A Review of Past Research and Some Future Perspectives Regarding Titanium Alloys in Biomedical Applications
Abstract
1. Introduction
2. A Brief History of Ti Alloys in Biomedical Applications
2.1. 1950s–1960s: Early Developments
2.2. 1970s: Introduction of Ti-6Al-4V Alloy
2.3. 1980s–1990s: Expanding Applications and Research
2.4. 2000s: Emerging Alloys and Innovations
2.5. 2010s–Present: Nanotechnology and Smart Materials
3. Specific Nanoparticles and Their Benefits in Ti Alloys
3.1. Powder Metallurgy vs. Additive Manufacturing (3D Printing)
3.2. Ti-6Al-4V with TiC-TiB2 Nanoparticles
3.3. Ti-6Al-4V with NanoAg Particles
4. Mechanisms of Enhancement
5. Current Applications of Biomedical Implants
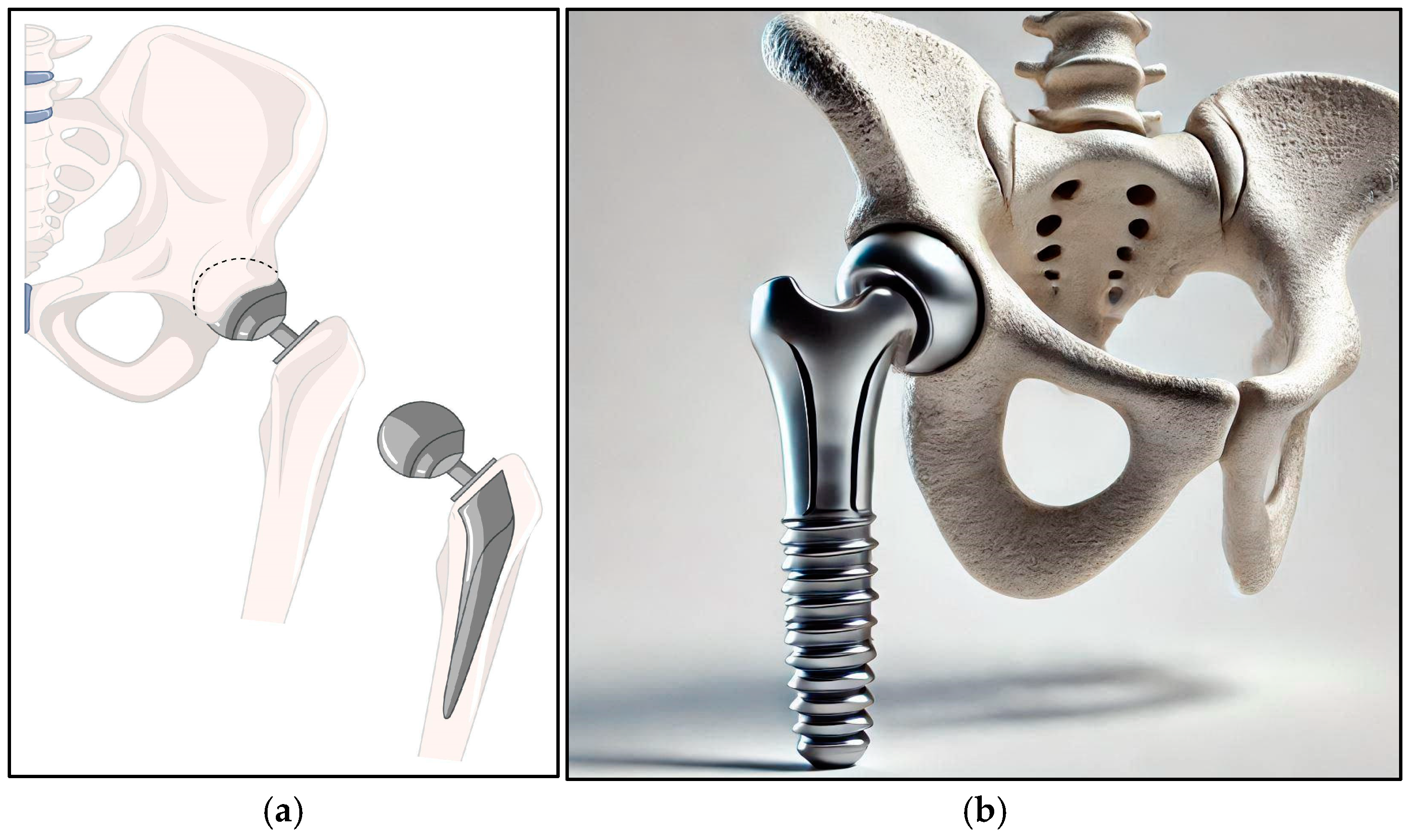
5.1. Orthopedic Implants
5.2. Dental Implants
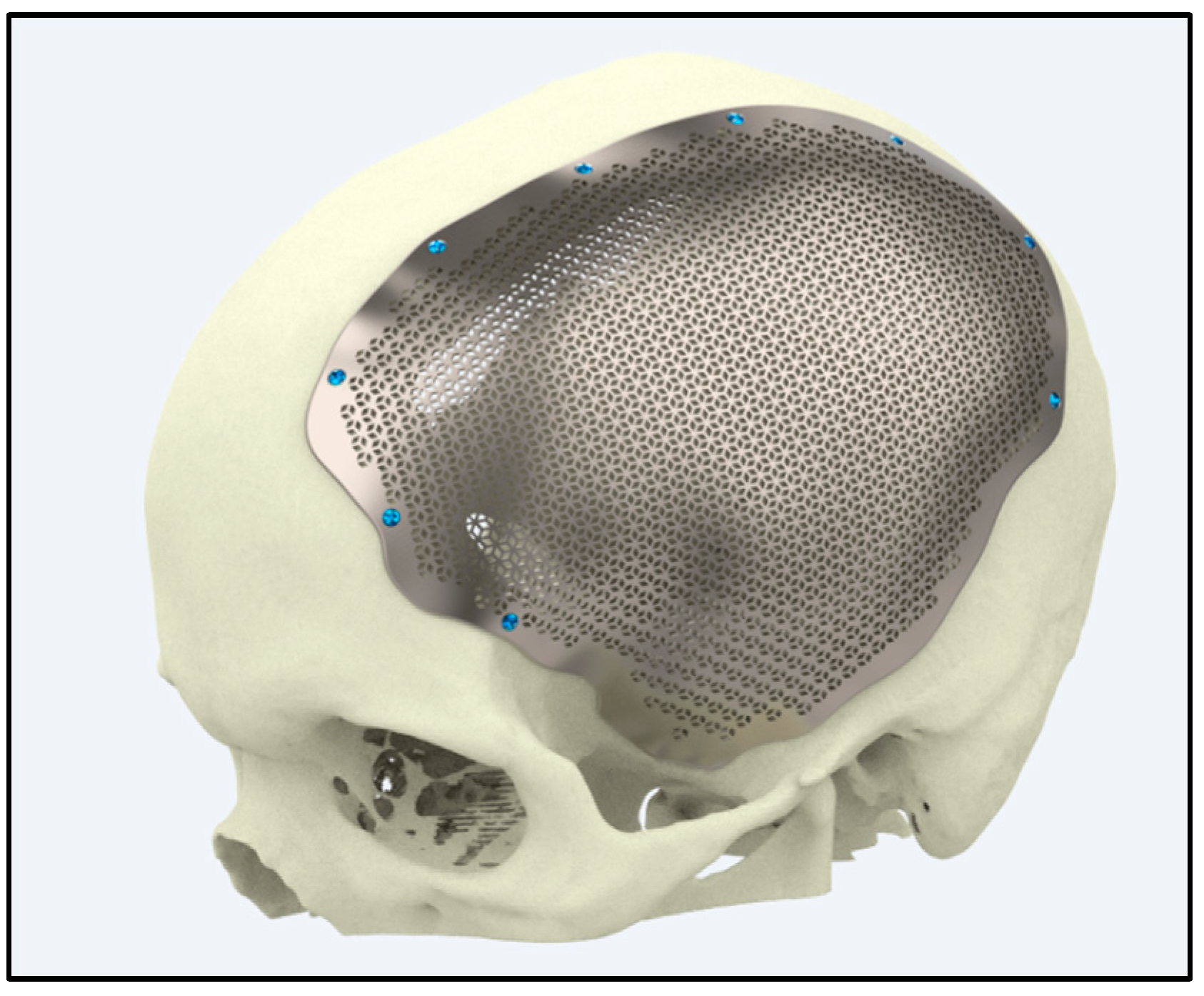
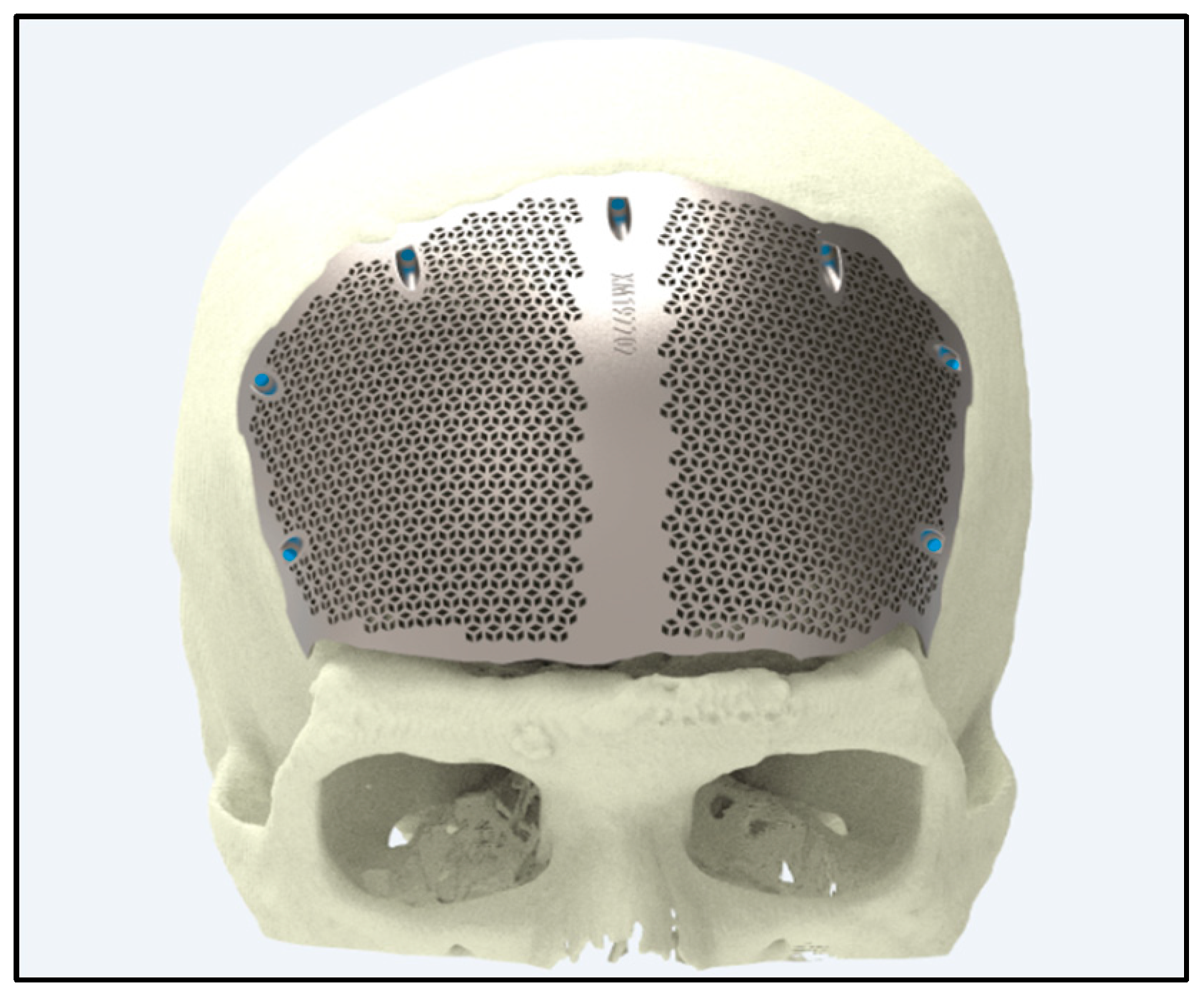
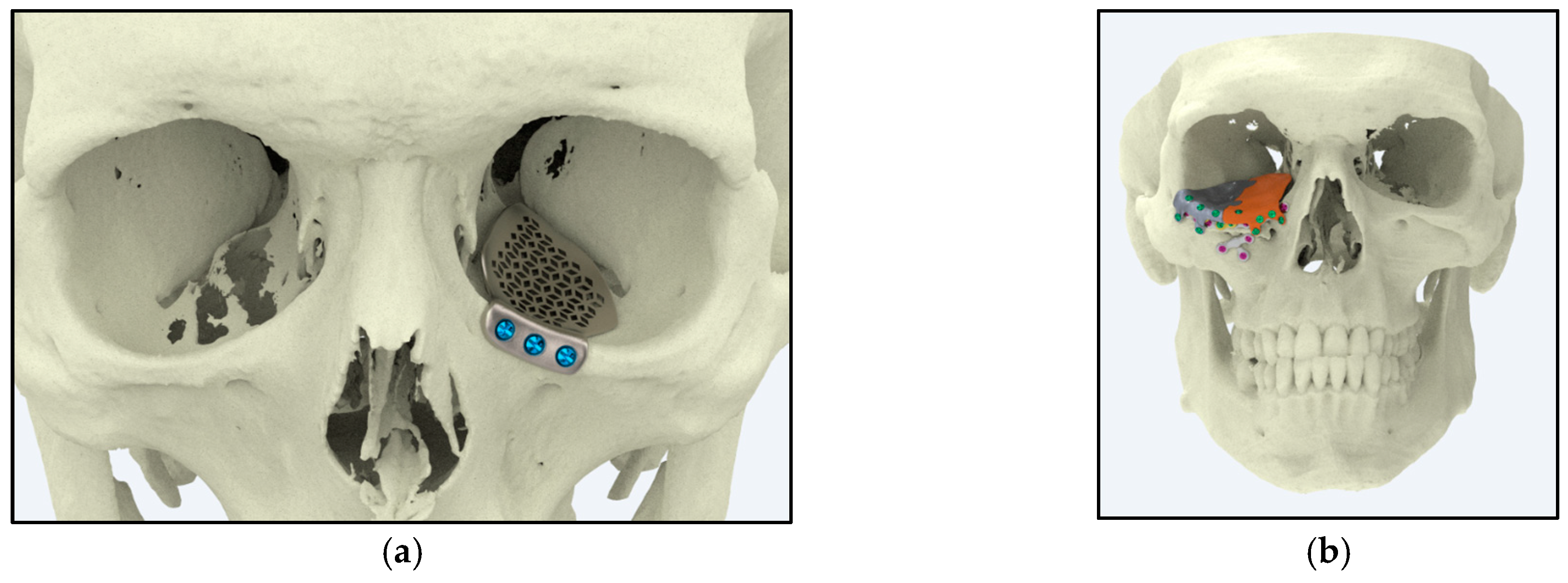
5.3. Craniofacial and Maxillofacial Implants
5.3.1. Reconstructive Surgery
5.3.2. Customized Implants
6. Study Perspectives
6.1. Advanced Material Development
6.2. Surface Modification Techniques
6.3. Smart Materials and Sensor Integration
6.4. Biocompatibility and Long-Term Performance
6.5. Additive Manufacturing and Customization
6.6. Sustainable and Cost-Effective Solutions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peter, I. Investigations into Ti-Based Metallic Alloys for Biomedical Purposes. Metals 2021, 11, 1626. [Google Scholar] [CrossRef]
- Thomas, N.G.; Varghese, N.; Kalarikkal, N.; Thomas, S.; Sreedharan, M.; George, S.S.; John, S.; Varghese, M.G.; George, V.T. Toxicity Evaluation and Biocompatibility of Nanostructured Biomaterials. In Cytotoxicity—Understanding Cellular Damage and Response; IntechOpen: London, UK, 2023. [Google Scholar]
- Tekade, R.K.; Maheshwari, R.; Jain, N.K. 9—Toxicity of nanostructured biomaterials. In Nanobiomaterials; Woodhead Publishing: Cambridge, UK, 2018; pp. 231–256. [Google Scholar]
- Scimeca, J.C.; Verron, E. Nano-engineered biomaterials: Safety matters and toxicity evaluation. Mater. Today Adv. 2022, 15, 100260. [Google Scholar] [CrossRef]
- Inayat-Hussain, S.; Rajab, N.F.; Siew, E.L. 20—In vitro testing of biomaterials toxicity and biocompatibility. In Cellular Response to Biomaterials; Woodhead Publishing: Cambridge, UK, 2009; pp. 508–537. [Google Scholar]
- Vranceanu, D.M.; Ungureanu, E.; Ionescu, I.C.; Parau, A.C.; Pruna, V.; Titorencu, I.; Badea, M.; Gălbău, C.-Ș.; Idomir, M.; Dinu, M.; et al. In Vitro Characterization of Hydroxyapatite-Based Coatings Doped with Mg or Zn Electrochemically Deposited on Nanostructured Titanium. Biomimetics 2024, 9, 244. [Google Scholar] [CrossRef]
- Supernak-Marczewska, M.; Ossowska, A.; Strąkowska, P.; Zieliński, A. Nanotubular Oxide Layers and Hydroxyapatite Coatings on Porous Titanium Alloy Ti13Nb13Zr. Adv. Mater. Sci. 2018, 18, 17–23. [Google Scholar] [CrossRef]
- Oliveira, N.T.C.; Biaggio, S.R.; Rocha-Filho, R.C.; Bocchi, N. Electrochemical Studies on Zirconium and Its Biocompatible Alloys Ti-50Zr at.% and Zr-2.5Nb wt.% in Simulated Physiologic Media. J. Biomed. Mater. Res. Part A 2005, 74A, 397–407. [Google Scholar] [CrossRef]
- Chopra, D.; Gulati, K.; Ivanovski, S. Micro + Nano: Conserving the Gold Standard Microroughness to Nanoengineer Zirconium Dental Implants. ACS Biomater. Sci. Eng. 2021, 7, 3069–3074. [Google Scholar] [CrossRef]
- Li, Q.; Huang, Q.; Li, J.; He, Q.; Nakai, M.; Zhang, K.; Niinomi, M.; Yamanaka, K.; Chiba, A.; Nakano, T. Microstructure and mechanical properties of Ti–Nb–Fe–Zr alloys with high strength and low elastic modulus. Trans. Nonferrous Met. Soc. China 2022, 32, 503–512. [Google Scholar] [CrossRef]
- Kopova, I.; Stráský, J.; Harcuba, P.; Landa, M.; Janeček, M.; Bačáková, L. Newly developed Ti–Nb–Zr–Ta–Si–Fe biomedical beta titanium alloys with increased strength and enhanced biocompatibility. Mater. Sci. Eng. C 2016, 60, 230–238. [Google Scholar] [CrossRef]
- Matekovits, L.; Mir, F.; Dassano, G.; Peter, I. Deeply Implanted Conformal Antenna for Real-Time Bio-Telemetry Applications. Sensors 2024, 24, 1170. [Google Scholar] [CrossRef]
- Peter, I.; Matekovits, L. Multidisciplinary investigations on the use of TiNb alloy orthopedic device equipped with low profile antenna as smart sensor. Procedia Manuf. 2020, 46, 828–837. [Google Scholar] [CrossRef]
- Sotniczuk, A.; Dou, B.; Liu, Y.; Gharbi, O.; Sun, F.; Garbacz, H.; Gilbert, J.L.; Ogle, K. Real-time monitoring of Ti-Nb-Ta-Zr and commercially pure Ti interaction with H2O2 using atomic force microscopy and atomic emission spectroelectrochemistry. Appl. Surf. Sci. 2024, 665, 160309. [Google Scholar] [CrossRef]
- Anju, M.S.; Raj, D.K.; Madathil, B.K.; Kasoju, N.; Kumar, P.R. Intelligent Biomaterials for Tissue Engineering and Biomedical Applications: Current Landscape and Future Prospects. Biomater. Tissue Eng. Regen. Med. 2021, 535–560. [Google Scholar] [CrossRef]
- Bîrsan, D.C.; Gurău, C.; Marin, F.-B.; Stefănescu, C.; Gurău, G. Modeling of Severe Plastic Deformation by HSHPT of As-Cast Ti-Nb-Zr-Ta-Fe-O Gum Alloy for Orthopedic Implant. Materials 2023, 16, 3188. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Y.; Li, R.; Tang, X.; Guo, D.; Qing, Y.; Qin, Y. Enhanced antibacterial properties of orthopedic implants by titanium nanotube surface modification: A review of current techniques. Int. J. Nanomed. 2019, 14, 7217–7236. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Chen, H.; Zhang, A.; Zhang, Y.; Zhang, J.; Chen, B.; Han, Q.; Wang, J. From Clinic to Lab: Advances in Porous Titanium-Based Orthopedic Implant Research. J. Mater. Res. Technol. 2024, 30, 3780–3806. [Google Scholar] [CrossRef]
- Nag, S.; Banerjee, R. Laser deposition and deformation behavior of Ti-Nb-Zr-Ta alloys for orthopedic implants. J. Mech. Behav. Biomed. Mater. 2012, 16, 21–28. [Google Scholar] [CrossRef]
- Samuel, S.; Nag, S.; Nasrazadani, S.; Ukirde, V.; El Bouanani, M.; Mohandas, A.; Nguyen, K.; Banerjee, R. Corrosion resistance and in vitro response of laser-deposited Ti-Nb-Zr-Ta alloys for orthopedic implant applications. J. Biomed. Mater. Res. Part A 2010, 94, 1251–1256. [Google Scholar] [CrossRef]
- Samuel, S.; Nag, S.; Scharf, T.W.; Banerjee, R. Wear resistance of laser-deposited boride reinforced Ti-Nb–Zr–Ta alloy composites for orthopedic implants. Mater. Sci. Eng. C 2008, 28, 414–420. [Google Scholar] [CrossRef]
- Nag, S.; Banerjee, R.; Fraser, H.L. Laser-deposited Ti-Nb-Zr-Ta orthopedic alloys. J. Biomed. Mater. Res. A 2006, 78, 298–305. [Google Scholar]
- Mishra, S.K.; Chowdhary, R. Evolution of dental implants through the work of Per-Ingvar Brånemark: A systematic review. Osseointegration 2020, 31, 930–956. [Google Scholar]
- Malinovschi, V.; Marin, A.; Andrei, V.; Coaca, E.; Mihailescu, C.N.; Lungu, C.P.; Radulescu, C.; Dulama, I.D. Obtaining and characterization of PEO layers prepared on CP-Ti in sodium dihydrogen phosphate dihydrate acidic electrolyte solution. Surf. Coat. Technol. 2019, 375, 621–636. [Google Scholar] [CrossRef]
- Beltrán-Partida, E.; Moreno-Ulloa, A.; Valdez-Salas, B.; Velasquillo, C.; Carrillo, M.; Escamilla, A.; Valdez, E.; Villarreal, F. Improved Osteoblast and Chondrocyte Adhesion and Viability by Surface-Modified Ti6Al4V Alloy with Anodized TiO2 Nanotubes Using a Super-Oxidative Solution. Materials 2015, 8, 867–883. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, S.; Sidhu, S.S.; Bains, P.S.; Bahraminasab, M. Mechanobiological Assessment of Ti-6Al-4V Fabricated via Selective Laser Melting Technique: A Review. Rapid Prototyp. J. 2019, 25, 1266–1284. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Dobrzański, L.B.; Achtelik-Franczak, A.; Dobrzańska, J. Application Solid Laser-Sintered or Machined Ti6Al4V Alloy in Manufacturing of Dental Implants and Dental Prosthetic Restorations According to Dentistry 4.0 Concept. Processes 2020, 8, 664. [Google Scholar] [CrossRef]
- Bains, P.S.; Bahraminasab, M.; Sidhu, S.S.; Singh, G. On the Machinability and Properties of Ti–6Al–4V Biomaterial with n-HAp Powder–Mixed ED Machining. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2019, 234, 232–242. [Google Scholar] [CrossRef]
- Walkowiak-Przybyło, M.; Klimek, L.; Okrój, W.; Jakubowski, W.; Chwiłka, M.; Czajka, A.; Walkowiak, B. Adhesion, activation, and aggregation of blood platelets and biofilm formation on the surfaces of titanium alloys Ti6Al4V and Ti6Al7Nb. J. Biomed. Mater. Res. Part A 2012, 100, 768–775. [Google Scholar] [CrossRef]
- Peuster, M.; Hesse, C.; Schloo, T.; Fink, C.; Beerbaum, P.; von Schnakenburg, C. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials 2006, 27, 4955–4962. [Google Scholar] [CrossRef]
- Yan, X.-H.; Ma, J.; Zhang, Y. High-throughput screening for biomedical applications in a Ti-Zr-Nb alloy system through masking co-sputtering. Sci. China Phys. Mech. Astron. 2019, 62, 996111. [Google Scholar] [CrossRef]
- Barilyuk, D.; Bazlov, A.; Arkharova, N.; Teplyakova, T.; Konopatsky, A.; Prokoshkin, S. Novel Zr-Rich Alloys of Ternary Ti-Zr-Nb System with Large Superelastic Recovery Strain. Metals 2022, 12, 185. [Google Scholar] [CrossRef]
- Arias-González, F.; Rodríguez-Contreras, A.; Punset, M.; Manero, J.M.; Barro, Ó.; Fernández-Arias, M.; Lusquiños, F.; Gil, F.J.; Pou, J. In-Situ Laser Directed Energy Deposition of Biomedical Ti-Nb and Ti-Zr-Nb Alloys from Elemental Powders. Metals 2021, 11, 1205. [Google Scholar] [CrossRef]
- Guo, A.X.Y.; Cheng, L.; Zhan, S.; Zhang, S.; Xiong, W.; Wang, Z.; Wang, G.; Cao, S.C. Biomedical applications of the powder-based 3D printed titanium alloys: A review. J. Mater. Sci. Technol. 2022, 125, 252–264. [Google Scholar] [CrossRef]
- Liu, J.; Chang, L.; Liu, H.; Li, Y.; Yang, H.; Ruan, J. Microstructure, mechanical behavior and biocompatibility of powder metallurgy Nb-Ti-Ta alloys as biomedical material. Mater. Sci. Eng. C 2017, 71, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xiao, Y.; Yi, D.; Liu, H.; Wu, L.; Wen, J. Corrosion behavior of Ti–Nb–Ta–Zr–Fe alloy for biomedical applications in Ringer’s solution. Trans. Nonferrous Met. Soc. China 2015, 25, 2556–2563. [Google Scholar] [CrossRef]
- Angelescu, R.M.; Cotruț, C.; Nocivin, A.; Cojocaru, V.D.; Răducanu, D.; Angelescu, M.L.; Cincă, I. Mechanical, Structural and Corrosion Analysis of a Ti-Nb-Zr-Fe Alloy Designated to Oral Implantology. Sci. Bull. 2015, 77, 3. [Google Scholar]
- Fatichi, A.Z.; Mello, M.G.; Caram, R.; Cremasco, A. Self-organized TiO2 nanotube layer on Ti–Nb–Zr alloys: Growth, characterization, and effect on corrosion behavior. J. Appl. Electrochem. 2019, 49, 1079–1089. [Google Scholar] [CrossRef]
- Angelescu, M.L.; Dan, A.; Ungureanu, E.; Zarnescu-Ivan, N.; Galbinasu, B.M. Effects of Cold Rolling Deformation and Solution Treatment on Microstructural, Mechanical, and Corrosion Properties of a Biocompatible Ti-Nb-Ta-Zr Alloy. Metals 2022, 12, 248. [Google Scholar] [CrossRef]
- Correa-Rossi, M.; Romero-Resendiz, L.; Leal-Bayerlein, D.; Garcia-Alves, A.L.; Segovia-López, F.; Amigó-Borrás, V. Mechanical, Corrosion, and Ion Release Studies of Ti-34Nb-6Sn Alloy with Comparable to the Bone Elastic Modulus by Powder Metallurgy Method. Powders 2022, 1, 3–17. [Google Scholar] [CrossRef]
- Gupta, S.K.; Gugulothu, S.B.; Ivanov, E.; Suwas, S.; Chatterjee, K. Additive Manufacturing of a Low Modulus Biomedical Ti-Nb-Ta-Zr Alloy by Directed Energy Deposition. Bioprinting 2024, 41, e00349. [Google Scholar] [CrossRef]
- Hua, N.; Wang, W.; Wang, Q.; Ye, Y.; Lin, S.; Zhang, L.; Guo, Q.; Brechtl, J.; Liaw, P.K. Mechanical, corrosion, and wear properties of biomedical Ti–Zr–Nb–Ta–Mo high entropy alloys. J. Alloys Compd. 2021, 861, 157997. [Google Scholar] [CrossRef]
- Izonin, I.; Tkachenko, R.; Duriagina, Z.; Shakhovska, N.; Kovtun, V.; Lotoshynska, N. Smart Web Service of Ti-Based Alloy’s Quality Evaluation for Medical Implants Manufacturing. Appl. Sci. 2022, 12, 5238. [Google Scholar] [CrossRef]
- Li, Q.; Sun, H.; Li, J.; Yuan, X.; Nakai, M.; Niinomi, M.; Nakano, T. Influence of Sintering Temperature on Mechanical Properties of Ti-Nb-Zr-Fe Alloys Prepared by Spark Plasma Sintering. J. Mater. Eng. Perform. 2021, 30, 5719–5727. [Google Scholar] [CrossRef]
- Jin, L.; Cui, W.; Song, X.; Zhou, L. The formation mechanisms of surface nanocrystallites in β-type biomedical TiNbZrFe alloy by surface mechanical attrition treatment. Appl. Surf. Sci. 2015, 347, 553–560. [Google Scholar] [CrossRef]
- Cojocaru, V.D.; Dan, A.; Șerban, N.; Cojocaru, E.M.; Zărnescu-Ivan, N.; Gălbinașu, B.M. Effect of Cold-Rolling Deformation on the Microstructural and Mechanical Properties of a Biocompatible Ti-Nb-Zr-Ta-Sn-Fe Alloy. Materials 2024, 17, 2312. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, V.D.; Nocivin, A.; Trisca-Rusu, C.; Dan, A.; Irimescu, R.; Raducanu, D.; Galbinasu, B.M. Improving the Mechanical Properties of a β-type Ti-Nb-Zr-Fe-O Alloy. Metals 2020, 10, 1491. [Google Scholar] [CrossRef]
- Li, Q.; Yuan, X.; Li, J.; Wang, P.; Nakai, M.; Niinomi, M.; Nakano, T.; Chiba, A.; Liu, X.; Pan, D. Effects of Fe on Microstructures and Mechanical Properties of Ti–15Nb–25Zr–(0, 2, 4, 8)Fe Alloys Prepared by Spark Plasma Sintering. Mater. Trans 2019, 60, 1763–1768. [Google Scholar] [CrossRef]
- Cojocaru, V.D.; Șerban, N.; Cojocaru, E.M.; Zărnescu-Ivan, N.; Gălbinașu, B.M. The Effect of Solution Treatment Duration on the Microstructural and Mechanical Properties of a Cold-Deformed-by-Rolling Ti-Nb-Zr-Ta-Sn-Fe Alloy. Materials 2024, 17, 864. [Google Scholar] [CrossRef]
- Cojocaru, V.D.; Șerban, N.; Cojocaru, E.M.; Nocivin, A.; Raducanu, D.; Cinca, I.; Angelescu, M.L.; Dan, I. Finding an Optimal Thermo-Mechanical Processing Scheme for a Gum-Type Ti-Nb-Zr-Fe-O Alloy. J. Mater. Eng. Perform. 2017, 26, 4373–4380. [Google Scholar]
- Niinomi, M. Recent research and development in titanium alloys for biomedical applications and healthcare goods. Sci. Technol. Adv. Mater. 2003, 4, 445–454. [Google Scholar] [CrossRef]
- Ciobotaru, I.-A.; Ismail, F.B.; Budei, R.; Cojocaru, A.; Vaireanu, D.-I. The Effect of Anodization and Thermal Treatment on Mixed-Oxide Layer Formation on Ti–Zr Alloy. Coatings 2024, 14, 1217. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, Y.; Zhou, W.; Chen, P.; Zhou, Z.; Kanetaka, H.; Ishimoto, T.; Koizumi, Y.; Nakano, T.; Nomura, N. Laser additive manufacturing of a carbon-supersaturated β-Ti alloy for biomaterial application. Addit. Manuf. Lett. 2024, 11, 100233. [Google Scholar] [CrossRef]
- Qi, J.; Chen, K.; Teng, S.; Li, X. Preparation and characterisation of gradient HA/TiN/Ti coatings for biomedical applications. Surf. Eng. 2024, 40, 826–837. [Google Scholar] [CrossRef]
- Zhang, X.; Hong, J.; Chen, G. Functionalized graphene nano-platelet reinforced magnesium-based biocomposites for potential implant applications. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2024, 09544054241277590. [Google Scholar] [CrossRef]
- Ding, C.; Lv, H.; Huang, S.; Hu, M.; Liao, Y.; Meng, X.; Gao, M.; Chen, H.; Feng, X.; Wu, Z. The Application Progress of Nonthermal Plasma Technology in the Modification of Bone Implant Materials. ACS Biomater. Sci. Eng. 2024, 10, 5893–5914. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, N.; Cheng, H.; Wang, Y.; Zhao, D. Tensile Deformation Mechanism of an In Situ Formed Ti-Based Bulk Metallic Glass Composites. Materials 2024, 17, 4486. [Google Scholar] [CrossRef]
- Calazans Neto, J.V.; Celles, C.A.S.; de Andrade, C.S.A.F.; Afonso, C.R.M.; Nagay, B.E.; Barao, V.A.R. Recent Advances and Prospects in β-type Titanium Alloys for Dental Implants Applications. ACS Biomater. Sci. Eng. 2024, 10, 6029–6060. [Google Scholar] [CrossRef]
- Rajabi, E.; Dehghani, K.; Shahmir, H. Tribological Behavior and In-Vitro Biocompatibility of a Newly Designed TiZrNbMoTa High-Entropy Alloy. Adv. Eng. Mater. 2024, 26, 2400763. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Kolli, R.P.; Devaraj, A. A Review of Metastable Beta Titanium Alloys. Metals 2018, 8, 506. [Google Scholar] [CrossRef]
- Elias, C.N.; Lima, J.H.C.; Valiev, R.; Meyers, M.A. Biomedical applications of titanium and its alloys. Biol. Mater. Sci. 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Alfred, T.S. Biocompatibility of Advanced Manufactured Titanium Implants—A Review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef]
- Nag, S.; Banerjee, R.; Fraser, H.L. Microstructural evolution and strengthening mechanisms in Ti–Nb–Zr–Ta, Ti–Mo–Zr–Fe and Ti–15Mo biocompatible alloys. Mater. Sci. Eng. C 2005, 25, 357–362. [Google Scholar] [CrossRef]
- Toualbia, K.; Fellah, M.; Hezil, N.; Milles, H.; Djafia, Z. Effect of milling time on structural, mechanical and tribological properties of nanostructured HIPed near type Ti-15Mo alloys. Tribol. Int. 2024, 197, 109731. [Google Scholar] [CrossRef]
- Srinivas Kumar, G.L.G.S.B.; Chinara, S.; Das, S.; Tiwari, C.; Nageswara Rao, P.G.V.S. Studies on Ti-29Nb-13Ta-4.6Zr alloy for use as a prospective biomaterial. Mater. Today Proc. 2019, 15, 11–20. [Google Scholar] [CrossRef]
- Lei, S.; Zhang, J.; An, X.; Guo, Y.; Xu, X.; Ma, Z.; Yao, W.; Kong, Q. Mechanical and corrosion properties of Ti–29Nb–13Ta-4.6Zr alloy prepared by cryomilling and spark plasma sintering. Vacuum 2023, 215, 112316. [Google Scholar] [CrossRef]
- Shen, X.; Shukla, P. A Review of Titanium Based Orthopaedic Implants (Part-I): Physical Characteristics, Problems and the need for Surface Modification. Int. J. Peen. Sci. Technol. 2020, 1, 301–332. [Google Scholar]
- Hsueh-Chuan, H.; Wong, K.-K.; Wu, S.-C.; Chang, H.-H.; Lu, Y.-C.; Ho, W.-F. Compositional-segregation-induced dual-length-scale nanotubes for enhanced surface bioactivity of Ti-rich Ti65–Zr18–Nb16–Mo1 medium-entropy alloy. Surf. Coat. Technol. 2024, 478, 130490. [Google Scholar]
- Zhang, G.; Khanlari, K.; Huang, S.; Li, X.; Zhao, D.; Wu, H.; Cao, Y.; Liu, B.; Huang, Q. Dual-structured oxide coatings with enhanced wear and corrosion resistance prepared by plasma electrolytic oxidation on Ti-Nb-Ta-Zr-Hf high-entropy alloy. Surf. Coat. Technol. 2023, 456, 129254. [Google Scholar] [CrossRef]
- Yuan, Y.; Ke, Z.; Zhang, L.; Jiang, Y.; He, Z. Mechanical, corrosion and antibacterial properties of Ti-13Nb-13Zr-based alloys with various Cu contents. Mater. Res. Express 2021, 8, 115403. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Demiroren, H. An Approximation to Determine Corrosion and Mechanical Behavior of Ti-Based Alloys. Surf. Rev. Lett. 2021, 28, 2150001. [Google Scholar] [CrossRef]
- Sutowo, C.; Supriadi, S.; Pramono, A.W.; Suharno, B. Microstructure, Mechanical Properties, and Corrosion Behavior of New βtype Ti–Mo–Nb Based Alloys by Mn Addition for Implant Material. East. -Eur. J. Enterp. Technol. 2020, 12, 30–37. [Google Scholar]
- Manam, N.S.; Harun, W.S.W.; Shri, D.N.A.; Ghani, S.A.C.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M.H.I. Study of corrosion in biocompatible metals for implants: A review. J. Alloys Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef]
- Mazare, A.; Totea, G.; Burnei, C.; Schmuki, P.; Demetrescu, I.; Ionita, D. Corrosion, antibacterial activity and haemocompatibility of TiO2 nanotubes as a function of their annealing temperature. Corros. Sci. 2016, 103, 215–222. [Google Scholar] [CrossRef]
- Stráský, J.; Harcuba, P.; Václavová, K.; Horváth, K.; Landa, M.; Srbac, O.; Janeček, M. Increasing strength of a biomedical Ti-Nb-Ta-Zr alloy by alloying with Fe, Si and O. J. Mech. Behav. Biomed. Mater 2017, 71, 329–336. [Google Scholar] [CrossRef]
- Ji, Q.Y.; Long, Y.; Yang, C.; Chen, W.G. Effect of Si addition on microstructure and performance of a biomedical Ti-Nb-Zr-Ta alloy prepared via powder hot extrusion. J. Alloys Compd. 2024, 977, 173326. [Google Scholar] [CrossRef]
- Meng, X.; Wang, X.; Guo, Y.; Ma, S.; Luo, W.; Xiang, X.; Zhao, J.; Zhou, Y. Biocompatibility Evaluation of a Newly Developed Ti–Nb–Zr–Ta–Si Alloy Implant. J. Biomater. Tissue Eng 2016, 6, 861–869. [Google Scholar] [CrossRef]
- Wang, X.; Meng, X.; Chu, S.; Xiang, X.; Liu, Z.; Zhao, J.; Zhou, Y. Osseointegration behavior of novel Ti–Nb–Zr–Ta–Si alloy for dental implants: An in vivo study. J. Mater. Sci. Mater. Med. 2016, 27, 139. [Google Scholar] [CrossRef]
- Lu, N.; Zhang, J.; Dan, Y.; Sun, M.; Gong, T.; Li, X.; Zhu, X. Growth of porous anodic TiO2 in silver nitrate solution without fluoride: Evidence against the field-assisted dissolution reactions of fluoride ions. Electrochem. Commun. 2021, 126, 107022. [Google Scholar] [CrossRef]
- Wu, Z.; Luo, J.; Zhang, J.; Huang, H.; Xie, Z.; Xie, X. Silver-Releasing Micro-/Nanoporous Coating on Additively Manufactured Macroporous Ti-Ta-Nb-Zr Scaffolds with High Osseointegration and Antibacterial Properties. Coatings 2021, 11, 716. [Google Scholar] [CrossRef]
- Li, Y.H.; Yang, C.; Kang, L.M.; Zhao, H.D.; Zhang, W.W.; Li, Y.Y. Biomedical porous TiNbZrFe alloys fabricated using NH4HCO3 as pore forming agent through powder metallurgy route. Powder Metall. 2015, 58, 228–234. [Google Scholar] [CrossRef]
- Çakmak, Ö.; Kaya, M. Effect of sintering procedure on microstructure and mechanical properties of biomedical TiNbSn alloy produced via powder metallurgy. Appl. Phys. A 2021, 127, 561. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Y.; Liu, B.; Zhang, W. Precipitation behavior during hot deformation of powder metallurgy Ti-Nb-Ta-Zr-Al high entropy alloys. Intermetallics 2018, 100, 95–103. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Q.; Liu, X.; Geng, R.; Wang, B.; Jiang, Q. Improved Strength-Ductility of Ti-6Al-4V Casting Alloys with Trace Addition of TiC-TiB2 Nanoparticles. Nanomaterials 2020, 10, 2330. [Google Scholar] [CrossRef] [PubMed]
- Atay, G.Y.; Uslu, G.; Amigo Borras, V. Manufacturing antibacterial Ti-6Al-4V alloys by using NanoAg particles synthesized by reduction method for biomedical applications. Discov. Mater. 2024, 4, 64. [Google Scholar] [CrossRef]
- Kheradmandfard, M.; Kashani-Bozorg, S.F.; Kang, K.H.; Penkov, O.V.; Hanzaki, A.Z.; Pyoun, Y.S.; Amanov, A.; Kim, D.E. Simultaneous grain refinement and nanoscale spinodal decomposition of β phase in Ti-Nb-Ta-Zr alloy induced by ultrasonic mechanical impacts. J. Alloys Compd. 2018, 738, 540–549. [Google Scholar] [CrossRef]
- Xu, X.; Ding, H.; Huang, H.; Liang, H.; Chen, R.; Guo, J.; Fu, H. Microstructure formation and columnar to equiaxed transition during cold crucible directional solidification of a high-Nb TiAl alloy. J. Mater. Res. Technol. 2021, 11, 2221–2234. [Google Scholar] [CrossRef]
- Pesode, P.; Barve, S. A review—Metastable β titanium alloy for biomedical applications. J. Eng. Appl. Sci. 2023, 70, 25. [Google Scholar] [CrossRef]
- Marczewski, M.; Wieczerzak, K.; Maeder, X.; Lapeyre, L.; Hain, C.; Jurczyk, M.; Nelis, T. Microstructure and mechanical properties of Ti-Nb alloys: Comparing conventional powder metallurgy, mechanical alloying, and high power impulse magnetron sputtering processes for supporting materials screening. J. Mater. Sci. 2024, 59, 9107–9125. [Google Scholar] [CrossRef]
- Nag, S.; Banerjee, R.; Fraser, H.L. Intra-granular alpha precipitation in Ti–Nb–Zr–Ta biomedical alloys. J. Mater. Sci. 2009, 44, 808–815. [Google Scholar] [CrossRef]
- Banerjee, R.; Nag, S.; Stechschulte, J.; Fraser, H.L. Strengthening mechanisms in Ti-Nb-Zr-Ta and Ti-Mo-Zr-Fe orthopaedic alloys. Biomaterials 2004, 25, 3413–3419. [Google Scholar] [CrossRef]
- Su, E.P.; Justin, D.F.; Pratt, C.R.; Sarin, V.K.; Nguyen, V.S.; Oh, S.; Jin, S. Effects of titanium nanotubes on the osseointegration, cell differentiation, mineralisation and antibacterial properties of orthopaedic implant surfaces. Bone Jt. J. 2018, 100-B, 9–16. [Google Scholar] [CrossRef]
- Rosenbaum, J.; Versace, D.L.; Abbad-Andallousi, S.; Pires, R.; Azevedo, C.; Cénédese, P.; Dubot, P. Antibacterial Properties of Nanostructured Cu-TiO2 Surfaces for Dental Implants. Biomater. Sci. 2017, 5, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; Sun, Y.-H.; Zhao, Y.; Liu, Y.-L.; Yao, X.-H.; Tang, B.; Hang, R.-Q. Antibacterial ability and cytocompatibility of Cu-incorporated Ni–Ti–O nanopores on NiTi alloy. Rare Met. 2019, 38, 552–560. [Google Scholar] [CrossRef]
- Rezayat, M.; Karamimoghadam, M.; Saghafi Yazdi, M.; Moradi, M.; Bodaghi, M. Statistical analysis of experimental factors for synthesis of copper oxide and tin oxide for antibacterial applications. Int. J. Adv. Manuf. Technol. 2023, 127, 3017–3030. [Google Scholar] [CrossRef]
- Yi, C.; Yuan, Y.; Zhang, L.; Jiang, Y.; He, Z. Antibacterial Ti-35Nb-7Zr-xCu alloy with excellent mechanical properties generated with a spark plasma sintering method for biological applications. J. Alloys Compd. 2021, 879, 160473. [Google Scholar] [CrossRef]
- Ueda, K.; Ueda, T.; Narushima, T. Antibacterial Functionalization of Ti-based Biomaterials Based on the Understanding of the Inactivation Mechanisms of Bacteria via Photocatalytic Activity of Titanium Oxide: Visible-light Responsive Reaction of Titanium Oxide Coating. Mater. Jpn. 2020, 59, 612–617. [Google Scholar] [CrossRef]
- Shen, X.; Al-Baadani, M.A.; He, H.; Cai, L.; Wu, Z.; Yao, L.; Wu, X.; Chen, S.M.; Zhang, H.; Liu, J. Antibacterial and osteogenesis performances of LL37-loaded titania nanopores in vitro and in vivo. Int. J. Nanomed. 2019, 14, 3043–3054. [Google Scholar] [CrossRef]
- Roguska, A.; Belcarz, A.; Suchecki, P.; Andrzejczuk, M.; Lewandowska, M. Antibacterial Composite Layers on Ti: Role of ZnO Nanoparticles. Arch. Metall. Mater. 2016, 61, 937–940. [Google Scholar] [CrossRef]
- Yu, S.R.; Zhang, X.P.; He, Z.M.; Liu, Y.H.; Liu, Z.H. Effects of Ce on the short-term biocompatibility of Ti–Fe–Mo–Mn–Nb–Zr alloy for dental materials. J. Mater. Sci. Mater. Med. 2004, 15, 687–691. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Beline, T.; Ribeiro, A.L.R.; Rangel, E.C.; da Cruz, N.C.; Landers, R.; Faverani, L.P.; Vaz, L.G.; Fais, L.M.G.; Vicente, F.B.; et al. Development of binary and ternary titanium alloys for dental implants. Dent. Mater. 2017, 33, 1244–1257. [Google Scholar] [CrossRef]
- Peter, I.; Rosso, M. Study of Ti-Enriched CoCrMo Alloy for Dental Application. IEEE Access 2015, 3, 73–80. [Google Scholar] [CrossRef]
- Peter, I.; Rosso, M.; Dan, I.; Toppi, A.; Ghiban, B. Investigation on cobalt based alloy modified by titanium for dental applications. J. Dent. Res. 2013, 61, 62–68. [Google Scholar]
- Ishfaq, K.; Rehman, M.; Khan, A.R.; Wang, Y. A review on the performance characteristics, applications, challenges and possible solutions in electron beam melted Ti-based orthopaedic and orthodontic implants. Rapid Prototyp. J. 2022, 28, 525–545. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on Titanium and Titanium Based Alloys as Biomaterials for Orthopaedic Applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.K. Surface Modifications in Ti-Based Orthopaedic Implants. Biomed. Eng. Its Appl. Healthc. 2019, 275–293. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Q.; Yu, Z.; Ren, L.; Zhao, X.; Wang, J. Study on Osseointegration Capability of β-Type Ti–Nb–Zr–Ta–Si Alloy for Orthopedic Implants. Materials 2024, 17, 472. [Google Scholar] [CrossRef]
- Zhang, T.; Ou, P.; Ruan, J.; Yang, H. Nb-Ti-Zr Alloys for Orthopedic Implants. J. Biomater. Appl. 2021, 35, 1284–1293. [Google Scholar] [CrossRef]
- Acharya, S.; Panicker, A.G.; Laxmi, D.V.; Suwas, S.; Chatterjee, K. Study of the Influence of Zr on the Mechanical Properties and Functional Response of Ti-Nb-Ta-Zr-O Alloy for Orthopedic Applications. Mater. Des. 2019, 164, 107555. [Google Scholar] [CrossRef]
- Li, B.Q.; Li, X.C.; Lu, X. Microstructure and compressive properties of porous Ti–Nb–Ta–Zr alloy for orthopedic applications. J. Mater. Res. 2019, 34, 4045–4055. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Gasik, M. Electrochemical and biological characterization of Ti–Nb–Zr–Si alloy for orthopedic applications. Sci. Rep. 2023, 13, 2312. [Google Scholar] [CrossRef]
- Du, P.; Wu, Z.; Li, K.; Xiang, T.; Xie, G. Porous Ti-based bulk metallic glass orthopedic biomaterial with high strength and low Young’s modulus produced by one step SPS. J. Mater. Sci. Technol. 2021, 13, 251–259. [Google Scholar] [CrossRef]
- Bărbînţă, A.C.; Chelariu, R.; Benchea, M.; Crimu, C.I.; Strugaru, S.I.; Munteanu, C. A Comparative Analysis of New Ti-Nb-Zr-Ta Orthopedic Alloys. Adv. Mater. Res. 2014, 837, 259–264. [Google Scholar] [CrossRef]
- Peter, I.; Andone, M.-C.; Singhwal, S.S.; Albu, S.-C. The Use of Materials for Dental Applications. Acta Marisiensis. Ser. Technol. 2022, 19, 6–11. [Google Scholar]
- Zhang, Y.; Gulati, K.; Li, Z.; Di, P.; Liu, Y. Dental Implant Nano-Engineering: Advances, Limitations and Future Directions. Nanomaterials 2021, 11, 2489. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, X.; Imai, K. Application of Zr and Ti-Based Bulk Metallic Glasses for Orthopaedic and Dental Device Materials. Metals 2020, 10, 203. [Google Scholar] [CrossRef]
- Dobrzański, L.A. Introductory Chapter: Multi-Aspect Bibliographic Analysis of the Synergy of Technical, Biological and Medical Sciences Concerning Materials and Technologies Used for Medical and Dental Implantable Devices. Biomater. Regen. Med. 2018, 1–15. [Google Scholar] [CrossRef]
- Biesiekierski, A.; Wang, J.; Gepreel, M.A.-H.; Wen, C. A new look at biomedical Ti-based shape memory alloys. Acta Biomater. 2012, 8, 1661–1669. [Google Scholar] [CrossRef]
- Wang, Y.; Venezuela, J.; Dargusch, M. Biodegradable shape memory alloys: Progress and prospects. Biomaterials 2021, 279, 121215. [Google Scholar] [CrossRef]
- Kelly, J.A. Craniofacial and Maxillofacial Implants. In Oral and Cranial Implants; Springer: Berlin/Heidelberg, Germany, 2012; pp. 29–39. [Google Scholar] [CrossRef]
- Chen, X.Y.; Yang, X.; Fan, X.L. The Evolution of Orbital Implants and Current Breakthroughs in Material Design, Selection, Characterization, and Clinical Use. Front. Bioeng. Biotechnol. 2022, 9, 800998. [Google Scholar] [CrossRef]
- Park, J.; Cimpean, A.; Tesler, A.B.; Mazare, A. Anodic TiO2 Nanotubes: Tailoring Osteoinduction via Drug Delivery. Nanomaterials 2021, 11, 2359. [Google Scholar] [CrossRef]
- Adeosun, S.O.; Ilomuanya, M.O.; Gbenebor, O.P.; Dada, M.O.; Odili, C.C. Biomaterials for Drug Delivery: Sources, Classification, Synthesis, Processing, and Applications. In Advanced Functional Materials; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Porter, J.R.; Ruckh, T.T.; Popat, K.C. Bone tissue engineering: A review in bone biomimetics and drug delivery strategies. Biotechnol. Prog. 2009, 25, 1539–1560. [Google Scholar] [CrossRef]
- Popat, K.C.; Eltgroth, M.; LaTempa, T.J.; Grimes, C.A.; Desai, T.A. Titania nanotubes: A novel platform for drug-eluting coatings for medical implants? Small 2007, 3, 1878–1881. [Google Scholar] [CrossRef] [PubMed]
- Stráský, J.; Janeček, M.; Harcuba, P.; Landa, M. Plastic Deformation and Elastic Properties of Ti-Nb-Zr-Ta(-Fe-Si) Biomedical Alloys. Adv. Mater. Res. 2014, 922, 734–739. [Google Scholar] [CrossRef]
- Gurau, C.; Gurau, G.; Mitran, V.; Dan, A.; Cimpean, A. The Influence of Severe Plastic Deformation on Microstructure and In Vitro Biocompatibility of the New Ti-Nb-Zr-Ta-Fe-O Alloy Composition. Materials 2020, 13, 4853. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Lai, T.-H.; Chen, C.-Y.; Niinomi, M.; Okada, K.; Chang, T.-F.M.; Hsieh, P.-Y.; Ozasa, K.; Matsushita, N.; Sone, M.; et al. Fully Depleted Ti-Nb-Ta-Zr-O Nanotubes: Interfacial Charge Dynamics and Solar Hydrogen Production. ACS Appl. Mater. Interfaces 2018, 10, 22997–23008. [Google Scholar] [CrossRef]
- de Carvalho, L.C.; da Silva, M.R.; Bolfarini, C.; Gargarella, P. The Influence of Aging Treatment on a Ti-Nb-Ta-Zr-O Alloy Processed by High-Pressure Torsion. JOM 2024, 76, 5302–5313. [Google Scholar] [CrossRef]
- Luo, J.P.; Lv, K.P.; Tang, J.C.; Wu, Z.Z.; Liu, Y.L.; Luo, J.T.; Lai, Y.X.; Yan, M. Electropolishing influence on biocompatibility of additively manufactured Ti-Nb-Ta-Zr: In vivo and in vitro. J. Mater. Sci. Mater. Med. 2023, 34, 25. [Google Scholar] [CrossRef]
- Aguilar, C.; Arancibia, M.; Alfonso, I.; Sancy, M.; Tello, K.; Salinas, V.; De Las Cuevas, F. Influence of Porosity on the Elastic Modulus of Ti-Zr-Ta-Nb Foams with a Low Nb Content. Metals 2019, 9, 176. [Google Scholar] [CrossRef]
- Sakaguchi, N.; Niinomi, M.; Akahori, T.; Saito, T.; Furuta, T. Effects of Alloying Elements on Elastic Modulus of Ti-Nb-Ta-Zr System Alloy for Biomedical Applications. Mater. Sci. Forum 2004, 449–452, 1269–1272. [Google Scholar] [CrossRef]
- Strnad, G.; Jakab-Farkas, L.; Gobber, F.S.; Peter, I. Synthesis and Characterization of Nanostructured Oxide Layers on Ti-Nb-Zr-Ta and Ti-Nb-Zr-Fe Biomedical Alloys. J. Funct. Biomater 2023, 14, 180. [Google Scholar] [CrossRef]
- Strnad, G.; Petrovan, C.; Russu, O.; Jakab-Farkas, L. TiO2 Nanostructured Surfaces for Biomedical Applications Developed by Electrochemical Anodization. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Tomsk, Russia, 1–3 June 2016; Volume 161, p. 012051. [Google Scholar]
- Strnad, G.; Portan, D.; Jakab-Farkas, L.; Petrovan, C.; Russu, O. Morphology of Nanostructured TiO2 Surfaces for Biomedical Implants Developed by Electrochemical Anodization. Mater. Sci. Forum 2017, 907, 91–98. [Google Scholar] [CrossRef]
- Russu, O.M.; Strnad, G.; Jakab-Farkas, L.; Cazacu, R.; Feier, A.; Gergely, I.; Trambitas, C.; Petrovan, C. Electrochemical Synthesis of Nanostructured Oxide Layers on Threaded Surfaces of Medical Implants. Rev. De Chim. 2018, 69, 1636–1639. [Google Scholar] [CrossRef]
- Yuda, A.W.; Supriadi, S.; Saragih, A.S. Surface modification of Ti-alloy based bone implant by sandblasting. In Proceedings of the 4th Biomedical Engineering’s Recent Progress in Biomaterials, Drugs Development, Health, and Medical Devices: Proceedings of the International Symposium of Biomedical Engineering (ISBE 2019), Padang, Indonesia, 22–24 July 2019; Volume 2193, p. 020015. [Google Scholar] [CrossRef]
- Răducanu, D.; Cojocaru, V.D.; Nocivin, A.; Cinca, I.; Șerban, N.; Cojocaru, E.M. Surface Modifications of Biomedical Gum-Metal-Type Alloy by Nano Surface—Severe Plastic Deformation. JOM 2019, 71, 4114–4124. [Google Scholar] [CrossRef]
- Gulati, K.; Moon, H.J.; Li, T.; Sudheesh Kumar, P.T.; Ivanovski, S. Titania nanopores with dual micro-/nano-topography for selective cellular bioactivity. Mater. Sci. Eng. C 2018, 91, 624–630. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, Y.; Cao, Y.; Liu, B.; Huang, Q.; Liu, Y. Superior Self-Lubricating Coatings with Heterogeneous Nanocomposite Structures on Ti–Nb–Zr–Ta–Hf Refractory Multi-Principal Element Alloy. Adv. Funct. Mater. 2024, 34, 2405657. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Nagay, B.E.; Ribeiro, A.L.R.; da Cruz, N.C.; Rangel, E.C.; Fais, L.M.G.; Vaz, L.G.; Barão, V.A.R. Functionalization of an experimental Ti-Nb-Zr-Ta alloy with a biomimetic coating produced by plasma electrolytic oxidation. J. Alloys Compd. 2019, 770, 1038–1048. [Google Scholar] [CrossRef]
- Zhao, F.; Gao, A.; Liao, Q.; Li, Y.; Ullah, I.; Zhao, Y.; Ren, X.; Tong, L.; Li, X.; Zheng, Y.; et al. Balancing the Anti-Bacterial and Pro-Osteogenic Properties of Ti-Based Implants by Partial Conversion of ZnO Nanorods into Hybrid Zinc Phosphate Nanostructures. Adv. Funct. Mater. 2024, 34, 2311812. [Google Scholar] [CrossRef]
- Ahlawat, S.; Kanaujia, B.K.; Rambabu, K.; Peter, I.; Matekovits, L. Circularly polarized differential intra-oral antenna design validation and characterization for tongue drive system. Sci. Rep. 2023, 13, 9935. [Google Scholar] [CrossRef]
- Kouhalvandi, L.; Matekovits, L.; Peter, I. Multi-band Implantable Microstrip Antenna on Large Ground Plane and TiO2 Substrate. In Proceedings of the 2021 IEEE 17th International Conference on Wearable and Implantable Body Sensor Networks (BSN 2021), Athens, Greece, 27–30 July 2021; pp. 1–4. [Google Scholar] [CrossRef]
- Abdullah, S.S.; Balci, E.; Qader, I.N.; Dagdelen, F. Assessment of Biocompatibility and Physical Properties of Ni–Ti–Zr–Nb Shape Memory Alloys. Trans. Indian Inst. Met. 2023, 76, 1237–1242. [Google Scholar] [CrossRef]
- Kim, D.G.; Woo, K.D.; Kang, D.S.; Lee, T.; Lee, M.H. Fabrication and biocompatibility evaluation of porous Ti-Nb-based biomaterials with space holder by rapid sintering. Mater. Res. Innov. 2015, 19, 301–304. [Google Scholar] [CrossRef]
- Dragan-Raileanu, L.A.; Munteanu, C.; Basescu, G.N.; Axinte, C.; Strugaru, S.C.; Papatoiu-Biniuc, C.; Barbinta, A. Biocompatibility Evaluation for Some New Ti-Nb-Zr-Ta Alloys. Ann. Rom. Soc. Cell Biol. 2013, 18, 192–197. [Google Scholar]
- Ding, X.; Zhou, L.; Wang, J.; Zhao, Q.; Lin, X.; Gao, Y.; Li, S.; Wu, J.; Rong, M.; Guo, Z.; et al. The effects of hierarchical micro/nanosurfaces decorated with TiO2 nanotubes on the bioactivity of titanium implants in vitro and in vivo. Int. J. Nanomed. 2015, 10, 6955–6973. [Google Scholar]
- Karazisis, D.; Ballo, A.; Petronis, S.; Agheli, H.; Emanuelsson, L.; Thomsen, P.; Omar, O. The role of well-defined nanotopography of titanium implants on osseointegration: Cellular and molecular events in vivo. Int. J. Nanomed. 2016, 11, 1367–1382. [Google Scholar]
- Hua, N.; Xu, Y.; Lin, B.; Zeng, D.; Liang, X.; Xiao, X.; Lin, H.; Zhang, L.; Lu, W.; Dai, P.; et al. Remarkable enhancement of the corrosive-wear resistance for Ti-Zr-Hf-Nb-Fe high-entropy alloys by a facile high-temperature oxidation treatment. Tribol. Int. 2024, 200, 110172. [Google Scholar] [CrossRef]
- Findik, F. Wear Properties of Ti-Based Biomaterials. Curr. Trends Biomed. Eng. Biosci. 2018, 12, 555837. [Google Scholar] [CrossRef]
- Sahasrabudhe, H. Characterization of Ti and Co Based Biomaterials Processed via Laser Based Additive Manufacturing. Ph.D. Thesis, Washington State University, Pullman, WA, USA, 2016. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Zhang, S.; Liu, H. Role of TiO2 Nanotubes on the Surface of Implants in Osseointegration in Animal Models: A Systematic Review and Meta-Analysis. J. Prosthodont. 2020, 29, 501–510. [Google Scholar] [CrossRef]
- Putra, N.E.; Mirzaali, M.J.; Apachitei, J.; Zhou, J.; Zadpoor, A.A. Multi-material additive manufacturing technologies for Ti-, Mg-, and Fe-based biomaterials for bone substitution. Acta Biomater. 2020, 109, 1–20. [Google Scholar] [CrossRef]
- Nakano, T.; Ishimoto, T.; Matsugaki, A.; Hagihara, K.; Koizumi, Y.; Ozasa, R. Control of crystallographic orientation by metal additive manufacturing process of β-type Ti alloys based on the bone tissue anisotropy. Mater. Trans. 2020, 321, 05002. [Google Scholar] [CrossRef]




| Alloy Type | Elements (% by Weight) | Reference |
|---|---|---|
| TNZF | Ti: 70–75, Nb: 15–20, Zr: 5–10, Fe: 2–5 | [1,6] |
| Ti13Nb13Zr | Ti: 74, Nb: 13, Zr: 13 | [8] |
| TNZT | Ti: 60, Nb: 30, Zr: 5, Ta: 5 | [12] |
| Property | TNZF Alloy | Ti13Nb13Zr Alloy | TNZT Alloy | References |
|---|---|---|---|---|
| Young’s modulus (GPa) | 40–110 | 65–75 | 50–65 | [2,8] |
| Tensile strength (MPa) | 600–1200 | 850–1100 | 700–850 | [10] |
| Corrosion rate (mm/year) | <0.01 | ~0.02 | ~0.015 | [1,11] |
| Mechanism | Benefits | References | |
|---|---|---|---|
| Grain Refinement | TiC and TiB2 nanoparticles (5–10%) reduce grain size during solidification. Ultrasonic impacts optimize the microstructure and mechanical properties of TNZT alloys. | Finer grains improve tensile strength by up to 30% and toughness by over 20%, enhancing durability for load-bearing implants. | [87] |
| Solid Solution Strengthening | Elements like Mo (2–5%), Nb (10–30%), and Zr (5–15%) dissolve in the Ti matrix to form a solid solution, limiting dislocation movement. For example, Ti-6Al-7Nb and Ti-35Nb-7Zr-5Ta. | This process increases yield strength by up to 25% and hardness by over 15%, improving resistance to deformation. | [27,88,89,90] |
| Precipitation Hardening | Elements such as Al and V (each 3–7%) precipitate in the Ti matrix, forming fine particles that obstruct dislocation motion. During hot deformation of high-entropy alloys, nanometric α plates form within the β matrix, improving mechanical properties. | This process boosts hardness by up to 20% and mechanical strength by over 15%, ensuring high load resistance for applications like joint replacements. | [84,91] |
| Dispersion Strengthening | Nanoparticles like HA and ZrO2 (approximately 5–12%) are uniformly dispersed within the Ti matrix, stabilizing grain boundaries and reducing dislocation movement. | This mechanism increases wear resistance by up to 25% and yield strength by over 20%, enhancing durability for dental and orthopedic applications. | [64,92] |
| Enhanced Osteointegration | HA nanoparticles (10–20%) mimic the mineral component of bone, facilitating better integration between the implant and bone tissue. | Effective osteointegration increases bone–implant contact by over 40%, ensuring the implant remains securely anchored, reducing the risk of loosening or failure. | [6,7] |
| Improved Corrosion Resistance | Adding elements like Nb, Ta, and Mo (combined total 15–25%), along with SiC nanoparticles (2–8%), enhances the protective oxide layer on Ti alloys. Treatment with PEO improves wear and corrosion resistance. | Enhanced corrosion resistance reduces degradation by up to 30%, significantly extending implant lifespan in physiological environments. | [20,70] |
| Antibacterial Properties | Ag nanoparticles (0.5–1.5%) release ions that prevent biofilm formation. Zinc oxide (ZnO) and copper-TiO2 (Cu-TiO2) nanoparticles (2–5%) demonstrate significant antibacterial activity. | Antibacterial properties reduce infection risks by up to 90%, promoting safer recovery and ensuring implant stability. | [17,71,75,81,93,94,95,96,97,98,99,100] |
| Real-Time Performance Monitoring | Sensors embedded in Ti alloys allow real-time monitoring of temperature, pH levels, and stress. Techniques such as AFM and AESEC analyze interactions of TNZT alloys with H2O2. | Real-time monitoring detects complications with over 90% accuracy, enabling timely intervention and improving clinical outcomes. | [12,14] |
| Key Focus | Examples of Advancements | Benefits | |
|---|---|---|---|
| Orthopedic Implants | Nanoparticle Reinforcement | Enhanced Ti alloys incorporating TiC, TiB2, and HA for hip and knee replacements. | Improved mechanical strength and durability; osteointegration yielding implant survival rates > 90% in long-term studies. |
| Advanced Manufacturing Process | Electron Beam Melting (EBM) increases wear resistance by up to 30% [105]. | Elevated tribomechanical performance and enhanced biocompatibility. | |
| Structural Properties Optimization | Advanced Ti alloys achieve yield strengths > 800 MPa, with inherent wear and corrosion resistance [106]. | Superior mechanical reliability across applications. | |
| Surface Modification | The application of TiO2 nanotube coatings boosts osseointegration by >40% and reduces bacterial colonization by >90% [93,107]. | Enhanced cellular differentiation and antibacterial properties, leading to a more stable, infection-resistant implant environment. | |
| New Alloy Compositions | The development of alloys such as Ti–Nb–Zr–Ta and Ti–Mo–Zr–Fe has yielded tensile strength increases of 20–30% compared to standard compositions [92]. | Optimized microstructural evolution that results in improved mechanical performance and overall implant function. | |
| Joint Replacements | Load-Bearing Materials and Alloy Selection | Use of Ti-6Al-4V and Ti-6Al-7Nb in hip and knee replacements; additionally, Ti–Nb–Zr–Ta exhibits a yield strength of ~900 MPa and elastic modulus in the range 50–60 GPa [16,18,108,109,110,111]. | Long-term stability with survival rates > 90% over 15 years; implant loosening reduced by >30%, ensuring superior load-bearing performance. |
| Porous Structural Design | Porous Ti alloys reduce stress shielding by up to 40% and promote bone ingrowth rates exceeding 80% [21,22]. | Enhanced osseointegration and load transfer, which minimizes the risk of implant loosening and associated complications. | |
| Bone Plates and Screws | Fracture Fixation Applications | Utilization of CP-Ti and advanced Ti alloys in plates and screws for fracture fixation. | High biocompatibility and effective bone integration drive rapid healing and reduced recovery times. |
| Alloy Modification for Corrosion and Biological Response | The Ti–Nb–Zr–Si (TNZS) alloy demonstrates superior corrosion resistance and enhanced biological responses [112,113]. | Improved implant stability and accelerated healing due to enhanced chemical resistance and cell compatibility. | |
| Alloy Composition Enhancement | Development of Ti–Nb–Ta–Zr–Fe alloys that deliver tensile strength improvements of 20–30% over CP-Ti [13,19,114]. | Enhanced mechanical performance and prolonged device longevity in bone fixation applications. | |
| Surface Engineering for Antibacterial Properties | Application of nanotube structures of TiO2 on implant surfaces [17]. | Marked reduction in bacterial colonization (>90%), significantly lowering the risk of postoperative infections. | |
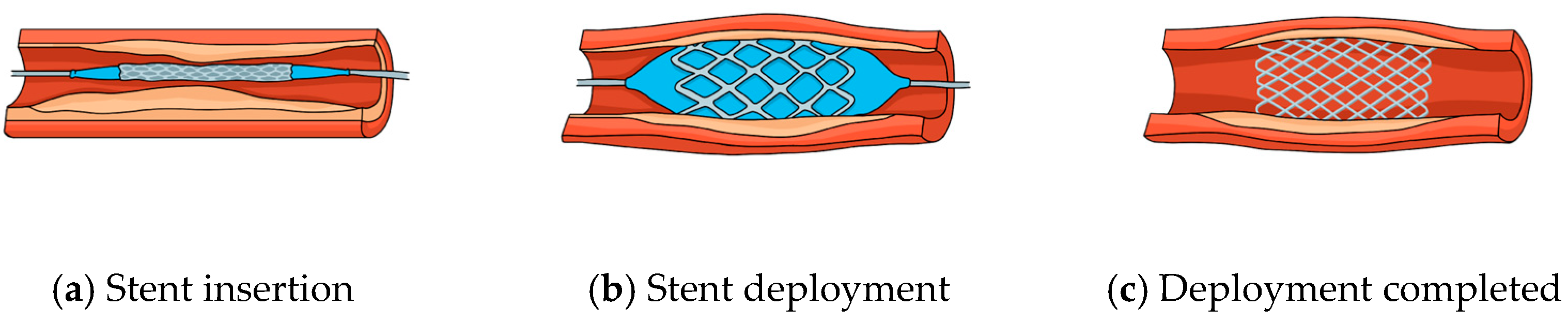
| Spinal Implants | Spinal Fusion Device Support | The use of Ti alloys in spinal fusion devices provides high strength, corrosion resistance, and biocompatibility. | Reliable structural support for effective spinal stabilization and fusion. |
| Customization via Additive Manufacturing | Additive manufacturing enables custom spinal implants with complex geometries and porosity levels up to 50% while retaining ~70% of the compressive strength of dense structures [18,111]. | Tailored implant designs that improve surgical outcomes and allow patient-specific fits. | |
| Porous Design for Optimal Mechanics | Engineered porous Ti alloys reduce stress shielding by ~40% compared to dense counterparts [18,111]. | Optimized balance between structural strength and flexibility, enhancing load transfer and minimizing bone resorption. | |
| Smart Technology Integration | Integration of low-profile antennas into spinal implants enables real-time monitoring, with sensor updates every 5 min and load sensitivity within ±5 N [19,20,22]. | Proactive postoperative management and enhanced safety through continuous implant performance tracking. | |
| Enhanced Alloy Formulation for Durability | Ti–Nb–Zr–Ta alloys exhibit ~25% improved corrosion and wear resistance over CP-Ti [20,21]. | Extended implant lifespan and robust performance under cyclic loading, ensuring reliable long-term spinal stabilization. |
| Device Type | Material Composition and Enhancements | Quantitative Properties and Performance | Clinical Impact | References |
|---|---|---|---|---|
| Stents | Ti-based shape memory alloys (e.g., Nitinol, Ti–Ni–Cu, Ti–Ni–Pd, Ti–Ni–Hf); nanoparticle-enhanced alloys (yield strength improvement up to 15%); biodegradable variants available |
|
| [30,119,120] |
| Heart Valves | Ti alloys; emerging TiNb alloys with integrated sensor technology for potential real-time monitoring |
|
| [13,118] |
| Key Focus | Examples of Advancements | Benefits | |
|---|---|---|---|
| Innovative Alloy Compositions | Develops new alloy compositions to meet clinical demands. | Severe plastic deformation techniques achieve grain sizes below 100 nm and tensile strength increases of 35% [128]. | Enhanced durability and performance. |
| Ti-Nb-Ta-Zr-O nanotubes improve corrosion resistance by over 90% [129]. | Greater reliability in biomedical environments. | ||
| Aging treatments yielded 20% increases in yield strength and enhanced fatigue resistance [130]. | Improved longevity and performance. | ||
| Electro-polishing enhances biocompatibility with cell viability rates exceeding 95% [131]. | Better preclinical outcomes and integration. | ||
| TNZT foams achieve up to 60% porosity and elastic moduli of 40–60 GPa, closely matching bone [132]. | Improved compatibility and stress mitigation. | ||
| Alloy element variations fine-tune the elastic modulus to mitigate stress shielding and improve implant performance [133]. | Enhanced long-term stability and reliability. | ||
| Nanostructured Alloys | Focuses on nanoscale grain sizes for superior material characteristics. | Nanostructured oxide layers enhance corrosion resistance and surface properties [134]. | Better integration with biological tissues. |
| Electrochemical anodization creates TiO2 surfaces for superior biocompatibility and tissue integration [135,136]. | Enhanced biological compatibility. | ||
| Threaded medical implants with nanostructured oxide layers ensure secure bone–implant interaction [137]. | Reliable and stable implant fixation. | ||
| Milling optimization improves tensile strength by 30% in nanostructured Ti-15Mo alloys [65]. | Improved mechanical properties. |
| Key Focus | Examples of Advancements | Benefits | |
|---|---|---|---|
| Bioactive Coatings | Promotes osseointegration by optimizing coatings made of materials like HA and bio-glass. | Nanopores with dual micro-/nano-roughness for cellular bioactivity [140]. | Enhanced bone–implant interaction and durability. |
| Heterogeneous nanocomposite coatings for lubrication and wear reduction [141]. | Improved wear resistance for long-term use. | ||
| Electrochemical oxidation of TNZT alloys for surface property enhancements [142]. | Advanced surface characteristics. | ||
| Antibacterial Surface Treatments | Reduces infection risks through coatings that inhibit bacterial adhesion or release antibacterial agents. | TiO2 nanostructured surfaces via electrochemical anodization [135,136]. | Antibacterial effectiveness and better tissue integration. |
| Nanostructured oxide layers for threaded implants [137]. | Secure implant interaction and reduced microbial activity. | ||
| ZnO nanorods hybridized with zinc phosphate for antibacterial and osteogenic balance [143]. | Balanced antibacterial and bone regeneration properties. |
| Key Focus | Examples of Advancements | Benefits | |
|---|---|---|---|
| Real-Time Monitoring | Enables continuous feedback on implant and physiological conditions. | Deeply implanted conformal antenna, achieving signal transmission rates exceeding 95% reliability [12]. | Accurate and timely data for personalized medical intervention. |
| Sensors monitoring factors like temperature and pH, enabling actionable insights in real time [14]. | Enhanced clinical outcomes through early detection of issues. | ||
| Sensor Miniaturization | Develops compact, energy-efficient sensors for seamless integration into implants. | Intra-oral differential circularly polarized antenna enabling signal detection with 90% sensitivity [144]. | Greater patient comfort and functionality for specialized applications. |
| Implantable multi-band microstrip antenna supporting biomedical signal transmission at frequencies of 2.4 GHz and 5 GHz [145]. | Reliable and efficient communication systems in implants. |
| Key Focus | Examples of Advancements | Benefits | |
|---|---|---|---|
| In Vivo Studies | Evaluates long-term performance and biocompatibility of Ti alloys in biological environments. | Long-term studies show improved tissue integration through advanced surface treatments and compositions [79,149]. | Helps identify optimal modifications for implant success. |
| Surface modifications reduce inflammation, with inflammation markers reduced by 25% [150]. | Ensures better long-term clinical outcomes. | ||
| Wear and Fatigue Resistance | Investigates the mechanisms of wear and fatigue to ensure implant longevity. | TNZT alloys with borides achieve a 30% improvement in wear resistance compared to Ti-6Al-4V ELI [21]. | Creates longer-lasting implants with reduced failure rates. |
| Fatigue resistance improved by over 20%, enabling implants to handle repetitive mechanical stress [151,152]. | Ensures reliability under high-stress conditions. |
| Key Focus | Examples of Advancements | Benefits | |
|---|---|---|---|
| Patient-Specific Implants | Utilizes additive manufacturing to create implants tailored to individual anatomy. | Hierarchical micro/nano surfaces decorated with TiO2 nanotubes improve bioactivity in vitro and in vivo [149]. | Superior osseointegration and reduced inflammation. |
| ZnO nanorods hybridized into zinc phosphate structures, balancing antibacterial and pro-osteogenic properties [143]. | Improved implant safety and biological activity. | ||
| Systematic review confirming TiO2 nanotubes enhance osseointegration capabilities by over 30% [154]. | Reliable tissue integration in clinical applications. | ||
| Complex Geometries | Enables the creation of intricate implant structures unattainable with traditional methods. | β-Ti alloys achieve tensile strength exceeding 1000 MPa and cell viability rates over 90% [41]. | Enhanced mechanical performance and biocompatibility. |
| Multi-material additive manufacturing producing biomaterials with elastic moduli matching bone (45–55 GPa) [155,156]. | Prevents stress shielding and ensures mechanical compatibility. | ||
| Laser-processed Ti and Co biomaterials achieve tensile strength over 1200 MPa and biocompatibility exceeding 95% [153]. | Durable and biologically compatible implants. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kacsó, A.-B.; Peter, I. A Review of Past Research and Some Future Perspectives Regarding Titanium Alloys in Biomedical Applications. J. Funct. Biomater. 2025, 16, 144. https://doi.org/10.3390/jfb16040144
Kacsó A-B, Peter I. A Review of Past Research and Some Future Perspectives Regarding Titanium Alloys in Biomedical Applications. Journal of Functional Biomaterials. 2025; 16(4):144. https://doi.org/10.3390/jfb16040144
Chicago/Turabian StyleKacsó, Alex-Barna, and Ildiko Peter. 2025. "A Review of Past Research and Some Future Perspectives Regarding Titanium Alloys in Biomedical Applications" Journal of Functional Biomaterials 16, no. 4: 144. https://doi.org/10.3390/jfb16040144
APA StyleKacsó, A.-B., & Peter, I. (2025). A Review of Past Research and Some Future Perspectives Regarding Titanium Alloys in Biomedical Applications. Journal of Functional Biomaterials, 16(4), 144. https://doi.org/10.3390/jfb16040144










