeting integrin pathways: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 1. [Google Scholar] [CrossRef]
- Zhong, Y. Research progress on the dermal-epidermal junction and its relationship with skin aging. Deterg. Cosmet. 2022, 45, 54–59. (In Chinese) [Google Scholar]
- Contet-Audonneau, J.L.; Jeanmarie, C.; Pauly, G. A histological study of human wrinkle structures: Comparison between sun-exposed areas of the face, with or without wrinkles, and sun-protected areas. Br. J. Dermatol. 1999, 140, 1038–1047. [Google Scholar] [CrossRef]
- Liu, N.; Matsumura, H.; Kato, T.; Ichinose, S.; Takada, A.; Namiki, T.; Asakawa, K.; Morinaga, H.; Mohri, Y.; De Arcangelis, A.; et al. Stem cell competition orchestrates skin homeostasis and ageing. Nature 2019, 568, 344–350. [Google Scholar] [CrossRef]
- Widel, M.; Krzywon, A.; Gajda, K.; Skonieczna, M.; Rzeszowska-Wolny, J. Induction of bystander effects by UVA, UVB, and UVC radiation in human fibroblasts and the implication of reactive oxygen species. Free Radic. Biol. Med. 2014, 68, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Ejiri, H.; Nomura, T.; Hasegawa, M.; Tatsumi, C.; Imai, M.; Sakakibara, S.; Terashi, H. Use of synthetic serum-free medium for culture of human dermal fibroblasts to establish an experimental system similar to living dermis. Cytotechnology 2015, 67, 507–514. [Google Scholar] [CrossRef]
- Sidney, L.E.; Branch, M.J.; Dua, H.S.; Hopkinson, A. Effect of culture medium on propagation and phenotype of corneal stroma-derived stem cells. Cytotherapy 2015, 17, 1706–1722. [Google Scholar] [CrossRef]
- Kim, H.; Kong, W.H.; Seong, K.Y.; Sung, D.K.; Jeong, H.; Kim, J.K.; Yang, S.Y.; Hahn, S.K. Hyaluronate-Epidermal Growth Factor Conjugate for Skin Wound Healing and Regeneration. Biomacromolecules 2016, 17, 3694–3705. [Google Scholar] [CrossRef]
- Takehara, K. Growth regulation of skin fibroblasts. J. Dermatol. Sci. 2000, 24, S70–S77. [Google Scholar] [CrossRef]
- Galluzzi, L.; Pedro, J.M.B.S.; Kepp, O.; Kroemer, G. Regulated cell death and adaptive stress responses. Cell. Mol. Life Sci. 2016, 73, 2405–2410. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, J.; Du, Y.; Shen, R.; Guo, F. The regulation of Estrogen and epidermal growth factor to fibroblast activity function and mechanism. Anat. Res. 2018, 40, 37–39+48. [Google Scholar]
- Quan, T.H.; Shao, Y.; He, T.Y.; Voorhees, J.J.; Fisher, G.J. Reduced Expression of Connective Tissue Growth Factor (CTGF/CCN2) Mediates Collagen Loss in Chronologically Aged Human Skin. J. Investig. Dermatol. 2010, 130, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Novelli, F.; Ganini, C.; Melino, G.; Nucci, C.; Han, Y.; Shi, Y.; Wang, Y.; Candi, E. p63 in corneal and epidermal differentiation. Biochem. Biophys. Res. Commun. 2022, 610, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Rosenbloom, J.; Jimenez, S.A. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem. J. 1987, 247, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Melino, G.; Memmi, E.M.; Pelicci, P.G.; Bernassola, F. Maintaining epithelial stemness with p63. Sci. Signal 2015, 8, re9. [Google Scholar] [CrossRef] [PubMed]
- Su, P.J.; Qiao, Q.; Ji, G.F.; Zhang, Z.B. CircAMD1 regulates proliferation and collagen synthesis via sponging miR-27a-3p in P63-mutant human dermal fibroblasts. Differentiation 2021, 119, 10–18. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, H.; Xu, Y.; Gao, Y.L.; Tan, P.J.; Zhao, R.; Liu, Z.H.; Tang, Y.J.; Zhu, X.D.; Bao, C.Y.; et al. The biological effect of recombinant humanized collagen on damaged skin induced by UV-photoaging: An study. Bioact. Mater. 2022, 11, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Miron-Mendoza, M.; Graham, E.; Manohar, S.; Petroll, W.M. Fibroblast-fibronectin patterning and network formation in 3D fibrin matrices. Matrix Biol. 2017, 64, 69–80. [Google Scholar] [CrossRef]
- Patten, J.; Wang, K. Fibronectin in development and wound healing. Adv. Drug Deliv. Rev. 2021, 170, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y. Laminin: Loss-of-function studies. Cell.. Mol. Life Sci. 2017, 74, 1095–1115. [Google Scholar] [CrossRef]
- Hwang, E.; Lee, D.G.; Park, S.H.; Oh, M.S.; Kim, S.Y. Coriander Leaf Extract Exerts Antioxidant Activity and Protects Against UVB-Induced Photoaging of Skin by Regulation of Procollagen Type I and MMP-1 Expression. J. Med. Food 2014, 17, 985–995. [Google Scholar] [CrossRef]
- Welgus, H.G.; Stricklin, G.P. Human skin fibroblast collagenase inhibitor. Comparative studies in human connective tissues, serum, and amniotic fluid. J. Biol. Chem. 1983, 258, 12259–12264. [Google Scholar] [CrossRef] [PubMed]


| Type of Group | RHC Dose | Number of Rats |
|---|---|---|
| Blank | 0 mg/mL | 6 |
| Control | 0.5 mg/mL rhCOLXVII | 6 |
| Experimental | 0.01 mg/mL RHC complex | 6 |
| 0.05 mg/mL RHC complex | 6 | |
| 0.1 mg/mL RHC complex | 6 | |
| 0.5 mg/mL RHC complex | 6 | |
| 2 mg/mL RHC complex | 6 |
| Type of Group | RHC Dose | UVC Radiation Dose |
|---|---|---|
| Blank | 0 mg/mL | 0.3 J/cm2 |
| Control | 0.5 mg/mL rhCOLXVII | 0.3 J/cm2 |
| Experimental | 0.01 mg/mL RHC complex | 0.3 J/cm2 |
| 0.05 mg/mL RHC complex | 0.3 J/cm2 | |
| 0.1 mg/mL RHC complex | 0.3 J/cm2 | |
| 0.5 mg/mL RHC complex | 0.3 J/cm2 | |
| 2 mg/mL RHC complex | 0.3 J/cm2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, S.; Chen, B.; Jeevithan, L.; Yang, H.; Kong, Y.; Diao, X.; Wu, W. Recombinant Humanized Collagen Enhances Secreted Protein Levels of Fibroblasts and Facilitates Rats’ Skin Basement Membrane Reinforcement. J. Funct. Biomater. 2025, 16, 47. https://doi.org/10.3390/jfb16020047
Ye S, Chen B, Jeevithan L, Yang H, Kong Y, Diao X, Wu W. Recombinant Humanized Collagen Enhances Secreted Protein Levels of Fibroblasts and Facilitates Rats’ Skin Basement Membrane Reinforcement. Journal of Functional Biomaterials. 2025; 16(2):47. https://doi.org/10.3390/jfb16020047
Chicago/Turabian StyleYe, Shijia, Boyu Chen, Lakshmi Jeevithan, Haoze Yang, Yaqi Kong, Xiaozhen Diao, and Wenhui Wu. 2025. "Recombinant Humanized Collagen Enhances Secreted Protein Levels of Fibroblasts and Facilitates Rats’ Skin Basement Membrane Reinforcement" Journal of Functional Biomaterials 16, no. 2: 47. https://doi.org/10.3390/jfb16020047
APA StyleYe, S., Chen, B., Jeevithan, L., Yang, H., Kong, Y., Diao, X., & Wu, W. (2025). Recombinant Humanized Collagen Enhances Secreted Protein Levels of Fibroblasts and Facilitates Rats’ Skin Basement Membrane Reinforcement. Journal of Functional Biomaterials, 16(2), 47. https://doi.org/10.3390/jfb16020047








