Commercial Biomaterial-Based Products for Tendon Surgical Augmentation: A Scoping Review on Currently Available Medical Devices
Abstract
1. Introduction
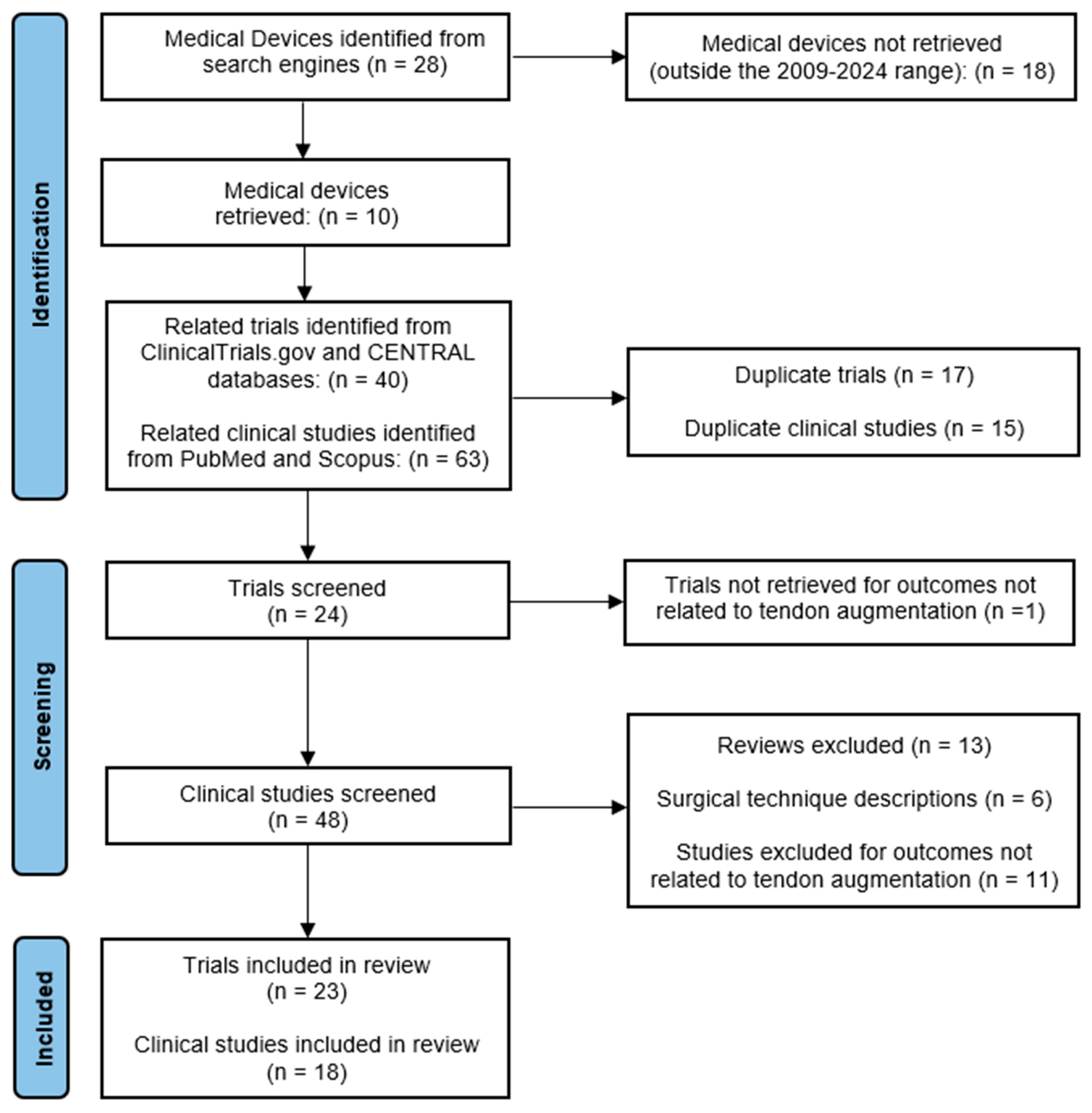
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection and Data Extraction
3. Results and Discussion
3.1. Natural Biomaterial-Based Products
| Manufacturer/ Distributor | Brand Name | Medical Device Launch (Year) | Material Type | Source | Clinical Indications | Retrieved Clinical Trials/Published Clinical Studies |
|---|---|---|---|---|---|---|
| Arthrex Inc., Naples, FL, USA | DX Reinforcement Matrix | 2015 | Dermal ECM | Porcine | Reinforcement of rotator cuff, patellar, Achilles, biceps, quadriceps and other tendons | 1/5 [29]/[30,31,33,34,35] |
| Alafair Biosciences, Austin, TX, USA | VersaWrap® | 2017 | Hyaluronic acid and alginate | Ocean seaweed | Peripheral nerve, tendon, ligament and skeletal muscle protection | 3/0 [52,53,54]/N.A. |
| Smith & Nephew, Memphis, TN, USA | REGENETEN™ Bioinductive Implant | 2023 * | Collagen type I | Bovine | Rotator cuff disease | 10/5 [36,37,38,39,40,41,42,43,44,45]/[46,47,48,49,50] |
3.2. Synthetic Biomaterial-Based Products
| Manufacturer/ Distributor | Brand Name | Material Type | Medical Device Launch (Year) | Clinical Indications | Retrieved Clinical Trials/Published Clinical Studies |
| Synthasome, San Diego, CA, USA | X-Repair | Poly-L-lactic acid (PLLA) | 2009 | Reinforcement of soft tissues and tendons | 0/1 N.A./[62] |
| Wright Medical Group, Memphis, TN, USA | BioFiber™ | Poly-4-hydroxybutyrate fibres (P4HB) | 2011 | Reinforcement of rotator cuff, patellar, Achilles, biceps and quadriceps tendons | 1/2 [63]/[64,65] |
| Xiros, Leeds, UK | Leeds–Kuff Patch | Polyethylene terephthalate (PET) | 2012 | Reinforcement of the rotator cuff following or during repair by suture or suture anchors | 1/1 [66]/[67] |
| Artelon, Sandy Springs, GA, USA | FLEXBAND™ | Co-polymer of polycaprolactone (PCL) and polyurethane-urea (PUU) | 2019 | Ankle tendon and ligament augmentation | 0/3 N.A./[68,69,70] |
| Xiros, Leeds, UK | Pitch–Patch | Polyethylene terephthalate (PET) | 2021 * | Reinforcement of the rotator cuff following or during repair by suture or suture anchors | 3/1 [71,72,73]/[74] |
3.3. Hybrid Biomaterial-Based Products
| Manufacturer/Distributor | Brand Name | Composition | Medical Device Launch (Year) | Clinical INDICATIONS | Retrieved Clinical trials/Published Clinical Studies |
|---|---|---|---|---|---|
| Wright Medical Group, Memphis, TN, USA | BioFiber™ CM | Bovine collagen type I and poly-4-hydroxybutyrate fibres (P4HB) | 2015 | Tendon and ligament repair | 1/0 [63]/N.A. |
| CONMED, Utica, NY, USA | BioBrace® | Bovine collagen type I and poly-L-lactic acid (PLLA) | 2021 | Knee, shoulder, hip, foot and ankle tendons augmentation | 3/0 [77,78,79]/N.A. |
3.4. Cost/Effectiveness Considerations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Market Insights. Tendon Repair Market Size. Available online: https://www.gminsights.com/industry-analysis/tendon-repair-market (accessed on 12 December 2024).
- Steinmann, S.; Pfeifer, C.G.; Brochhausen, C.; Docheva, D. Spectrum of Tendon Pathologies: Triggers, Trails and End-State. Int. J. Mol. Sci. 2020, 21, 844. [Google Scholar] [CrossRef] [PubMed]
- DelveInsight’s Analysis. Tendinopathy Incidence in the United States in 2022. Available online: https://www.globenewswire.com/news-release/2023/11/29/2788013/0/en/Tendinopathy-Market-to-Register-Incremental-Growth-During-the-Study-Period-2019-2032-Estimates-DelveInsight-Leading-Companies-in-the-Market-Novartis-SynOx-Abbisko-AmMax-Deciphera.html (accessed on 7 February 2025).
- Bergamin, F.; Civera, M.; Reinoso, M.R.; Burgio, V.; Ruiz, O.G.; Surace, C. Worldwide Incidence and Surgical Costs of Tendon Injuries: A Systematic Review and Meta-Analysis. Muscles Ligaments Tendons J. 2023, 13, 31–45. [Google Scholar] [CrossRef]
- Millar, N.L.; Silbernagel, K.G.; Thorborg, K.; Kirwan, P.D.; Galatz, L.M.; Abrams, G.D.; Murrell, G.A.C.; McInnes, I.B.; Rodeo, S.A. Tendinopathy. Nat. Rev. Dis. Primers 2021, 7, 1. [Google Scholar] [CrossRef]
- Jarvinen, M.; Jozsa, L.; Kannus, P.; Jarvinen, T.L.; Kvist, M.; Leadbetter, W. Histopathological findings in chronic tendon disorders. Scand. J. Med. Sci. Sports 1997, 7, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Backman, L.J.; Speed, C. Tendinopathy: Update on Pathophysiology. J. Orthop. Sports Phys. Ther. 2015, 45, 833–841. [Google Scholar] [CrossRef]
- Andres, B.M.; Murrell, G.A. Treatment of tendinopathy: What works, what does not, and what is on the horizon. Clin. Orthop. Relat. Res. 2008, 466, 1539–1554. [Google Scholar] [CrossRef]
- Loppini, M.; Maffulli, N. Conservative management of tendinopathy: An evidence-based approach. Muscles Ligaments Tendons J. 2011, 1, 134–137. [Google Scholar]
- Rawson, S.; Cartmell, S.; Wong, J. Suture techniques for tendon repair; a comparative review. Muscles Ligaments Tendons J. 2013, 3, 220–228. [Google Scholar]
- Hoeffner, R.; Svensson, R.B.; Bjerregaard, N.; Kjær, M.; Magnusson, S.P. Persistent Deficits after an Achilles Tendon Rupture: A Narrative Review. Transl. Sports Med. 2022, 2022, 7445398. [Google Scholar] [CrossRef]
- Tissues and Cells Directive (2004/23/EC). Available online: https://eur-lex.europa.eu/eli/dir/2004/23/oj/eng (accessed on 7 February 2025).
- 21 CFR Part 1271—Human Cells, Tissues, and Cellular and Tissue-Based Products. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-L/part-1271 (accessed on 7 February 2025).
- Costa-Almeida, R.; Calejo, I.; Gomes, M.E. Mesenchymal Stem Cells Empowering Tendon Regenerative Therapies. Int. J. Mol. Sci. 2019, 20, 3002. [Google Scholar] [CrossRef]
- K, N.; CA, V.; Joseph, J.; U, A.; John, A.; Abraham, A. Mesenchymal Stem Cells Seeded Decellularized Tendon Scaffold for Tissue Engineering. Curr. Stem Cell Res. Ther. 2021, 16, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yin, H.; Nerlich, M.; Pfeifer, C.G.; Docheva, D. Boosting tendon repair: Interplay of cells, growth factors and scaffold-free and gel-based carriers. J. Exp. Orthop. 2018, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Nerem, R.M. Tissue engineering: The hope, the hype, and the future. Tissue Eng. 2006, 12, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.M.; Liew, V.; Marine, B. Updates in Tendinopathy Treatment Options. Clin. Podiatr. Med. Surg. 2019, 36, 543–552. [Google Scholar] [CrossRef]
- Medical Device Regulation (EU) 2017/745. Available online: https://eur-lex.europa.eu/eli/reg/2017/745/oj/eng (accessed on 7 February 2025).
- Clinical Evaluation—Equivalence: A Guide for Manufacturers and Notified Bodies. Available online: https://health.ec.europa.eu/system/files/2020-09/md_mdcg_2020_5_guidance_clinical_evaluation_equivalence_en_0.pdf (accessed on 7 February 2025).
- The 510(k) Program: Evaluating Substantial Equivalence in Premarket Notifications. Available online: https://www.fda.gov/media/82395/download?attachment (accessed on 7 February 2025).
- Alexeev, S.O.; Buckley, S.E.; Hewitt, M.A.; Hunt, K.J. Publication trends in ligament augmentation techniques: Current concepts. J. ISAKOS 2023, 8, 232–238. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Wallace, S.S.; Barak, G.; Truong, G.; Parker, M.W. Hierarchy of Evidence Within the Medical Literature. Hosp. Pediatr. 2022, 12, 745–750. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Wang, A.; Zheng, M. Scaffolds for tendon and ligament repair: Review of the efficacy of commercial products. Expert. Rev. Med. Devices 2009, 6, 61–73. [Google Scholar] [CrossRef]
- Cissell, D.D.; Hu, J.C.; Griffiths, L.G.; Athanasiou, K.A. Antigen removal for the production of biomechanically functional, xenogeneic tissue grafts. J. Biomech. 2014, 47, 1987–1996. [Google Scholar] [CrossRef]
- Arthrex Inc. DX Reinforcement Matrix. Available online: https://www.arthrex.com/orthobiologics/arthrex-dx-reinforcement-matrix?currenttab=resources (accessed on 6 December 2024).
- Smith & Nephew. REGENETEN™ Bioinductive Implant. Available online: https://www.smith-nephew.com/en/health-care-professionals/products/sports-medicine/regeneten-bioinductive-implant#overview (accessed on 6 December 2024).
- ClinicalTrials.gov Identifier NCT01586351. Rotator Cuff Reconstruction with Xenologous Dermis-patch Augmentation and ACP®—Injection. Available online: https://clinicaltrials.gov/study/NCT01586351?tab=results (accessed on 6 December 2024).
- Flury, M.; Rickenbacher, D.; Jung, C.; Schneider, M.M.; Endell, D.; Audige, L. Porcine Dermis Patch Augmentation of Supraspinatus Tendon Repairs: A Pilot Study Assessing Tendon Integrity and Shoulder Function 2 Years After Arthroscopic Repair in Patients Aged 60 Years or Older. Arthroscopy 2018, 34, 24–37. [Google Scholar] [CrossRef]
- Ferrando, A.; Kingston, R.; Delaney, R.A. Superior capsular reconstruction using a porcine dermal xenograft for irreparable rotator cuff tears: Outcomes at minimum two-year follow-up. J. Shoulder Elbow Surg. 2021, 30, 1053–1059. [Google Scholar] [CrossRef]
- Barber, F.A. Editorial Commentary: Don’t Pig Out When Selecting a Shoulder, Rotator Cuff Augmentation Graft! Xenografts Are Not the Way to Go. Arthroscopy 2018, 34, 38–40. [Google Scholar] [CrossRef]
- Kalina, R.; Neoral, P.; Holibka, R.; Gallo, J. Arthroscopic Superior Capsule Reconstruction Using the DX Reinforcement Matrix in Patients with Irreparable Rotator Cuff Tears—Pilot Data. Acta Chir. Orthop. Traumatol. Cech. 2019, 86, 264–270. [Google Scholar]
- Colosio, A.; Bergomi, A.; Pratobevera, A.; Paderno, M.; Saccomanno, M.F.; Milano, G. Combined Biologic Augmentation Strategies with Collagen Patch Graft, Microfractures, and Platelet Concentrate Injections Improve Functional and Structural Outcomes of Arthroscopic Revision Rotator Cuff Repair. J. Clin. Med. 2023, 12, 5694. [Google Scholar] [CrossRef] [PubMed]
- Fallah, J.-M.; Resende Sousa, M.; Vide, J.; Mendes, D. Interpositional arthroplasty with Dx Reinforcement Matrix®: A possible solution in the surgical treatment of lateral TMT joints arthritis. Foot Ankle Surg. Tech. Rep. Cases 2023, 3, 100267. [Google Scholar] [CrossRef]
- ClinicalTrials.gov Identifier NCT04444076. Clinical Trial on the Effect of REGENETEN Bioinductive Implant in the Supraspinatus Tendon Repair. Available online: https://clinicaltrials.gov/study/NCT04444076?term=NCT04444076&rank=1 (accessed on 6 December 2024).
- ClinicalTrials.gov Identifier NCT04450342. REGENETEN™ Bioinductive Implant System in Full-Thickness Tears (REGENETEN). Available online: https://clinicaltrials.gov/study/NCT04450342?term=NCT04450342&rank=1 (accessed on 6 December 2024).
- ClinicalTrials.gov Identifier NCT04861714. Evaluation of Regeneten Augmentation for Subscapularis Healing After Total Shoulder Arthroplasty (RESTOR). Available online: https://clinicaltrials.gov/study/NCT04861714?term=NCT04861714&rank=1 (accessed on 6 December 2024).
- ClinicalTrials.gov Identifier NCT03734536. Treatment of Partial-Thickness Rotator Cuff Tears (REGEN PUB 2018). Available online: https://clinicaltrials.gov/study/NCT03734536?term=NCT03734536&rank=1 (accessed on 6 December 2024).
- ClinicalTrials.gov Identifier NCT06252389. Degenerative Achilles Tendon Rupture Repair with Regeneten Augmentation. Available online: https://clinicaltrials.gov/study/NCT06252389?term=NCT06252389&rank=1 (accessed on 6 December 2024).
- ClinicalTrials.gov Identifier NCT04673344. Regeneten Patch vs. Standard Care in Partial Thickness Rotator Cuff Repair. Available online: https://clinicaltrials.gov/study/NCT04673344?term=NCT04673344&rank=1 (accessed on 6 December 2024).
- ClinicalTrials.gov Identifier NCT06269965. Assessment of Collagen Membrane Interest in Distal Cuff Lesions Repair (REGENECUFF). Available online: https://clinicaltrials.gov/study/NCT06269965?term=NCT06269965&rank=1 (accessed on 6 December 2024).
- ClinicalTrials.gov Identifier NCT04248751. Evaluating the Use of a Bioinductive Graft in Treating Massive Rotator Cuff Tears. Available online: https://clinicaltrials.gov/study/NCT04248751?term=NCT04248751&rank=1 (accessed on 6 December 2024).
- ClinicalTrials.gov Identifier NCT05444465. Use of the REGENETEN™ Bioinductive Implant System in High Grade Partial-Thickness Tears (IMPACT). Available online: https://clinicaltrials.gov/study/NCT05444465?term=NCT05444465&rank=1 (accessed on 6 December 2024).
- ClinicalTrials.gov Identifier NCT06215417. Rotator Cuff Augmentation with Human Dermal Allograft Versus Bovine Collagen Xenograft Patch: A Randomized Controlled Trial (HDA v Regen). Available online: https://clinicaltrials.gov/study/NCT06215417?term=NCT06215417&rank=1 (accessed on 6 December 2024).
- Ruiz Iban, M.A.; Garcia Navlet, M.; Moros Marco, S.; Diaz Heredia, J.; Hernando Sanchez, A.; Ruiz Diaz, R.; Vaquero Comino, C.; Rosas Ojeda, M.L.; Del Monte Bello, G.; Avila Lafuente, J.L. Augmentation of a Transosseous-Equivalent Repair in Posterosuperior Nonacute Rotator Cuff Tears With a Bioinductive Collagen Implant Decreases the Retear Rate at 1 Year: A Randomized Controlled Trial. Arthroscopy 2024, 40, 1760–1773. [Google Scholar] [CrossRef]
- Schlegel, T.F.; Abrams, J.S.; Angelo, R.L.; Getelman, M.H.; Ho, C.P.; Bushnell, B.D. Isolated bioinductive repair of partial-thickness rotator cuff tears using a resorbable bovine collagen implant: Two-year radiologic and clinical outcomes from a prospective multicenter study. J. Shoulder Elbow Surg. 2021, 30, 1938–1948. [Google Scholar] [CrossRef]
- Yeazell, S.; Lutz, A.; Bohon, H.; Shanley, E.; Thigpen, C.A.; Kissenberth, M.J.; Pill, S.G. Increased stiffness and reoperation rate in partial rotator cuff repairs treated with a bovine patch: A propensity-matched trial. J. Shoulder Elbow Surg. 2022, 31, S131–S135. [Google Scholar] [CrossRef] [PubMed]
- Looney, A.M.; Fortier, L.M.; Leider, J.D.; Bryant, B.J. Bioinductive Collagen Implant Augmentation for the Repair of Chronic Lower Extremity Tendinopathies: A Report of Two Cases. Cureus 2021, 13, e15567. [Google Scholar] [CrossRef]
- Valk, J.; Wilk, M.J.; Murdock, K.; Saad, M.A. Bioinductive Collagen Implant Augmentation for Myotendinous Achilles Rupture in a Teenage Competitive Gymnast: A Case Report. JBJS Case Connect. 2023, 13, e22.00383. [Google Scholar] [CrossRef]
- Alafair Biosciences. VersaWrap®. Available online: https://www.alafairbiosciences.com/versawrap (accessed on 6 December 2024).
- ClinicalTrials.gov Identifier NCT05598801. A Single-Center, Prospective, Clinical Study of VersaWrap Utilization in the Hand. Available online: https://clinicaltrials.gov/study/NCT05598801?term=NCT05598801&rank=1 (accessed on 6 December 2024).
- ClinicalTrials.gov Identifier NCT04976335. Quantitative and Clinical Assessment of Flexor Tendon Gliding Following Application of a Bioresorbable Hydrogel: A Prospective, Randomized Study in Patients Undergoing Distal Radius Fracture Repair. Available online: https://clinicaltrials.gov/study/NCT04976335?term=NCT04976335&rank=1 (accessed on 6 December 2024).
- ClinicalTrials.gov Identifier NCT04322370. Prospective Randomized Blinded Trial of VersaWrap Tendon Protector for Zone 2 Flexor Tendon Injuries. Available online: https://clinicaltrials.gov/study/NCT04322370?term=NCT04322370&rank=1 (accessed on 6 December 2024).
- Hones, K.M.; Nichols, D.S.; Barker, H.; Cox, E.; Hones, J.A.; Chim, H. Outcomes following use of VersaWrap nerve protector in treatment of patients with recurrent compressive neuropathies. Front. Surg. 2023, 10, 1123375. [Google Scholar] [CrossRef] [PubMed]
- Thakker, A.; Sharma, S.C.; Hussain, N.M.; Devani, P.; Lahiri, A. Nerve wrapping for recurrent compression neuropathy: A systematic review. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 549–559. [Google Scholar] [CrossRef]
- Synthasome. X-Repair. Available online: https://www.synthasome.com/xRepair.php (accessed on 6 December 2024).
- Wright Medical Group. BioFiber™. Available online: https://www.wright.com/footandanklebrand/biofiber (accessed on 6 December 2024).
- Xiros. Leeds-Kuff Patch. Available online: https://komak.pl/wp-content/uploads/PDF/leeds.pdf (accessed on 6 December 2024).
- Artelon. FLEXBAND™. Available online: https://www.artelon.com/artelon-science (accessed on 6 December 2024).
- Xiros. Pitch-Patch. Available online: https://xiros.co.uk/product/rotator-cuff-repair/ (accessed on 4 December 2024).
- Proctor, C.S. Long-term successful arthroscopic repair of large and massive rotator cuff tears with a functional and degradable reinforcement device. J. Shoulder Elbow Surg. 2014, 23, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov Identifier NCT01849458. BioFiber Scaffold Post-Market Observational Study. Available online: https://clinicaltrials.gov/study/NCT01849458?term=NCT01849458&rank=1 (accessed on 6 December 2024).
- Barbash, S.; Denny, C.; Collin, P.; Reish, T.; Hart, J.M.; Brockmeier, S.F. Clinical Outcomes and Structural Healing After Arthroscopic Rotator Cuff Repair Reinforced With A Novel Absorbable Biologic Scaffold: A Prospective, Multicenter Trial. Orthop. J. Sports Med. 2015, 3, 2325967115S2325900071. [Google Scholar] [CrossRef]
- Burkhard, M.D.; Dietrich, M.; Andronic, O.; Nikolic, N.; Grueninger, P. Arthroscopic repair of posterosuperior rotator cuff tears with bioabsorbable patch augmentation: A magnetic resonance-controlled case series with 1-year follow-up. JSES Int. 2020, 4, 860–868. [Google Scholar] [CrossRef]
- ISRCTN Registry Identifier ISRCTN79844053. Shoulder Patch for Rotator Cuff Tears. Available online: https://www.isrctn.com/ISRCTN79844053 (accessed on 6 December 2024).
- Cowling, P.; Hackney, R.; Dube, B.; Grainger, A.J.; Biglands, J.D.; Stanley, M.; Song, D.; Conaghan, P.G.; Kingsbury, S.R. The use of a synthetic shoulder patch for large and massive rotator cuff tears—A feasibility study. BMC Musculoskelet. Disord. 2020, 21, 213. [Google Scholar] [CrossRef]
- Kelly, M.J.; Dean, D.M.; Hussaini, S.H.; Neufeld, S.K.; Cuttica, D.J. Safety Profile of Synthetic Elastic Degradable Matrix for Soft Tissue Reconstruction in Foot & Ankle Surgery. Foot Ankle Spec. 2024, 17, 201–207. [Google Scholar] [CrossRef]
- Alencar Mendes de Carvalho, K.; Schmidt, E.; Ehret, A.; Chun Kim, K.; Barbachan Mansur, N.S.; de César Netto, C. Achilles tendon reconstruction using a biosynthetic graft: A case report. J. Foot Ankle 2022, 16, 288–294. [Google Scholar] [CrossRef]
- Solan, C.; Starring, H.; Reese, J.; Leithead, C.; Galli, S. Simultaneous extensor hallicus longus tendon laceration and Dorsalis Pedis artery pseudoaneurysm: A case report and a review of the literature. Foot Ankle Surg. Tech. Rep. Cases 2023, 3, 100313. [Google Scholar] [CrossRef]
- ClinicalTrials.gov Identifier NCT05906004. Retrospective Data Collection and Prospective Clinical Investigation for the Augmentation or Reinforcement of the Rotator Cuff Using the Pitch-Patch. Available online: https://clinicaltrials.gov/study/NCT05906004?term=NCT05906004&rank=1 (accessed on 6 December 2024).
- ClinicalTrials.gov Identifier NCT06076902. Patch Study (Patch-Augmented Rotator Cuff Repair). Available online: https://clinicaltrials.gov/study/NCT06076902?term=NCT06076902&rank=1 (accessed on 6 December 2024).
- ClinicalTrials.gov Identifier NCT03511547. Comparison of Arthroscopic Supraspinatus Tendon Tear Repair in Patients Over 60 Years with and Without Patch Augmentation. Available online: https://clinicaltrials.gov/study/NCT03511547?term=NCT03511547&rank=1 (accessed on 6 December 2024).
- Smolen, D.; Haffner, N.; Mittermayr, R.; Hess, F.; Sternberg, C.; Leuzinger, J. Application of a new polyester patch in arthroscopic massive rotator cuff repair-a prospective cohort study. J. Shoulder Elbow Surg. 2020, 29, e11–e21. [Google Scholar] [CrossRef]
- Wright Medical Group. BioFiber™ CM. Available online: https://www.wright.com/foot_self/biofiber-cm-collagen-coated-absorbable-scaffold (accessed on 6 December 2024).
- CONMED. BioBrace®. Available online: https://www.conmed.com/-/media/conmed/documents/literature/biobrace_productbrochure.ashx (accessed on 4 December 2024).
- ClinicalTrials.gov Identifier NCT05997381. BioBrace® Implant for Arthroscopic Repair of Full Thickness Rotator Cuff Tears (REinForce). Available online: https://clinicaltrials.gov/study/NCT05997381?term=NCT05997381&rank=1 (accessed on 6 December 2024).
- ClinicalTrials.gov Identifier NCT05959733. Maximal Repair Versus Bridging Reconstruction with BioBrace®. Available online: https://clinicaltrials.gov/expert-search?term=NCT05959733 (accessed on 6 December 2024).
- ClinicalTrials.gov Identifier NCT05487677. Subscapularis Repair Augmentation for Total Shoulder Arthroplasty. Available online: https://clinicaltrials.gov/expert-search?term=NCT05487677 (accessed on 6 December 2024).
- Cheesman, Q.T.; Szukics, P.F.; Stark, M.; Kramer, S.C.; McMillan, S. Arthroscopic Rotator Cuff Repair Technique Using a Bio-Composite Scaffold for Tissue Augmentation. Arthrosc. Tech. 2022, 11, e517–e522. [Google Scholar] [CrossRef] [PubMed]
- Geers, B.A.; Bishai, S.K. Chronic Midsubstance Patellar Tendon and Retinacular Rupture: Primary Repair Enhancement Using Bioinductive Implant Augmentation. Arthrosc. Tech. 2023, 12, e1595–e1600. [Google Scholar] [CrossRef] [PubMed]
- Le, D.; McMillan, S. A Novel Distal Biceps Rupture Repair Technique Utilizing a Biocomposite Scaffold. Surg. Technol. Int. 2023, 42, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Patrizio, H.A.; Lutz, R.W.; Middleton, C.J.; Bianco, L.; McMillan, S. A Novel Achilles Tendon Repair Technique Utilizing a Bio-Composite Scaffold for a Sub-Acute Tear. Surg. Technol. Int. 2023, 42, 375–378. [Google Scholar] [CrossRef]
- Civera, M.; Devietti Goggia, E.; De Ros, M.; Burgio, V.; Bergamin, F.; Rodriguez Reinoso, M.; Surace, C. Implantable medical devices for tendon and ligament repair: A review of patents and commercial products. Expert. Rev. Med. Devices 2022, 19, 825–845. [Google Scholar] [CrossRef]
- Losina, E.; Wright, J.; Katz, J.N. Clinical trials in orthopaedics research. Part III. Overcoming operational challenges in the design and conduct of randomized clinical trials in orthopaedic surgery. J. Bone Joint Surg. Am. 2012, 94, e35. [Google Scholar] [CrossRef]
- Quigley, R.; Verma, N.; Evuarherhe, A., Jr.; Cole, B.J. Rotator Cuff Repair with Graft Augmentation Improves Function, Decreases Revisions, and Is Cost-Effective. Arthroscopy 2022, 38, 2166–2174. [Google Scholar] [CrossRef]
- Rognoni, C.; Nherera, L.M.; Garofalo, R.; Guerra, E.; Longo, U.G.; Taverna, E.; Tarricone, R. Economic Evaluation of a Bioinductive Implant for the Repair of Rotator Cuff Tears Compared with Standard Surgery in Italy. Adv. Ther. 2023, 40, 5271–5284. [Google Scholar] [CrossRef]
- Russo, M.; Dirkx, G.K.; Rosso, C. Patch Augmentation in Arthroscopic Rotator Cuff Surgery-Review of Current Evidence and Newest Trends. J. Clin. Med. 2024, 13, 5066. [Google Scholar] [CrossRef]
- Longo, U.G.; Lamberti, A.; Maffulli, N.; Denaro, V. Tendon augmentation grafts: A systematic review. Br. Med. Bull. 2010, 94, 165–188. [Google Scholar] [CrossRef]
- Galatz, L.M.; Gerstenfeld, L.; Heber-Katz, E.; Rodeo, S.A. Tendon regeneration and scar formation: The concept of scarless healing. J. Orthop. Res. 2015, 33, 823–831. [Google Scholar] [CrossRef]
- Prestwich, G.D.; Bhatia, S.; Breuer, C.K.; Dahl, S.L.; Mason, C.; McFarland, R.; McQuillan, D.J.; Sackner-Bernstein, J.; Schox, J.; Tente, W.E.; et al. What is the greatest regulatory challenge in the translation of biomaterials to the clinic? Sci. Transl. Med. 2012, 4, 160cm114. [Google Scholar] [CrossRef]


| Medical Device (Brand Name) | Clinical Trial ID | Study Focus | Intervention | Study Design | Actual Estimated Enrolment | Clinical Trial Status | Posted Results | Conflicts of Interest * |
|---|---|---|---|---|---|---|---|---|
| DX Reinforcement Matrix | NCT01586351 [29] | Rotator cuff tears | Patch implant and autologous conditioned plasma injection | Observational | 20 20 | Completed | N.A. | Likely |
| NCT04444076 [36] | Supraspinatus tear | Bioinductive implant on supraspinatus tendon repair | Interventional, randomised | 124 120 | Active, not recruiting | N.A. | Yes | |
| REGENETEN™ | NCT04450342 [37] | Rotator cuff tears | Bioinductive implant augmentation | Interventional, prospective, multi-centre, randomised | 119 300 | Terminated | N.A. | Likely |
| NCT04861714 [38] | Subscapularis tendon | Augmentation for subscapularis healing after total shoulder arthroplasty | Interventional, randomised | 75 50 | Active, not recruiting | N.A. | No | |
| NCT03734536 [39] | Rotator cuff tear | Surgical treatment of partial-thickness rotator cuff tears the bioinductive implant | Interventional, non-randomised | 118 118 | Terminated | N.A. | Yes | |
| NCT06252389 [40] | Achilles rupture | Achilles Tendon repair augmented with bioinductive collagen patch | Observational, retrospective case series | N.A. 9 | Not yet recruiting | N.A. | No | |
| NCT04673344 [41] | Rotator cuff tear | Partial rotator cuff repair surgery with the addition of the bioinductive collagen patch | Interventional, randomised | N.A. 80 | Unknown | N.A. | No | |
| NCT06269965 [42] | Rotator cuff syndrome | Arthroscopic shoulder repair, in double row, with complete coverage of the foot print and addition of the bioinductive collagen patch | Interventional, randomised | N.A. 204 | Not yet recruiting | N.A. | No | |
| NCT04248751 [43] | Massive rotor cuff tear | Bioinductive implant augmentation | Interventional, prospective, randomised | N.A. 76 | Recruiting | N.A. | Likely | |
| NCT05444465 [44] | High grade partial-thickness tear | Isolated Bioinductive repair with the Bioinductive Implant | Interventional, randomised | N.A. 156 | Recruiting | N.A. | Yes | |
| NCT06215417 [45] | Rotator cuff tear | Arthroscopic rotator cuff repair augmented with graft | Interventional, randomised | N.A. 102 | Not yet recruiting | N.A. | No | |
| VersaWrap® | NCT05598801 [52] | Hand/fingers tendon repair | Graft applied to the affected tendon to allow post-operative gliding. | Prospective, observational | N.A. 20 | Enrolling by invitation | N.A. | No |
| NCT04976335 [53] | Flexor tendon | Membrane placed between distal radius plate and flexor tendons | Interventional, randomised | N.A. 100 | Recruiting | N.A. | No | |
| NCT04322370 [54] | Zone 2 flexor tendon | Graft applied to the flexor tendon where there is no significant loss of tendon tissue | Interventional, prospective, randomised | 42 52 | Recruiting | N.A. | No | |
| X-Repair | / | / | / | / | / | / | / | / |
| BioFiber™ | NCT01849458 [63] | Full thickness rotator cuff tears | Subjects implanted with BioFiber | Post-market observational | 50 50 | Completed | Improvements in clinical functional outcomes | Yes |
| Leeds–Kuff-Patch | ISRCTN79844053 [66] | Rotator cuff tears | Patch implant | Interventional, non-randomised | 68 60 | Completed | Improvements in outcome scores | No |
| Pitch–Patch | NCT05906004 [71] | Rotator cuff tears | Patch device used for rotator cuff augmentation/ reinforcement | Observational, perspective | N.A. 32 | Not yet recruiting | N.A. | Yes |
| NCT06076902 [72] | Rotator cuff tears | Pitch–Patch device used for rotator cuff augmentation/ reinforcement | Interventional, prospective, randomised | N.A. 300 | Recruiting | N.A. | No | |
| NCT03511547 [73] | Supraspinatus tendon tear | Pitch–Patch device used for rotator cuff augmentation | Interventional, randomised | 0 N.A. | Withdrawn | N.A. | No | |
| FLEXBAND™ | / | / | / | / | / | / | / | / |
| BioBrace® | NCT05997381 [77] | Full thickness rotator cuff tears | Implant augmentation | Interventional, randomised | N.A. 268 | Enrolling by invitation | N.A. | Yes |
| NCT05959733 [78] | Rotator cuff tears | Implant augmentation | Interventional, randomised | N.A. 60 | Recruiting | N.A. | Likely | |
| NCT05487677 [79] | Subscapularis repair | Implant augmentation | Interventional, randomised | N.A. 100 | Recruiting | N.A. | No | |
| BioFiber™ CM | NCT01849458 [63] | Full thickness rotator cuff tears | Implant augmentation | Post-market observational | 50 50 | Completed | Improvements in clinical functional outcomes | Yes |
| Medical Device (Brand Name) | Clinical Trial ID | Study Focus | Intervention | Control/Comparator | Study Design | Subjects (Gender, No., Age) | Results | Conflicts of Interest ** | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| DX Reinforcement Matrix | NCT01586351 [29] | Rotator cuff repair | ARCR + patch implant | Group without patch | Observational study | F: 28, M: 12; 60–74 | No significant group differences | Potential | [30] |
| / | Superior capsular reconstruction | SCR + patch implant | / | Retrospective study | F: 17, M: 39; 56–74 | No significant improvement; 25% graft failure (34 months f.u.) | Yes | [31] | |
| / | Superior capsular reconstruction | SCR + patch implant | / | Pilot study | F: 8, M: 12; 48–73 | Significant pain relief and a considerable improvement in the range of motion | N.A. | [33] | |
| / | Revision rotator cuff repair | ARRCR + patch implant | Group without patch | Retrospective comparative study | F:22, M: 18; 56–70 | ↑ CMS ↔ DASH | Yes | [34] | |
| / | The 5th TMT joint | Interpositional arthroplasty + patch implant | / | Case report | M: 1; 22 years | Asymptomatic patient at 3 years f.u., symmetric mobility, AOFAS of 100/100 | Likely | [35] | |
| REGENETEN™ | NCT04444076 [36] | Medium-to-large posterosuperior rotator cuff tears | TOE repair + Patch implant | Group without patch | Randomised controlled trial | F: 63, M: 61; 49–62 | Two-third reduction of the retear rate at 12 month f.u. Similar improvements in clinical outcomes. No increase in complication rates | Potential | [46] |
| / | Intermediate- and high-grade partial-thickness rotator cuff tears | Arthroplasty with bioinductive implant | / | Prospective study | F: 14, M: 19; 33–74 | ↑ ASES and CMS | Yes | [47] | |
| / | High-grade partial-thickness rotator cuff tears | Arthroscopic debridement + bioinductive collagen patch | Group without patch | Propensity-matched trial | F: 28, M: 36; 54.4 | ↑ Postoperative stiffness | No | [48] | |
| / | Case 1: chronic patellar tendinopathy Case 2: chronic proximal hamstring tendinopathy | Cases 1 and 2: bioaugmentation in the surgical treatment of chronic tendinopathies | / | Report of two cases | Case 1: M, 22 Case 2: F, 40 | Cases 1 and 2: an accelerated rate of return to patient pre-injury activity levels | No | [49] | |
| / | Acute Achilles tendon rupture | Bioaugmentation | / | Case report | F: 1; 16 years | Full range of motion, strength, and MRI evidence of increased tendon thickness | No | [50] | |
| VersaWrap® | / | / | / | / | / | / | / | / | / |
| X-Repair | / | Large to massive rotator cuff tears | Arthroscopic repairs with patch reinforcement and fixation | / | Case series | Gender: N.A. No.: 18 Age: 52–89 | ↑ ASES | No | [62] |
| BioFiber™ | / | Arthroscopic rotator cuff repair | Arthroscopic repairs with patch reinforcement | / | Prospective trial | F: 27, M: 23; 52–70 | ↑ CMS, WORC, ROM, and strength testing | N.A. | [64] |
| / | Posterosuperior rotator cuff repair | Arthroscopic repairs with patch reinforcement | / | Controlled case series | F: 4, M: 12; 45–76 | ↑ CMS, Muscular strength | No | [65] | |
| Leeds–Kuff-Patch | ISRCTN79844053 [66] | Large and massive rotator cuff tears | Arthroscopic repairs with patch reinforcement | Group without patch | Feasibility study | F: 42, M: 26; 56–74 | ↑ OSS and SPADI, ↔ CMS | Yes | [67] |
| Pitch–Patch | / | Massive rotator cuff tears | Arthroscopic repairs with patch reinforcement | / | Prospective cohort study | F: 16, M: 34; 57–73 | ↑ CMS, ↓ retear rate | No | [74] |
| FLEXBAND™ | / | Soft tissue reconstruction in foot and ankle surgery | Tissue reconstruction with patch reinforcement | / | Retrospective study | F: 65, M: 40; 34–66 | ↓ VAS, low compication rate | Potential | [68] |
| / | Achilles tendon reconstruction | Tissue reconstruction with patch reinforcement | / | Case report | M: 1, 56 years | ↑ PROMIS GPH T-score, ↓ PROMIS Pain T-score, ↓ ALS | No | [69] | |
| / | Extensor hallicus longus tendon laceration | Tissue reconstruction with patch reinforcement | / | Case report | M: 1, 29 years | ↑ Functional outcomes | No | [70] | |
| BioBrace® | / | / | / | / | / | / | / | / | / |
| BioFiber™ CM | / | / | / | // | / | / | / | / | / |
| Medical Device (Brand Name) | Category | Claimed Biomechanical Properties | Clinical Indication and Use | Shape | Dimensions | ||||
|---|---|---|---|---|---|---|---|---|---|
| Rotator Cuff | Patellar Tendon | Achilles Tendon | Other Tendons/Ligaments | Arthroscopy Use | |||||
| DX Reinforcement Matrix | Natural Biomaterial | Maintains strength (mean 137.5 N/cm) greater than native fascial tissue and the empty control throughout the healing process | Yes | Yes | Yes | Yes | Yes | Patch | 5 × 5 cm–6 × 8 cm |
| REGENETEN™ Bioinductive Implant | Natural Biomaterial | N.A. | Yes | Yes | Membrane | Size of a postage stamp | |||
| VersaWrap® | Natural Biomaterial | Reduction of 46% in friction (peak gliding resistance analysis); the analysis of rupture strength showed that it does not interrupt the repair process | Yes | Gel only | Ultrathin membrane or gel | Membrane: 2.5 × 5 cm–5 × 5 cm Gel: 1 mL | |||
| X-Repair | Synthetic Biomaterial | Tensile modulus: 500 MPa | Yes | Yes | Patch | 1.2 × 4.3 cm–2.5 × 2.5 cm–2.5 × 3 cm–2.5 × 3.5 cm–2.5 × 4.3 cm–4 × 3 cm–4 × 3.5 cm–4 × 4.3 cm | |||
| BioFiber™ | Synthetic Biomaterial | Tensile strength: 2500 N | Yes | Yes | Yes | Yes | Yes | Strip or disc | Strips: 1.3 × 2.3 cm–2 × 3 cm–2.5 × 5 cm; Disc: ⌀ 0.8 cm |
| Leeds–Kuff Patch | Synthetic Biomaterial | Suture retention strength: 550 N | Yes | Yes | Patch | 2 × 2 cm–3 × 3 cm–3.5 × 4 cm | |||
| Pitch–Patch | Synthetic Biomaterial | N.A. | Yes | Yes | Patch | 3 × 2 cm–3.5 × 2.5 cm | |||
| FLEXBAND™ | Synthetic Biomaterial | Provides a high suture retention strength compared to other commercially available products | Yes | No | Strip | 0.3 × 8 cm–0.3 × 16 cm–0.3 × 32 cm–0.5 × 8 cm–0.5 × 16 cm–0.5 × 32 cm–0.7 × 8 cm–0.7 × 16 cm–0.7 × 32 cm | |||
| BioBrace® | Hybrid Biomaterial | Strength of 355 N when fully sutured along the medial and lateral edges | Yes | Yes | Patch or cord | Patch: 2.3 × 3 cm–4 × 6 cm; Cord: ⌀ 0.5 cm × 25 cm | |||
| BioFiber™ CM | Hybrid Biomaterial | Ultimate tensile strength of 172.2 N, similar to ankle ligaments | Yes | Yes | Strip | 2 × 3 cm | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pluchino, M.; Vivarelli, L.; Giavaresi, G.; Dallari, D.; Govoni, M. Commercial Biomaterial-Based Products for Tendon Surgical Augmentation: A Scoping Review on Currently Available Medical Devices. J. Funct. Biomater. 2025, 16, 130. https://doi.org/10.3390/jfb16040130
Pluchino M, Vivarelli L, Giavaresi G, Dallari D, Govoni M. Commercial Biomaterial-Based Products for Tendon Surgical Augmentation: A Scoping Review on Currently Available Medical Devices. Journal of Functional Biomaterials. 2025; 16(4):130. https://doi.org/10.3390/jfb16040130
Chicago/Turabian StylePluchino, Marta, Leonardo Vivarelli, Gianluca Giavaresi, Dante Dallari, and Marco Govoni. 2025. "Commercial Biomaterial-Based Products for Tendon Surgical Augmentation: A Scoping Review on Currently Available Medical Devices" Journal of Functional Biomaterials 16, no. 4: 130. https://doi.org/10.3390/jfb16040130
APA StylePluchino, M., Vivarelli, L., Giavaresi, G., Dallari, D., & Govoni, M. (2025). Commercial Biomaterial-Based Products for Tendon Surgical Augmentation: A Scoping Review on Currently Available Medical Devices. Journal of Functional Biomaterials, 16(4), 130. https://doi.org/10.3390/jfb16040130











