Application of Hydroxyapatite Composites in Bone Tissue Engineering: A Review
Abstract
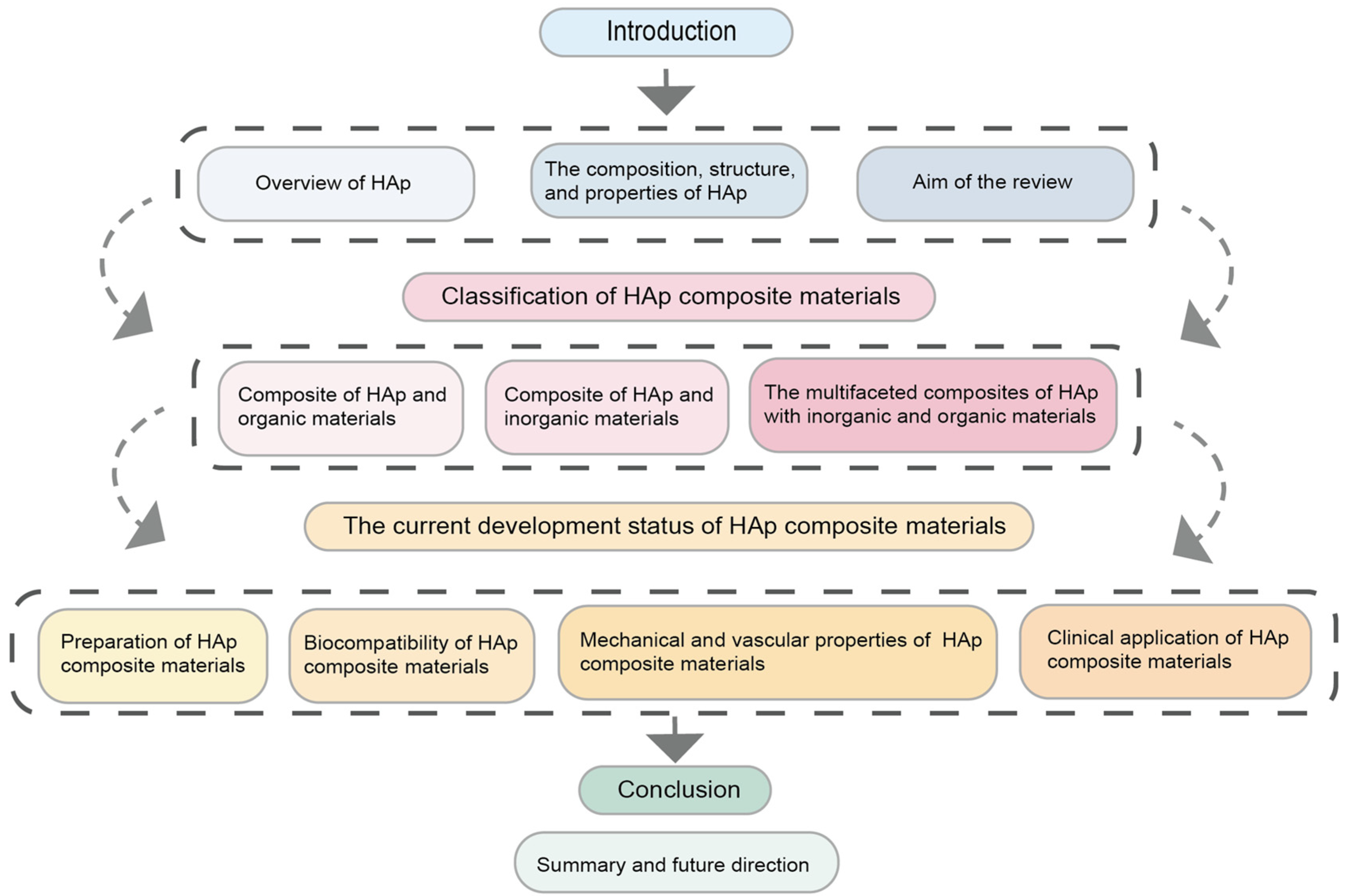
:1. Introduction
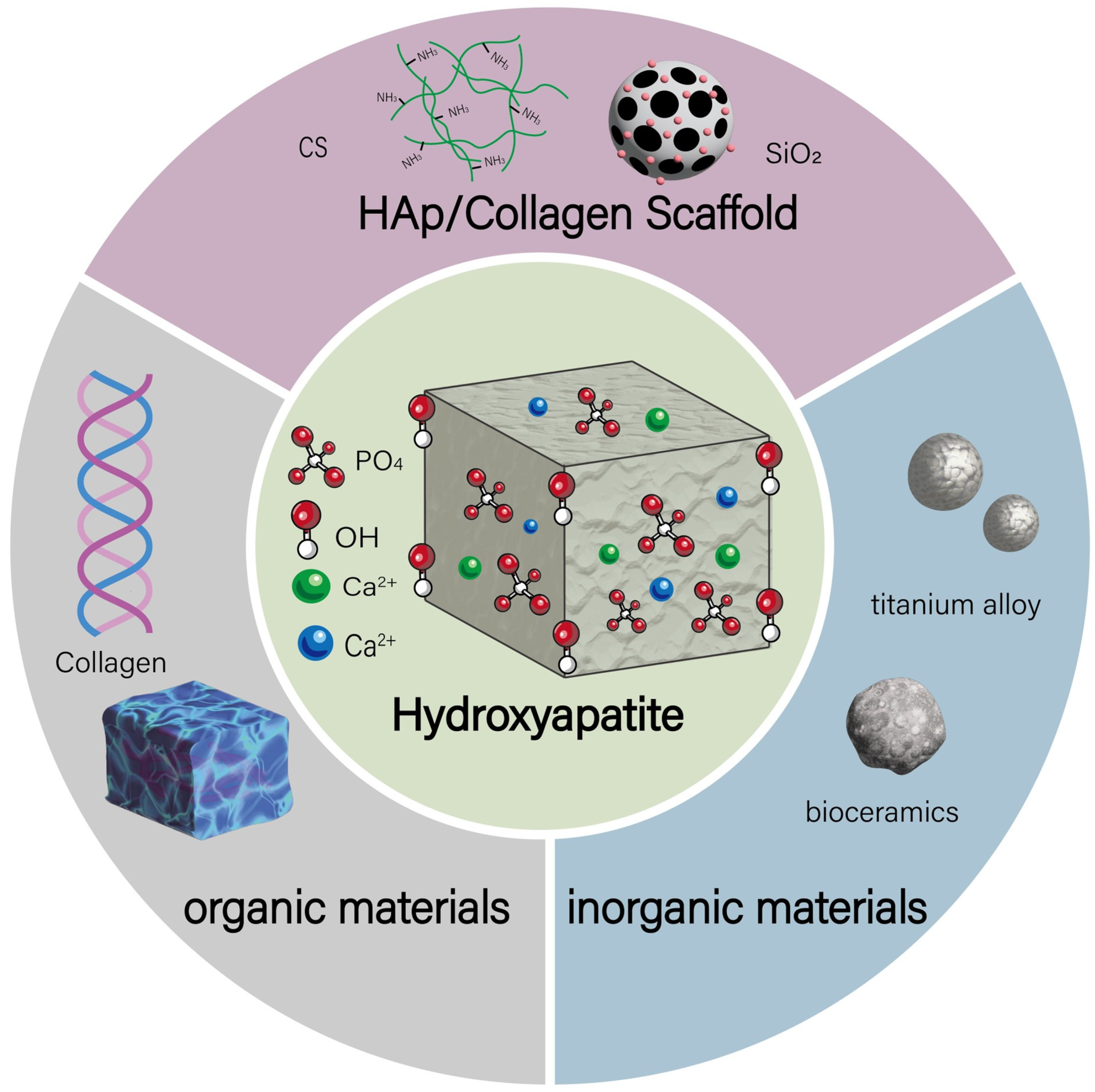
2. HAp Composites Classification
2.1. Inorganic-Based HAp Composites
2.2. Organic-Based HAp Composites
2.3. Hybrid HAp Composites
| Classification of Composite Materials | Combination of HAp with Types of Reinforcements | HAp Sintering Temperature (°C) | Preparation Method | Function | Refs |
|---|---|---|---|---|---|
| Inorganic-based HAp Composites | HAp-Ti/Al alloy | 900 | High-velocity suspension flame spray (HVSFS) technique | Improves bioactivity, biodegradability, and bone conduction capability | [32,65,66] |
| HAp/SiO2 | 1200 | Mechanochemical synthesis followed by sintering; sol–gel method | Improves the hardness, toughness, compressive strength, and thermal stability of the composite material, and serves as a drug carrier | [27,34,67] | |
| HAp/CNT | 1100 | In situ growth method; sol–gel method; mechanical mixing method | Improves material strength, toughness, electrical properties, induces bone formation, serves as a drug carrier, and exhibits antimicrobial performance | [37,41,68] | |
| Ag+/Si-HAp | 600 | High-temperature and high-pressure hydrothermal method | Inhibits multiple pathogenic yeast species | [35,69] | |
| Organic-based HAp Composites | Col/HAp | 900 | Microwave-assisted co-titration method | Improves the mechanical modulus of the scaffold material | [28,42] |
| PLA/HAp | / | Preparation of electrospinning solution | Promotes bone cell adhesion/proliferation, increases fiber size/roughness, slows degradation, enhances bioactivity | [48,49] | |
| SNF/nHAp | / | HAp formation via in situ precipitation from calcium phosphate solution on SNF template | Vascularization and bone regeneration | [29,67,70] | |
| HAp/PCL | 800 | Liquid deposition modeling (LDM); 3D printing technology | Compatible with structurally complex shapes, high porosity, and tailored for personalized treatment | [61,71,72] | |
| Hybrid HAp Composites | HAp/CS/SiO2 | 1200 | Sol–gel method; 3D printing technology | Drug and protein loading capability, and osteogenic effect | [51,52,53,73] |
| HAp/CS/Zn | 800 | Co-precipitation method | Antibacterial effect and improvement of scaffold morphology | [54,55,56,57,74] | |
| HAp/Collagen/Sr | / | Polymer-induced liquid precursor (PILP) | Enhances bone cell differentiation and osseointegration of the scaffold, and improves mechanical properties | [58,59,60] | |
| HAp/CaP/Col | / | Preparation by vacuum infusion process and biomimetic mineralization method | Improves mechanical performance and accelerates bone formation | [26,75] |
3. Biomedical Advances in Hydroxyapatite Scaffold Design
3.1. Preparation of Hydroxyapatite Composites
3.2. Biocompatibility of Hydroxyapatite Composites
3.3. Mechanical Properties and Vascularization Performance of Hydroxyapatite Composites
3.4. Clinical Applications of Hydroxyapatite Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deplaigne, V.; Rochefort, G.Y. Bone tissue engineering at a glance. AIMS Bioeng. 2022, 9, 22–25. [Google Scholar]
- Song, J.E.; Lee, D.H.; Khang, G.; Yoon, S.J. Accelerating bone regeneration using poly(lactic-co-glycolic acid)/hydroxyapatite scaffolds containing duck feet-derived collagen. Int. J. Biol. Macromol. 2023, 229, 486–495. [Google Scholar] [PubMed]
- Yuan, X.; Zhu, W.; Yang, Z.; He, N.; Chen, F.; Han, X.; Zhou, K. Recent Advances in 3D Printing of Smart Scaffolds for Bone Tissue Engineering and Regeneration. Adv. Mater. 2024, 36, e2403641. [Google Scholar] [PubMed]
- Bhushan, S.; Singh, S.; Maiti, T.K.; Sharma, C.; Dutt, D.; Sharma, S.; Li, C.; Tag Eldin, E.M. Scaffold Fabrication Techniques of Biomaterials for Bone Tissue Engineering: A Critical Review. Bioengineering 2022, 9, 728. [Google Scholar] [CrossRef]
- Costa, J.B.; Pereira, H.; Espregueira-Mendes, J.; Khang, G.; Oliveira, J.M.; Reis, R.L. Tissue engineering in orthopaedic sports medicine: Current concepts. J. ISAKOS 2017, 2, 60–66. [Google Scholar]
- Huang, B.; Li, S.; Dai, S.; Lu, X.; Wang, P.; Li, X.; Zhao, Z.; Wang, Q.; Li, N.; Wen, J.; et al. Ti3C2Tx MXene-Decorated 3D-Printed Ceramic Scaffolds for Enhancing Osteogenesis by Spatiotemporally Orchestrating Inflammatory and Bone Repair Responses. Adv. Sci. 2024, 11, e2400229. [Google Scholar]
- Kang, M.; Lee, C.-S.; Lee, M. Bioactive Scaffolds Integrated with Liposomal or Extracellular Vesicles for Bone Regeneration. Bioengineering 2021, 8, 137. [Google Scholar] [CrossRef]
- He, S.; Fang, J.; Zhong, C.; Wang, M.; Ren, F. Spatiotemporal Delivery of pBMP2 and pVEGF by a Core-Sheath Structured Fiber-Hydrogel Gene-Activated Matrix Loaded with Peptide-Modified Nanoparticles for Critical-Sized Bone Defect Repair. Adv. Health Mater. 2022, 11, e2201096. [Google Scholar]
- Tsiklin, I.L.; Shabunin, A.V.; Kolsanov, A.V.; Volova, L.T. In Vivo Bone Tissue Engineering Strategies: Advances and Prospects. Polymers 2022, 14, 3222. [Google Scholar] [CrossRef]
- George, S.M.; Nayak, C.; Singh, I.; Balani, K. Multifunctional Hydroxyapatite Composites for Orthopedic Applications: A Review. ACS Biomater. Sci. Eng. 2022, 8, 3162–3186. [Google Scholar]
- Mohd Pu’ad, N.A.S.; Abdul Haq, R.H.; Mohd Noh, H.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Synthesis method of hydroxyapatite: A review. Mater. Today Proc. 2020, 29, 233–239. [Google Scholar]
- Girón, J.; Kerstner, E.; Medeiros, T.; Oliveira, L.; Machado, G.M.; Malfatti, C.F.; Pranke, P. Biomaterials for bone regeneration: An orthopedic and dentistry overview. Braz. J. Med. Biol. Res. 2021, 54, e11055. [Google Scholar]
- Tariq, U.; Haider, Z.; Chaudhary, K.; Hussain, R.; Ali, J. Calcium to phosphate ratio measurements in calcium phosphates using LIBS. J. Phys. Conf. Ser. 2018, 1027, 012015. [Google Scholar]
- Tzavellas, A.-N.; Katrilaka, C.; Karipidou, N.; Kanari, M.; Pitou, M.; Koliakos, G.; Cheva, A.; Choli-Papadopoulou, T.; Aggeli, A.; Tsiridis, E. The “Forgotten” Hydroxyapatite Crystals in Regenerative Bone Tissue Engineering: A Critical Review. Crystals 2024, 14, 448. [Google Scholar] [CrossRef]
- Kribaa, O.K.; Labidi, C.E.; Djouama, H.; Laacher, L. Coupled experimental and theoretical study of hydroxyapatite ceramics. Bioinspired Biomim. Nanobiomater. 2023, 12, 104–114. [Google Scholar]
- Delpierre, A.; Savard, G.; Renaud, M.; Rochefort, G.Y. Tissue Engineering Strategies Applied in Bone Regeneration and Bone Repair. Bioengineering 2023, 10, 644. [Google Scholar] [CrossRef]
- Mondal, S.; Park, S.; Choi, J.; Vu, T.T.H.; Doan, V.H.M.; Vo, T.T.; Lee, B.; Oh, J. Hydroxyapatite: A journey from biomaterials to advanced functional materials. Adv. Colloid Interface Sci. 2023, 321, 103013. [Google Scholar]
- Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. Hydroxyapatite for Biomedical Applications: A Short Overview. Ceramics 2021, 4, 542–563. [Google Scholar] [CrossRef]
- Poddar, D.; Singh, A.; Rao, P.; Mohanty, S.; Jain, P. Modified-Hydroxyapatite-Chitosan Hybrid Composite Interfacial Coating on 3D Polymeric Scaffolds for Bone Tissue Engineering. Macromol. Biosci. 2023, 23, e2300243. [Google Scholar]
- Alkaron, W.; Almansoori, A.; Balázsi, K.; Balázsi, C. Hydroxyapatite-Based Natural Biopolymer Composite for Tissue Regeneration. Materials 2024, 17, 4117. [Google Scholar] [CrossRef]
- Yang, R.; Chen, B.; Zhang, X.; Bao, Z.; Yan, Q.; Luan, S. Degradable Nanohydroxyapatite-Reinforced Superglue for Rapid Bone Fixation and Promoted Osteogenesis. ACS Nano 2024, 18, 8517–8530. [Google Scholar]
- Cowan, C.M.; Soo, C.; Ting, K.; Wu, B. Evolving concepts in bone tissue engineering. Curr. Top. Dev. Biol. 2005, 66, 239–285. [Google Scholar] [PubMed]
- Salah, M.; Tayebi, L.; Moharamzadeh, K.; Naini, F.B. Three-dimensional bio-printing and bone tissue engineering: Technical innovations and potential applications in maxillofacial reconstructive surgery. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 18. [Google Scholar]
- Dec, P.; Modrzejewski, A.; Pawlik, A. Existing and Novel Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2022, 24, 529. [Google Scholar] [CrossRef]
- Shi, J.; Dai, W.; Gupta, A.; Zhang, B.; Wu, Z.; Zhang, Y.; Pan, L.; Wang, L. Frontiers of Hydroxyapatite Composites in Bionic Bone Tissue Engineering. Materials 2022, 15, 8475. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Ye, X.; Fan, Y.; Ma, L.; Tan, Y.; Qing, F.; Zhang, X. Biomimetic fabrication of a three-level hierarchical calcium phosphate/collagen/hydroxyapatite scaffold for bone tissue engineering. Biofabrication 2014, 6, 035013. [Google Scholar]
- Taha, M.A.; Youness, R.A.; Ibrahim, M. Biocompatibility, physico-chemical and mechanical properties of hydroxyapatite-based silicon dioxide nanocomposites for biomedical applications. Ceram. Int. 2020, 46, 23599–23610. [Google Scholar]
- Wang, J.; Liu, C. Biomimetic Collagen/Hydroxyapatite Composite Scaffolds: Fabrication and Characterizations. J. Bionic Eng. 2014, 11, 600–609. [Google Scholar]
- Cheng, W.; Ding, Z.; Zheng, X.; Lu, Q.; Kong, X.; Zhou, X.; Lu, G.; Kaplan, D.L. Injectable hydrogel systems with multiple biophysical and biochemical cues for bone regeneration. Biomater. Sci. 2020, 8, 2537–2548. [Google Scholar]
- Bollino, F.; Armenia, E.; Tranquillo, E. Zirconia/Hydroxyapatite Composites Synthesized Via Sol-Gel: Influence of Hydroxyapatite Content and Heating on Their Biological Properties. Materials 2017, 10, 757. [Google Scholar] [CrossRef]
- Eyidogan, B.; Kirca, M. Mechanical enhancement of hydroxyapatite via carbon and boron nitride nanotubes: A molecular dynamics study. J. Mol. Model. 2025, 31, 88. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, E.M.; Sayed, M.; Awaad, M.; El-Zomor, S.T.; Blum, M.; Killinger, A.; Gadow, R.; Naga, S.M. Evaluation of Ti/Al alloy coated with biogenic hydroxyapatite as an implant device in dogs’ femur bones. J. Mater. Sci. Mater. Med. 2021, 32, 119. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.C.; Treccani, L.; Rezwan, K. Effect of silica on porosity, strength, and toughness of pressureless sintered calcium phosphate-zirconia bioceramics. Biomed. Mater. 2015, 10, 045020. [Google Scholar] [CrossRef]
- Garibay-Alvarado, J.A.; Herrera-Ríos, E.B.; Vargas-Requena, C.L.; de Jesús Ruíz-Baltazar, Á.; Reyes-López, S.Y. Cell behavior on silica-hydroxyapatite coaxial composite. PLoS ONE 2021, 16, e0246256. [Google Scholar] [CrossRef]
- Piecuch, A.; Targońska, S.; Rewak-Sorczyńska, J.; Ogórek, R.; Wiglusz, R.J. New silicate-substituted hydroxyapatite materials doped with silver ions as potential antifungal agents. BMC Microbiol. 2023, 23, 193. [Google Scholar] [CrossRef] [PubMed]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of carbon nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef]
- Lekshmi, G.; Sana, S.S.; Nguyen, V.-H.; Nguyen, T.H.C.; Nguyen, C.C.; Le, Q.V.; Peng, W. Recent Progress in Carbon Nanotube Polymer Composites in Tissue Engineering and Regeneration. Int. J. Mol. Sci. 2020, 21, 6440. [Google Scholar] [CrossRef]
- Nayak, C.; Singh, P.; Balani, K. Contact stress and sliding wear damage tolerance of hydroxyapatite and carbon nanotube reinforced polyethylene cup liner against zirconia femoral head. J. Mech. Behav. Biomed. Mater. 2022, 136, 105435. [Google Scholar] [CrossRef]
- Dalili, F.; Aghdam, R.M.; Soltani, R.; Saremi, M. Corrosion, mechanical and bioactivity properties of HA-CNT nanocomposite coating on anodized Ti6Al4V alloy. J. Mater. Sci. Mater. Med. 2022, 33, 34. [Google Scholar] [CrossRef]
- Shankar, D.; Jambagi, S.C.; Gowda, N.; Lakshmi, K.S.; Jayanthi, K.J.; Chaudhary, V.K. Effect of Surface Chemistry on Hemolysis, Thrombogenicity, and Toxicity of Carbon Nanotube Doped Thermally Sprayed Hydroxyapatite Implants. ACS Biomater. Sci. Eng. 2024, 10, 1403–1417. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, X.; Yeung, K.W.K.; Chu, P.K.; Wu, S. Biofunctionalization of carbon nanotubes/chitosan hybrids on Ti implants by atom layer deposited ZnO nanostructures. Appl. Surf. Sci. 2017, 400, 14–23. [Google Scholar]
- Yang, W.; Ni, W.; Yu, C.; Gu, T.; Ye, L.; Sun, R.; Ying, X.; Yik, J.H.N.; Haudenschild, D.R.; Yao, S.; et al. Biomimetic Bone-Like Composite Hydrogel Scaffolds Composed of Collagen Fibrils and Natural Hydroxyapatite for Promoting Bone Repair. ACS Biomater. Sci. Eng. 2024, 10, 2385–2397. [Google Scholar]
- Mulazzi, M.; Campodoni, E.; Bassi, G.; Montesi, M.; Panseri, S.; Bonvicini, F.; Gentilomi, G.A.; Tampieri, A.; Sandri, M. Medicated Hydroxyapatite/Collagen Hybrid Scaffolds for Bone Regeneration and Local Antimicrobial Therapy to Prevent Bone Infections. Pharmaceutics 2021, 13, 1090. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Du, T.; Liu, Y. Biomechanical Characteristics and Analysis Approaches of Bone and Bone Substitute Materials. J. Funct. Biomater. 2023, 14, 212. [Google Scholar] [CrossRef]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [PubMed]
- Tabrizian, P.; Sun, H.; Jargalsaikhan, U.; Sui, T.; Davis, S.; Su, B. Biomimetic Nacre-like Hydroxyapatite/Polymer Composites for Bone Implants. J. Funct. Biomater. 2023, 14, 393. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Markel, D.C.; Wang, S.; Shi, T.; Mao, G.; Ren, W. Electrospun polyvinyl alcohol-collagen-hydroxyapatite nanofibers: A biomimetic extracellular matrix for osteoblastic cells. Nanotechnology 2012, 23, 115101. [Google Scholar]
- Zhang, Y.; Wang, J.; Ma, Y.; Han, B.; Niu, X.; Liu, J.; Gao, L.; Wang, J.; Zhai, X.; Chu, K.; et al. Preparation of poly(lactic acid)/sintered hydroxyapatite composite biomaterial by supercritical CO2. Biomed. Mater. Eng. 2018, 29, 67–79. [Google Scholar]
- Kareem, M.M.; Tanner, K.E. Optimising micro-hydroxyapatite reinforced poly(lactide acid) electrospun scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Med. 2020, 31, 38. [Google Scholar]
- Wan, Y.; Wu, C.; Xiong, G.; Zuo, G.; Jin, J.; Ren, K.; Zhu, Y.; Wang, Z.; Luo, H. Mechanical properties and cytotoxicity of nanoplate-like hydroxyapatite/polylactide nanocomposites prepared by intercalation technique. J. Mech. Behav. Biomed. Mater. 2015, 47, 29–37. [Google Scholar]
- Dong, Y.; Liang, J.; Cui, Y.; Xu, S.; Zhao, N. Fabrication of novel bioactive hydroxyapatite-chitosan-silica hybrid scaffolds: Combined the sol-gel method with 3D plotting technique. Carbohydr. Polym. 2018, 197, 183–193. [Google Scholar]
- Abdian, N.; Etminanfar, M.; Hamishehkar, H.; Sheykholeslami, S.O.R. Incorporating mesoporous SiO2-HA particles into chitosan/hydroxyapatite scaffolds: A comprehensive evaluation of bioactivity and biocompatibility. Int. J. Biol. Macromol. 2024, 260 Pt 2, 129565. [Google Scholar]
- Jun, S.H.; Lee, E.J.; Jang, T.S.; Kim, H.E.; Jang, J.H.; Koh, Y.H. Bone morphogenic protein-2 (BMP-2) loaded hybrid coating on porous hydroxyapatite scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Med. 2013, 24, 773–782. [Google Scholar] [PubMed]
- Bhowmick, A.; Banerjee, S.L.; Pramanik, N.; Jana, P.; Mitra, T.; Gnanamani, A.; Das, M.; Kundu, P.P. Organically modified clay supported chitosan/hydroxyapatite-zinc oxide nanocomposites with enhanced mechanical and biological properties for the application in bone tissue engineering. Int. J. Biol. Macromol. 2018, 106, 11–19. [Google Scholar] [PubMed]
- Bhushan, S.; Singh, S.; Maiti, T.K.; Das, A.; Barui, A.; Chaudhari, L.R.; Joshi, M.; Dutt, D. Zinc-doped hydroxyapatite loaded chitosan gelatin nanocomposite scaffolds as a promising platform for bone regeneration. Biomed. Mater. 2024, 20, 025006. [Google Scholar]
- Sukhodub, L.B.; Kumeda, M.; Bielai, V.; Sukhodub, L.F. Hydroxyapatite-biopolymers-ZnO composite with sustained Ceftriaxone release as a drainage system for treatment of purulent cavities. Carbohydr. Polym. 2021, 266, 118137. [Google Scholar] [PubMed]
- Tripathi, A.; Saravanan, S.; Pattnaik, S.; Moorthi, A.; Partridge, N.C.; Selvamurugan, N. Bio-composite scaffolds containing chitosan/nano-hydroxyapatite/nano-copper-zinc for bone tissue engineering. Int. J. Biol. Macromol. 2012, 50, 294–299. [Google Scholar]
- Ye, Z.; Qi, Y.; Zhang, A.; Karels, B.J.; Aparicio, C. Biomimetic Mineralization of Fibrillar Collagen with Strontium-doped Hydroxyapatite. ACS Macro Lett. 2023, 12, 408–414. [Google Scholar]
- Kontogianni, G.I.; Coelho, C.; Gauthier, R.; Fiorilli, S.; Quadros, P.; Vitale-Brovarone, C.; Chatzinikolaidou, M. Osteogenic Potential of Nano-Hydroxyapatite and Strontium-Substituted Nano-Hydroxyapatite. Nanomaterials 2023, 13, 1881. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, H.; Sun, X.Y.; Xia, W.; Deng, C. In vitro and in vivo study on the osseointegration of magnesium and strontium ion with two different proportions of mineralized collagen and its mechanism. J. Biomater. Appl. 2021, 36, 528–540. [Google Scholar]
- Babaei, M.; Ebrahim-Najafabadi, N.; Mirzadeh, M.; Abdali, H.; Farnaghi, M.; Gharavi, M.K.; Kheradmandfard, M.; Kharazi, A.Z.; Poursamar, S.A. A comprehensive bench-to-bed look into the application of gamma-sterilized 3D-printed polycaprolactone/hydroxyapatite implants for craniomaxillofacial defects, an in vitro, in vivo, and clinical study. Biomater. Adv. 2024, 161, 213900. [Google Scholar]
- Soleymani, S.; Naghib, S.M. 3D and 4D printing hydroxyapatite-based scaffolds for bone tissue engineering and regeneration. Heliyon 2023, 9, e19363. [Google Scholar]
- Ma, H.; Feng, C.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018, 79, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Han, S.; Wu, W.; Wu, Z.; Yuan, Y.; Wu, J.; Liu, C. Harnessing 4D Printing Bioscaffolds for Advanced Orthopedics. Small 2022, 18, e2106824. [Google Scholar] [PubMed]
- Ma, L.; Li, M.; Komasa, S.; Hontsu, S.; Hashimoto, Y.; Okazaki, J.; Maekawa, K. Effect of Er:YAG Pulsed Laser-Deposited Hydroxyapatite Film on Titanium Implants on M2 Macrophage Polarization In Vitro and Osteogenesis In Vivo. Int. J. Mol. Sci. 2023, 25, 349. [Google Scholar] [CrossRef]
- Hussain, S.; Shah, Z.A.; Sabiruddin, K.; Keshri, A.K. Characterization and tribological behaviour of Indian clam seashell-derived hydroxyapatite coating applied on titanium alloy by plasma spray technique. J. Mech. Behav. Biomed. Mater. 2023, 137, 105550. [Google Scholar]
- Herrera-Alonso, A.E.; Ibarra-Alonso, M.C.; Esparza-González, S.C.; Estrada-Flores, S.; García-Cerda, L.A.; Martínez-Luévanos, A. Biomimetic Growth of Hydroxyapatite on SiO2 Microspheres to Improve Its Biocompatibility and Gentamicin Loading Capacity. Materials 2021, 14, 6941. [Google Scholar] [CrossRef]
- Correia, J.V.F.B.; Correia, M.F.B. Properties of chitosan matrix composites with hydroxyapatite and carbon nanotubes, and their use in bone tissue engineering. Arch. Biosci. Health 2019, 1, 139–148. [Google Scholar]
- Farmani, M.; Mirahmadi-Zare, S.Z.; Masaeli, E.; Tabatabaei, F.; Houreh, A.B. Macroporous coating of silver-doped hydroxyapatite/silica nanocomposite on dental implants by EDTA intermediate to improve osteogenesis, antibacterial, and corrosion behavior. Biomed. Mater. 2025, 20, 025010. [Google Scholar]
- Huang, Y.; Xie, H.; Fang, W.; Zou, Z.; Fu, Z. Silk fibroin directs the formation of monetite nanocrystals and their assembly into hierarchical composites. J. Mater. Chem. B 2021, 9, 9136–9141. [Google Scholar] [CrossRef]
- Liu, J.; Yu, P.; Wang, D.; Chen, Z.; Cui, Q.; Hu, B.; Zhang, D.; Li, Y.; Chu, H.; Li, J. Wood-Derived Hybrid Scaffold with Highly Anisotropic Features on Mechanics and Liquid Transport toward Cell Migration and Alignment. ACS Appl. Mater. Interfaces 2020, 12, 17957–17966. [Google Scholar] [CrossRef]
- Iwamoto, T.; Hieda, Y.; Kogai, Y. Effect of hydroxyapatite surface morphology on cell adhesion. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.; Bhaskar, R.; Gupta, M.K.; Sharma, S.; Dasgupta, S.; Kumar, A.; Kumar, P. Mechanical, Electrical, and Biological Properties of Mechanochemically Processed Hydroxyapatite Ceramics. Nanomaterials 2021, 11, 2216. [Google Scholar] [CrossRef] [PubMed]
- Trzaskowska, M.; Vivcharenko, V.; Przekora, A. The Impact of Hydroxyapatite Sintering Temperature on Its Microstructural, Mechanical, and Biological Properties. Int. J. Mol. Sci. 2023, 24, 5083. [Google Scholar] [CrossRef] [PubMed]
- Bahir, M.M.; Rajendran, A.; Pattanayak, D.; Lenka, N. Fabrication and characterization of ceramic-polymer composite 3D scaffolds and demonstration of osteoinductive propensity with gingival mesenchymal stem cells. RSC Adv. 2023, 13, 26967–26982. [Google Scholar] [CrossRef]
- Shaikh, S.; Gupta, S.; Mishra, A.; Sheikh, P.A.; Singh, P.; Kumar, A. Laser-assisted synthesis of nano-hydroxyapatite and functionalization with bone active molecules for bone regeneration. Colloids Surf. B. Biointerfaces 2024, 237, 113859. [Google Scholar] [CrossRef]
- Hashemi, N.; Vaezi, Z.; Khanmohammadi, S.; Naderi Sohi, A.; Masoumi, S.; Hruschka, V.; Wolbank, S.; Redl, H.; Marolt Presen, D.; Naderi-Manesh, H. A novel fluorescent hydroxyapatite based on iron quantum cluster template to enhance osteogenic differentiation. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110775. [Google Scholar] [CrossRef]
- Wang, Z.; Shang, J.; Zhang, Z. Composite or Modified Hydroxyapatite Microspheres as Drug Delivery Carrier for Bone and Tooth Tissue Engineering. Curr. Med. Chem. 2024, 32, 974–981. [Google Scholar] [CrossRef]
- Ferraz, M.P. Bone Grafts in Dental Medicine: An Overview of Autografts, Allografts and Synthetic Materials. Materials 2023, 16, 4117. [Google Scholar] [CrossRef]
- Okuda, Y.; Sasaki, R.; Kido, E.; Hirota, K.; Mizutani, T. One-Pot Hybridization of Microfibrillated Cellulose and Hydroxyapatite as a Versatile Route to Eco-Friendly Mechanical Materials. ACS Omega 2024, 9, 44457–44464. [Google Scholar] [CrossRef]
- Costa, W.B.; Félix Farias, A.F.; Silva-Filho, E.C.; Osajima, J.A.; Medina-Carrasco, S.; Del Mar Orta, M.; Fonseca, M.G. Polysaccharide Hydroxyapatite (Nano)composites and Their Biomedical Applications: An Overview of Recent Years. ACS Omega 2024, 9, 30035–30070. [Google Scholar] [CrossRef]
- Wu, M.Y.; Kuo, Y.T.; Kao, I.F.; Yen, S.K. Porous Chitosan/Hydroxyapatite Composite Microspheres for Vancomycin Loading and Releasing. Pharmaceutics 2024, 16, 730. [Google Scholar] [CrossRef]
- Sathiyavimal, S.; Vasantharaj, S.; Mattheos, N.; Pugazhendhi, A.; Subbalekha, K. Mussel shell-derived biogenic hydroxyapatite as reinforcement on chitosan-loaded gentamicin composite for antibacterial activity and bone regeneration. Int. J. Biol. Macromol. 2024, 278 Pt 2, 134143. [Google Scholar]
- Pul, M.; Erdem, Ü.; Bozer, B.M.; Şimşek, T.; Yılmazel, R.; Erten, M.Y. Synthesis of biocompatible Ti-6Al-4V composite reinforced with ZrO(2) and bioceramic produced by powder metallurgy: Morphological, structural, and biocompatibility analysis. Microsc. Res. Tech. 2024, 87, 2728–2744. [Google Scholar] [PubMed]
- Costa, J.P.; Sousa, S.A.; Leitão, J.H.; Marques, F.; Alves, M.M.; Carvalho, M. Insights into the Dual Anticancer and Antibacterial Activities of Composites Based on Silver Camphorimine Complexes. J. Funct. Biomater. 2024, 15, 240. [Google Scholar] [CrossRef]
- Zhou, Y.; Deng, J.; Zhang, Y.; Li, C.; Wei, Z.; Shen, J.; Li, J.; Wang, F.; Han, B.; Chen, D.; et al. Engineering DNA-Guided Hydroxyapatite Bulk Materials with High Stiffness and Outstanding Antimicrobial Ability for Dental Inlay Applications. Adv. Mater. 2022, 34, e2202180. [Google Scholar] [CrossRef] [PubMed]
- Jaita, P.; Chokethawai, K.; Randorn, C.; Boonsri, K.; Pringproa, K.; Thongkorn, K.; Watcharapasorn, A.; Jarupoom, P. Enhancing bioactivity and mechanical performances of hydroxyapatite-calcium sulfate bone cements for bone repair: In vivo histological evaluation in rabbit femurs. RSC Adv. 2024, 14, 23286–23302. [Google Scholar]
- Jaita, P.; Randorn, C.; Watcharapasorn, A.; Jarupoom, P. In vitro bioactivity, mechanical, and cell interaction of sodium chloride-added calcium sulfate-hydroxyapatite composite bone cements. RSC Adv. 2024, 14, 35460–35474. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, L.; Niu, B.; Zhao, H.; Hu, Y.; Wei, Y.; Huang, D.; Wang, T.; Lian, X. The study of self-regulating α-TCP based composite by micro/nano scaled silk fibroin and α-CSH on physicochemical and biological properties of bone cement. J. Biomater. Appl. 2023, 37, 1801–1812. [Google Scholar]
- Meng, Y.; Li, X.; Yun, B. Preparation and Characterization of Mechanical Properties of HAP/45S5 Bioglass Laminated Ceramic Composites via Spark Plasma Sintering. Materials 2024, 17, 5413. [Google Scholar] [CrossRef]
- Elango, J.; Bushin, R.; Lijnev, A.; De Aza, P.N.; Martínez, C.P.; Marín, J.M.G.; Hernandez, A.B.; Olmo, L.R.M.; Val, J. The Effect of Germanium-Loaded Hydroxyapatite Biomaterials on Bone Marrow Mesenchymal Stem Cells Growth. Cells 2022, 11, 2993. [Google Scholar] [CrossRef] [PubMed]
- Shendage, S.S.; Kachare, K.; Gaikwad, K.; Kashte, S.; Ghule, A.V. Porous calcium silicate bioactive material-alginate composite for bone regeneration. RSC Adv. 2024, 14, 25740–25749. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Wu, J.; Xu, F.; Zuo, Y.; Jansen, J.A. In vitro and in vivo study to the biocompatibility and biodegradation of hydroxyapatite/poly(vinyl alcohol)/gelatin composite. J. Biomed. Mater. Res. Part A 2008, 85, 418–426. [Google Scholar] [CrossRef]
- Ratnayake, J.; Gould, M.; Ramesh, N.; Mucalo, M.; Dias, G.J. A Porous Fluoride-Substituted Bovine-Derived Hydroxyapatite Scaffold Constructed for Applications in Bone Tissue Regeneration. Materials 2024, 17, 1107. [Google Scholar] [CrossRef] [PubMed]
- Dias, G.J.; Mahoney, P.; Hung, N.A.; Sharma, L.A.; Kalita, P.; Smith, R.A.; Kelly, R.J.; Ali, A. Osteoconduction in keratin-hydroxyapatite composite bone-graft substitutes. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2034–2044. [Google Scholar]
- Han, D.; Wang, W.; Gong, J.; Ma, Y.; Li, Y. Collagen-hydroxyapatite based scaffolds for bone trauma and regeneration: Recent trends and future perspectives. Nanomedicine 2024, 19, 1689–1709. [Google Scholar] [CrossRef]
- Ramesh, N.; Moratti, S.C.; Dias, G.J. Hydroxyapatite-polymer biocomposites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2046–2057. [Google Scholar]
- Nocchetti, M.; Pietrella, D.; Antognelli, C.; Di Michele, A.; Russo, C.; Giulivi, E.; Ambrogi, V. Alginate microparticles containing silver@hydroxyapatite functionalized calcium carbonate composites. Int. J. Pharm. 2024, 661, 124393. [Google Scholar]
- Mucalo, M.R. Special Issue: Novel Advances and Approaches in Biomedical Materials Based on Calcium Phosphates. Materials 2019, 12, 405. [Google Scholar] [CrossRef]
- Silva Marques, J.M.; Gomes, P.S.; Silva, M.A.; Silvério Cabrita, A.M.; Santos, J.D.; Fernandes, M.H. Growth and phenotypic expression of human endothelial cells cultured on a glass-reinforced hydroxyapatite. J. Mater. Sci. Mater. Med. 2009, 20, 725–731. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, J.; Kim, J.P.; Shen, H.; Yang, F.; Zhang, Q.; Xu, M.; Bi, W.; Wang, X.; Yang, J.; et al. Ionic Colloidal Molding as a Biomimetic Scaffolding Strategy for Uniform Bone Tissue Regeneration. Adv. Mater. 2017, 29, 1605546. [Google Scholar]
- Cipriano, J.; Lakshmikanthan, A.; Buckley, C.; Mai, L.; Patel, H.; Pellegrini, M.; Freeman, J.W. Characterization of a prevascularized biomimetic tissue engineered scaffold for bone regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1655–1668. [Google Scholar]
- Li, J.; Ma, J.; Feng, Q.; Xie, E.; Meng, Q.; Shu, W.; Wu, J.; Bian, L.; Han, F.; Li, B. Building Osteogenic Microenvironments with a Double-Network Composite Hydrogel for Bone Repair. Research 2023, 6, 0021. [Google Scholar]
- Godoy-Gallardo, M.; Portolés-Gil, N.; López-Periago, A.M.; Domingo, C.; Hosta-Rigau, L. Immobilization of BMP-2 and VEGF within Multilayered Polydopamine-Coated Scaffolds and the Resulting Osteogenic and Angiogenic Synergy of Co-Cultured Human Mesenchymal Stem Cells and Human Endothelial Progenitor Cells. Int. J. Mol. Sci. 2020, 21, 6418. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shi, Y.; Zhang, X.; Ma, J. Evaluation of BMP-2 and VEGF loaded 3D printed hydroxyapatite composite scaffolds with enhanced osteogenic capacity in vitro and in vivo. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110893. [Google Scholar]
- Kocak, F.Z.; Yar, M.; Rehman, I.U. Hydroxyapatite-Integrated, Heparin- and Glycerol-Functionalized Chitosan-Based Injectable Hydrogels with Improved Mechanical and Proangiogenic Performance. Int. J. Mol. Sci. 2022, 23, 5370. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xiong, Y.; Zheng, J.; Kongling, W.; Chen, J.; Li, C.; Hu, P.; Yang, S.; Wang, X. A multifaceted biomimetic periosteum with a lamellar architecture and osteogenic/angiogenic dual bioactivities. Biomater. Sci. 2023, 11, 3878–3892. [Google Scholar]
- Targońska, S.; Dobrzyńska-Mizera, M.; Di Lorenzo, M.L.; Knitter, M.; Longo, A.; Dobrzyński, M.; Rutkowska, M.; Barnaś, S.; Czapiga, B.; Stagraczyński, M.; et al. Design, clinical applications and post-surgical assessment of bioresorbable 3D-printed craniofacial composite implants. Biomater. Sci. 2024, 12, 3374–3388. [Google Scholar]
- Taufik, S.A.; Wiweko, A.; Yudhanto, D.; Rizki, M.; Habib, P.; Dirja, B.T.; Rosyidi, R.M. Treatment of bone defects with bovine hydroxyapatite xenograft and platelet rich fibrin (PRF) to accelerate bone healing. Int. J. Surg. Case Rep. 2022, 97, 107370. [Google Scholar]
- Artzi, Z.; Weinreb, M.; Carmeli, G.; Lev-Dor, R.; Dard, M.; Nemcovsky, C.E. Histomorphometric assessment of bone formation in sinus augmentation utilizing a combination of autogenous and hydroxyapatite/biphasic tricalcium phosphate graft materials: At 6 and 9 months in humans. Clin. Oral Implants Res. 2008, 19, 686–692. [Google Scholar]
- Xiong, Y.; Ren, C.; Zhang, B.; Yang, H.; Lang, Y.; Min, L.; Zhang, W.; Pei, F.; Yan, Y.; Li, H.; et al. Analyzing the behavior of a porous nano-hydroxyapatite/polyamide 66 (n-HA/PA66) composite for healing of bone defects. Int. J. Nanomed. 2014, 9, 485–494. [Google Scholar]
- Acharya, N.K.; Kumar, R.J.; Varma, H.K.; Menon, V.K. Hydroxyapatite-bioactive glass ceramic composite as stand-alone graft substitute for posterolateral fusion of lumbar spine: A prospective, matched, and controlled study. J. Spinal Disord. Tech. 2008, 21, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Milovac, D.; Gallego Ferrer, G.; Ivankovic, M.; Ivankovic, H. PCL-coated hydroxyapatite scaffold derived from cuttlefish bone: Morphology, mechanical properties and bioactivity. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 34, 437–445. [Google Scholar] [PubMed]
- Santoso, A.; Utomo, P.; Mahyudin, F.; Utomo, D.N.; Budhiparama, N.C.; Anwar, I.B.; Sibarani, T.; Warman, F.I. The use of bovine-derived hydroxyapatite xenograft for defect filling in opening-wedge high tibial osteotomy. Ann. Med. Surg. 2023, 85, 3339–3346. [Google Scholar]
- Yang, C.; Zhuo, W.; Li, Q.; Huang, C.; Yan, H.; Jin, D. Preliminary outcomes of allograft and hydroxyapatite as substitutes for autograft in anterior cervical discectomy and fusion with self-locking standalone cages. J. Orthop. Surg. Res. 2021, 16, 123. [Google Scholar]
- Fieux, M.; Tournegros, R.; Hermann, R.; Tringali, S. Allograft bone vs. bioactive glass in rehabilitation of canal wall-down surgery. Sci. Rep. 2023, 13, 17945. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Cheong, N.; He, Z.; Zhang, T. Application of Hydroxyapatite Composites in Bone Tissue Engineering: A Review. J. Funct. Biomater. 2025, 16, 127. https://doi.org/10.3390/jfb16040127
Liu W, Cheong N, He Z, Zhang T. Application of Hydroxyapatite Composites in Bone Tissue Engineering: A Review. Journal of Functional Biomaterials. 2025; 16(4):127. https://doi.org/10.3390/jfb16040127
Chicago/Turabian StyleLiu, Weijie, Nalini Cheong, Zhuling He, and Tonghan Zhang. 2025. "Application of Hydroxyapatite Composites in Bone Tissue Engineering: A Review" Journal of Functional Biomaterials 16, no. 4: 127. https://doi.org/10.3390/jfb16040127
APA StyleLiu, W., Cheong, N., He, Z., & Zhang, T. (2025). Application of Hydroxyapatite Composites in Bone Tissue Engineering: A Review. Journal of Functional Biomaterials, 16(4), 127. https://doi.org/10.3390/jfb16040127







