Piezoelectric Nanomaterials for Cancer Therapy: Current Research and Future Perspectives on Glioblastoma
Abstract
:1. Nanotechnology in Cancer Treatment
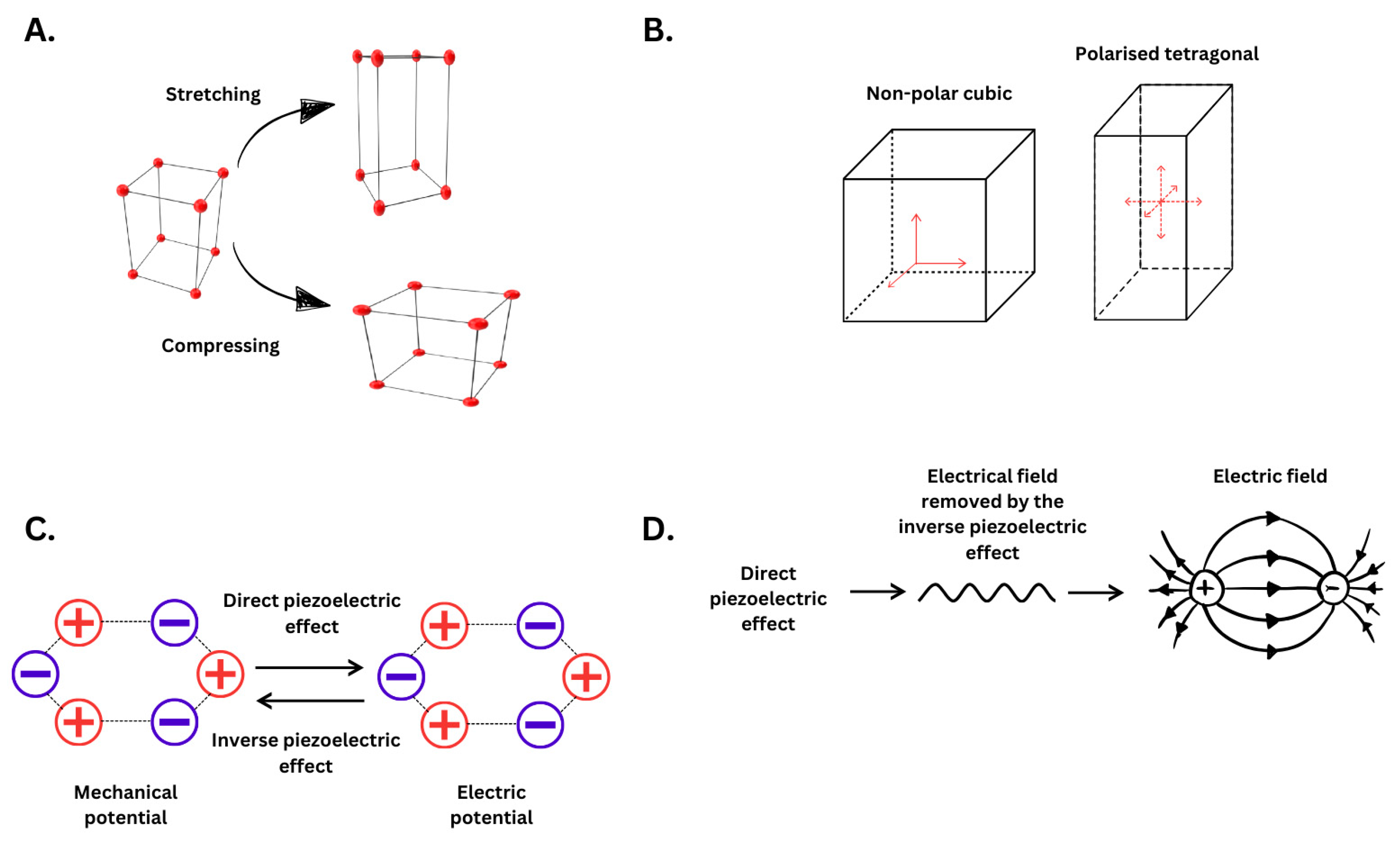
2. Piezoelectricity and Its Relevance in Physiology and Health
2.1. Piezoelectricity Principles and Mechanism
2.2. Piezoelectricity in Biological Systems and Its Function
3. Applications of Piezoelectric Nanomaterials in Cancer Therapy
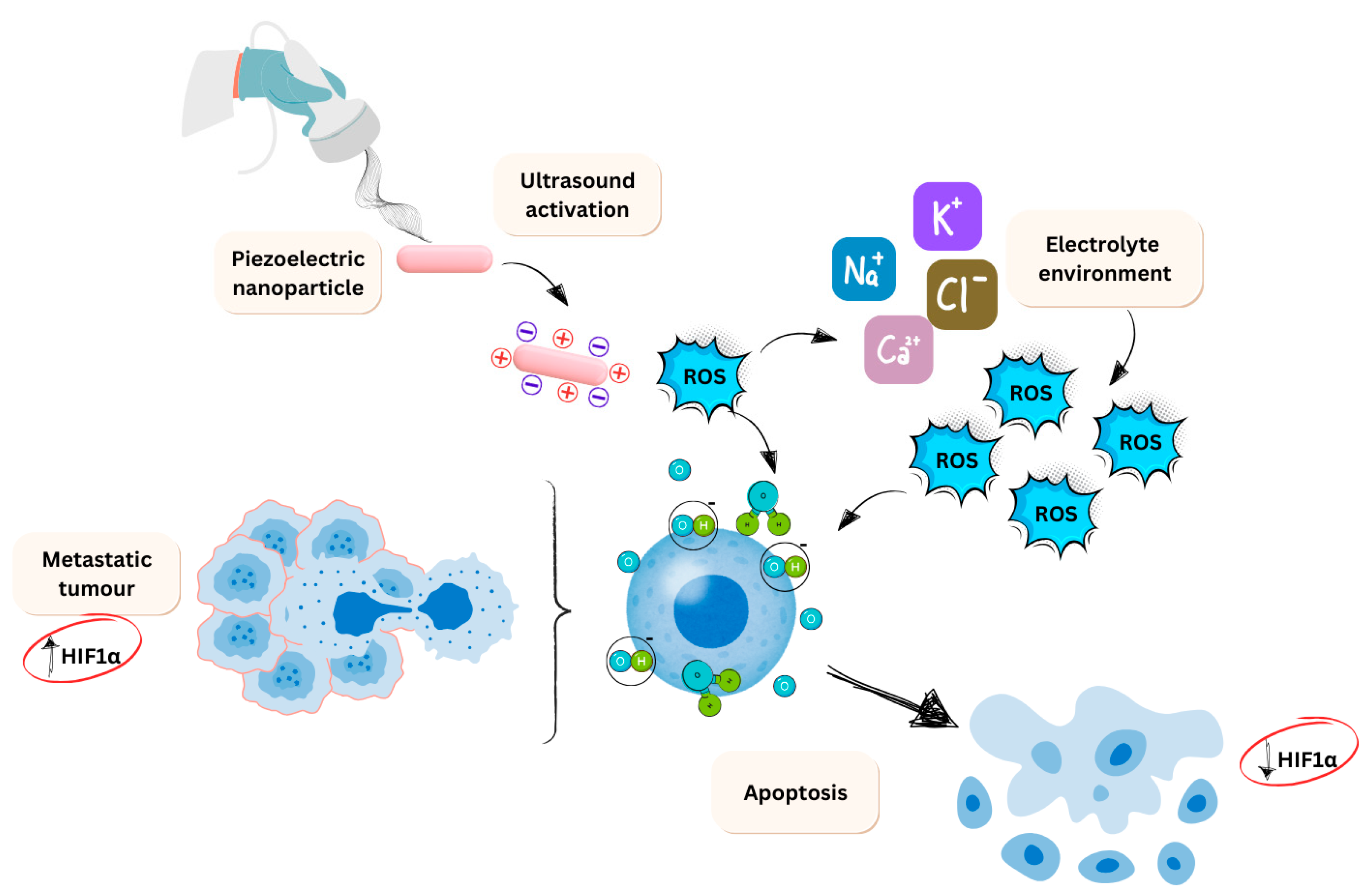
3.1. Piezo-Catalysis
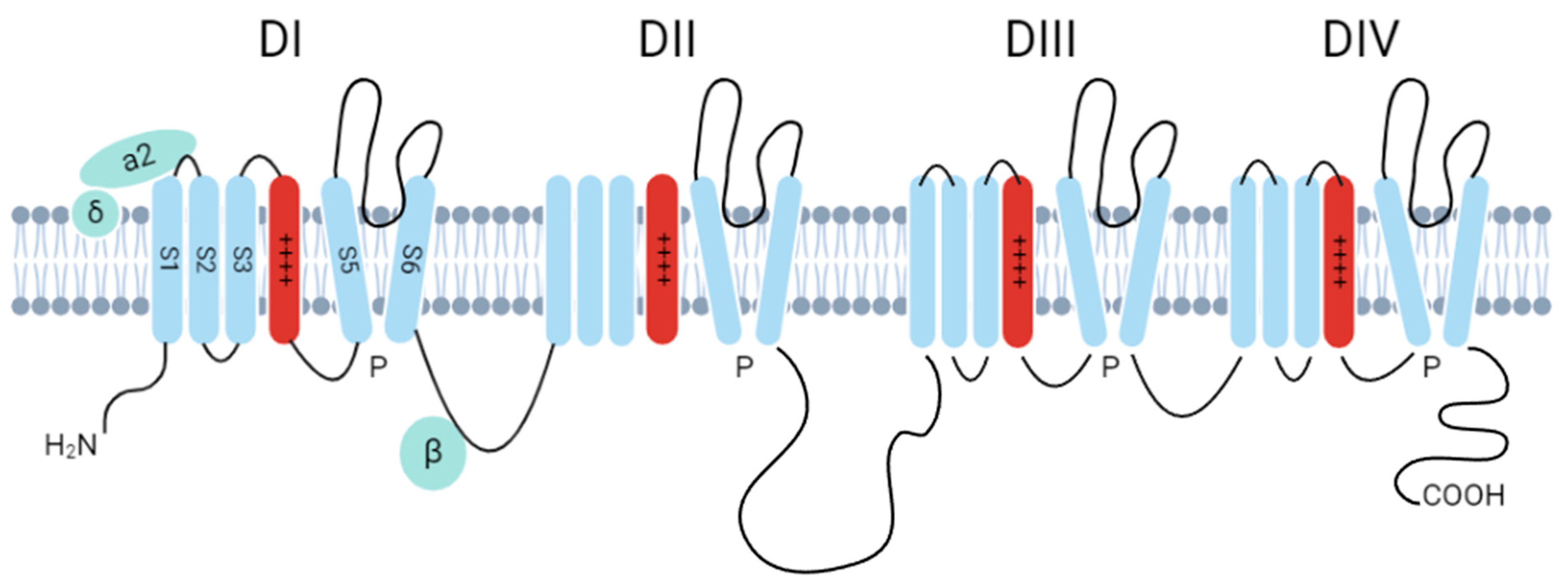
3.2. Ion Channel Activation
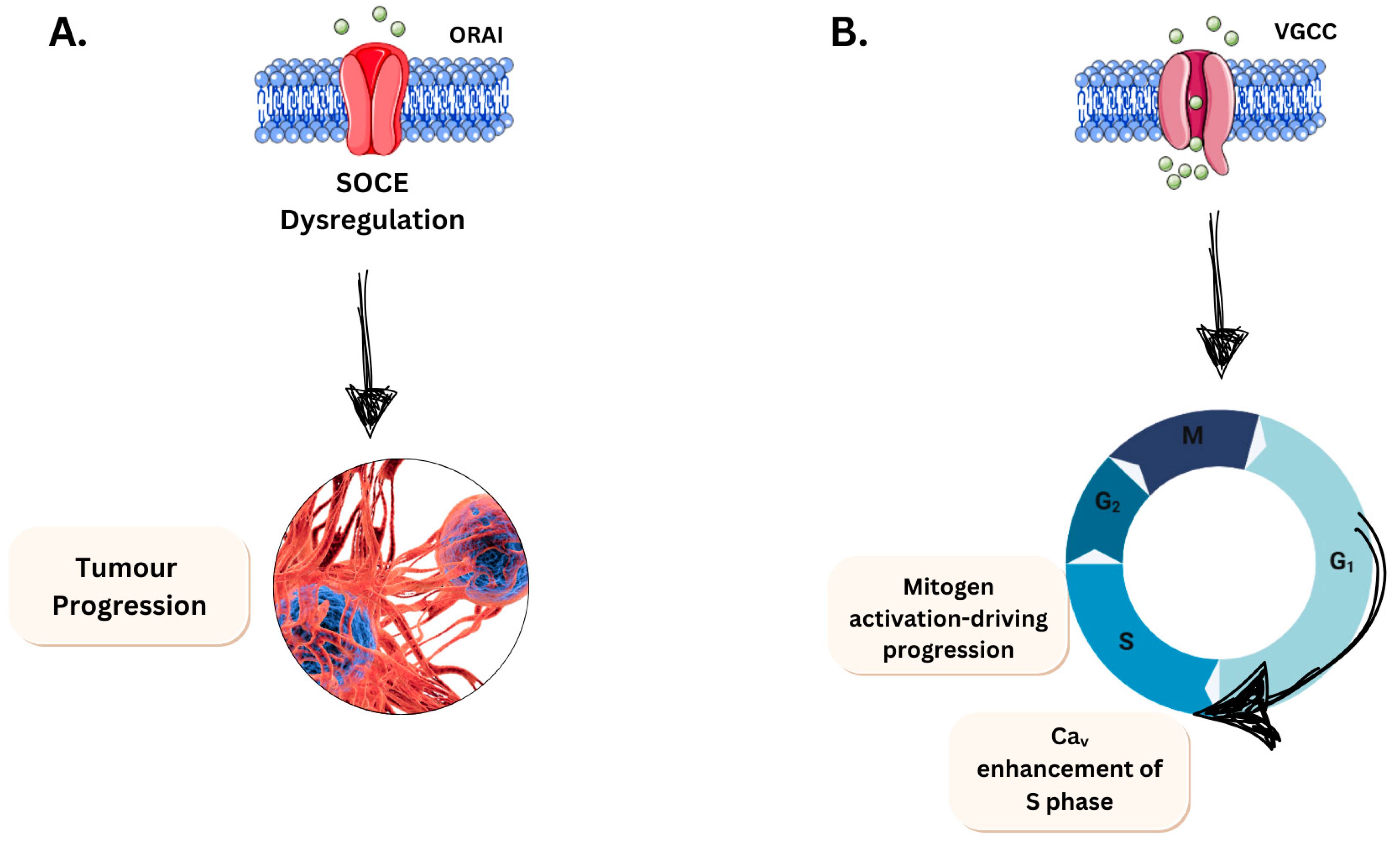
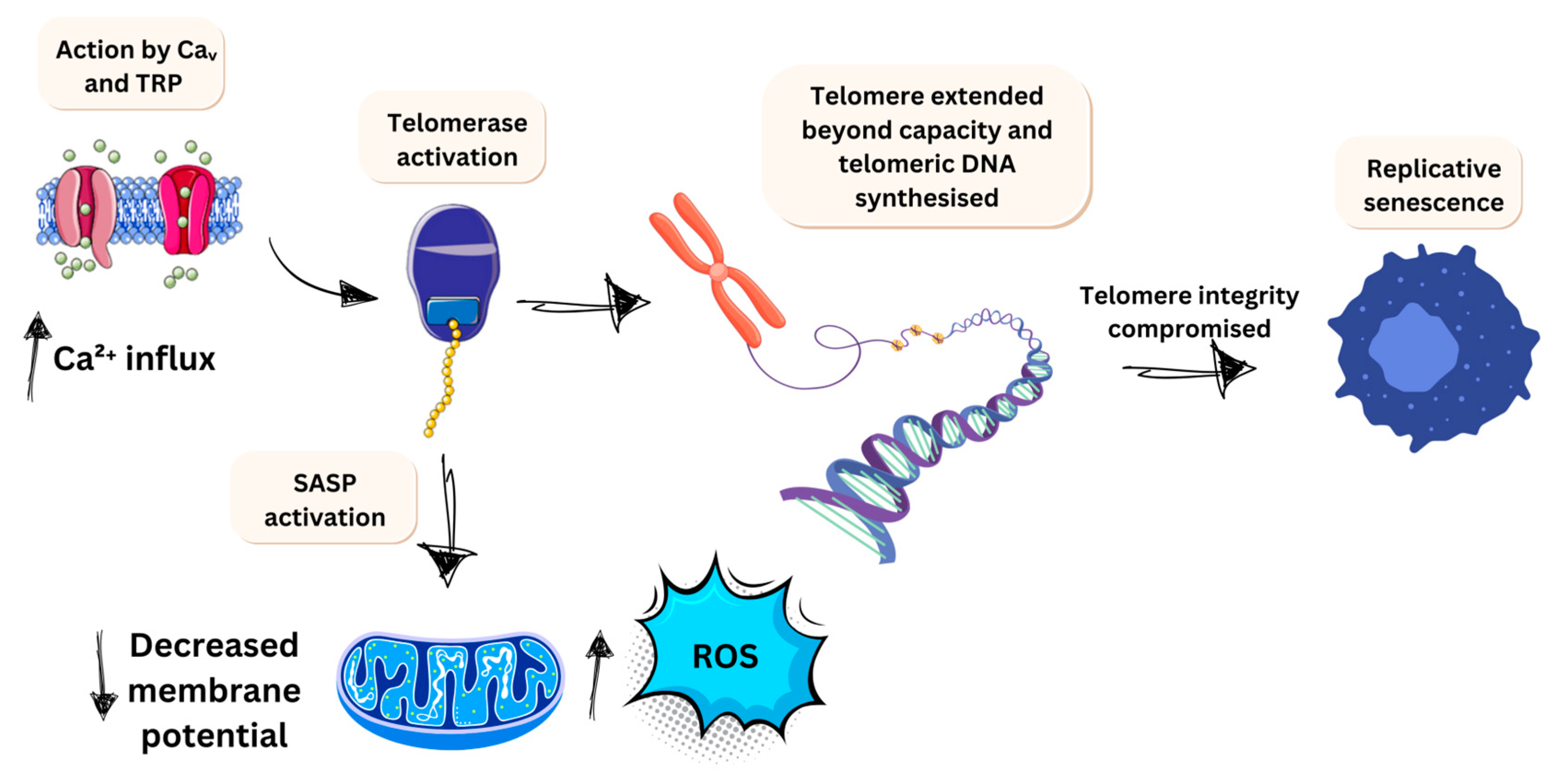
3.2.1. Evading Apoptosis
3.2.2. Limitless Growth Potential
3.3. Drug Delivery
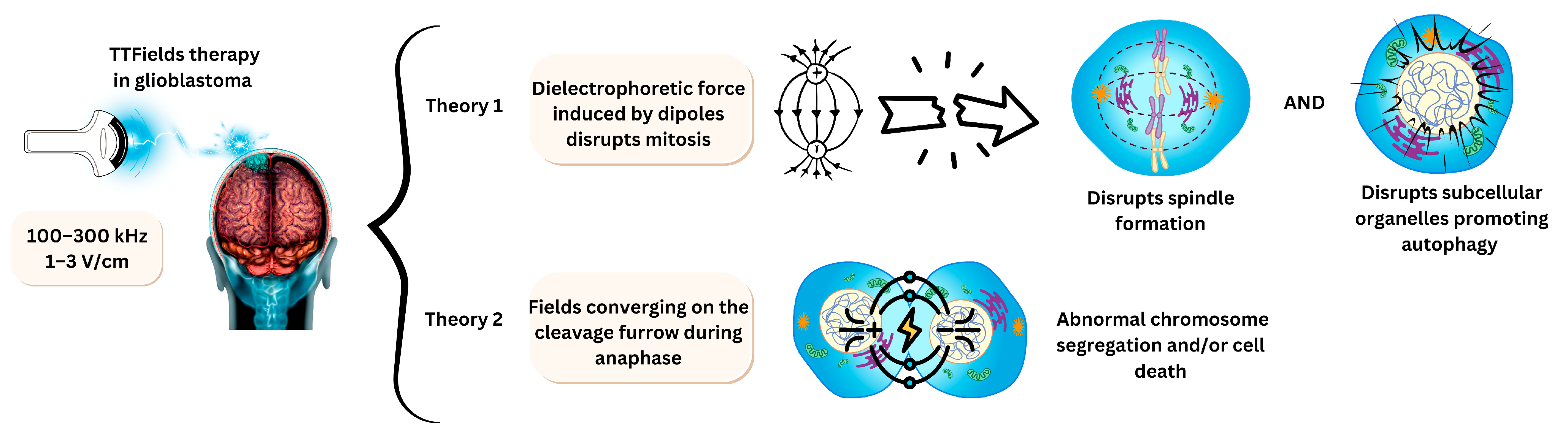
3.4. Electrodes for Tumour Treating Fields
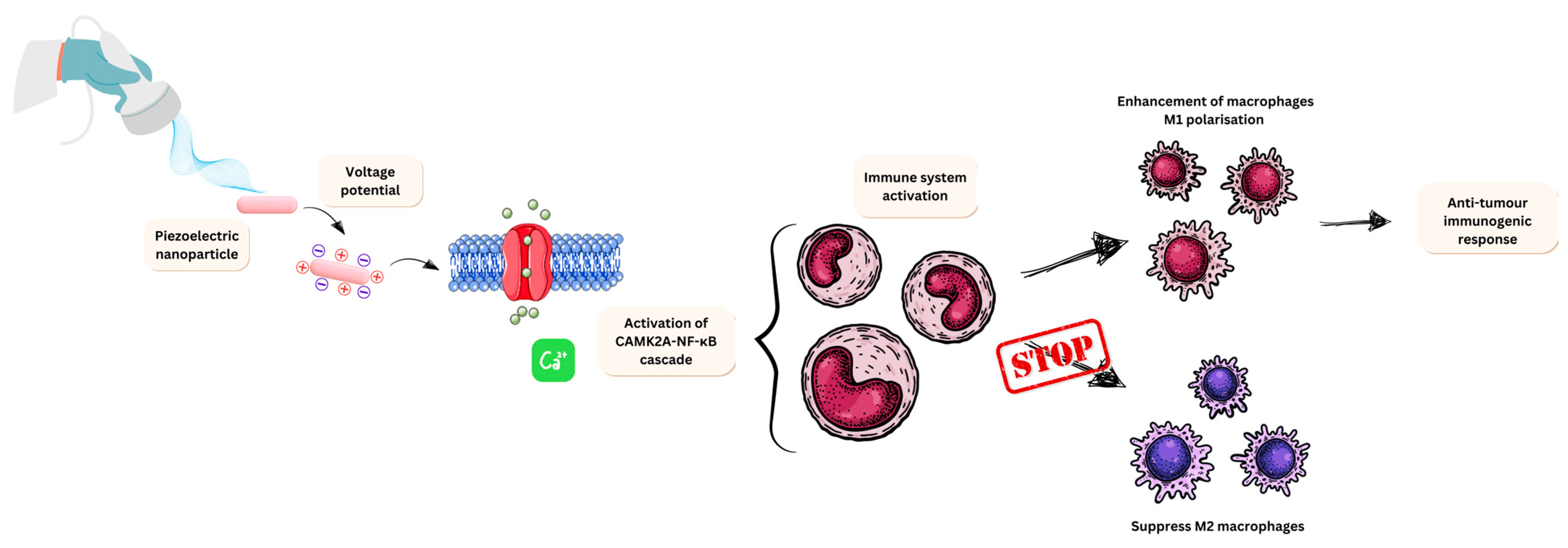
3.5. Nano-Piezoelectric Immunotherapy
3.6. Piezoelectric Nanomaterials for Glioblastoma Treatment
4. Advantages and Current Challenges
4.1. Challenges of Using Piezoelectric Nanomaterials in Glioblastoma
4.2. Therapeutic Benefit of Using Tumour-Treating Fields
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Păduraru, D.N.; Ion, D.; Niculescu, A.-G.; Mușat, F.; Andronic, O.; Grumezescu, A.M.; Bolocan, A. Recent Developments in Metallic Nanomaterials for Cancer Therapy, Diagnosing and Imaging Applications. Pharmaceutics 2022, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Cancer Burden Growing, Amidst Mounting Need for Services. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 7 November 2024).
- Macmillan. Cancer Statistics in the UK. Available online: https://www.macmillan.org.uk/about-us/what-we-do/research/cancer-statistics-fact-sheet (accessed on 7 November 2024).
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhou, P.-K. DNA damage repair: Historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 254. [Google Scholar] [CrossRef]
- Zeien, J.; Qiu, W.; Triay, M.; Dhaibar, H.A.; Cruz-Topete, D.; Cornett, E.M.; Urits, I.; Viswanath, O.; Kaye, A.D. Clinical implications of chemotherapeutic agent organ toxicity on perioperative care. Biomed. Pharmacother. 2022, 146, 112503. [Google Scholar] [CrossRef]
- Wopat, H.; Harrod, T.; Brem, R.F.; Kaltman, R.; Anderson, K.; Robien, K. Body composition and chemotherapy toxicity among women treated for breast cancer: A systematic review. J. Cancer Surviv. 2024, 18, 1356–1369. [Google Scholar] [CrossRef]
- van den Boogaard, W.M.C.; Komninos, D.S.J.; Vermeij, W.P. Chemotherapy Side-Effects: Not All DNA Damage Is Equal. Cancers 2022, 14, 627. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Chen, H.-A.; Lu, Y.-J.; Dash, B.S.; Chao, Y.-K.; Chen, J.-P. Hyaluronic Acid-Modified Cisplatin-Encapsulated Poly(Lactic-co-Glycolic Acid) Magnetic Nanoparticles for Dual-Targeted NIR-Responsive Chemo-Photothermal Combination Cancer Therapy. Pharmaceutics 2023, 15, 290. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.-B.; Cai, L. Smart nanoparticles for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef]
- Xu, J.; Song, M.; Fang, Z.; Zheng, L.; Huang, X.; Liu, K. Applications and challenges of ultra-small particle size nanoparticles in tumor therapy. J. Control. Release 2023, 353, 699–712. [Google Scholar] [CrossRef]
- Yu, W.; Liu, R.; Zhou, Y.; Gao, H. Size-Tunable Strategies for a Tumor Targeted Drug Delivery System. ACS Central Sci. 2020, 6, 100. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Abrishami, A.; Bahrami, A.R.; Nekooei, S.; Saljooghi, A.S.; Matin, M.M. Hybridized quantum dot, silica, and gold nanoparticles for targeted chemo-radiotherapy in colorectal cancer theranostics. Commun. Biol. 2024, 7, 393. [Google Scholar] [CrossRef]
- Aggarwal, V.; Solanki, S.; Malhotra, B.D. Applications of metal-organic framework-based bioelectrodes. Chem. Sci. 2022, 13, 8727–8743. [Google Scholar] [CrossRef]
- Shano, L.B.; Karthikeyan, S.; Kennedy, L.J.; Chinnathambi, S.; Pandian, G.N. MOFs for next-generation cancer therapeutics through a biophysical approach-a review. Front. Bioeng. Biotechnol. 2024, 12, 1397804. [Google Scholar] [CrossRef]
- Singhal, M.; Riches-Suman, K.; Pors, K.; Addicoat, M.A.; Ruiz, A.; Nayak, S.; Elies, J. Encapsulation and Delivery of Mitoxantrone Using Zirconium-Based Metal–Organic Frameworks (MOFs) and Their Cytotoxic Potential in Breast Cancer Cells. Appl. Sci. 2024, 14, 1902. [Google Scholar] [CrossRef]
- Bairagi, S.; Islam, S.U.; Shahadat, M.; Mulvihill, D.M.; Ali, W. Mechanical energy harvesting and self-powered electronic applications of textile-based piezoelectric nanogenerators: A systematic review. Nano Energy 2023, 111, 108414. [Google Scholar] [CrossRef]
- Zaka, A.; Liaqat, R.; Mehmood, S.; Haider, A.; Iqbal, M.; Mansoor, M.A. Single Source Precursor Synthesis of Lead Titanate (PbTiO3) for the Electrochemical Detection of Nitric Oxide (NO). J. Electrochem. Soc. 2024, 171, 057518. [Google Scholar]
- Vijayakanth, T.; Shankar, S.; Finkelstein-Zuta, G.; Rencus-Lazar, S.; Gilead, S.; Gazit, E. Perspectives on recent advancements in energy harvesting, sensing and bio-medical applications of piezoelectric gels. Chem. Soc. Rev. 2023, 52, 6191–6220. [Google Scholar] [CrossRef]
- Wu, L.; Gao, H.; Han, Q.; Guan, W.; Sun, S.; Zheng, T.; Liu, Y.; Wang, X.; Huang, R.; Li, G. Piezoelectric materials for neuroregeneration: A review. Biomater. Sci. 2023, 11, 7296–7310. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, R.; Chen, Z.; Chen, J.; Shung, K.K.; Zhou, Q. Transparent Lead lanthanum zirconate titanate (PLZT) ceramic fibers for High-frequency Ultrasonic Transducer Applications. Ceram. Int. 2016, 42, 18554–18559. [Google Scholar] [CrossRef] [PubMed]
- Rathee, G.; Bartwal, G.; Rathee, J.; Mishra, Y.K.; Kaushik, A.; Solanki, P.R. Emerging Multimodel Zirconia Nanosystems for High-Performance Biomedical Applications. Adv. NanoBiomed Res. 2021, 1, 2100039. [Google Scholar] [CrossRef]
- Su, L.; Zou, L.; Fong, C.C.; Wong, W.L.; Wei, F.; Wong, K.Y.; Wu, R.S.; Yang, M. Detection of cancer biomarkers by piezoelectric biosensor using PZT ceramic resonator as the transducer. Biosens. Bioelectron. 2013, 46, 155–161. [Google Scholar] [CrossRef]
- Guan, Y.; Bai, M.; Wang, Q.; Liu, L.; Yu, S.; Kong, B.; Lv, F.; Guo, M.; Liu, G.; Li, L.; et al. A self-powered wearable piezoelectric nanogenerator for physiological monitoring based on lead zirconate titanate/microfibrillated cellulose@polyvinyl alcohol (PZT/MFC@PVA) composition. Chem. Eng. J. 2023, 460, 141598. [Google Scholar] [CrossRef]
- Wang, J.-B.; Wu, J.; Zhang, J.; Guan, L.-A.; Feng, H.-B.; Zhu, K.-Y.; Zhang, Y.; Sun, H.; Cheng, Y.-D.; Zhang, L. Bibliometric and Visualized Analysis of Piezoelectric Materials in Biomedical Application. ACS Appl. Electron. Mater. 2024, 6, 1562–1573. [Google Scholar] [CrossRef]
- Zhou, Q.; Lam, K.H.; Zheng, H.; Qiu, W.; Shung, K.K. Piezoelectric single crystal ultrasonic transducers for biomedical applications. Prog. Mater. Sci. 2014, 66, 87–111. [Google Scholar] [CrossRef]
- Park, J.S.; Huh, K.Y.; Kim, M.-S.; Jung, S.Y.; Park, J.H.; Kim, S.J.; Jang, H.W.; Hwang, K.S.; Kim, H.N.; Kim, T.G.; et al. A relaxor-ferroelectric PMN-PZT thin-film-based drop-on-demand printhead for bioprinting applications with high piezoelectricity and low heat dissipation. Sens. Actuators B Chem. 2024, 417, 136194. [Google Scholar] [CrossRef]
- Sood, A.; Desseigne, M.; Dev, A.; Maurizi, L.; Kumar, A.; Millot, N.; Han, S.S. A Comprehensive Review on Barium Titanate Nanoparticles as a Persuasive Piezoelectric Material for Biomedical Applications: Prospects and Challenges. Small 2023, 19, 2206401. [Google Scholar] [CrossRef]
- Gong, Z.; Mao, Y.; Liu, Y.; Hu, X.; Zhang, Y.; Zhu, L.; Guo, S.; Ding, Z.; Zhang, L. Sono-promoted piezocatalysis and low-dose drug penetration for personalized therapy via tumor organoids. J. Colloid. Interface Sci. 2024, 675, 192–206. [Google Scholar] [CrossRef]
- Xiang, Z.; Xu, L.; Shan, Y.; Cui, X.; Shi, B.; Xi, Y.; Ren, P.; Zheng, X.; Zhao, C.; Luo, D.; et al. Tumor microenviroment-responsive self-assembly of barium titanate nanoparticles with enhanced piezoelectric catalysis capabilities for efficient tumor therapy. Bioact. Mater. 2024, 33, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Liang, S.; Si, J.; Xu, Q.; Zhang, H.; Ma, L.; Yang, C.; Zhang, X. Performance of LiTaO3 Crystals and Thin Films and Their Application. Crystals 2023, 13, 1233. [Google Scholar] [CrossRef]
- DeCeanne, A.V.; Fry, A.L.; Wilkinson, C.J.; Dittmer, M.; Ritzberger, C.; Rampf, M.; Mauro, J.C. Experimental analysis and modeling of the Knoop hardness of lithium disilicate glass-ceramics containing lithium tantalate as a secondary phase. J. Non-Cryst. Solids 2022, 585, 121540. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Montiel Schneider, M.G.; Martín, M.J.; Otarola, J.; Vakarelska, E.; Simeonov, V.; Lassalle, V.; Nedyalkova, M. Biomedical Applications of Iron Oxide Nanoparticles: Current Insights Progress and Perspectives. Pharmaceutics 2022, 14, 204. [Google Scholar] [CrossRef]
- Aisida, S.O.; Akpa, P.A.; Ahmad, I.; Zhao, T.-K.; Maaza, M.; Ezema, F.I. Bio-inspired encapsulation and functionalization of iron oxide nanoparticles for biomedical applications. Eur. Polym. J. 2020, 122, 109371. [Google Scholar] [CrossRef]
- Garg, J.; Chiu, M.N.; Krishnan, S.; Kumar, R.; Rifah, M.; Ahlawat, P.; Jha, N.K.; Kesari, K.K.; Ruokolainen, J.; Gupta, P.K. Emerging Trends in Zinc Ferrite Nanoparticles for Biomedical and Environmental Applications. Appl. Biochem. Biotechnol. 2024, 196, 1008–1043. [Google Scholar] [CrossRef]
- Haghniaz, R.; Rabbani, A.; Vajhadin, F.; Khan, T.; Kousar, R.; Khan, A.R.; Montazerian, H.; Iqbal, J.; Libanori, A.; Kim, H.-J.; et al. Anti-bacterial and wound healing-promoting effects of zinc ferrite nanoparticles. J. Nanobiotechnol. 2021, 19, 38. [Google Scholar] [CrossRef]
- Aisida, S.O.; Ali, A.; Oyewande, O.E.; Ahmad, I.; Ul-Hamid, A.; Zhao, T.-K.; Maaza, M.; Ezema, F.I. Biogenic synthesis enhanced structural, morphological, magnetic and optical properties of zinc ferrite nanoparticles for moderate hyperthermia applications. J. Nanopart. Res. 2021, 23, 47. [Google Scholar] [CrossRef]
- Concha, V.O.C.; Timóteo, L.; Duarte, L.A.N.; Bahú, J.O.; Munoz, F.L.; Silva, A.P.; Lodi, L.; Severino, P.; León-Pulido, J.; Souto, E.B. Properties, characterization and biomedical applications of polyvinylidene fluoride (PVDF): A review. J. Mater. Sci. 2024, 59, 14185–14204. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Ismail, A.M.; El-Newehy, M.H.; El-Naggar, M.E.; Meera Moydeen, A.; Menazea, A.A. Enhancement the electrical conductivity of the synthesized polyvinylidene fluoride/polyvinyl chloride composite doped with palladium nanoparticles via laser ablation. J. Mater. Res. Technol. 2020, 9, 11178–11188. [Google Scholar] [CrossRef]
- Shakiba, M.; Rezvani Ghomi, E.; Khosravi, F.; Jouybar, S.; Bigham, A.; Zare, M.; Abdouss, M.; Moaref, R.; Ramakrishna, S. Nylon—A material introduction and overview for biomedical applications. Polym. Adv. Technol. 2021, 32, 3368–3383. [Google Scholar] [CrossRef]
- Eom, K.; Na, S.; Kim, J.-K.; Ko, H.; Jin, J.; Kang, S.J. Engineering crystal phase of Nylon-11 films for ferroelectric device and piezoelectric sensor. Nano Energy 2021, 88, 106244. [Google Scholar] [CrossRef]
- Danagody, B.; Bose, N.; Rajappan, K. Electrospun polyacrylonitrile-based nanofibrous membrane for various biomedical applications. J. Polym. Res. 2024, 31, 119. [Google Scholar] [CrossRef]
- Tao, J.; Wang, Y.; Zheng, X.; Zhao, C.; Jin, X.; Wang, W.; Lin, T. A review: Polyacrylonitrile as high-performance piezoelectric materials. Nano Energy 2023, 118, 108987. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Szewczyk, P.K.; Berniak, K.; Knapczyk-Korczak, J.; Karbowniczek, J.E.; Marzec, M.M.; Bernasik, A.; Stachewicz, U. Mimicking natural electrical environment with cellulose acetate scaffolds enhances collagen formation of osteoblasts. Nanoscale 2023, 15, 6890–6900. [Google Scholar]
- Rakesh, M.; Babu, B.R.N.; Prakash, A.P.G.; Prema, N.S.; Gowda, A.C.; Madhukar, B.S.; Kashimatt, M.G.V.; Pradeep, T.M.; Kumar, B.V.S.; Madhusudan, P. Fabrication of lead zirconate titanate-based polyvinylidene fluoride polymer nano-composites: Microcrystalline, morphological and electrical studies. J. Mater. Sci. Mater. Electron. 2023, 34, 372. [Google Scholar] [CrossRef]
- Chang, G.; Pan, X.; Hao, Y.; Du, W.; Wang, S.; Zhou, Y.; Yang, J.; He, Y. PVDF/ZnO piezoelectric nanofibers designed for monitoring of internal micro-pressure. RSC Adv. 2024, 14, 11775–11783. [Google Scholar] [CrossRef]
- Shuai, C.; Liu, G.; Yang, Y.; Yang, W.; He, C.; Wang, G.; Liu, Z.; Qi, F.; Peng, S. Functionalized BaTiO3 enhances piezoelectric effect towards cell response of bone scaffold. Colloids Surf. B Biointerfaces 2020, 185, 110587. [Google Scholar] [CrossRef]
- Islam, M.N.; Rupom, R.H.; Adhikari, P.R.; Demchuk, Z.; Popov, I.; Sokolov, A.P.; Wu, H.F.; Advincula, R.C.; Dahotre, N.; Jiang, Y.; et al. Boosting Piezoelectricity by 3D Printing PVDF-MoS2 Composite as a Conformal and High-Sensitivity Piezoelectric Sensor. Adv. Funct. Mater. 2023, 33, 2302946. [Google Scholar] [CrossRef]
- Jandas, P.J.; Prabakaran, K.; Luo, J.; Derry Holaday, M.G. Effective utilization of quartz crystal microbalance as a tool for biosensing applications. Sens. Actuators A Phys. 2021, 331, 113020. [Google Scholar] [CrossRef]
- He, Q.; Zeng, Y.; Jiang, L.; Wang, Z.; Lu, G.; Kang, H.; Li, P.; Bethers, B.; Feng, S.; Sun, L.; et al. Growing recyclable and healable piezoelectric composites in 3D printed bioinspired structure for protective wearable sensor. Nat. Commun. 2023, 14, 6477. [Google Scholar] [CrossRef]
- Khan, A.; Joshi, R.; Sharma, M.K.; Huang, C.-J.; Yu, J.-H.; Wang, Y.-L.; Lin, Z.-H. The potential of organic piezoelectric materials for next-generation implantable biomedical devices. Nano Trends 2024, 6, 100032. [Google Scholar] [CrossRef]
- Sahoo, J.K.; Hasturk, O.; Falcucci, T.; Kaplan, D.L. Silk chemistry and biomedical material designs. Nat. Rev. Chem. 2023, 7, 302–318. [Google Scholar] [CrossRef]
- Lee, J.C.; Suh, I.W.; Park, C.H.; Kim, C.S. Polyvinylidene fluoride/silk fibroin-based bio-piezoelectric nanofibrous scaffolds for biomedical application. J. Tissue Eng. Regen. Med. 2021, 15, 869–877. [Google Scholar] [CrossRef]
- Khare, D.; Basu, B.; Dubey, A.K. Electrical stimulation and piezoelectric biomaterials for bone tissue engineering applications. Biomaterials 2020, 258, 120280. [Google Scholar]
- Joo, S.; Gwon, Y.; Kim, S.; Park, S.; Kim, J.; Hong, S. Piezoelectrically and Topographically Engineered Scaffolds for Accelerating Bone Regeneration. ACS Appl. Mater. Interfaces 2024, 16, 1999–2011. [Google Scholar] [CrossRef]
- Liu, H.; Shi, Y.; Zhu, Y.; Wu, P.; Deng, Z.; Dong, Q.; Wu, M.; Cai, L. Bioinspired Piezoelectric Periosteum to Augment Bone Regeneration via Synergistic Immunomodulation and Osteogenesis. ACS Appl. Mater. Interfaces 2023, 15, 12273–12293. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Liang, X. Piezoelectric Nanomaterials Activated by Ultrasound in Disease Treatment. Pharmaceutics 2023, 15, 1338. [Google Scholar] [CrossRef] [PubMed]
- Dolai, J.; Biswas, A.; Jana, N.R. Piezoelectric Nanoparticles for Ultrasound-Based Wireless Therapies. ACS Appl. Nano Mater. 2022, 5, 14038–14050. [Google Scholar] [CrossRef]
- Mikolajick, T.; Park, M.H.; Begon-Lours, L.; Slesazeck, S. From Ferroelectric Material Optimization to Neuromorphic Devices. Adv. Mater. 2023, 35, 2206042. [Google Scholar] [CrossRef]
- Nataf, G.F.; Guennou, M.; Gregg, J.M.; Meier, D.; Hlinka, J.; Salje, E.K.H.; Kreisel, J. Domain-wall engineering and topological defects in ferroelectric and ferroelastic materials. Nat. Rev. Phys. 2020, 2, 634–648. [Google Scholar] [CrossRef]
- Wang, J.; Chu, Y.; Zhao, Z.; Zhang, C.; Chen, Q.; Ran, H.; Cao, Y.; Wu, C. Piezoelectric enhanced sulfur doped graphdiyne nanozymes for synergistic ferroptosis–apoptosis anticancer therapy. J. Nanobiotechnol. 2023, 21, 311. [Google Scholar] [CrossRef]
- Kamel, N.A. Bio-piezoelectricity: Fundamentals and applications in tissue engineering and regenerative medicine. Biophys. Rev. 2022, 14, 717–733. [Google Scholar] [CrossRef]
- Potnis, P.R.; Tsou, N.-T.; Huber, J.E.; Potnis, P.R.; Tsou, N.-T.; Huber, J.E. A Review of Domain Modelling and Domain Imaging Techniques in Ferroelectric Crystals. Materials 2011, 4, 417–447. [Google Scholar] [CrossRef]
- Murugan, C.; Lee, H.; Park, S. Tumor-targeted molybdenum disulfide@barium titanate core–shell nanomedicine for dual photothermal and chemotherapy of triple-negative breast cancer cells. J. Mater. Chem. B 2023, 11, 1044–1056. [Google Scholar] [CrossRef]
- Catalano, E. Recent and future applications of ultrasound-activated piezoelectric nanoparticles for anticancer treatment: Mini-review. J. Phys. Conf. Ser. 2023, 2579, 012005. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, P.; Mao, L.; Wu, W.; Lin, H.; Xu, D.; Lu, X.; Shi, J. Piezocatalytic Medicine: An Emerging Frontier using Piezoelectric Materials for Biomedical Applications. Adv. Mater. 2023, 35, 2208256. [Google Scholar] [CrossRef]
- Wang, Y.; Zang, P.; Yang, D.; Zhang, R.; Gai, S.; Yang, P. The fundamentals and applications of piezoelectric materials for tumor therapy: Recent advances and outlook. Mater. Horiz. 2023, 10, 1140–1184. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, Z.; Zhao, J.; Zhang, Z.; Li, X.; Zhang, J. Recent Advances of Ferro-, Piezo-, and Pyroelectric Nanomaterials for Catalytic Applications. ACS Appl. Nano Mater. 2020, 3, 1063–1079. [Google Scholar] [CrossRef]
- Wu, Y.; Zou, J.; Tang, K.; Xia, Y.; Wang, X.; Song, L.; Wang, J.; Wang, K.; Wang, Z. From electricity to vitality: The emerging use of piezoelectric materials in tissue regeneration. Burns Trauma 2024, 12, tkae013. [Google Scholar] [CrossRef]
- Yang, J.; Chen, M.; Lee, H.; Xu, Z.; Zhou, Z.; Feng, S.-P.; Kim, J.T. Three-Dimensional Printing of Self-Assembled Dipeptides. ACS Appl. Mater. Interfaces 2021, 13, 20573–20580. [Google Scholar] [CrossRef]
- Sencadas, V.; Garvey, C.; Mudie, S.; Kirkensgaard, J.J.K.; Gouadec, G.; Hauser, S. Electroactive properties of electrospun silk fibroin for energy harvesting applications. Nano Energy 2019, 66, 104106. [Google Scholar] [CrossRef]
- Jiang, P.; Yan, F.; Nasr Esfahani, E.; Xie, S.; Zou, D.; Liu, X.; Zheng, H.; Li, J. Electromechanical Coupling of Murine Lung Tissues Probed by Piezoresponse Force Microscopy. ACS Biomater. Sci. Eng. 2017, 3, 1827–1835. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Boutis, G.S. The coupled bio-chemo-electro-mechanical behavior of glucose exposed arterial elastin. J. Phys. D Appl. Phys. 2017, 50, 133001. [Google Scholar]
- Liu, W.-L.; Zou, M.-Z.; Qin, S.-Y.; Cheng, Y.-J.; Ma, Y.-H.; Sun, Y.-X.; Zhang, X.-Z. Recent Advances of Cell Membrane-Coated Nanomaterials for Biomedical Applications. Adv. Funct. Mater. 2020, 30, 2003559. [Google Scholar] [CrossRef]
- Jacob, J.; More, N.; Kalia, K.; Kapusetti, G. Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm. Regen. 2018, 38, 2. [Google Scholar] [CrossRef]
- Kang, Y.; Kang, Y.; Lei, L.; Lei, L.; Zhu, C.; Zhu, C.; Zhang, H.; Zhang, H.; Mei, L.; Mei, L.; et al. Piezo-photocatalytic effect mediating reactive oxygen species burst for cancer catalytic therapy. Mater. Horiz. 2021, 8, 2273–2285. [Google Scholar] [CrossRef]
- Ran, M.; Xu, H.; Bao, Y.; Zhang, Y.; Zhang, J.; Xing, M. Selective Production of CO from Organic Pollutants by Coupling Piezocatalysis and Advanced Oxidation Processes. Angew. Chem. Int. Ed. 2023, 62, 202303728. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Q.; Wang, W.; Peng, M.; Zhang, X.-Z. Free radicals for cancer theranostics. Biomaterials 2021, 266, 120474. [Google Scholar] [CrossRef]
- Wang, K.; Han, C.; Li, J.; Qiu, J.; Sunarso, J.; Liu, S. The Mechanism of Piezocatalysis: Energy Band Theory or Screening Charge Effect? Angew. Chem. 2022, 134, 202110429. [Google Scholar] [CrossRef]
- Jin, C.-C.; Liu, D.-M.; Zhang, L.-X. An Emerging Family of Piezocatalysts: 2D Piezoelectric Materials. Small 2023, 19, 2303586. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Dong, S.; Wang, P.; Chen, W.; Lu, Z.; Ye, D.; Pan, B.; Wu, D.; Vecitis, C.D.; et al. Ultrasonic activation of inert poly(tetrafluoroethylene) enables piezocatalytic generation of reactive oxygen species. Nat. Commun. 2021, 12, 3508. [Google Scholar] [CrossRef]
- Duranti, C.; Arcangeli, A. Ion Channel Targeting with Antibodies and Antibody Fragments for Cancer Diagnosis. Antibodies 2019, 8, 33. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Montllor-Albalate, C.; Kim, H.; Thompson, A.E.; Jonke, A.P.; Torres, M.P.; Reddi, A.R. Sod1 integrates oxygen availability to redox regulate NADPH production and the thiol redoxome. Proc. Natl. Acad. Sci. USA 2022, 119, 2023328119. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, P.-J.; Liu, J.-H.; Dong, M.; Shen, X.-Q.; Chen, Y.-X.; Shen, Q.-D. Electromagnetized-Nanoparticle-Modulated Neural Plasticity and Recovery of Degenerative Dopaminergic Neurons in the Mid-Brain. Adv. Mater. 2020, 32, 2003800. [Google Scholar] [CrossRef]
- Truong Hoang, Q.; Huynh, K.A.; Nguyen Cao, T.G.; Kang, J.H.; Dang, X.N.; Ravichandran, V.; Kang, H.C.; Lee, M.; Kim, J.-E.; Ko, Y.T.; et al. Piezocatalytic 2D WS2 Nanosheets for Ultrasound-Triggered and Mitochondria-Targeted Piezodynamic Cancer Therapy Synergized with Energy Metabolism-Targeted Chemotherapy. Adv. Mater. 2023, 35, 2300437. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Structure and function of voltage-gated ion channels. Annu. Rev. Biochem. 1995, 64, 493–531. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Droogmans, G. Ion channels and their functional role in vascular endothelium. Physiol. Rev. 2001, 81, 1415–1459. [Google Scholar] [CrossRef]
- Bates, E. Ion Channels in Development and Cancer. Annu. Rev. Cell Dev. Biol. 2015, 31, 231–247. [Google Scholar] [CrossRef]
- Kefauver, J.M.; Ward, A.B.; Patapoutian, A. Discoveries in structure and physiology of mechanically activated ion channels. Nature 2020, 587, 567–576. [Google Scholar] [CrossRef]
- Huang, J.; Pan, X.; Yan, N. Structural biology and molecular pharmacology of voltage-gated ion channels. Nat. Rev. Mol. Cell Biol. 2024, 25, 904–925. [Google Scholar]
- Palmisano, V.F.; Anguita-Ortiz, N.; Faraji, S.; Nogueira, J.J. Voltage-Gated Ion Channels: Structure, Pharmacology and Photopharmacology. ChemPhysChem 2024, 25, e202400162. [Google Scholar] [CrossRef]
- Fang, X.-Z.; Zhou, T.; Xu, J.-Q.; Wang, Y.-X.; Sun, M.-M.; He, Y.-J.; Pan, S.-W.; Xiong, W.; Peng, Z.-K.; Gao, X.-H.; et al. Structure, kinetic properties and biological function of mechanosensitive Piezo channels. Cell Biosci. 2021, 11, 1–20. [Google Scholar] [CrossRef]
- Levin, M.; Martyniuk, C.J. The bioelectric code: An ancient computational medium for dynamic control of growth and form. Biosystems 2018, 164, 76–93. [Google Scholar] [CrossRef]
- Wright, S.H. Generation of resting membrane potential. Adv. Physiol. Educ. 2004, 28, 139–142. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Natour, Z.A.; Mustafa, F.; Rizvi, T.A. Electrical Characterization of Normal and Cancer Cells. IEEE Access 2018, 6, 25979–25986. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion channels and the hallmarks of cancer. Trends Mol. Med. 2010, 16, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion Channels in Cancer: Are Cancer Hallmarks Oncochannelopathies? Physiol. Rev. 2018, 98, 559–621. [Google Scholar] [CrossRef]
- Payne, S.L.; Levin, M.; Oudin, M.J. Bioelectric Control of Metastasis in Solid Tumors. Bioelectricity 2019, 1, 114–130. [Google Scholar] [CrossRef]
- Ohkubo, T.; Yamazaki, J. T-type voltage-activated calcium channel Cav3.1, but not Cav3.2, is involved in the inhibition of proliferation and apoptosis in MCF-7 human breast cancer cells. Int. J. Oncol. 2012, 41, 267–275. [Google Scholar] [CrossRef]
- Lopez-Charcas, O.; Benouna, O.; Lemoine, R.; Rosendo-Pineda, M.J.; Anguheven-Ledezma, T.G.; Sandoval-Vazquez, L.; Gallegos-Gomez, M.L.; Robles-Martinez, L.; Herrera-Carrillo, Z.; Ramírez-Aragón, M.; et al. Blockade of CaV3 calcium channels and induction of G0/G1 cell cycle arrest in colon cancer cells by gossypol. Br. J. Pharmacol. 2024, 181, 4546–4570. [Google Scholar] [CrossRef]
- Motiani, R.K.; Hyzinski-García, M.C.; Zhang, X.; Henkel, M.M.; Abdullaev, I.F.; Kuo, Y.-H.; Matrougui, K.; Mongin, A.A.; Trebak, M. STIM1 and Orai1 mediate CRAC channel activity and are essential for human glioblastoma invasion. Pflüg. Arch.-Eur. J. Physiol. 2013, 465, 1249–1260. [Google Scholar] [CrossRef]
- Roger, S.; Besson, P.; Le Guennec, J.Y. Involvement of a novel fast inward sodium current in the invasion capacity of a breast cancer cell line. Biochim. Biophys. Acta 2003, 1616, 107–111. [Google Scholar] [CrossRef]
- Huber, S.M. Oncochannels. Cell Calcium 2013, 53, 241–255. [Google Scholar] [CrossRef]
- Williams, S.; Bateman, A.; O’Kelly, I. Altered Expression of Two-Pore Domain Potassium (K2P) Channels in Cancer. PLoS ONE 2013, 8, e74589. [Google Scholar] [CrossRef]
- Litan, A.; Langhans, S.A. Cancer as a channelopathy: Ion channels and pumps in tumor development and progression. Front. Cell Neurosci. 2015, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Gandini, M.A.; Zamponi, G.W. Voltage-gated calcium channel nanodomains: Molecular composition and function. FEBS J. 2022, 289, 614–633. [Google Scholar] [CrossRef] [PubMed]
- Pallone, T.L.; Khurana, S.; Cao, C. Voltage-Gated Calcium Channels: Structure and Function (CACNA). In Encyclopedia of Signaling Molecules; Choi, S., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 5942–5949. [Google Scholar] [CrossRef]
- Marino, A.; Arai, S.; Hou, Y.; Sinibaldi, E.; Pellegrino, M.; Chang, Y.-T.; Mazzolai, B.; Mattoli, V.; Suzuki, M.; Ciofani, G. Piezoelectric Nanoparticle-Assisted Wireless Neuronal Stimulation. ACS Nano 2015, 9, 7678–7689. [Google Scholar] [CrossRef] [PubMed]
- Phan, N.N.; Wang, C.Y.; Chen, C.F.; Sun, Z.; Lai, M.D.; Lin, Y.C. Voltage-gated calcium channels: Novel targets for cancer therapy. Oncol. Lett. 2017, 14, 2059–2074. [Google Scholar] [CrossRef]
- McKerr, N.; Mohd-Sarip, A.; Dorrian, H.; Breen, C.; James, J.A.; McQuaid, S.; Mills, I.G.; McCloskey, K.D. CACNA1D overexpression and voltage-gated calcium channels in prostate cancer during androgen deprivation. Sci. Rep. 2023, 13, 4683. [Google Scholar] [CrossRef]
- Bhargava, A.; Saha, S. T-Type voltage gated calcium channels: A target in breast cancer? Breast Cancer Res. Treat. 2019, 173, 11–21. [Google Scholar] [CrossRef]
- Ikeda, S.; Matsushima, S.; Okabe, K.; Ikeda, M.; Ishikita, A.; Tadokoro, T.; Enzan, N.; Yamamoto, T.; Sada, M.; Deguchi, H.; et al. Blockade of L-type Ca2+ channel attenuates doxorubicin-induced cardiomyopathy via suppression of CaMKII-NF-κB pathway. Sci. Rep. 2019, 9, 9850. [Google Scholar] [CrossRef]
- Dalavaikodihalli Nanjaiah, N.; Ramaswamy, P.; Goswami, K.; Fathima, K.H.; Borkotokey, M. Survival of glioblastoma cells in response to endogenous and exogenous oxidative challenges: Possible implication of NMDA receptor-mediated regulation of redox homeostasis. Cell Biol. Int. 2019, 43, 1443–1452. [Google Scholar] [CrossRef]
- North, W.G.; Liu, F.; Dragnev, K.H.; Demidenko, E. Small-cell lung cancer growth inhibition: Synergism between NMDA receptor blockade and chemotherapy. Clin. Pharmacol. Adv. Appl. 2019, 11, 15–23. [Google Scholar] [CrossRef]
- Ramaswamy, P.; Aditi Devi, N.; Hurmath Fathima, K.; Dalavaikodihalli Nanjaiah, N. Activation of NMDA receptor of glutamate influences MMP-2 activity and proliferation of glioma cells. Neurol. Sci. 2014, 35, 823–829. [Google Scholar] [CrossRef]
- González-Cota, A.L.; Martínez-Flores, D.; Rosendo-Pineda, M.J.; Vaca, L. NMDA receptor-mediated Ca(2+) signaling: Impact on cell cycle regulation and the development of neurodegenerative diseases and cancer. Cell Calcium 2024, 119, 102856. [Google Scholar] [CrossRef]
- Angus, M.; Ruben, P. Voltage gated sodium channels in cancer and their potential mechanisms of action. Channels 2019, 13, 400–409. [Google Scholar] [CrossRef]
- Sheth, M.; Esfandiari, L. Bioelectric Dysregulation in Cancer Initiation, Promotion, and Progression. Front. Oncol. 2022, 12, 846917. [Google Scholar] [CrossRef]
- Guéguinou, M.; Harnois, T.; Crottes, D.; Uguen, A.; Deliot, N.; Gambade, A.; Chantôme, A.; Haelters, J.P.; Jaffrès, P.A.; Jourdan, M.L.; et al. SK3/TRPC1/Orai1 complex regulates SOCE-dependent colon cancer cell migration: A novel opportunity to modulate anti-EGFR mAb action by the alkyl-lipid Ohmline. Oncotarget 2016, 7, 36168–36184. [Google Scholar] [CrossRef]
- Tajada, S.; Villalobos, C. Calcium Permeable Channels in Cancer Hallmarks. Front. Pharmacol. 2020, 11, 968. [Google Scholar] [CrossRef]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef]
- Guo, Y.R.; MacKinnon, R. Structure-based membrane dome mechanism for Piezo mechanosensitivity. Elife 2017, 6, e33660. [Google Scholar] [CrossRef]
- Saotome, K.; Murthy, S.E.; Kefauver, J.M.; Whitwam, T.; Patapoutian, A.; Ward, A.B. Structure of the mechanically activated ion channel Piezo1. Nature 2018, 554, 481–486. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, H.; Chi, S.; Wang, Y.; Wang, J.; Geng, J.; Wu, K.; Liu, W.; Zhang, T.; Dong, M.-Q.; et al. Structure and mechanogating mechanism of the Piezo1 channel. Nature 2018, 554, 487–492. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.; Zhang, M.; Liu, W.; Deng, T.; Zhao, Q.; Li, Y.; Lei, J.; Li, X.; Xiao, B. Structure and mechanogating of the mammalian tactile channel PIEZO2. Nature 2019, 573, 225–229. [Google Scholar] [CrossRef]
- De Felice, D.; Alaimo, A. Mechanosensitive Piezo Channels in Cancer: Focus on altered Calcium Signaling in Cancer Cells and in Tumor Progression. Cancers 2020, 12, 1780. [Google Scholar] [CrossRef] [PubMed]
- Karska, J.; Kowalski, S.; Saczko, J.; Moisescu, M.G.; Kulbacka, J. Mechanosensitive Ion Channels and Their Role in Cancer Cells. Membranes 2023, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, Z.; Niu, Y.; Tang, Y.; Wang, Y.; Huang, J.; Leung, E.L. TRP channels in cancer: Therapeutic opportunities and research strategies. Pharmacol. Res. 2024, 209, 107412. [Google Scholar] [CrossRef]
- Marini, M.; Titiz, M.; Souza Monteiro de Araújo, D.; Geppetti, P.; Nassini, R.; De Logu, F. TRP Channels in Cancer: Signaling Mechanisms and Translational Approaches. Biomolecules 2023, 13, 1557. [Google Scholar] [CrossRef]
- Fan, H.; Shen, Y.X.; Yuan, Y.-F. Expression and prognostic roles of TRPV5 and TRPV6 in non-small cell lung cancer after curative resection. Asian Pac. J. Cancer Prev. 2014, 15, 2559–2563. [Google Scholar]
- Berrout, J.; Kyriakopoulou, E.; Moparthi, L.; Hogea, A.S.; Berrout, L.; Ivan, C.; Lorger, M.; Boyle, J.; Peers, C.; Muench, S.; et al. TRPA1-FGFR2 binding event is a regulatory oncogenic driver modulated by miRNA-142-3p. Nat. Commun. 2017, 8, 947. [Google Scholar] [CrossRef]
- Hu, S.; Li, L.; Huang, W.; Liu, J.; Lan, G.; Yu, S.; Peng, L.; Xie, X.; Yang, L.; Nian, Y.; et al. CAV3.1 knockdown suppresses cell proliferation, migration and invasion of prostate cancer cells by inhibiting AKT. Cancer Manag. Res. 2018, 10, 4603–4614. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, Y.; Zhang, L.; Yee, P.P.; Johnson, M.; Zhang, X.; Gulley, M.; Atkinson, J.M.; Trebak, M.; Wang, H.-G.; et al. Induction of store-operated calcium entry (SOCE) suppresses glioblastoma growth by inhibiting the Hippo pathway transcriptional coactivators YAP/TAZ. Oncogene 2019, 38, 120–139. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, J.J.; Huang, X.Y. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell 2009, 15, 124–134. [Google Scholar] [CrossRef]
- Chen, Y.F.; Chiu, W.T.; Chen, Y.T.; Lin, P.Y.; Huang, H.J.; Chou, C.Y.; Chang, H.C.; Tang, M.J.; Shen, M.R. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 15225–15230. [Google Scholar] [CrossRef]
- Takayasu, T.; Kurisu, K.; Esquenazi, Y.; Ballester, L.Y. Ion Channels and Their Role in the Pathophysiology of Gliomas. Mol. Cancer Ther. 2020, 19, 1959–1969. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Yuan, H.; Soifer, I.; Maile, T.M.; Wang, R.Y.; Ireland, A.; O’Brien, J.J.; Goudeau, J.; Chan, L.J.G.; Vijay, T.; et al. Novel insights from a multiomics dissection of the Hayflick limit. eLife 2022, 11, e70283. [Google Scholar] [CrossRef] [PubMed]
- Fletcher-Sananikone, E.; Kanji, S.; Tomimatsu, N.; Di Cristofaro, L.F.M.; Kollipara, R.K.; Saha, D.; Floyd, J.R.; Sung, P.; Hromas, R.; Burns, T.C.; et al. Elimination of Radiation-Induced Senescence in the Brain Tumor Microenvironment Attenuates Glioblastoma Recurrence. Cancer Res. 2021, 81, 5935–5947. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.; Bernard, D. Calcium signaling and cellular senescence. Cell Calcium 2018, 70, 16–23. [Google Scholar] [CrossRef]
- Martin, N.; Zhu, K.; Czarnecka-Herok, J.; Vernier, M.; Bernard, D. Regulation and role of calcium in cellular senescence. Cell Calcium 2023, 110, 102701. [Google Scholar] [CrossRef]
- Rosenbaum, T.; Morales-Lázaro, S.L.; Islas, L.D. TRP channels: A journey towards a molecular understanding of pain. Nat. Rev. Neurosci. 2022, 23, 596–610. [Google Scholar] [CrossRef]
- Koivisto, A.-P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef]
- Lin, X.; Song, J.; Chen, X.; Yang, H. Ultrasound-Activated Sensitizers and Applications. Angew. Chem. Int. Ed. 2020, 59, 14212–14233. [Google Scholar] [CrossRef]
- Leinenga, G.; Langton, C.; Nisbet, R.; Götz, J. Ultrasound treatment of neurological diseases—Current and emerging applications. Nat. Rev. Neurol. 2016, 12, 161–174. [Google Scholar] [CrossRef]
- Shi, X.; Chen, Y.; Zhao, Y.; Ye, M.; Zhang, S.; Gong, S. Ultrasound-activable piezoelectric membranes for accelerating wound healing. Biomater. Sci. 2022, 10, 692–701. [Google Scholar]
- Ramedani, A.; Sabzevari, O.; Simchi, A. Hybrid ultrasound-activated nanoparticles based on graphene quantum dots for cancer treatment. Int. J. Pharm. 2022, 629, 122373. [Google Scholar] [CrossRef] [PubMed]
- Barcena, A.J.R.; Dhal, K.; Patel, P.; Ravi, P.; Kundu, S.; Tappa, K. Current Biomedical Applications of 3D-Printed Hydrogels. Gels 2024, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Cheng, Y.; Chen, G.; Zhao, Y. 3D-Printed Janus Piezoelectric Patches for Sonodynamic Bacteria Elimination and Wound Healing. Research 2023, 6, 0022. [Google Scholar] [CrossRef] [PubMed]
- Wenger, C.; Miranda, P.C.; Salvador, R.; Thielscher, A.; Bomzon, Z.; Giladi, M.; Mrugala, M.M.; Korshoej, A.R. A Review on Tumor-Treating Fields (TTFields): Clinical Implications Inferred from Computational Modeling. IEEE Rev. Biomed. Eng. 2018, 11, 195–207. [Google Scholar] [CrossRef]
- Kirson, E.D.; Gurvich, Z.; Schneiderman, R.; Dekel, E.; Itzhaki, A.; Wasserman, Y.; Schatzberger, R.; Palti, Y. Disruption of Cancer Cell Replication by Alternating Electric Fields. Cancer Res. 2004, 64, 3288–3295. [Google Scholar] [CrossRef]
- Ornelas, A.S.; Porter, A.B.; Sharma, A.; Knox, M.G.; Marks, L.A.; Wingerchuk, D.M.; O’Carroll, C.B. What is the Role of Tumor-treating Fields in Newly Diagnosed Glioblastoma? Neurologist 2019, 24, 71. [Google Scholar] [CrossRef]
- Foster, K.R.; Ziskin, M.C.; Balzano, Q. Time-temperature Thresholds and Safety Factors for Thermal Hazards from Radiofrequency Energy above 6 GHz. Health Phys. 2021, 121, 234–247. [Google Scholar] [CrossRef]
- Arvind, R.; Chandana, S.R.; Borad, M.J.; Pennington, D.; Mody, K.; Babiker, H. Tumor-Treating Fields: A fourth modality in cancer treatment, new practice updates. Crit. Rev. Oncol./Hematol. 2021, 168, 103535. [Google Scholar] [CrossRef]
- Moser, J.C.; Salvador, E.; Deniz, K.; Swanson, K.; Tuszynski, J.; Carlson, K.W.; Karanam, N.K.; Patel, C.B.; Story, M.; Lou, E.; et al. The Mechanisms of Action of Tumor Treating Fields. Cancer Res. 2022, 82, 3650–3658. [Google Scholar] [CrossRef]
- Shams, S.; Patel, C.B. Anti-cancer mechanisms of action of therapeutic alternating electric fields (tumor treating fields [TTFields]). J. Mol. Cell Biol. 2022, 14, mjac047. [Google Scholar] [CrossRef]
- Rominiyi, O.; Vanderlinden, A.; Clenton, S.J.; Bridgewater, C.; Al-Tamimi, Y.; Collis, S.J. Tumour treating fields therapy for glioblastoma: Current advances and future directions. Br. J. Cancer 2021, 124, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Le, H.T.; Staelens, M.; Lazzari, D.; Chan, G.; Tuszyński, J.A. Real-Time Monitoring of the Effect of Tumour-Treating Fields on Cell Division Using Live-Cell Imaging. Cells 2022, 11, 2712. [Google Scholar] [CrossRef] [PubMed]
- Karanam, N.K.; Ding, L.; Aroumougame, A.; Story, M.D. Tumor treating fields cause replication stress and interfere with DNA replication fork maintenance: Implications for cancer therapy. Transl. Res. 2020, 217, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Jo, Y.; Oh, G.; Gi, Y.; Kim, H.; Park, S.; Seo, J.; Yoon, M. Technical note: Evaluation of methods for reducing edge current density under electrode arrays for tumor-treating fields therapy. Med. Phys. 2022, 49, 4837–4844. [Google Scholar] [CrossRef]
- Kong, Y.; Liu, F.; Ma, B.; Duan, J.; Yuan, W.; Sang, Y.; Han, L.; Wang, S.; Liu, H. Wireless Localized Electrical Stimulation Generated by an Ultrasound-Driven Piezoelectric Discharge Regulates Proinflammatory Macrophage Polarization. Adv. Sci. 2021, 8, 2100962. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Ye, T.; Zhang, X.; Dong, Y.; Liu, J.; Zhang, W.; Wu, F.; Bo, H.; Shao, H.; Zhang, R.; Shen, H. Chemokine CCL17 Affects Local Immune Infiltration Characteristics and Early Prognosis Value of Lung Adenocarcinoma. Front. Cell Dev. Biol. 2022, 10, 816927. [Google Scholar] [CrossRef]
- Miao, Y.-B.; Ren, H.-X.; Zhang, G.; Song, F.-X.; Liu, W.; Shi, Y. Achieving precise non-invasive ROS spatiotemporal manipulation for colon cancer immunotherapy. Chem. Eng. J. 2024, 481, 148520. [Google Scholar] [CrossRef]
- Marino, A.; Battaglini, M.; De Pasquale, D.; Degl’Innocenti, A.; Ciofani, G. Ultrasound-Activated Piezoelectric Nanoparticles Inhibit Proliferation of Breast Cancer Cells. Sci. Rep. 2018, 8, 6257. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Khan, M.A.M.; Alhadlaq, H.A.; Alshamsan, A. Barium Titanate (BaTiO3) Nanoparticles Exert Cytotoxicity through Oxidative Stress in Human Lung Carcinoma (A549) Cells. Nanomaterials 2020, 10, 2309. [Google Scholar] [CrossRef]
- Yoon, Y.N.; Lee, D.-S.; Park, H.J.; Kim, J.-S. Barium Titanate Nanoparticles Sensitise Treatment-Resistant Breast Cancer Cells to the Antitumor Action of Tumour-Treating Fields. Sci. Rep. 2020, 10, 2560. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Tang, Q.; Zhang, L.; Xu, M.; Sun, L.; Sun, S.; Zhang, J.; Wang, S.; Liang, X. Ultrasmall Barium Titanate Nanoparticles for Highly Efficient Hypoxic Tumor Therapy via Ultrasound Triggered Piezocatalysis and Water Splitting. ACS Nano 2021, 15, 11326–11340. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Xiao, C.; Li, C.; Zhai, J.; Yang, F.; Piao, J.; Ning, C.; Zhou, Z.; Yu, P.; Qi, S. Internal Wireless Electrical Stimulation from Piezoelectric Barium Titanate Nanoparticles as a New Strategy for the Treatment of Triple-Negative Breast Cancer. ACS Appl. Mater. Interfaces 2022, 14, 45032–45041. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Almici, E.; Migliorin, S.; Tapeinos, C.; Battaglini, M.; Cappello, V.; Marchetti, M.; De Vito, G.; Cicchi, R.; Pavone, F.S.; et al. Piezoelectric barium titanate nanostimulators for the treatment of glioblastoma multiforme. J. Colloid. Interface Sci. 2019, 538, 449–461. [Google Scholar] [CrossRef]
- Racca, L.; Limongi, T.; Vighetto, V.; Dumontel, B.; Ancona, A.; Canta, M.; Canavese, G.; Garino, N.; Cauda, V. Zinc Oxide Nanocrystals and High-Energy Shock Waves: A New Synergy for the Treatment of Cancer Cells. Front. Bioeng. Biotechnol. 2020, 8, 577. [Google Scholar]
- Truong Hoang, Q.; Ravichandran, V.; Nguyen Cao, T.G.; Kang, J.H.; Ko, Y.T.; Lee, T.I.; Shim, M.S. Piezoelectric Au-decorated ZnO nanorods: Ultrasound-triggered generation of ROS for piezocatalytic cancer therapy. Chem. Eng. J. 2022, 435, 135039. [Google Scholar] [CrossRef]
- Han, Q.; Fang, Z.; Lin, R.; Chen, J.; Wei, X.; Gong, C.; Yang, Z.; Zou, P.; Zhu, J.; Xing, L.; et al. Piezo-photodynamic therapy of Au@PEG-ZnO nanostructures enabled with a battery-free wireless cancer therapeutic dot. Nano Energy 2024, 125, 109530. [Google Scholar] [CrossRef]
- Xie, C.; Ding, R.; Wang, X.; Hu, C.; Yan, J.; Zhang, W.; Wang, Y.; Qu, Y.; Zhang, S.; He, P.; et al. A disulfiram-loaded electrospun poly(vinylidene fluoride) nanofibrous scaffold for cancer treatment. Nanotechnology 2020, 31, 115101. [Google Scholar] [CrossRef]
- Milenković, S.; Virijević, K.; Živić, F.; Radojević, I.; Grujović, N. Composite Nanoarchitectonics of Electrospun Piezoelectric PVDF/AgNPs for Biomedical Applications, Including Breast Cancer Treatment. Materials 2024, 17, 3872. [Google Scholar] [CrossRef]
- Silva Pedraza, Z.; Wang, Y.; Carlos, C.; Tang, Z.; Li, J.; Cai, W.; Wang, X. Development of Ferroelectric P(VDF-TrFE) Microparticles for Ultrasound-Driven Cancer Cell Killing. ACS Appl. Mater. Interfaces 2023, 15, 54304–54311. [Google Scholar] [CrossRef]
- Montorsi, M.; Pucci, C.; De Pasquale, D.; Marino, A.; Ceccarelli, M.C.; Mazzuferi, M.; Bartolucci, M.; Petretto, A.; Prato, M.; Debellis, D.; et al. Ultrasound-Activated Piezoelectric Nanoparticles Trigger Microglia Activity Against Glioblastoma Cells. Adv. Healthc. Mater. 2024, 13, 2304331. [Google Scholar] [CrossRef] [PubMed]
- Pucci, C.; Marino, A.; Şen, Ö.; De Pasquale, D.; Bartolucci, M.; Iturrioz-Rodríguez, N.; Di Leo, N.; De Vito, G.; Debellis, D.; Petretto, A.; et al. Ultrasound-responsive nutlin-loaded nanoparticles for combined chemotherapy and piezoelectric treatment of glioblastoma cells. Acta Biomater. 2022, 139, 218–236. [Google Scholar] [CrossRef] [PubMed]
- Shah, S. Novel Therapies in Glioblastoma Treatment: Review of Glioblastoma; Current Treatment Options; and Novel Oncolytic Viral Therapies. Med. Sci. 2023, 12, 1. [Google Scholar] [CrossRef]
- Perry, J.R.; Laperriere, N.; O’Callaghan, C.J.; Brandes, A.A.; Menten, J.; Phillips, C.; Fay, M.; Nishikawa, R.; Cairncross, J.G.; Roa, W.; et al. Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N. Engl. J. Med. 2017, 376, 1027–1037. [Google Scholar] [CrossRef]
- Kim, T.; Kim, H.J.; Choi, W.; Lee, Y.M.; Pyo, J.H.; Lee, J.; Kim, J.; Kim, J.; Kim, J.-H.; Kim, C.; et al. Deep brain stimulation by blood–brain-barrier-crossing piezoelectric nanoparticles generating current and nitric oxide under focused ultrasound. Nat. Biomed. Eng. 2023, 7, 149–163. [Google Scholar] [CrossRef]
- Wang, J.; Cao, M.; Han, L.; Shangguan, P.; Liu, Y.; Zhong, Y.; Chen, C.; Wang, G.; Chen, X.; Lin, M.; et al. Blood–Brain Barrier-Penetrative Fluorescent Anticancer Agents Triggering Paraptosis and Ferroptosis for Glioblastoma Therapy. J. Am. Chem. Soc. 2024, 146, 28783–28794. [Google Scholar] [CrossRef]
- Srinivasa Rao, K.; Hamza, M.; Ashok Kumar, P.; Girija Sravani, K. Design and optimization of MEMS based piezoelectric actuator for drug delivery systems. Microsyst. Technol. 2020, 26, 1671–1679. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Seker-Polat, F.; Pinarbasi Degirmenci, N.A.-O.X.; Solaroglu, I.; Bagci-Onder, T.A.-O. Tumor Cell Infiltration into the Brain in Glioblastoma: From Mechanisms to Clinical Perspectives. Cancer 2022, 14, 443. [Google Scholar] [CrossRef]
- Noorani, I.; de la Rosa, J. Breaking barriers for glioblastoma with a path to enhanced drug delivery. Nat. Commun. 2023, 14, 5909. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. Jama 2017, 318, 2306–2316. [Google Scholar] [PubMed]
- Olatunji, G.; Aderinto, N.A.-O.; Adefusi, T.; Kokori, E.; Akinmoju, O.; Yusuf, I.; Olusakin, T.; Muzammil, M.A. Efficacy of tumour-treating fields therapy in recurrent glioblastoma: A narrative review of current evidence. Medicine 2023, 102, e36421. [Google Scholar] [PubMed]
- Yu, Q.; Shi, W.; Li, S.; Liu, H.; Zhang, J. Emerging Advancements in Piezoelectric Nanomaterials for Dynamic Tumor Therapy. Molecules 2023, 28, 3170. [Google Scholar] [CrossRef]


| Type of Nanomaterial | Specific Material | Potential Application in Biomedicine | Refs. | ||
|---|---|---|---|---|---|
| Synthetic Materials | Inorganic ceramics | Lead-based | Lead titanate | Electrochemical detection of nitrous oxide | [21,22,23] |
| Lead lanthanum zirconate | Electro-optic applications. Ultrasound imaging, reduction in aperture, wireless device applications, stabilising additive. | [24,25] | |||
| Lead zirconate titanate | Ceramic resonator for cancer biomarker detection and wireless monitoring applications. | [26,27,28] | |||
| Lead magnesium niobate | Medical ultrasound imaging (biomedical measuring). | [29,30] | |||
| Lead-free | Barium titanate | Tissue engineering, bioelectrical sensing, cancer therapy, drug delivery, gene engineering. | [31,32,33] | ||
| Lithium tantalate | Infrared detection and acoustic wave devices, glass and or bone engineering. | [34,35] | |||
| Iron oxide | Drug delivery, iron supplementation, cancer therapy, gene carriers, macrophage polarisation. | [36,37,38] | |||
| Zinc ferrite | Considered non-toxic. Cancer therapy (thermal ablation), antibacterial/antifungal, and inorganic/organic pollutant removal. | [39,40,41] | |||
| Organic polymers | Poly (vinylidene difluoride) | Multifilament for vascular grafts, ligaments, and corneas. Also, drug and diffusion media. It can also be used as an encapsulation material. | [42,43,44] | ||
| Nylon | Hybridised materials such as skin dressings and composites for piezoelectric bone stimulation. | [45,46] | |||
| Polyacrylonitrile | Hybridisation with metallic materials as an antibacterial agent. It could be used in wound healing and prevention. Hydrophilic and highly elastic material. | [47,48] | |||
| Cellulose acetate | Tissue engineering as a primary scaffold or scaffold structures to osteoblast/fibroblast anchoring. | [49,50] | |||
| Polymer composites | PVDF/PZT | It could be used for portable devices, mobile imaging, and electrical storage. | [51] | ||
| PVDF/ZnO | Organic electro-spun piezoelectric nanofibers. Self-powered sensors to monitor the cardiovascular walls of the heart. | [52] | |||
| PVDF/BT NPs | Increased electrical wireless stimulation in bone scaffolds for wound healing and oestogeneration. | [53] | |||
| PVDF/MOS2 | Next-generation sensors for wireless biomedical applications. | [54] | |||
| Natural Materials | Quartz crystals | Used in quartz crystal microbalance systems, which are used as wide-range biosensors. | [50,55] | ||
| Rochelle salt | To be developed as a 3D composite for prosthetics and smart sensing impact-prone professions (self-generative material). | [56,57] | |||
| Silk | Tissue engineering, cell coating, drug delivery, microfluidics, and formation of composite biomaterials. | [58,59] | |||
| Bone | An inherent piezoelectric material. Piezoelectrics contribute towards the promotion of regeneration and repair. | [60,61,62] | |||
| Ion Channel Class | Cellular Role | Mechanism of Action |
|---|---|---|
| Voltage-gated potassium channels | Regulate excitability and control the action potential waveform. Help with the secretion of hormones. | Activity regulated by calcium, voltage, and neurotransmitters |
| Voltage-gated chloride channels | Cell volume regulation, salt transport, and importantly, acidification of intracellular and extracellular components with cell cycle signalling. | Anion-selective and activated by intracellular calcium. |
| Acid-sensing ion channels | Permeable by Na+ and serves to detect extracellular PH from neuronal response. | Gated by membrane depolarisation by transmembrane PH gradient |
| Volage-gated sodium channels | Allow movement of electrically charged particles. Dysfunctions of these, change the charge potentials of cells | Gated by depolarisation, and rapid inactivation, and are the first channels to respond to changes in voltage. |
| Voltage-gated calcium channels | Present in the membrane of highly excitable cells. Works as a secondary messenger with many functions across the cell. | Form hetero-oligomeric complexes with the α1 subunit providing extracellular binding sites. Cav3 type (T types) are low voltage-activated, unlike L types. |
| Piezo channels | Expressed in mechanically sensitive cells. Allow Ca2+ influx and are observed in a variety of endothelial cells to sense physiological shear stress. | Allow the Ca2+ influx in response to external force. The main function and action of this channel is that of mechanical transduction. |
| Piezoelectric Nanomaterial | Size Range | Advantage | Limitation | Reference |
|---|---|---|---|---|
| Barium Titanate nanoparticles | 50–300 nm | -High Piezoelectric coefficient -Can be surface functionalised | -Possible inflammatory response | [155,172,173,174,175,176,177] |
| Zinc Oxide nanoparticles | 30–150 nm | -Biocompatible and biodegradable -Good piezoelectric responses | -Potential toxicity at high concentration | [155,178,179,180] |
| Polyvinylidene Fluoride (PVDF) nanofibers and nanoparticles | 20–100 nm | -Flexible and biocompatible | -Limited piezoelectric response compared to inorganic materials | [181,182] |
| Polyvinylidene fluoride-trifluoroethylene (PVDF-TrFE) micro particles | 2–6 µm | -Flexible, high piezoelectric output, | - Requires specific processing to enhance piezoelectric response | [183,184,185] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knight, Z.; Ruiz, A.; Elies, J. Piezoelectric Nanomaterials for Cancer Therapy: Current Research and Future Perspectives on Glioblastoma. J. Funct. Biomater. 2025, 16, 114. https://doi.org/10.3390/jfb16040114
Knight Z, Ruiz A, Elies J. Piezoelectric Nanomaterials for Cancer Therapy: Current Research and Future Perspectives on Glioblastoma. Journal of Functional Biomaterials. 2025; 16(4):114. https://doi.org/10.3390/jfb16040114
Chicago/Turabian StyleKnight, Zayne, Amalia Ruiz, and Jacobo Elies. 2025. "Piezoelectric Nanomaterials for Cancer Therapy: Current Research and Future Perspectives on Glioblastoma" Journal of Functional Biomaterials 16, no. 4: 114. https://doi.org/10.3390/jfb16040114
APA StyleKnight, Z., Ruiz, A., & Elies, J. (2025). Piezoelectric Nanomaterials for Cancer Therapy: Current Research and Future Perspectives on Glioblastoma. Journal of Functional Biomaterials, 16(4), 114. https://doi.org/10.3390/jfb16040114







