Keeping an Eye on Decellularized Corneas: A Review of Methods, Characterization and Applications
Abstract
:1. Introduction
| Corneal replacement | Advantages | Disadvantages |
|---|---|---|
| Keratoprostheses: (KPros) an acellular artificial implant. | Currently the only synthetic corneal replacements with market approval [14]. An alternative treatment for patients considered untreatable by conventional corneal allografting [25,26]. KPro implantation procedure is no more invasive or complex than routine corneal transplantation [27]. KPro procedure is reversible [27]. Clinical data is being accumulated. Scheduling independent of human donor availability. | Success is dependent upon patient maintenance of the device [16]. Many KPro materials are non-cell adhesive and require modification to allow for cell adhesion and migration [28]. Several complications have been seen including: wound leaks [3,20]; inflammation and infection due to protein adhesion [29,30]; increased glaucoma [27,31,32]; extrusion or protrusion of the implant [3,25,29]; tissue melting [3,32]. Some common eye drugs are also harmful to certain types of KPros [19]. Limited long-term success [3]. Limited clinical use [27]. |
| Xenograft: A cellular or acellular tissue graft derived from another species [33]. | A virtually unlimited organ, tissue and cell source. Scheduling independent of human donor availability [34]. Porcine corneas are most commonly used and have a similar physiology and refractive properties compared to human corneas and are relatively easy to obtain in large numbers [21] thus, are commercially advantageous [9,11]. Clinical trials using porcine xenografts currently underway. | Commonly used porcine corneas may be unacceptable based on religious beliefs (Islam, Judaism, Jainism) [11]. All xenografts eventually fail due to immune response. Xenografts are rejected more quickly than allograft tissues when similar tissues and circumstances are compared [35]. Risk of cross-species disease transmission. Poor public perception. |
| Tissue Engineered (TE) constructs: a manufactured biological or semi-synthetic constructs that can be cellular or acellular. | Compelling advances in the development of synthetic corneal replacements and culture of human corneal cells onto and within supporting substrates. It has already been shown that the three main corneal layers can be recreated in vitro using collagen-based scaffolds and immortalized cell lines [36]. Success in Phase 1 clinical trials have been reported for acellular corneal matrices [14]. | Gross measurable results of TE corneas are poor [9,16,37]. Lack of tensile strength to permit surgical manipulation and attachment of the corneal equivalent. Failure to mimic native surface curvature [38]. Lack of the native stromal architecture [39]. Biomechanical and optical properties of the cornea models are often compromised [40]. Presently, there is no cellularized TE corneal equivalent in routine clinical use. No standardized cell sources available. |
2. Human Decellularized Corneas—A more Promising Alternative?
3. Methods of Decellularization
| Method/Technique | Mechanism of action | Advantages/Disadvantages | References | |||
|---|---|---|---|---|---|---|
| Biological | ||||||
| Enzymatic Agents | ||||||
| Trypsin | Hydrolyzes protein and disrupts protein-protein interactions. | Disruptive to collagen structure. Not suited to corneal tissue. | [6,64] | |||
| Dispase | Cleaves peptides associated with basement membrane proteins. | Can aid decellularization process by initially removing epithelium and endothelium. May cause damage to basement membrane. | [6,62,64] | |||
| Phospholiphases A2 (PLA2) | Hydrolyzes phospholipid components of cells. | No interaction with collagen or proteoglycans. | [68,71,73,74] | |||
| Nucleases (RNase and DNase) | Cleaves nucleic acids and aid in their removal. | Effective at removal of DNA and residual cellular components that have a tendency to adhere to ECM proteins. Incomplete removal of the enzymes may impede recellularization and successful transplantation. | [6,44,64] | |||
| Sera | Serum nucleases degrade DNA and RNA. | Effectively removes cells while maintaining tissue transparency. Use of non-human sera carries risk of cross-species transmission of pathogens. | [12] | |||
| Non-enzymatic Agents | ||||||
| EDTA | Dissociates cells by separating metal ions. | Ineffective at cell removal when used unaccompanied. | [47,69,75] | |||
| Chemical | ||||||
| Alcohols | ||||||
| Ethanol | Dehydrates and lyses cells. Removes lipids from tissues. | Can cause damage to ultrastructure of tissue. | [76] | |||
| Glycerol | Dehydrates and lyses cells. Removes lipids from tissues | Antimicrobial, antifungal, and antiviral properties. Cryoprotectant for long-term tissue storage. Can maintain or restore corneal transparency. | [45,47,64,69,77,78] | |||
| Acids and Alkalis | ||||||
| Peracetic acid | Solubilizes cytoplasmic components of cells. Removes nucleic acids via hydrolytic degradation. | Acts to simultaneously sterilize tissue. Poor results in DCs. Can disrupt ECM. | [76] | |||
| Ammonium hydroxide | Hydrolytic degradation of biomolecules. | Can eliminate GFs and reduce mechanical properties. | [46,78] | |||
| Ionic Detergents | ||||||
| Sodium dodecyl sulfate (SDS) | Solubilizes cell membranes and dissociate DNA from protein. Disrupts protein-protein interactions. | Complete removal of cells can be achieved. Can be highly detrimental to ECM structure including disorganization of collagen fibrils and loss of GAGs. Loss of tissue transparency. | [47,61,64,65,69,72,75,76,79] | |||
| Sodium deoxycholate (SD) | Solubilizes cell membranes and dissociates DNA from protein. Disrupts protein-protein interactions. | Less effective at removal of cells but can be effective when used with other agents. | [68,79] | |||
| Non-ionic Detergents | ||||||
| Triton X-100 | Breaks up lipid-lipid and lipid-protein interactions. | Mild and non-denaturing. Less effective than ionic detergent treatments. Can cause damage to ECM structure. | [6,44,46,78,80] | |||
| Zwitterionic Detergents | ||||||
| 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) | Has properties of non-ionic and ionic detergents. | Poor cellular removal. Very disruptive to stromal architecture. | [79] | |||
| Hypo- and Hypertonic Solutions | ||||||
| Sodium Chloride (NaCl) | Detaches DNA from proteins. | Remains optically clear. Ability to maintain stromal architecture and retain GAG content. Mixed reports on success of cell removal. | [62,64] | |||
| Tris-HCL | Lyses cells by osmotic shock. | Reduces time required in harsh decellularizing agents. | [6,44,69,75] | |||
| Physical | ||||||
| Freeze-thawing | Ice crystal formation causes cell lysis. | Requires subsequent treatment to remove cellular content. Causes pore formation. Disruptions to ECM architecture. | [64,74,79] | |||
| Hydrostatic Pressure | Increase in pressure results in cell lysis. | Effectively decellularizes whilst maintaining collagen fibril structure. Kills bacteria and viruses. Expensive. | [63,66] | |||
3.1. Biological Decellularization Techniques
3.1.1. Enzymatic Agents
3.1.2. Non-Enzymatic Agents
3.2. Chemical
3.2.1. Acid and Alkali Treatment
3.2.2. Alcohols
3.2.3. Detergents
3.2.4. Hyper- and Hypo-tonic Solutions
3.3. Physical Decellularization Techniques

4. Characterization of Decellularized Corneas
4.1. Assessment of Removal of Cellular Materials and Retention of ECM Architecture
4.1.1. Removal of Cellular Materials

4.1.2. Biological Assessment of ECM Architecture
4.1.3. Toxicity and Immunogenicity of Decellularized Corneas
4.2. Imaging of Structural Architecture and Transparency

4.2.1. Light Microscopy Techniques
4.2.2. Electron Microscopy

4.2.3. Second Harmonic Imaging
4.2.4. High Frequency Ultrasound
4.2.5. Optical Coherence Tomography
4.2.6. X-Ray
4.2.7. Atomic Force Microscopy
4.3. Characterization of Mechanical Properties
| Method/technique | Description/applications | Advantages | Disadvantages |
|---|---|---|---|
| Bulge/inflation testing | Involves inflation of the whole tissue/membrane/film through a window in the substrate and measuring the displacement as a function of the applied pressure [146,146,150]. Used to measure mechanical strength of thin films, membranes and corneal tissue. Can determine constitutive relationships of corneal tissue [151]. | No gripping problems. Maintains corneal integrity [148]. Reliable technique. Enables intrinsic properties on a layer-by-layer basis to be determined [152]. Can be used to simulate intraocular pressure [148]. Can be performed under physiological conditions [148,153]. Whole tissues can be measured. Previously used to characterize DCs [55] and the biomechanical stability of xeno-tissues for human transplantation [148]. | Complex procedure [152]. Difficulties in controlling the applied pressure; i.e., leaking or trapping of dissolved air. Most inflation tests do not account for corneal anisotropy [148], inhomogeneity or viscoelasticity [153]. |
| Compression testing | Test materials are compressed between two plates and deformed under a known load. Used to determine the mechanical behavior of materials under crushing loads [154,155]. | Regularly used in TE applications [156]. Confined and unconfined tests can be performed. Gives a comprehensive evaluation of a materials load-bearing capacity [155]. | Does not account for corneal curvature. Involves flattening of the tissue. Difficulties associated with applying pressure evenly. Destructive [157]. |
| Holographic interferometry | Uses laser light to create an image. Can be used to compare pressure changes in healthy and diseased corneas [158]. Previously used to determine differences between intact, incised [159] and laser ablated [160] corneas. Measures the elastic modulus [161] and extensibility of in vivo corneas [162]. | Very sensitive, precise method. Allows for direct comparison of two adjacent areas in a single sample. Non-destructive. Allows for repeated measures of a sample [158]. | Rarely used by researchers. Limited to use in linear elastic materials under small deformation [146]. |
| Indentation testing | A well-defined indenter is used to deform test materials and measure their force-displacement curves; this can be used to calculate the elastic modulus. Traditionally used to measure the hardness of materials. | Can be adapted to be non-destructive. Can be adapted to test for prolonged culture periods under sterile conditions [146,163]. Fast, online real-time measurements. Can be performed on a nanometric scale. Suspending the materials eliminates problems associated with backing substrates. | Cannot be used to test high stiffness materials. |
| In vivo mechanical testing | Pulses of air or poking mechanisms are used to test materials. Used to measure corneal hysteresis by comparing inward and outward pressure values [164]. | Can be performed on live patients. Changes in mechanical properties can be directly linked to medical conditions [165]. | In vivo tests are difficult to apply to in vitro models. Unsuitable for prolonged culture periods. Sample contamination. Creep, stress-relaxation and stress-strain relationships are yet to be assessed. |
| Strip extensiometry (coupon testing) | Involves applying a tensile force to dissected strips with constant width of corneal tissue that are gripped and stretched via the application of a tensile force. Is used to calculate the Young’s modulus, yield strength and ultimate tensile strength of the cornea and equivalents. | A relatively simple technique [151]. Inexpensive. Can be used to compare corneas of different species with each other [152,166]. Commonly used to determine the properties of engineering materials [151]. Has been previously used to characterize DCs [55] | Unreliable [151]. Does not account for corneal curvature unless complex calculations are employed [162]. Stress distribution of corneal tissue is not uniform. Destructive [151]. Cannot be used to study whole tissues. Problems associated with sample gripping. Complex calculations involved [151]. |
| Ultrasound | A biomicroscopy technique which utilizes high frequency transducers, creating 2D images from backscattered ultrasonic waves [167]. Used to visualize numerous ocular structures and to detect in vivo foreign bodies. | Allows for detailed surface imaging up to 5 mm in depth. Allows for quantitative assessments of the anterior ocular surface to be made [168]. Non-invasive technique. Can be applied in vivo and in vitro. | Expensive. Yields results that are too high when compared to known measurements [168]. |
5. Recellularization Techniques
5.1. In Vivo Recellularization
5.1.1. Intra-Lamellar Grafting
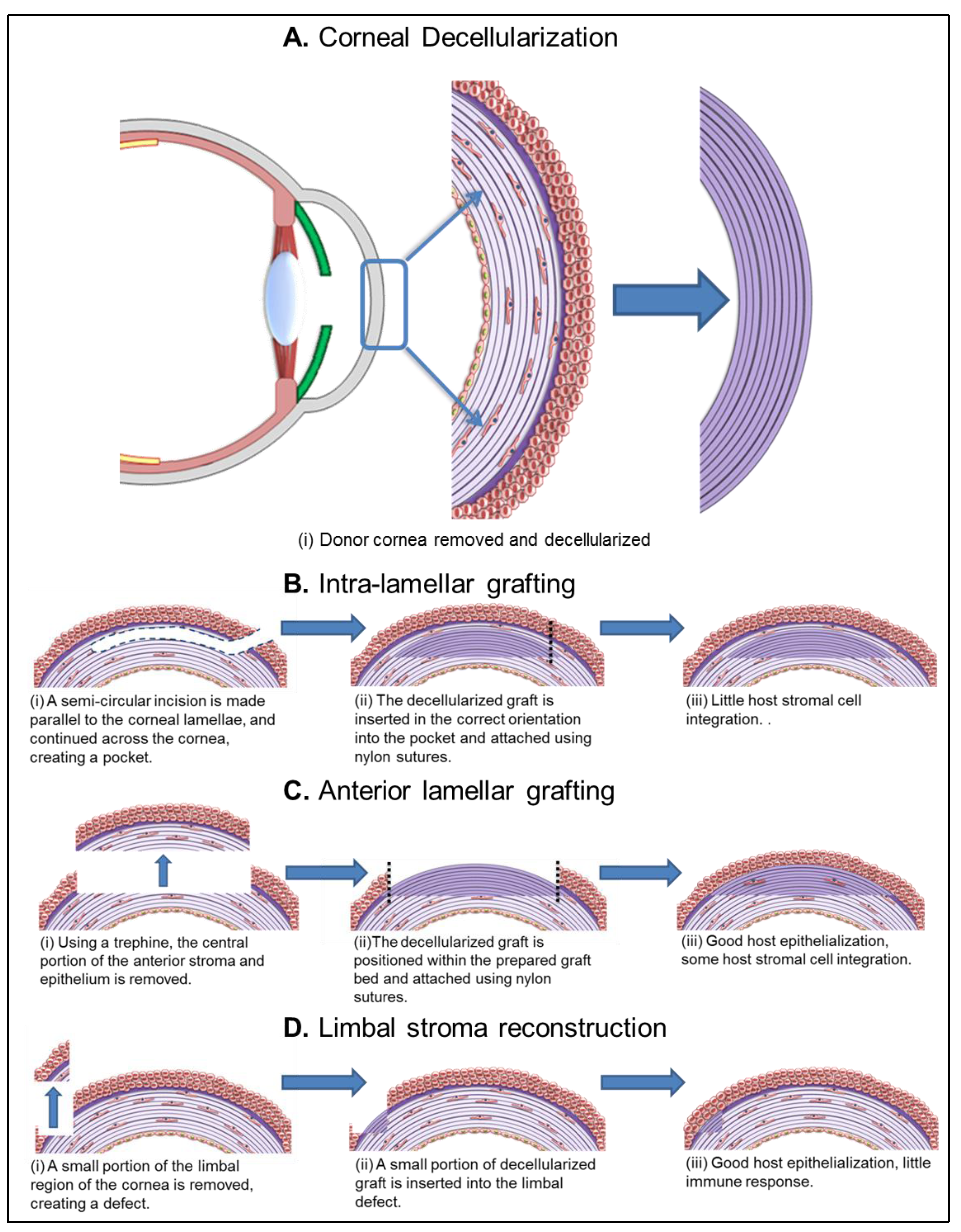
5.1.2. Anterior Lamellar Grafting
5.1.3. Limbal Stroma Reconstruction
5.2. Ex Vivo Recellularization and Cell Sources
5.2.1. Epithelial Cells

5.2.2. Corneal Stromal Cells
5.2.3. Endothelial Cells
5.3. In Vivo versus ex Vivo Recellularization
6. Alternative Use of Human Decellularized Tissues for Toxicity Testing
7. Conclusions
8. Perspectives
Acknowledgements
Conflict of Interest
References
- Huang, Y.-X.; Li, Q.-H. An active artificial cornea with the function of inducing new corneal tissue generation in vivo—A new approach to corneal tissue engineering. Biomed. Mater. 2007, 2, 121–125. [Google Scholar] [CrossRef]
- Carlsson, D.J.; Li, F.; Shimmura, S.; Griffith, M. Bioengineered corneas: How close are we? Curr. Opin. Ophthalmol. 2003, 14, 192–197. [Google Scholar] [CrossRef]
- Chirila, T.V.; Hicks, C.R.; Dalton, P.D.; Vijayasekaran, S.; Lou, X.; Hong, Y.; Clayton, A.B.; Ziegelaar, B.W.; Fitton, J.H.; Platten, S.; et al. Artificial cornea. Prog. Polym. Sci. 1998, 23, 447–473. [Google Scholar] [CrossRef]
- Oliva, M.S.; Schottman, T.; Gulati, M. Turning the tide of corneal blindness. Indian. J. Othalmol. 2012, 60, 423–427. [Google Scholar] [CrossRef]
- Keenan, T.D.L.; Carley, F.; Yeates, D.; Jones, M.N.A.; Rushton, S.; Goldacre, M.J. Trends in corneal graft surgery in the UK. Br. J. Ophthalmol. 2011, 95, 468–472. [Google Scholar] [CrossRef]
- Zhang, C.; Nie, X.; Hu, D.; Liu, Y.; Deng, Z.; Dong, R.; Zhang, Y.; Jin, Y. Survival and integration of tissue-engineered corneal stroma in a model of corneal ulcer. Cell Tissue Res. 2007, 329, 249–257. [Google Scholar] [CrossRef]
- Wilkemeyer, I.; Pruss, A.; Kalus, U.; Schroeter, J. Comparative infectious serology testing of pre- and post-mortem blood samples from cornea donors. Cell Tissue Bank 2012, 13, 447–452. [Google Scholar] [CrossRef]
- Khodadoust, A.A. The allograft rejection reaction: The leading cause of late failure of clinical corneal grafts. In Ciba foundation symposium 15—Corneal graft failure; Porter, R., Knight, D., Eds.; John Wiley & Sons, Ltd: Chichester, UK, 2008; pp. 151–167. [Google Scholar]
- Amano, S.; Shimomura, N.; Yokoo, S.; Araki-Sasaki, K.; Yamagami, S. Decellularizing corneal stroma using N2 gas. Mol. Vis. 2008, 14, 878–882. [Google Scholar]
- Ekser, B.; Ezzelarab, M.; Hara, H.; van der Windt, D.J.; Wijkstrom, M.; Bottino, R.; Trucco, M.; Cooper, D.K.C. Clinical xenotransplantation: The next medical revolution? Lancet 2012, 379, 672–683. [Google Scholar]
- Hara, H.; Cooper, D.K. Xenotransplantation—The future of corneal transplantation? Cornea 2011, 30, 371–378. [Google Scholar] [CrossRef]
- Shao, Y.; Yu, Y.; Pei, C.G.; Zhou, Q.; Liu, Q.P.; Tan, G.; Li, J.M.; Gao, G.P.; Yang, L. Evaluation of novel decellularizing corneal stroma for cornea tissue engineering applications. Int. J. Ophthalmol. 2012, 5, 415–418. [Google Scholar]
- Stevenson, W.; Cheng, S.-F.; Emami-Naeini, P.; Hua, J.; Paschalis, E.I.; Dana, R.; Saban, D.R. Gamma-irradiation reduces the allogenicity of donor corneas. Invest. Ophthalmol. Vis. Sci. 2012, 53, 7151–7158. [Google Scholar] [CrossRef]
- Fagerholm, P.; Lagali, N.S.; Merrett, K.; Jackson, W.B.; Munger, R.; Liu, Y.; Polarek, J.W.; Söderqvist, M.; Griffith, M. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci. Transl. Med. 2010, 2, 46–61. [Google Scholar]
- Pintucci, S.; Pintucci, F.; Cecconi, M.; Caiazza, S. New dacron tissue colonisable keratoprosthesis—Clinical-experience. Br. J. Ophthalmol. 1995, 79, 825–829. [Google Scholar] [CrossRef]
- Ruberti, J.W.; Zieske, J.D.; Trinkaus-Randall, V. Corneal-tissue replacement. In Principles of tissue engineering; Lanza, R.P., Langer, R., Vacanti, J., Eds.; Elsevier Academic Press: London, UK, 2007; Volume 3, pp. 1025–1047. [Google Scholar]
- Hicks, C.R.; Fitton, J.H.; Chirila, T.V.; Crawford, G.J.; Constable, I.J. Keratoprostheses: Advancing toward a true artificial cornea. Sur. Ophthalmol. 1997, 42, 175–189. [Google Scholar] [CrossRef]
- Eguchi, H.; Hicks, C.R.; Crawford, G.J.; Tan, D.T.; Sutton, G.R. Cataract surgery with the alphacor artificial cornea. J. Cataract. Refract. Surg. 2004, 30, 1486–1491. [Google Scholar]
- Chirila, T.V. An overview of the development of artificial corneas with porous skirts and the use of phema for such an application. Biomaterials 2001, 22, 3311–3317. [Google Scholar] [CrossRef]
- Zerbe, B.L.; Belin, M.W.; Ciolino, J.B. Results from the multicenter Boston type 1 keratoprosthesis study. Ophthalmology 2006, 113, 1779–1784. [Google Scholar] [CrossRef]
- Pan, Z.; Sun, C.; Jie, Y.; Wang, N.; Wang, L. Wzs-pig is a potential donor alternative in corneal xenotransplantation. Xenotransplantation 2007, 14, 603–611. [Google Scholar] [CrossRef]
- Van Essen, T.H.; Lin, C.C.; Hussain, A.K.; Maas, S.; Lai, H.J.; Linnartz, H.; van den Berg, T.J.T.P.; Salvatori, D.C.F.; Luyten, G.P.M.; Jager, M.J. A fish scale-derived collagen matrix as artificial cornea in rats: Properties and potential. Invest. Ophthalmol. Vis. Sci. 2013, 7, 3224–3233. [Google Scholar]
- Suuronen, E.J.; McLaughlin, C.R.; Stys, P.K.; Nakamura, M.; Munger, R.; Griffith, M. Functional innervation in tissue engineered models for in vitro study and testing purposes. Toxicol. Sci. 2004, 82, 525–533. [Google Scholar] [CrossRef]
- Ahearne, M.; Yang, Y.; Liu, K.K. Mechanical characterisation of hydrogels for tissue engineering applications. In Topics in Tissue Engineering; Ashammakhi, N., Reis, R.L., Chielini, F., Eds.; Kluwer Academic Publisher: Dordrecht, the Netherlands, 2008; Volume 4. [Google Scholar]
- Allan, B. Artificial corneas—Risks of complications are high now, but better materials are on the way. Bri. Med. J. 1999, 318, 821–822. [Google Scholar] [CrossRef]
- Hicks, C.R.; Crawford, G.J.; Lou, X.; Tan, D.T.; Snibson, G.R.; Sutton, G.; Downie, N.; Werner, L.; Chirila, T.V.; Constable, I.J. Corneal replacement using a synthetic hydrogel cornea, alphacorTM: Device, preliminary outcomes and complications. Eye 2003, 17, 385–392. [Google Scholar] [CrossRef]
- Hicks, C.; Crawford, G.; Chirila, T.; Wiffen, S.; Vijayasekaran, S.; Lou, X.; Fitton, J.; Maley, M.; Clayton, A.; Dalton, P.; et al. Development and clinical assessment of an artificial cornea. Prog. Retin. Eye Res. 2000, 19, 149–170. [Google Scholar]
- McLaughlin, C.R.; Tsai, R.J.F.; Latorre, M.A.; Griffith, M. Bioengineered corneas for transplantation and in vitro toxicology. Front. Biosci. 2009, 14, 3326–3337. [Google Scholar]
- Orwin, E.J.; Hubel, A. In vitro culture characteristics of corneal epithelial, endothelial, and keratocyte cells in a native collagen matrix. Tissue Eng. 2000, 6, 307–319. [Google Scholar] [CrossRef]
- Sandeman, S.R.; Lloyd, A.W.; Tighe, B.J.; Franklin, V.; Li, J.; Lydon, F.; Liu, C.S.C.; Mann, D.J.; James, S.E.; Martin, R. A model for the preliminary biological screening of potential keratoprosthetic biomaterials. Biomaterials 2003, 24, 4729–4739. [Google Scholar] [CrossRef]
- Netland, P.A.; Terada, H.; Dohlman, C.H. Glaucoma associated with keratoprosthesis. Ophthalmology 1998, 105, 751–757. [Google Scholar]
- Chew, H.F.; Ayres, B.D.; Hammersmith, K.M.; Rapuano, C.J.; Laibson, P.R.; Myers, J.S.; Jin, Y.-P.; Cohen, E.J. Boston keratoprosthesis outcomes and complications. Cornea 2009, 28, 989–996. [Google Scholar] [CrossRef]
- Wilson, S.L.; El Haj, A.J.; Yang, Y. Control of scar tissue formation in the cornea: Strategies in clinical and corneal tissue engineering. J. Funct. Biomat. 2012, 3, 642–687. [Google Scholar] [CrossRef]
- Boneva, R.S.; Folks, T.M. Xenotransplantation and risks of zoonotic infections. Ann. Med. 2004, 36, 504–517. [Google Scholar] [CrossRef]
- Larkin, D.F.P.; Williams, K.A. The host response in experimental corneal xenotransplantation. Eye 1995, 9, 254–260. [Google Scholar] [CrossRef]
- Griffith, M.; Osborne, R.; Munger, R.; Xiong, X.J.; Doillon, C.J.; Laycock, N.L.C.; Hakim, M.; Song, Y.; Watsky, M.A. Functional human corneal equivalents constructed from cell lines. Science 1999, 286, 2169–2172. [Google Scholar] [CrossRef]
- Vrana, N.E.; Builles, N.; Justin, V.; Bednarz, J.; Pellegrini, G.; Ferrari, B.; Damour, O.; Hulmes, D.J.S.; Hasirci, V. Development of a reconstructed cornea from collagen-chondroitin sulfate foams and human cell cultures. Invest. Ophthalmol. Vis. Sci. 2008, 49, 5325–5331. [Google Scholar] [CrossRef]
- Roujeau, J.C.; Kelly, J.P.; Naldi, L.; Rzany, B.; Stern, R.S.; Anderson, T.; Auquier, A.; Bastujigarin, S.; Correia, O.; Locati, F.; et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N. Engl. J. Med. 1995, 333, 1600–1607. [Google Scholar] [CrossRef]
- Torbet, J.; Malbouyres, M.; Builles, N.; Justin, V.; Roulet, M.; Damour, O.; Oldberg, A.; Ruggieo, F.; Hulmes, D.J.S. Orthogonal scaffold of magnetically aligned collagen lamellae for corneal stroma reconstruction. Biomaterials 2007, 28, 4268–4276. [Google Scholar] [CrossRef]
- Boote, C.; Dennis, S.; Huang, Y.F.; Quantock, A.J.; Meek, K.M. Lamellar orientation in human cornea in relation to mechanical properties. J. Struct. Biol. 2005, 149, 1–6. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar]
- Tegtmeyer, S.; Papantoniou, I.; Muller-Goymann, C.C. Reconstruction of an in vitro cornea and its use for drug permeation studies from different formulations containing pilocarpine hydrochloride. Eur. J. Pharm. Biopharm. 2001, 51, 119–125. [Google Scholar]
- Xu, Y.-G.; Xu, Y.-S.; Huang, C.; Feng, Y.; Li, Y.; Wang, W. Development of a rabbit corneal equivalent using an acellular corneal matrix of a porcine substrate. Mol. Vis. 2008, 14, 2180–2189. [Google Scholar]
- Chen, W.; Lin, Y.; Zhang, X.; Wang, L.; Liu, M.; Liu, J.; Ye, Y.; Sun, L.; Ma, H.; Qu, J. Comparison of fresh corneal tissue versus glycerin-cryopreserved corneal tissue in deep anterior lamellar keratoplasty. Invest. Ophthalmol. Vis. Sci. 2010, 51, 775–781. [Google Scholar] [CrossRef]
- Choi, J.S.; Williams, J.K.; Greven, M.; Walter, K.A.; Laber, P.W.; Khang, G.; Soker, S. Bioengineering endothelialized neo-corneas using donor-derived corneal endothelial cells and decellularized corneal stroma. Biomaterials 2010, 31, 6738–6745. [Google Scholar] [CrossRef]
- Bayyoud, T.; Thaler, S.; Hofmann, J.; Maurus, C.; Spitzer, M.S.; Bartz-Schmidt, K.U.; Szurman, P.; Yoeruek, E. Decellularized bovine corneal posterior lamellae as carrier matrix for cultivated human corneal endothelial cells. Curr. Eye. Res. 2012, 37, 179–186. [Google Scholar] [CrossRef]
- Badylak, S.F. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl. Immunol. 2004, 12, 367–377. [Google Scholar] [CrossRef]
- Lakshman, N.; Petroll, W.M. Growth factor regulation of corneal keratocyte mechanical phenotypes in 3D collagen matrices. Invest. Ophthalmol. Vis. Sci. 2012, 53, 1077–1086. [Google Scholar] [CrossRef]
- Imanishi, J.; Kamiyama, K.; Iguchi, I.; Kita, M.; Sotozono, C.; Kinoshita, S. Growth factors: Importance in wound healing and maintenance of transparency of the cornea. Prog. Retin. Eye Res. 2000, 19, 113–129. [Google Scholar]
- Klenkler, B.; Sheardown, H. Growth factors in the anterior segment: Role in tissue maintenance, wound healing and ocular pathology. Exp. Eye Res. 2004, 79, 677–688. [Google Scholar] [CrossRef]
- Karamichos, D.; Hutcheon, A.E.K.; Zieske, J.D. Transforming growth factor-beta 3 regulates assembly of a non-fibrotic matrix in a 3D corneal model. J. Tissue Eng. Regen. Med. 2011, 5, 228–238. [Google Scholar] [CrossRef]
- Bussolino, F.; Direnzo, M.F.; Ziche, M.; Bocchietto, E.; Olivero, M.; Naldini, L.; Gaudino, G.; Tamagnone, L.; Coffer, A.; Comoglio, P.M. Hepatocyte growth-factor is a potent angiogenic factor which stimulates endothelial-cell motility and growth. J. Cell Biol. 1992, 119, 629–641. [Google Scholar] [CrossRef]
- NHS UK transplant registry. Available online: http://www.organdonation.nhs.uk/ (accessed on 4 February 2013).
- Lynch, A.P.; Ahearne, M. Strategies for developing decellularized corneal scaffolds. Exp. Eye Res. 2012, 108, 42–47. [Google Scholar] [CrossRef]
- Musselmann, K.; Kane, B.; Alexandrou, B.; Hassell, J.R. Stimulation of collagen synthesis by insulin and proteoglycan accumulation by ascorbate in bovine keratocytes in vitro. Invest. Ophthalmol. Vis. Sci. 2006, 47, 5260–5266. [Google Scholar] [CrossRef]
- Fullwood, N.J. Collagen fibril orientation and corneal curvature. Structure 2004, 12, 169–170. [Google Scholar]
- Meek, K.M.; Boote, C. The organization of collagen in the corneal stroma. Exp. Eye. Res. 2004, 78, 503–512. [Google Scholar] [CrossRef]
- Wray, L.S.; Orwin, E.J. Recreating the microenvironment of the native cornea for tissue engineering applications. Tissue Eng. Part A 2009, 15, 1463–1472. [Google Scholar] [CrossRef]
- Bron, A.J. The architecture of the corneal stroma. Br. J. Ophthalmol. 2001, 85, 379–381. [Google Scholar] [CrossRef]
- Du, L.; Wu, X. Development and characterization of a full-thickness acellular porcine cornea matrix for tissue engineering. Artif. Organs 2011, 35, 691–705. [Google Scholar] [CrossRef]
- Gonzalez-Andrades, M.; de la Cruz Cardona, J.; Ionescu, A.M.; Campos, A.; Del Mar Perez, M.; Alaminos, M. Generation of bioengineered corneas with decellularized xenografts and human keratocytes. Invest. Ophthalmol. Vis. Sci. 2011, 52, 215–222. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Funamoto, S.; Sasaki, S.; Honda, T.; Hattori, S.; Nam, K.; Kimura, T.; Mochizuki, M.; Fujisato, T.; Kobayashi, H.; et al. Preparation and characterization of decellularized cornea using high-hydrostatic pressurization for corneal tissue engineering. Biomaterials 2010, 31, 3941–3948. [Google Scholar] [CrossRef]
- Oh, J.Y.; Kim, M.K.; Lee, H.J.; Ko, J.H.; Wee, W.R.; Lee, J.H. Processing porcine cornea for biomedical applications. Tissue Eng. Part C Methods 2009, 15, 635–645. [Google Scholar]
- Pang, K.; Du, L.; Wu, X. A rabbit anterior cornea replacement derived from acellular porcine cornea matrix, epithelial cells and keratocytes. Biomaterials 2010, 31, 7257–7265. [Google Scholar] [CrossRef]
- Sasaki, S.; Funamoto, S.; Hashimoto, Y.; Kimura, T.; Honda, T.; Hattori, S.; Kobayashi, H.; Kishida, A.; Mochizuki, M. In vivo evaluation of a novel scaffold for artificial corneas prepared by using ultrahigh hydrostatic pressure to decellularize porcine corneas. Mol. Vis. 2009, 15, 2022–2028. [Google Scholar]
- Shao, Y.; Quyang, L.; Zhou, Y.; Tang, J.; Tan, Y.; Liu, Q.; Lin, Z.; Yin, T.; Qiu, F.; Liu, Z. Preparation and physical properties of a novel biocompatible porcine corneal acellularized matrix. In Vitro Cell Dev. Biol. Anim. 2010, 46, 600–605. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, Y.; Li, N.; Huang, M.; Duan, H.; Ge, J.; Xiang, P.; Wang, Z. The use of phospholipase a(2) to prepare acellular porcine corneal stroma as a tissue engineering scaffold. Biomaterials 2009, 30, 3513–3522. [Google Scholar] [CrossRef]
- Yoeruek, E.; Bayyoud, T.; Maurus, C.; Hofmann, J.; Spitzer, M.S.; Bartz-Schmidt, K.-U.; Szurman, P. Decellularization of porcine corneas and repopulation with human corneal cells for tissue-engineered xenografts. Acta Ophthalmol. 2012, 90, 125–131. [Google Scholar]
- Proulx, S.; Audet, C.; Uwamaliya, J.; Deschambeault, A.; Carrier, P.; Giasson, C.J.; Brunette, I.; Germain, L. Tissue engineering of feline corneal endothelium using a devitalized human cornea as carrier. Tiss. Eng. Part A 2009, 15, 1709–1718. [Google Scholar]
- Li, N.; Wang, X.; Wan, P.; Huang, M.; Wu, Z.; Liang, X.; Liu, Y.; Ge, J.; Huang, J.; Wang, Z. Tectonic lamellar keratoplasty with acellular corneal stroma in high-risk corneal transplantation. Mol. Vis. 2011, 17, 1909–1917. [Google Scholar]
- Shafiq, M.A.; Gemeinhart, R.A.; Yue, B.Y.; Djalilian, A.R. Decellularized human cornea for reconstructing the corneal epithelium and anterior stroma. Tissue Eng. Part C Methods 2012, 18, 340–348. [Google Scholar]
- Huang, M.; Li, N.; Wu, Z.; Wan, P.; Liang, X.; Zhang, W.; Wang, X.; Li, C.; Xiao, J.; Zhou, Q.; et al. Using acellular porcine limbal stroma for rabbit limbal stem cell microenvironment reconstruction. Biomaterials 2011, 32, 7812–7821. [Google Scholar]
- Xiao, J.; Duan, H.; Liu, Z.; Wu, Z.; Lan, Y.; Zhang, W.; Li, C.; Chen, F.; Zhou, Q.; Wang, X.; et al. Construction of the recellularized corneal stroma using porous acellular corneal scaffold. Biomaterials 2011, 32, 6962–6971. [Google Scholar] [CrossRef]
- Yoeruek, E.; Bayyoud, T.; Maurus, C.; Hofmann, J.; Spitzer, M.S.; Bartz-Schmidt, K.-U.; Szurman, P. Reconstruction of corneal stroma with decellularized porcine xenografts in a rabbit model. Acta Ophthalmol. 2011, 90, 206–210. [Google Scholar]
- Ponce Márquez, S.; Martínez, V.S.; McIntosh Ambrose, W.; Wang, J.; Gantxegui, N.G.; Schein, O.; Elisseeff, J. Decellularization of bovine corneas for tissue engineering applications. Acta biomat. 2009, 5, 1839–1847. [Google Scholar] [CrossRef]
- Li, J.; Shi, S.; Zhang, X.; Ni, S.; Wang, Y.; Curcio, C.A.; Chen, W. Comparison of different methods of glycerol preservation for deep anterior lamellar keratoplasty eligible corneas. Invest. Ophthalmol.Vis. Sci. 2012, 53, 5675–5685. [Google Scholar] [CrossRef]
- Dai, Y.; Chen, J.; Li, H.; Li, S.; Chen, J.; Ding, Y.; Wu, J.; Wang, C.; Tan, M. Characterizing the effects of VPA, VC and RCCS on rabbit keratocytes onto decellularized bovine cornea. PLoS One 2012, 7, 1–10. [Google Scholar]
- Du, L.; Wu, X.; Pang, K.; Yang, Y. Histological evaluation and biomechanical characterisation of an acellular porcine cornea scaffold. Br. J. Ophthalmol. 2011, 95, 410–414. [Google Scholar] [CrossRef]
- Fu, Y.; Fan, X.; Chen, P.; Shao, C.; Lu, W. Reconstruction of a tissue-engineered cornea with porcine corneal acellular matrix as the scaffold. Cells Tissues Organs 2010, 191, 193–202. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Z.; Sun, X.; Wang, F.; Xu, X.; Zhang, X. Safety and efficacy of dispase and plasmin in pharmacologic vitreolysis. Invest. Ophthalmol. Vis. Sci. 2004, 45, 3286–3290. [Google Scholar] [CrossRef]
- Yang, M.; Chen, C.Z.; Wang, X.N.; Zhu, Y.B.; Gu, Y.J. Favorable effects of the detergent and enzyme extraction method for preparing decellularized bovine pericardium scaffold for tissue engineered heart valves. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 354–361. [Google Scholar]
- Gui, L.; Chan, S.A.; Breuer, C.K.; Niklason, L.E. Novel utilization of serum in tissue decellularization. Tissue Eng. Part C Methods 2010, 16, 173–184. [Google Scholar]
- Klebe, R.J. Isolation of a collagen-dependent cell attachment factor. Nature 1974, 250, 248–251. [Google Scholar]
- Gailit, J.; Ruoslahti, E. Regulation of the fibronectin receptor affinity by divalent cations. J. Biol. Chem. 1988, 263, 12927–12932. [Google Scholar]
- Reing, J.E.; Brown, B.N.; Daly, K.A.; Freund, J.M.; Gilbert, T.W.; Hsiong, S.X.; Huber, A.; Kullas, K.E.; Tottey, S.; Wolf, M.T.; et al. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials 2010, 31, 8626–8633. [Google Scholar] [CrossRef]
- Meyer, S.R.; Chiu, B.; Churchill, T.A.; Zhu, L.; Lakey, J.R.; Ross, D.B. Comparison of aortic valve allograft decellularization techniques in the rat. J. Biomed. Mater. Res. A 2006, 79, 254–262. [Google Scholar]
- Yang, B.; Zhang, Y.; Zhou, L.; Sun, Z.; Zheng, J.; Chen, Y.; Dai, Y. Development of a porcine bladder acellular matrix with well-preserved extracellular bioactive factors for tissue engineering. Tissue Eng. Part C Methods 2010, 16, 1201–1211. [Google Scholar]
- Dong, X.; Wei, X.; Yi, W.; Gu, C.; Kang, X.; Liu, Y.; Li, Q.; Yi, D. RGD-modified acellular bovine pericardium as a bioprosthetic scaffold for tissue engineering. J. Mater. Sci. Mater. Med. 2009, 20, 2327–2336. [Google Scholar] [CrossRef]
- Baptista, P.M.; Siddiqui, M.M.; Lozier, G.; Rodriguez, S.R.; Atala, A.; Soker, S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology 2011, 53, 604–617. [Google Scholar] [CrossRef]
- Badylak, S.; Liang, A.; Record, R.; Tullius, R.; Hodde, J. Endothelial cell adherence to small intestinal submucosa: An acellular bioscaffold. Biomaterials 1999, 20, 2257–2263. [Google Scholar]
- Dejardin, L.M.; Arnoczky, S.P.; Clarke, R.B. Use of small intestinal submucosal implants for regeneration of large fascial defects: An experimental study in dogs. J. Biomed. Mater. Res. 1999, 46, 203–211. [Google Scholar]
- Huang, Q.; Dawson, R.A.; Pegg, D.E.; Kearney, J.N.; Macneil, S. Use of peracetic acid to sterilize human donor skin for production of acellular dermal matrices for clinical use. Wound Repair Regen. 2004, 12, 276–287. [Google Scholar]
- Prasertsung, I.; Kanokpanont, S.; Bunaprasert, T.; Thanakit, V.; Damrongsakkul, S. Development of acellular dermis from porcine skin using periodic pressurized technique. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 85, 210–219. [Google Scholar]
- Jamur, M.C.; Oliver, C. Cell fixatives for immunostaining. Methods Mol. Biol. 2010, 588, 55–61. [Google Scholar] [CrossRef]
- Suthipintawong, C.; Leong, A.S.; Vinyuvat, S. Immunostaining of cell preparations: A comparative evaluation of common fixatives and protocols. Diagn. Cytopathol. 1996, 15, 167–174. [Google Scholar] [CrossRef]
- King, J.H., Jr.; Mc, T.J.; Meryman, H.T. Preservation of corneas for lamellar keratoplasty: A simple method of chemical glycerine-dehydration. Trans. Am. Ophthalmol. Soc. 1961, 59, 194–201. [Google Scholar]
- King, J.H., Jr.; Mc, T.J.; Meryman, H.T. A simple method of preservation of corneas for lamellar keratoplasty. Am. J. Ophthalmol. 1962, 53, 445–449. [Google Scholar]
- King, J.H., Jr.; Townsend, W.M. The prolonged storage of donor corneas by glycerine dehydration. Trans. Am. Ophthalmol. Soc. 1984, 82, 106–110. [Google Scholar]
- Cox, B.; Emili, A. Tissue subcellular fractionation and protein extraction for use in mass-spectrometry-based proteomics. Nat. Protoc. 2006, 1, 1872–1878. [Google Scholar] [CrossRef]
- Giusti, S.; Bogetti, M.E.; Bonafina, A.; de Plazas, F.S. An improved method to obtain a soluble nuclear fraction from embryonic brain tissue. Neurochem. Res. 2009, 34, 2022–2029. [Google Scholar] [CrossRef]
- Elder, B.D.; Kim, D.H.; Athanasiou, K.A. Developing an articular cartilage decellularization process toward facet joint cartilage replacement. Neurosurgery 2010, 66, 722–727. [Google Scholar] [CrossRef]
- Xu, C.C.; Chan, R.W.; Tirunagari, N. A biodegradable, acellular xenogeneic scaffold for regeneration of the vocal fold lamina propria. Tissue Eng. 2007, 13, 551–566. [Google Scholar]
- Nagata, S.; Hanayama, R.; Kawane, K. Autoimmunity and the clearance of dead cells. Cell 2010, 140, 619–630. [Google Scholar] [CrossRef]
- Brown, B.N.; Valentin, J.E.; Stewart-Akers, A.M.; McCabe, G.P.; Badylak, S.F. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 2009, 30, 1482–1491. [Google Scholar]
- Keane, T.J.; Londono, R.; Turner, N.J.; Badylak, S.F. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 2012, 33, 1771–1781. [Google Scholar]
- Zheng, M.H.; Chen, J.; Kirilak, Y.; Willers, C.; Xu, J.; Wood, D. Porcine small intestine submucosa (sis) is not an acellular collagenous matrix and contains porcine DNA: Possible implications in human implantation. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 73, 61–67. [Google Scholar]
- Ju, C.; Gao, L.; Wu, X.; Pang, K. A human corneal endothelium equivalent constructed with acellular porcine corneal matrix. Indian J. Med. Res. 2012, 135, 887–894. [Google Scholar]
- Liu, Z.; Ji, J.; Zhang, J.; Huang, C.; Meng, Z.; Qiu, W.; Li, X.; Wang, W. Corneal reinforcement using an acellular dermal matrix for an analysis of biocompatibility, mechanical properties, and transparency. Acta Biomater. 2012, 8, 3326–3332. [Google Scholar] [CrossRef]
- Chen, R.H.; Kadner, A.; Mitchell, R.N.; Adams, D.H. Mechanism of delayed rejection in transgenic pig-to-primate cardiac xenotransplantation. J. Surg. Res. 2000, 90, 119–125. [Google Scholar] [CrossRef]
- Galili, U.; Clark, M.R.; Shohet, S.B.; Buehler, J.; Macher, B.A. Evolutionary relationship between the natural anti-gal antibody and the gal alpha 1–3gal epitope in primates. Proc. Natl. Acad. Sci. USA 1987, 84, 1369–1373. [Google Scholar] [CrossRef]
- Simon, P.; Kasimir, M.T.; Seebacher, G.; Weigel, G.; Ullrich, R.; Salzer-Muhar, U.; Rieder, E.; Wolner, E. Early failure of the tissue engineered porcine heart valve synergraft in pediatric patients. Eur. J Cardiothorac. Surg. 2003, 23, 1002–1006. [Google Scholar] [CrossRef]
- Daly, K.A.; Stewart-Akers, A.M.; Hara, H.; Ezzelarab, M.; Long, C.; Cordero, K.; Johnson, S.A.; Ayares, D.; Cooper, D.K.; Badylak, S.F. Effect of the alphagal epitope on the response to small intestinal submucosa extracellular matrix in a nonhuman primate model. Tissue Eng. Part A 2009, 15, 3877–3888. [Google Scholar] [CrossRef]
- Vitova, A.; Kuffova, L.; Klaska, I.P.; Holan, V.; Cornall, R.J.; Forrester, J.V. The high-risk corneal regraft model: A justification for tissue matching in humans. Transpl. Int. 2013, 26, 453–461. [Google Scholar] [CrossRef]
- Streilein, J.W.; Arancibia-Caracamo, C.; Osawa, H. The role of minor histocompatibility alloantigens in penetrating keratoplasty. Dev. Ophthalmol. 2003, 36, 74–88. [Google Scholar]
- Jenke, D. Evaluation of the chemical compatibility of plastic contact materials and pharmaceutical products, safety considerations related to extractables and leachables. J. Pharm. Sci. 2007, 96, 2566–2581. [Google Scholar] [CrossRef]
- Teng, S.W.; Tan, H.Y.; Peng, J.L.; Lin, H.H.; Kim, K.H.; Lo, W.; Sun, Y.; Lin, W.C.; Lin, S.J.; Jee, S.H.; et al. Multiphoton autofluorescence and second-harmonic generation imaging of the ex vivo porcine eye. Invest. Ophthalmol. Vis. Sci. 2006, 47, 1216–1224. [Google Scholar] [CrossRef]
- Morishige, N.; Petroll, W.M.; Nishida, T.; Kenney, M.C.; Jester, J.V. Noninvasive corneal stromal collagen imaging using two-photon-generated second-harmonic signals. J. Cataract Refrac. Surg. 2006, 32, 1784–1791. [Google Scholar]
- Meek, K.M.; Fullwood, N.J. Corneal and scleral collagens—A microscopist’s perspective. Micron 2001, 32, 261–272. [Google Scholar] [CrossRef]
- Jalbert, I.; Stapleton, F.; Papas, E.; Sweeney, D.F.; Coroneo, M. In vivo confocal microscopy of the human cornea. Br. J. Ophthalmol. 2003, 87, 225–236. [Google Scholar]
- Erie, J.C.; McLaren, J.W.; Patel, S.V. Confocal microscopy in ophthalmology. Am. J. Ophthalmol. 2009, 148, 639–646. [Google Scholar]
- Newton, R.H.; Haffegee, J.P.; Ho, M.W. Polarized light microscopy of weakly birefringent biological specimens. J. Microsc. 1995, 180, 127–130. [Google Scholar] [CrossRef]
- Patel, D.V.; McGhee, C.N. Contemporary in vivo confocal microscopy of the living human cornea using white light and laser scanning techniques: A major review. Clin. Exp. Ophthalmol. 2007, 35, 71–88. [Google Scholar]
- Guthoff, R.F.; Zhivov, A.; Stachs, O. In vivo confocal microscopy, an inner vision of the cornea—A major review. Clin. Exp. Ophthalmol. 2009, 37, 100–117. [Google Scholar] [CrossRef]
- Meek, K.M.; Boote, C. The use of X-ray scattering techniques to quantify the orientation and distribution of collagen in the corneal stroma. Prog. Retin. Eye Res. 2009, 28, 369–392. [Google Scholar] [CrossRef]
- Doughty, M.J.; Bergmanson, J.P. Resolution and reproducibility of measures of the diameter of small collagen fibrils by transmission electron microscopy-application to the rabbit corneal stroma. Micron 2005, 36, 331–343. [Google Scholar] [CrossRef]
- Akhtar, S. Effect of processing methods for transmission electron microscopy on corneal collagen fibrils diameter and spacing. Microsc. Res. Tech. 2012, 75, 1420–1424. [Google Scholar] [CrossRef]
- Hayashi, S.; Osawa, T.; Tohyama, K. Comparative observations on corneas, with special reference to Bowman's layer and Descemet’s membrane in mammals and amphibians. J. Morphol. 2002, 254, 247–258. [Google Scholar] [CrossRef]
- Gusnard, D.; Kirschner, R.H. Cell and organelle shrinkage during preparation for scanning electron microscopy: Effects of fixation, dehydration and critical point drying. J. Microsc. 1977, 110, 51–57. [Google Scholar] [CrossRef]
- Morishige, N.; Wahlert, A.J.; Kenney, M.C.; Brown, D.J.; Kawamoto, K.; Chikama, T.; Nishida, T.; Jester, J.V. Second-harmonic imaging microscopy of normal human and keratoconus cornea. Invest. Ophthalmol. Vis. Sci. 2007, 48, 1087–1094. [Google Scholar] [CrossRef]
- Han, M.; Zickler, L.; Giese, G.; Walter, M.; Loesel, F.H.; Bille, J.F. Second-harmonic imaging of cornea after intrastromal femtosecond laser ablation. J. Biomed. Opt. 2004, 9, 760–766. [Google Scholar] [CrossRef]
- Zoumi, A.; Yeh, A.; Tromberg, B.J. Imaging cells and extracellular matrix in vivo by using second-harmonic generation and two-photon excited fluorescence. Proc. Natl. Acad. Sci. USA 2002, 99, 11014–11019. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Silverman, R.H.; Sutton, H.F.; Coleman, D.J. Very high-frequency ultrasound corneal analysis identifies anatomic correlates of optical complications of lamellar refractive surgery: Anatomic diagnosis in lamellar surgery. Ophthalmology 1999, 106, 474–482. [Google Scholar] [CrossRef]
- Aslanides, I.M.; Reinstein, D.Z.; Silverman, R.H.; Lazzaro, D.R.; Rondeau, M.J.; Rodriguez, H.S.; Coleman, D.J. High-frequency ultrasound spectral parameter imaging of anterior corneal scars. CLAO 1995, 21, 268–272. [Google Scholar]
- Denoyer, A.; Ossant, F.; Arbeille, B.; Fetissof, F.; Patat, F.; Pourcelot, L.; Pisella, P.J. Very-high-frequency ultrasound corneal imaging as a new tool for early diagnosis of ocular surface toxicity in rabbits treated with a preserved glaucoma drug. Ophthalmic. Res. 2008, 40, 298–308. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Archer, T.J.; Gobbe, M.; Silverman, R.H.; Coleman, J. Epithelial thickness in the normal cornea: Three-dimensional display with artemis very high-frequency digital ultrasound. J. Refrac. Surg. 2008, 24, 571–581. [Google Scholar]
- Drexler, W.; Morgner, U.; Ghanta, R.K.; Kartner, F.X.; Schuman, J.S.; Fujimoto, J.G. Ultrahigh-resolution ophthalmic optical coherence tomography. Nat. Med. 2001, 7, 502–507. [Google Scholar] [CrossRef]
- Bagnaninchi, P.O.; Yang, Y.; Zghoul, N.; Maffulli, N.; Wang, R.K.; El Haj, A.J. Chitosan microchannel scaffolds for tendon tissue engineering characterized using optical coherence tomography. Tissue Eng. 2007, 13, 323–331. [Google Scholar]
- Yang, Y.; Dubois, A.; Qin, X.P.; Li, J.; El Haj, A.; Wang, R.K. Investigation of optical coherence tomography as an imaging modality in tissue engineering. Phys. Med. Biol. 2006, 51, 1649–1659. [Google Scholar]
- Yang, Y.; Bagnaninchi, P.O.; Ahearne, M.; Wang, R.K.; Liu, K.-K. A novel optical coherence tomography-based micro-indentation technique for mechanical characterization of hydrogels. J. R. Soc. Interface 2007, 4, 1169–1173. [Google Scholar] [CrossRef]
- Rodriguez-Padilla, J.A.; Hedges, T.R.; Monson, B.; Srinivasan, V.; Wojtkowski, M.; Reichel, E.; Duker, J.S.; Schuman, J.S.; Fujimoto, J.G. High-speed ultra-high-resolution optical coherence tomography findings in hydroxychloroquine retinopathy. Arch. Ophthalmol. 2007, 125, 775–780. [Google Scholar]
- Maurice, D.M. The structure and transparency of the cornea. J. Physiol. 1957, 136, 263–286. [Google Scholar]
- Daxer, A.; Fratzl, P. Collagen fibril orientation in the human corneal stroma and its implication in keratoconus. Invest. Ophthalmol. Vis. Sci. 1997, 38, 121–129. [Google Scholar]
- Daxer, A.; Misof, K.; Grabner, B.; Ettl, A.; Fratzl, P. Collagen fibrils in the human corneal stroma: Structure and aging. Invest. Ophthalmol. Vis. Sci. 1998, 39, 644–648. [Google Scholar]
- Yamamoto, S.; Hitomi, J.; Sawaguchi, S.; Abe, H.; Shigeno, M.; Ushiki, T. Observation of human corneal and scleral collagen fibrils by atomic force microscopy. Jpn. J. Ophthalmol. 2002, 46, 496–501. [Google Scholar] [CrossRef]
- Ahearne, M. Mechanical Characterisation of Cornea and Corneal Stromal Equivalents. Ph.D. Dissertation, Keele University, Staffordshire, UK, 2007. [Google Scholar]
- Glass, D.H.; Roberts, C.J.; Litsky, A.S.; Weber, P.A. A viscoelastic biomechanical model of the cornea describing the effect of viscosity and elasticity on hysteresis. Invest. Ophthalmol. Vis. Sci. 2008, 49, 3919–3926. [Google Scholar] [CrossRef]
- Bao, F.; Jiang, L.; Wang, X.; Zhang, D.; Wang, Q.; Zeng, Y. Assessment of the ex vivo biomechanical properties of porcine cornea with inflation test for corneal xenotransplantation. J. Med. Eng. Technol. 2012, 36, 17–21. [Google Scholar] [CrossRef]
- Ahearne, M.; Siamantouras, E.; Yang, Y.; Liu, K.K. Mechanical characterization of biomimetic membranes by micro-shaft poking. J. R. Soc. Interface 2009, 6, 471–478. [Google Scholar] [CrossRef]
- Tsakalakos, T. The bulge test—A comparison of theory and experiment for isotropic and anisotropic films. Thin Solid Films 1981, 75, 293–305. [Google Scholar]
- Elsheikh, A.; Anderson, K. Comparative study of corneal strip extensometry and inflation tests. J. R. Soc. Interface 2005, 2, 177–185. [Google Scholar] [CrossRef]
- Hoeltzel, D.A.; Altman, P.; Buzard, K.; Choe, K.I. Strip extensiometry for comparison of the mechanical response of bovine, rabbit, and human corneas. J. Biomech. Eng. 1992, 114, 202–215. [Google Scholar] [CrossRef]
- Boyce, B.L.; Grazier, J.M.; Jones, R.E.; Nguyen, T.D. Full-field deformation of bovine cornea under constrained inflation conditions. Biomaterials 2008, 29, 3896–3904. [Google Scholar] [CrossRef]
- Krupa, I.; Nedelcev, T.; Racko, D.; Lacik, I. Mechanical properties of silica hydrogels prepared and aged at physiological conditions: Testing in the compression mode. J. Sol-Gel. Sci. Techn. 2010, 53, 107–114. [Google Scholar] [CrossRef]
- Stammen, J.A.; Williams, S.; Ku, D.N.; Guldberg, R.E. Mechanical properties of a novel PVA hydrogel in shear and unconfined compression. Biomaterials 2001, 22, 799–806. [Google Scholar]
- Korhonen, R.K.; Laasanen, M.S.; Toyras, J.; Rieppo, J.; Hirvonen, J.; Helminen, H.J.; Jurvelin, J.S. Comparison of the equilibrium response of articular cartilage in unconfined compression, confined compression and indentation. J. Biomech. 2002, 35, 903–909. [Google Scholar] [CrossRef]
- Koob, T.J.; Hernandez, D.J. Mechanical and thermal properties of novel polymerized NDGA—Gelatin hydrogels. Biomaterials 2003, 24, 1285–1292. [Google Scholar] [CrossRef]
- Calkins, J.L.; Hochheimer, B.F.; Stark, W.J. Corneal wound-healing—Holographic stress-test analysis. Invest. Ophthalmol. Vis. Sci. 1981, 21, 322–334. [Google Scholar]
- Smolek, M.K. Holographic-interferometry of intact and radially incised human eye-bank corneas. J. Cataract Refract. Surg. 1994, 20, 277–286. [Google Scholar]
- Kasprzak, H.; Jaronski, J.; Forster, W.; Vonbally, G. Analysis of holographic interferograms of the expanded cornea after refractive surgery procedure. Proc. SPIE 1994, 2340, 480–486. [Google Scholar]
- Kasprzak, H.; Forster, W.; Vonbally, G. Measurement of elastic-modulus of the bovine cornea by means of holographic-interferometry 1. Method and experiment. Optom. Vis. Sci. 1993, 70, 535–544. [Google Scholar] [CrossRef]
- Maurice, D.M. Mechanics of the cornea. In The cornea: Transactions of the World Congress on the Cornea III; Cavanagh, H.D., Ed.; Raven Press Ltd: New York, NY, USA, 1988; Volume 8, p. 187. [Google Scholar]
- Ahearne, M.; Yang, Y.; Then, K.Y.; Liu, K.K. Non-destructive mechanical characterisation of UVA/riboflavin crosslinked collagen hydrogels. Br. J. Ophthalmol. 2008, 92, 268–271. [Google Scholar] [CrossRef]
- Sullivan-Mee, M.; Billingsley, S.C.; Patel, A.D.; Halverson, K.D.; Alldredge, B.R.; Qualls, C. Ocular response analyzer in subjects with and without glaucoma. Optom. Vis. Sci. 2008, 85, 463–470. [Google Scholar] [CrossRef]
- Abitbol, O.; Bouden, J.; Doan, S.; Hoang-Xuan, T.; Gatinel, D. Corneal hysteresis measured with the ocular response analyzer in normal and glaucomatous eyes. Acta Ophthalmol. 2010, 88, 116–119. [Google Scholar] [CrossRef]
- Zeng, Y.J.; Yang, J.; Huang, K.; Lee, Z.H.; Lee, X.Y. A comparison of biomechanical properties between human and porcine cornea. J. Biomech. 2001, 34, 533–537. [Google Scholar] [CrossRef]
- Ozdal, M.P.C.; Mansour, M.; Deschenes, J. Ultrasound biomicroscopic evaluation of the traumatized eyes. Eye 2003, 17, 467–472. [Google Scholar] [CrossRef]
- Urbak, S.F. Ultrasound biomicroscopy. III. Accuracy and agreement of measurements. Acta Ophthalmol. Scand. 1999, 77, 293–297. [Google Scholar]
- Schneider, C.K.; Salmikangas, P.; Jilma, B.; Flamion, B.; Todorova, L.R.; Paphitou, A.; Haunerova, I.; Maimets, T.; Trouvin, J.H.; Flory, E.; et al. Challenges with advanced therapy medicinal products and how to meet them. Nat. Rev. Drug Discov. 2010, 9, 195–201. [Google Scholar]
- Li, J.; Yu, L.; Deng, Z.; Wang, L.; Sun, L.; Ma, H.; Chen, W. Deep anterior lamellar keratoplasty using acellular corneal tissue for prevention of allograft rejection in high-risk corneas. Am. J. Ophthalmol. 2011, 152, 762–770. [Google Scholar] [CrossRef]
- Edelhauser, H.F. The balance between corneal transparency and edema the proctor lecture. Invest. Ophthalmol. Vis. Sci. 2006, 47, 1755–1767. [Google Scholar] [CrossRef]
- Dua, H.S.; Shanmuganathan, V.A.; Powell-Richards, A.O.; Tighe, P.J.; Joseph, A. Limbal epithelial crypts: A novel anatomical structure and a putative limbal stem cell niche. Br. J. Ophthalmol. 2005, 89, 529–532. [Google Scholar]
- Dua, H.S.; Azuara-Blanco, A. Limbal stem cells of the corneal epithelium. Surv. Ophthalmol. 2000, 44, 415–425. [Google Scholar] [CrossRef]
- Fernandes, M.; Sangwan, V.S.; Rao, S.K.; Basti, S.; Sridhar, M.S.; Bansal, A.K.; Dua, H.S. Limbal stem cell transplantation. Indian J. Ophthalmol. 2004, 52, 5–22. [Google Scholar]
- Jenkins, C.; Tuft, S.; Liu, C.; Buckley, R. Limbal transplantation in the management of chronic contact-lens-associated epitheliopathy. Eye 1993, 7, 629–633. [Google Scholar] [CrossRef]
- Kim, H.S.; Jun Song, X.; de Paiva, C.S.; Chen, Z.; Pflugfelder, S.C.; Li, D.Q. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp. Eye Res. 2004, 79, 41–49. [Google Scholar] [CrossRef]
- Zito-Abbad, E.; Borderie, V.M.; Baudrimont, M.; Bourcier, T.; Laroche, L.; Chapel, C.; Uzel, J.L. Corneal epithelial cultures generated from organ-cultured limbal tissue: Factors influencing epithelial cell growth. Curr. Eye Res. 2006, 31, 391–399. [Google Scholar] [CrossRef]
- Lopez-Paniagua, M.; Nieto-Miguel, T.; de la Mata, A.; Galindo, S.; Herreras, J.M.; Corrales, R.M.; Calonge, M. Consecutive expansion of limbal epithelial stem cells from a single limbal biopsy. Curr. Eye Res. 2013, 38, 537–549. [Google Scholar] [CrossRef]
- Loureiro, R.R.; Cristovam, P.C.; Martins, C.M.; Covre, J.L.; Sobrinho, J.A.; Ricardo, J.R.; Hazarbassanov, R.M.; Hofling-Lima, A.L.; Belfort, R., Jr.; Nishi, M.; et al. Comparison of culture media for ex vivo cultivation of limbal epithelial progenitor cells. Mol. Vis. 2013, 19, 69–77. [Google Scholar]
- Yu, D.; Chen, M.; Sun, X.; Ge, J. Differentiation of mouse induced pluripotent stem cells into corneal epithelial-like cells. Cell Biol. Int. 2013, 37, 87–94. [Google Scholar]
- Hayashi, R.; Ishikawa, Y.; Ito, M.; Kageyama, T.; Takashiba, K.; Fujioka, T.; Tsujikawa, M.; Miyoshi, H.; Yamato, M.; Nakamura, Y.; et al. Generation of corneal epithelial cells from induced pluripotent stem cells derived from human dermal fibroblast and corneal limbal epithelium. Plos One 2012, 7, 1–10. [Google Scholar]
- Ahmed, N.; Vogel, B.; Rohde, E.; Strunk, D.; Grifka, J.; Schulz, M.B.; Grassel, S. CD45-positive cells of haematopoietic origin enhance chondrogenic marker gene expression in rat marrow stromal cells. Int. J. Mol. Med. 2006, 18, 233–240. [Google Scholar]
- Homma, R.; Yoshikawa, H.; Takeno, M.; Kurokawa, M.S.; Masuda, C.; Takada, E.; Tsubota, K.; Ueno, S.; Suzuki, N. Induction of epithelial progenitors in vitro from mouse embryonic stem cells and application for reconstruction of damaged cornea in mice. Invest. Ophthalmol. Vis. Sci. 2004, 45, 4320–4326. [Google Scholar] [CrossRef]
- Notara, M.; Hernandez, D.; Mason, C.; Daniels, J.T. Characterization of the phenotype and functionality of corneal epithelial cells derived from mouse embryonic stem cells. Regen. Med. 2012, 7, 167–178. [Google Scholar] [CrossRef]
- Gomes, J.A.; Geraldes Monteiro, B.; Melo, G.B.; Smith, R.L.; Cavenaghi Pereira da Silva, M.; Lizier, N.F.; Kerkis, A.; Cerruti, H.; Kerkis, I. Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Invest. Ophthalmol. Vis. Sci. 2010, 51, 1408–1414. [Google Scholar] [CrossRef]
- Reza, H.M.; Ng, B.Y.; Gimeno, F.L.; Phan, T.T.; Ang, L.P. Umbilical cord lining stem cells as a novel and promising source for ocular surface regeneration. Stem Cell Rev. 2011, 7, 935–947. [Google Scholar]
- Jiang, T.S.; Cai, L.; Ji, W.Y.; Hui, Y.N.; Wang, Y.S.; Hu, D.; Zhu, J. Reconstruction of the corneal epithelium with induced marrow mesenchymal stem cells in rats. Mol. Vis. 2010, 16, 1304–1316. [Google Scholar]
- Hoar, R.M. Embryology of the eye. Environ. Health Perspect. 1982, 44, 31–34. [Google Scholar] [CrossRef]
- Forrester, J.V.; Dick, A.D.; McMenamin, P.G.; Roberts, F. The eye: Basic sciences in practice, 3rd ed.; Saunders: Aberdeen, UK, 2008; p. 540. [Google Scholar]
- West-Mays, J.A.; Dwivedi, D.J. The keratocyte: Corneal stromal cell with variable repair phenotypes. Int. J. Biochem. Cell Biol. 2006, 38, 1625–1631. [Google Scholar] [CrossRef]
- Du, Y.; Funderburgh, M.L.; Mann, M.M.; SundarRaj, N.; Funderburgh, J.L. Multipotent stem cells in human corneal stroma. Stem Cells 2005, 23, 1266–1275. [Google Scholar] [CrossRef]
- Poole, C.A.; Brookes, N.H.; Clover, G.M. Keratocyte networks visualised in the living cornea using vital dyes. J. Cell Sci. 1993, 106, 685–691. [Google Scholar]
- Ueda, A.; Nishida, T.; Otori, T.; Fujita, H. Electron-microscopic studies on the presence of gap junctions between corneal fibroblasts in rabbits. Cell Tissue Res. 1987, 249, 473–475. [Google Scholar]
- Marshall, G.E.; Konstas, A.G.; Lee, W.R. Immunogold fine structural localization of extracellular matrix components in aged human cornea. I. Types I-IV collagen and laminin. Graefes Arch. Clin. Exp. Ophthalmol. 1991, 229, 157–163. [Google Scholar] [CrossRef]
- Chakravarti, S.; Petroll, W.M.; Hassell, J.R.; Jester, J.V.; Lass, J.H.; Paul, J.; Birk, D.E. Corneal opacity in lumican-null mice: Defects in collagen fibril structure and packing in the posterior stroma. Invest. Ophthalmol. Vis. Sci. 2000, 41, 3365–3373. [Google Scholar]
- Scott, J.E.; Thomlinson, A.M. The structure of interfibrillar proteoglycan bridges (shape modules’) in extracellular matrix of fibrous connective tissues and their stability in various chemical environments. J. Anat. 1998, 192, 391–405. [Google Scholar]
- Nakamura, M.; Kimura, S.; Kobayashi, M.; Hirano, K.; Hoshino, T.; Awaya, S. Type VI collagen bound to collagen fibrils by chondroitin/dermatan sulfate glycosaminoglycan in mouse corneal stroma. Jpn. J. Ophthalmol. 1997, 41, 71–76. [Google Scholar] [CrossRef]
- Michelacci, Y.M. Collagens and proteoglycans of the corneal extracellular matrix. Braz. J. Med. Biol. Res. 2003, 36, 1037–1046. [Google Scholar] [CrossRef] [Green Version]
- Fini, M.E.; Stramer, B.M. How the cornea heals: Cornea-specific repair mechanisms affecting surgical outcomes. Cornea 2005, 24, 2–11. [Google Scholar] [CrossRef]
- Hassell, J.R.; Birk, D.E. The molecular basis of corneal transparency. Exp. Eye Res. 2010, 91, 326–335. [Google Scholar] [CrossRef]
- Jester, J.V.; Brown, D.; Pappa, A.; Vasiliou, V. Myofibroblast differentiation modulates keratocyte crystallin protein expression, concentration, and cellular light scattering. Invest. Ophthalmol. Vis. Sci. 2012, 53, 770–778. [Google Scholar] [CrossRef]
- Jester, J.V.; Moller-Pedersen, T.; Huang, J.; Sax, C.M.; Kays, W.T.; Cavangh, H.D.; Petroll, W.M.; Piatigorsky, J. The cellular basis of corneal transparency: Evidence for “corneal crystallins”. J. Cell Sci. 1999, 112, 613–622. [Google Scholar]
- Sax, C.M.; Kays, W.T.; Salamon, C.; Chervenak, M.M.; Xu, Y.S.; Piatigorsky, J. Transketolase gene expression in the cornea is influenced by environmental factors and developmentally controlled events. Cornea 2000, 19, 833–841. [Google Scholar] [CrossRef]
- Joseph, A.; Hossain, P.; Jham, S.; Jones, R.E.; Tighe, P.; McIntosh, R.S.; Dua, H.S. Expression of CD34 and l-selectin on human corneal keratocytes. Invest. Ophthalmol. Vis. Sci. 2003, 44, 4689–4692. [Google Scholar] [CrossRef]
- Perrella, G.; Brusini, P.; Spelat, R.; Hossain, P.; Hopkinson, A.; Dua, H.S. Expression of haematopoietic stem cell markers, CD133 and CD34 on human corneal keratocytes. Br. J. Ophthalmol. 2007, 91, 94–99. [Google Scholar] [CrossRef]
- Fini, M.E. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog. Retin. Eye Res. 1999, 18, 529–551. [Google Scholar] [CrossRef]
- Beales, M.P.; Funderburgh, J.L.; Jester, J.V.; Hassell, J.R. Proteoglycan synthesis by bovine keratocytes and corneal fibroblasts: Maintenance of the keratocyte phenotype in culture. Invest. Ophthalmol. Vis. Sci. 1999, 40, 1658–1663. [Google Scholar]
- Masur, S.K.; Dewal, H.S.; Dinh, T.T.; Erenburg, I.; Petridou, S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc. Natl. Acad. Sci. USA 1996, 93, 4219–4223. [Google Scholar]
- Long, C.J.; Roth, M.R.; Tasheva, E.S.; Funderburgh, M.; Smit, R.; Conrad, G.W.; Funderburgh, J.L. Fibroblast growth factor-2 promotes keratan sulfate proteoglycan expression by keratocytes in vitro. J. Biol. Chem. 2000, 275, 13918–13923. [Google Scholar]
- Ahearne, M.; Yang, Y.; El Haj, A.J.; Then, K.Y.; Liu, K.K. Characterizing the viscoelastic properties of thin hydrogel-based constructs for tissue engineering applications. J. R. Soc. Interface 2005, 2, 455–463. [Google Scholar] [CrossRef]
- Barbaro, V.; Di Iorio, E.; Ferrari, S.; Bisceglia, L.; Ruzza, A.; De Luca, M.; Pellegrini, G. Expression of VSX1 in human corneal keratocytes during differentiation into myofibroblasts in response to wound healing. Invest. Ophthalmol. Vis. Sci. 2006, 47, 5243–5250. [Google Scholar]
- Park, S.H.; Kim, K.W.; Chun, Y.S.; Kim, J.C. Human mesenchymal stem cells differentiate into keratocyte-like cells in keratocyte-conditioned medium. Exp. Eye Res. 2012, 101, 16–26. [Google Scholar] [CrossRef]
- Du, Y.; Carlson, E.C.; Funderburgh, M.L.; Birk, D.E.; Pearlman, E.; Guo, N.; Kao, W.W.; Funderburgh, J.L. Stem cell therapy restores transparency to defective murine corneas. Stem Cells 2009, 27, 1635–1642. [Google Scholar]
- Choong, P.F.; Mok, P.L.; Cheong, S.K.; Then, K.Y. Mesenchymal stromal cell-like characteristics of corneal keratocytes. Cytotherapy 2007, 9, 252–258. [Google Scholar] [CrossRef]
- Funderburgh, J.L.; Mann, M.M.; Funderburgh, M.L. Keratocyte phenotype mediates proteoglycan structure: A role for fibroblasts in corneal fibrosis. J. Biol. Chem. 2003, 278, 45629–45637. [Google Scholar] [CrossRef]
- Helary, C.; Ovtracht, L.; Coulomb, B.; Godeau, G.; Giraud-Guille, M.M. Dense fibrillar collagen matrices: A model to study myofibroblast behaviour during wound healing. Biomaterials 2006, 27, 4443–4452. [Google Scholar]
- Jester, J.V.; Petroll, W.M.; Barry, P.A.; Cavanagh, H.D. Expression of alpha-smooth muscle (alpha-sm) actin during corneal stromal wound healing. Invest. Ophthalmol. Vis. Sci. 1995, 36, 809–819. [Google Scholar]
- Wilson, S.L.; Wimpenny, I.; Ahearne, M.; Rauz, S.; El Haj, A.J.; Yang, Y. Chemical and topographical effects on cell differentiation and matrix elasticity in a corneal stromal layer model. Adv. Func. Mater. 2012, 22, 3641–3649. [Google Scholar] [CrossRef]
- Ren, R.; Hutcheon, A.E.; Guo, X.Q.; Saeidi, N.; Melotti, S.A.; Ruberti, J.W.; Zieske, J.D.; Trinkaus-Randall, V. Human primary corneal fibroblasts synthesize and deposit proteoglycans in long-term 3-D cultures. Dev. Dyn. 2008, 237, 2705–2715. [Google Scholar]
- Funderburgh, M.L.; Du, Y.; Mann, M.M.; SundarRaj, N.; Funderburgh, J.L. PAX6 expression identifies progenitor cells for corneal keratocytes. FASEB J. 2005, 19, 1371–1373. [Google Scholar]
- Branch, M.J.; Hashmani, K.; Dhillon, P.; Jones, D.R.; Dua, H.S.; Hopkinson, A. Mesenchymal stem cells in the human corneal limbal stroma. Invest. Ophthalmol. Vis. Sci. 2012, 53, 5109–5166. [Google Scholar]
- Bray, L.J.; Heazlewood, C.F.; Atkinson, K.; Hutmacher, D.W.; Harkin, D.G. Evaluation of methods for cultivating limbal mesenchymal stromal cells. Cytotherapy 2012, 14, 936–947. [Google Scholar] [Green Version]
- Waring, G.O.; Bourne, W.M.; Edelhauser, H.F.; Kenyon, K.R. The corneal endothelium. Normal and pathologic structure and function. Ophthalmology 1982, 89, 531–590. [Google Scholar]
- Kaufman, H.E.; Capella, J.A.; Robbins, J.E. The human corneal endothelium. Am. J. Ophthalmol. 1966, 61, 835–841. [Google Scholar]
- Fabricant, R.N.; Alpar, A.J.; Centifanto, Y.M.; Kaufman, H.E. Epidermal growth factor receptors on corneal endothelium. Arch. Ophthalmol. 1981, 99, 305–308. [Google Scholar]
- Hyldahl, L. Primary cell cultures from human embryonic corneas. J. Cell Sci. 1984, 66, 343–351. [Google Scholar]
- Pistsov, M.Y.; Sadovnikova, E.; Danilov, S.M. Human corneal endothelial cells: Isolation, characterization and long-term cultivation. Exp. Eye Res. 1988, 47, 403–414. [Google Scholar] [CrossRef]
- Samples, J.R.; Binder, P.S.; Nayak, S.K. Propagation of human corneal endothelium in vitro effect of growth factors. Exp. Eye Res. 1991, 52, 121–128. [Google Scholar] [CrossRef]
- Engelmann, K.; Bednarz, J.; Valtink, M. Prospects for endothelial transplantation. Exp. Eye Res. 2004, 78, 573–578. [Google Scholar] [CrossRef]
- Engelmann, K.; Bohnke, M.; Friedl, P. Isolation and long-term cultivation of human corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 1988, 29, 1656–1662. [Google Scholar]
- Hempel, B.; Bednarz, J.; Engelmann, K. Use of a serum-free medium for long-term storage of human corneas. Influence on endothelial cell density and corneal metabolism. Graefes Arch. Clin. Exp. Ophthalmol. 2001, 239, 801–805. [Google Scholar] [CrossRef]
- Proulx, S.; Bourget, J.M.; Gagnon, N.; Martel, S.; Deschambeault, A.; Carrier, P.; Giasson, C.J.; Auger, F.A.; Brunette, I.; Germain, L. Optimization of culture conditions for porcine corneal endothelial cells. Mol. Vis. 2007, 13, 524–533. [Google Scholar]
- Zhu, C.; Joyce, N.C. Proliferative response of corneal endothelial cells from young and older donors. Invest. Ophthalmol. Vis. Sci. 2004, 45, 1743–1751. [Google Scholar]
- Peh, G.S.; Beuerman, R.W.; Colman, A.; Tan, D.T.; Mehta, J.S. Human corneal endothelial cell expansion for corneal endothelium transplantation: An overview. Transplantation 2011, 91, 811–819. [Google Scholar]
- Whikehart, D.R.; Parikh, C.H.; Vaughn, A.V.; Mishler, K.; Edelhauser, H.F. Evidence suggesting the existence of stem cells for the human corneal endothelium. Mol. Vis. 2005, 11, 816–824. [Google Scholar]
- Yu, W.Y.; Sheridan, C.; Grierson, I.; Mason, S.; Kearns, V.; Lo, A.C.; Wong, D. Progenitors for the corneal endothelium and trabecular meshwork: A potential source for personalized stem cell therapy in corneal endothelial diseases and glaucoma. J. Biomed. Biotech. 2011, 2011, 1–13. [Google Scholar]
- McGowan, S.L.; Edelhauser, H.F.; Pfister, R.R.; Whikehart, D.R. Stem cell markers in the human posterior limbus and corneal endothelium of unwounded and wounded corneas. Mol. Vis. 2007, 13, 1984–2000. [Google Scholar]
- Joyce, N.C.; Harris, D.L.; Markov, V.; Zhang, Z.; Saitta, B. Potential of human umbilical cord blood mesenchymal stem cells to heal damaged corneal endothelium. Mol. Vis. 2012, 18, 547–564. [Google Scholar]
- Shao, C.; Fu, Y.; Lu, W.; Fan, X. Bone marrow-derived endothelial progenitor cells: A promising therapeutic alternative for corneal endothelial dysfunction. Cells Tissues Organs 2011, 193, 253–263. [Google Scholar] [CrossRef]
- Ju, C.; Zhang, K.; Wu, X. Derivation of corneal endothelial cell-like cells from rat neural crest cells in vitro. PLoS One 2012, 7, 1–7. [Google Scholar]
- Hatou, S.; Yoshida, S.; Higa, K.; Miyashita, H.; Inagaki, E.; Okano, H.; Tsubota, K.; Shimmura, S. Functional corneal endothelium derived from corneal stroma stem cells of neural crest origin by retinoic acid and wnt/beta-catenin signaling. Stem Cells Dev. 2013, 22, 828–839. [Google Scholar]
- Griffith, M.; Jackson, W.B.; Lagali, N.; Merrett, K.; Li, F.; Fagerholm, P. Artificial corneas: A regenerative medicine approach. Eye 2009, 23, 1985–1989. [Google Scholar]
- Wilhelmus, K.R. The Draize eye test. Surv. Ophthalmol. 2001, 45, 493–515. [Google Scholar]
- Jester, J.V.; Li, L.; Molai, A.; Maurer, J.K. Extent of initial corneal injury as a basis for alternative eye irritation tests. Toxicol. in Vitro 2001, 15, 115–130. [Google Scholar]
- NC3Rs. Available online: http://www.nc3rs.org.uk/ (accessed on 27 March 2013).
- European Parliment Council. Directive 2010/63/EU, The European parliment and of the council. Off. J. Eur. Union 2012, 53, 33–79.
- Burton, A.B.G.; York, M.; Lawrence, R.S. The in vitro assessment of severe eye irritants. Food Cosmet. Toxicol. 1981, 19, 471–480. [Google Scholar] [CrossRef]
- Prinsen, M.K. The chicken enucleated eye test (CEET): A practical (pre)screen for the assessment of eye irritation/corrosion potential of test materials. Food Chem. Toxicol. 1996, 34, 291–296. [Google Scholar] [CrossRef]
- Reichl, S.; Muller-Goymann, C.C. Development of an organotypical cornea construct as an in vitro model for permeation studies. Ophthalmologe 2001, 98, 853–858. [Google Scholar]
- Matsuda, S.; Hisama, M.; Shibayama, H.; Itou, N.; Iwaki, M. Application of the reconstructed rabbit corneal epithelium model to assess the in vitro eye irritancy test of chemicals. Yakugaku Zasshi 2009, 129, 1113–1120. [Google Scholar]
- Reichl, S.; Muller-Goymann, C.C. The use of a porcine organotypic cornea construct for permeation studies from formulations containing befunolol hydrochloride. Int. J. Pharm. 2003, 250, 191–201. [Google Scholar] [CrossRef]
- Reichl, S.; Bednarz, J.; Muller-Goymann, C.C. Human corneal equivalent as cell culture model for in vitro drug permeation studies. Br. J. Ophthalmol. 2004, 88, 560–565. [Google Scholar] [CrossRef]
- Mazzoleni, G.; Di Lorenzo, D.; Steimberg, N. Modelling tissues in 3D: The next future of pharmaco-toxicology and food research? Genes Nutr. 2009, 4, 13–22. [Google Scholar]
- Reichl, S.; Dohring, S.; Bednarz, J.; Muller-Goymann, C.C. Human cornea construct HCC—An alternative for in vitro permeation studies? A comparison with human donor corneas. Eur. J. Pharm. Biopharm. 2005, 60, 305–308. [Google Scholar]
- Sheasgreen, J.; Klausner, M.; Kandarova, H.; Ingalls, D. The mattek story—How the three rs principles led to 3D tissue success! Altern. Lab. Anim. 2009, 37, 611–622. [Google Scholar]
- NIH. Available online: http://www.clinicaltrials.gov/ct2/results?term=acellular+cornea &Search=Search &Search=Search (accessed on 5 March 2013).
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wilson, S.L.; Sidney, L.E.; Dunphy, S.E.; Rose, J.B.; Hopkinson, A. Keeping an Eye on Decellularized Corneas: A Review of Methods, Characterization and Applications. J. Funct. Biomater. 2013, 4, 114-161. https://doi.org/10.3390/jfb4030114
Wilson SL, Sidney LE, Dunphy SE, Rose JB, Hopkinson A. Keeping an Eye on Decellularized Corneas: A Review of Methods, Characterization and Applications. Journal of Functional Biomaterials. 2013; 4(3):114-161. https://doi.org/10.3390/jfb4030114
Chicago/Turabian StyleWilson, Samantha L., Laura E. Sidney, Siobhán E. Dunphy, James B. Rose, and Andrew Hopkinson. 2013. "Keeping an Eye on Decellularized Corneas: A Review of Methods, Characterization and Applications" Journal of Functional Biomaterials 4, no. 3: 114-161. https://doi.org/10.3390/jfb4030114
APA StyleWilson, S. L., Sidney, L. E., Dunphy, S. E., Rose, J. B., & Hopkinson, A. (2013). Keeping an Eye on Decellularized Corneas: A Review of Methods, Characterization and Applications. Journal of Functional Biomaterials, 4(3), 114-161. https://doi.org/10.3390/jfb4030114




